Abstract
Studies suggest that tomato and soy foods may contribute to a lower risk of certain cancers. We developed a novel soy germ tomato juice to be used in controlled cancer prevention trials. This study describes an initial test of compliance, phytochemical bioavailability, and effects on biomarkers of blood lipids. Healthy men and women (n = 18) consumed a soy germ-fortified juice daily (300 mL supplying 66 mg isoflavones and 22 mg lycopene) for 8 wk. A single-dose bioavailability study was completed on day 1 and isoflavones in plasma and urine, and lycopene in the plasma, were measured. All subjects completed the trial, with 97.7% ± 3.5% (mean ± SD) of the scheduled juice consumed. No adverse effects were documented. The postprandial study indicated that 3.1% ± 2.3% of lycopene was absorbed and that 49.3% ± 12.1% isoflavones ingested were recovered in 24-h urines. Lycopene plasma concentration changed from 0.60 ± 0.22 to 1.24 ± 0.30 μmol/L during 8 wk of consumption. Juice consumption significantly improved resistance of LDL+VLDL-C to Cu2+-mediated oxidation (P = 0.039), HDL-C (47.3 ± 15.8 to 51.7 ± 14.8 mg/dL, P < 0.001), and the ratio of total-C/HDL-C (4.25 ± 1.59 to 3.63 ± 1.16, P < 0.001) at 8 wk. A well-characterized soy-fortified tomato juice can be produced in large scale for multiinstitutional long-term cancer prevention trials and showed excellent compliance with no toxicity, while demonstrating absorption of biologically active phytochemicals.
INTRODUCTION
Based on extensive epidemiologic and laboratory investigations, governmental agencies and expert committees recommend a diet rich in fruit and vegetables as a foundation for public health guidelines for cancer prevention. Among the plant food items that have received increased attention are tomatoes and their products, as their consumption has been associated with a number of health benefits, such as decreased incidence of cardiovascular diseases (1,2) and prostate and other cancers (3–5). Among the possible bioactive agents responsible for these observations is lycopene, the predominant carotenoid in tomatoes. This lipophilic antioxidant compound has also been discussed for its use as a pharmacologic agent and examined as a chemopreventive drug; however, stronger evidence supports an anticancer benefit of whole tomato products with their diverse array of phytochemicals. For example, rodent studies suggested that whole tomato powder may be more effective for the inhibition of prostate carcinogenesis (3,6) and tumorigenesis (7) than pure lycopene, perhaps due to added or synergistic effects with other compounds present in tomato. In addition to their potential beneficial health effects, tomato and its products can mask undesirable flavors, such as found in soy (8) or other potential anticancer ingredients. Processed and homogenized tomato products such as soup, sauce, and juice provide also relatively high phytochemical bioavailability, particularly for carotenoids when provided with a source of lipid (9,10).
Similar as for tomatoes, it has been hypothesized that consumption of isoflavone-rich soy foods are associated with reduced risk for certain cancers, especially prostate cancer (11–13), as well as other diseases (14,15); albeit more recently, adverse effects have also been reported, especially with respect to tumor progression in breast cancer (16). However, consumption of soy foods in Western cultures remains very low compared to Asian countries, where consumption of soy isoflavones is about 10 to 100 times higher, providing approximately 20–100 mg/d (17). Attempts to increase soy consumption by making them more appealing include soy incorporation into bread (18), beverages (19), and cookies (20). Unfortunately, addition of high amounts of soy to foods often results in a characteristic bitter soy flavor, which is not desirable (21).
Thus, both soy and tomato have been discussed for their employment in cancer prevention trials. Unfortunately, randomized intervention studies for cancer prevention using specific fruits and vegetables or their components targeting specific cancers are rare, and quantitative estimates of individual or societal benefits remain speculative. This is in contrast to chemoprevention strategies using established principles of clinical pharmacology, with pure agents, precise dosing, and pharmacokinetic validation of exposure, some of which have shown great promise in carefully controlled human studies (22,23). Dietary intervention studies suffer from obstacles such as participant education to achieve compliance with assigned dietary changes, variations in bioactive compounds, and their imprecise quantification. Our laboratory has considered many of these concerns during the development of a phytochemical rich tomato product, combined with soy components, designed to serve as an intervention agent for clinical trials.
In this study, we investigated the combination of soy and tomato components in a single juice-based product for its use in future clinical trials targeting disease endpoints and safety. Juice was chosen as it showed to be a popular approach to increasing tomato intake (24), able to provide 1) precisely defined components, 2) convenient size that can be incorporated into a daily routine, and 3) stability over time. The present study describes the short-term compliance and safety of a soy-rich tomato juice. We describe the bioavailability of tomato carotenoids based upon postprandial examinations and the overall plasma response over 8 wk. Soy components were measured in blood and urine as an indicator of absorption and metabolism. In addition, changes in blood lipids and oxidative stress markers over the 8-wk intervention period were monitored to assess biological activity.
MATERIALS AND METHODS
Production of Soy-Fortified Tomato Juice
The soy-fortified tomato juice (Table 1) was developed and produced at The Ohio State University (OSU) pilot plant of the Food Science and Technology Department, following guidelines of good manufacturing practice. A series of preliminary studies were conducted using a variety of food components in order to establish a juice product with acceptable sensory characteristics and properties that would allow future large-scale production for clinical studies. All individual ingredients used were considered “generally recommended as safe” by regulatory agencies and the food industry (25). After screening and sensory testing of a variety of soy components, soy germ was chosen as a source of phytochemcials due to its lower viscosity compared to soy protein or similar components available, thus making the product more palatable in our taste-testing panels (data not shown). In addition, we observed that adding soy germ to tomato juice improved its rheological properties (26).
TABLE 1.
Isoflavone and carotenoid content of the developed soy germ-fortified tomato juice
| Phytochemical | Concentration* (μmol/100 mL) |
|---|---|
| Soy isoflavones | |
| Daidzein | 3.16 ± 0.03 |
| Daidzin | 29.05 ± 0.33 |
| Acetyldaidzin | 9.99 ± 0.01 |
| Malonyldaidzin | 1.06 ± 0.04 |
| Glycitein | 8.92 ± 0.11 |
| Glycitin | 15.69 ± 0.17 |
| Acetylglycitin | 4.78 ± 0.13 |
| Malonylglycitin | 0.22 ± 0.01 |
| Genistein | 0.97 ± 0.03 |
| Genistin | 8.25 ± 0.08 |
| Acetylgenistin | 0.05 ± 0.01 |
| Malonylgenistin | 0.32 ± 0.02 |
| Total Isoflavone Content | 82.46 ± 1.20 |
| Tomato carotenoids | |
| All-trans-lycopene | 12.10 ± 0.29 |
| Total-cis-lycopene | 1.54 ± 0.12 |
| Total lycopene | 13.64 ± 0.21 |
| β-carotene | 0.49 ± 0.04 |
| Sum (lycopene + β-carotene) | 14.13 ± 0.25 |
Mean ± SD (n = 6).
The tomato juice was prepared from tomatoes grown by the Department of Horticulture and Crop Science at OSU. Among the hundreds of varieties of tomatoes available, we selected the high carotenoid tomato variety FG99-218, homozygous for the dark green gene and for the old-gold crimson gene, which has excellent juice-producing properties and grows successfully at locations in Ohio. Plants were grown with 30 cm within-row spacing and 1.5 m between-row spacing at OSU North Central Agricultural Research Station (near Fremont, OH), following standard practices. Tomato juice was produced by a hot-break treatment (27), using fully ripe, sorted, washed, chopped, and blended tomatoes that were filled in salt-added cans (white/pln, 300 × 407; Ball Corporation, Columbus, OH), with 1 salt tablet (Canning Tablets for Tomatoes, Morton, Chicago, IL) added per can. Cans were then steam sealed and retorted (Food Machine and Chemical Corporation, Hoopeston, IL) at 105°C for 30 min. The final product was produced following the addition of 0.2% lemon juice powder (Blue California, Tomas, CA), 1.2% sucrose, and 1% extra virgin olive oil (Bertolli, Secaucus, NJ) to enhance taste and palatability, and 1.5% soy germ (Soylife Complex SG, Acatris, Inc., Minneapolis, MN) on weight basis. The mixture was then homogenized at 60°C for 10 min and then retorted at 100°C for 15 min in an agitated steam-jacketed vessel and hot-filled into small cans (6-oz cans). All constituents used for the juice production originated from single batches of ingredients. After sealing and cooling in an upside-down position, this product was stored at room temperature until the beginning of the study. The final juice product, per 100 mL volume, contained 96.2% tomato juice and contained 34 kcal, 1.1 g protein, 1.1 g fat, 5.6 g carbohydrate, and <0.6 g dietary fiber. The final isoflavone and lycopene content of the soy-tomato juice were 22 mg/100 mL and 7.3 mg/100 mL, respectively. Thus, two 6-oz cans containing 294 mL of juice provided 66 mg isoflavones as aglycon equivalents and 22 mg lycopene.
Subjects
The human trial was approved by the Institutional Review Board (IRB) of OSU. Healthy subjects (9 men, 9 women) were recruited for the study. Mean age was 28 ± 5 yr (range 21–40) and mean body mass index (BMI) 25.4 ± 4.1 kg/m2 (range 17.5–33.9). Exclusion criteria for participating in the study were as follows: pregnancy, lactation, any history of chronic malabsorption or metabolic disease causing digestive disturbances, smoking, following a therapeutic diet, taking antibiotics within the past 6 mo, consuming dietary supplements containing carotenoids or soy isoflavones, history of alcohol or drug abuse, consumption of medications other than for oral contraception, thyroid disease, and anemia (hematocrit <35% and/or hemoglobin <12.0 g/100 mL).
Study Design
The 9-wk study was a single-arm phase II trial with a 1-wk washout and a subsequent 8-wk intervention period. During washout (Week -1 to Week 0), individuals consumed a carotenoid and isoflavone restricted diet devoid of colored fruits and vegetables, and foods or processed products containing soy isoflavones. During Week 0 to 8, each subject consumed 2 of the 6-oz cans of soy-fortified tomato juice/day, a total of 66 mg isoflavones as aglycon equivalents and 22 mg lycopene per day. The subjects were permitted to consume their usual diets but were asked to refrain from foods containing soy and lycopene for the duration of the study. Blood samples were collected on the morning starting the washout (Week 1), at the end of the washout (Week 0), and after Weeks 4 and 8 of consuming the juice. Blood samples were procured and processed at the General Clinical Research Center (GCRC) at OSU after a 12-h overnight fast. In addition, complete 24-h urine samples were collected starting the day prior to the subject’s appointment at the GCRC on Week -1, 4, and 8, and for the first 24 hrs after starting consuming the product (Week 0). In addition, weight, pulse, and blood pressure were measured at each appointment at the GCRC.
The postprandial kinetic component of the study was performed on the first day of product consumption after washout. Subjects were monitored at OSU GCRC for 10 h, starting at 8 a.m. Venous blood samples were drawn immediately before consumption of the test meal, and at 2, 3, 4, 5, 6, 8, and 10 h following the test meal. Test meals served as breakfast (8 a.m.) after an overnight fast, consisted of 259 ± 15 mL soy-fortified tomato juice (with variation due to residual juice in glasses), 50 g white wheat bread with 22 g cream cheese, and 200 g water. The meal provided 369 kcal, 11 g protein, 19 g fat, and 41 g carbohydrates and was consumed within 20 min under observation. After 4 h, lunch was provided, consisting of 200 mL mushroom soup, 1 banana, 1 can caffeine-free soft drink, 1 turkey sandwich (50 g white wheat bread, 85 g turkey, 1 portion Italian dressing of 12 g), providing 718 kcal, 27 g protein, 23 g fat, and 103 g carbohydrates. No additional foods or beverages, besides water, were allowed during the 10-h stay.
Compliance
A screening questionnaire was filled out by each subject prior to the study to assess anthropometric data, age, race, exercise habits, allergies and chronic diseases, typical lifestyle and dietary habits, which included smoking and drinking habits, taking dietary supplements, use of antibiotics, consumption of isoflavone and carotenoid containing fruits and vegetables, or following specific diets, as described previously (28). A notebook, with calendar and appointments, was provided to participants along with a compliance form called “Keeping Score” that was filled out daily during the study, to record the time and amount of soy-fortified tomato juice consumed. In addition, participants were educated regarding foods containing soy components and were instructed to avoid foods containing soy isoflavones during the entire study. The daily compliance worksheet provided participants a mechanism to record the frequency and amount of consumption of the soy and tomato product.
Blood and Urine Sampling
Venous blood samples (3 × 10 mL) were placed (Week 0) into prelabeled 10 mL EDTA vacutainer tubes (BD, Franklin Lakes, NJ), and plasma was immediately separated from blood cells by centrifugation at approximately 1000 × g for 10 min at 4°C (Bamon/IEC division, Needham, MA). Blood cells for isoflavone analysis were washed 3 times with physiological saline (0.9%) to obtain the erythrocyte fraction and stored at −80°C for further analysis. Aliquots of plasma for determination of carotenoids in the TRL fraction, and Cu2+-mediated oxidation of the LDL+VLDL-C were used immediately for analyses. The remaining plasma was stored at −80°C for analysis.
Complete 24-h urine pools were collected in preweighed 3-L polyethylene terephthalate (PET) containers (Fisher Scientific, Pittsburgh, PA). Each container contained 1 g ascorbic acid and 2 g boric acid as preservatives to prevent bacterial growth and oxidation of unstable compounds. Following collection, containers were reweighed, volume derived, and 50 mL aliquots of each 24-h urine pool were stored at −80°C for later analysis.
Isoflavone Analysis
All chemicals used were of analytical grade or superior. Plasma and urine were analyzed based on a modified published method (29). In brief, 100 μL ascorbic acid solution (10 g/100 mL), 0.4 mL acetate buffer (pH 5.5), and 100 μL of a suspension of glucuronidase/arylsulfatase (Cat. No. 127060, Hoffman La Roche, Basel, Switzerland) were added to 2.0 mL urine and 1.5 mL plasma aliquots. This was followed by 3-h incubation in a shaking water bath at 37°C under light exclusion. After extracting the aglycones twice with 5 mL diethylether, the extracts were evaporated under nitrogen and reconstituted in 350 μL methanol.
Juice samples (2 mL) and washed red blood cells (0.5 mL) were extracted as described previously (18) with 7 and 4.5 mL acetonitrile, respectively, in graded centrifuge vials, to which 100 μL 1 mol/L HCl was added. In addition, 100 μL of ascorbic acid solution (10 g/100 mL) was also added to the red blood cells. Dried blood cell sample extracts were reconstituted in 0.9 mL acetate buffer (pH 5.5), 100 μL ascorbic acid (10 g/100 mL), and 100 μL glucuronidase/arylsulfatase and treated as described above for plasma samples. Juice extracts were reconstituted in 350 μL methanol/water 80:20 (v/v). All samples were stored at −80°C until analysis.
Isoflavones were separated by HPLC (Waters 2996, Milford, MA) and detected either by photo-diode-array detector (Waters, 2996), or (for plasma and red blood cells) by mass spectrometry (Quattro Ultima, Micromass, Ltd., Manchester, UK). Standards were purchased from Sigma [daidzein, genistein, glycitein, equol, daidzin, genistin, glycitin (St. Louis, MO); Plantech: dihydrodaidzein (DHD), dihydrogenistein (DHG), and O-desmethylangolensin (O-DMA) (Reading, UK)] or LC-Labs [acetyldaidzin, acetylgenistein, acetylglyciten, malonlylgenistein, malonylgicitin, malonyldaidzin (Woburn, MA)]. Quantification was based on external standard calibration curves of each respective compound. An internal standard, 2′,4′ dihydroxy-2-phenylacetophenone (Indofine, Hillsborough, NJ) was used during extraction and analysis as a control. The internal standard (50 μL containing 8.2 nmol) was added to individual samples prior to sample preparation. Isoflavones in juice were analyzed as described previously (18). For all other samples, a Hydrobond RP-18 (150 × 3 mm, 3-μm particle size, Mac-Mod, Chadds Ford, PA) HPLC column was used. Mobile phase consisted of a mixture of MeOH/ACN/1% aqueous acetic acid (10:15:75, v/v), which was changed to 15:20:65 within 5 min, then to 20:20:60 at min 10, then to 25:25:50 at min 13, changed to 45:45:10 at min 14, changed to 45:50:5 at min 18, changed to 10:15:75 at min 20, holding this for the next 5 min (total 25 min). Flow rate was 0.55 mL/min, injection volume was 15 μL, and temperature was 30°C.
For LC-MS/MS analyses, selected reaction monitoring was conducted using the following transitions that had been determined during positive ion electrospray ionization: DHD (257 → 123), daidzein (255 → 199), glycitein (285 → 270), DHG (273 → 123), equol (243 → 123), genistein (271 → 153), and O-DMA (259 →121). An ionization energy of 25 eV was used for all analytes.
Carotenoid and Tocopherol Analysis
Lycopene, its cis-isomers, beta-carotene, and α-, γ-, and δ-tocopherols in plasma samples from the 8-wk intervention period, juice, and standards (Sigma) were analyzed following a modified method described by Ferruzzi et al. (30). Lycopene in the TRL fractions of plasma obtained during the postprandial part of the study was isolated by ultracentrifugation and extracted as described earlier (31). Detection and quantification were achieved by HPLC in combination with a coulometric detector (Coul Array Detector, ESA, Chelmsford, MA). Eight coulometric channels were set to 60-mV increments, starting from 200 mV. For HPLC separation, a YMC carotenoid C-30 column (150 × 4.6 mm, 5-μm particle size, Waters) was used in combination with gradient elution: Mobile phase A was methanol:MTBE:water:1 mol/L ammonium acetate (88:5:5:2), B was methanol:MTBE:water:1 mol/L ammonium acetate (17:78:3:2), starting with 100%A, switching to 85% B within 9 min, switching to 100% B until min 14, keeping this until min 20.5, switching back to A until min 22, holding this until min 27. Quantification was based on external calibration curves, using their specific absorption coefficients.
Analysis of Blood Biomarkers
Total cholesterol (total-C), HDL-C, LDL-C, triacylglycerols (TG), and urine creatinine were determined based on spectrophotometric methods using a Synchron LX 20 (Beckman Coulter, Inc., Fullerton, CA). C-reactive protein was measured by a Beckman Coulter Image based on nephelometry. Hematocrit and hemoglobin were measured using a Beckman Coulter LH755. All analyses followed the manufacture’s recommendations. A–I and B apolipoproteins were analyzed only prior to washout and at the end of the study and sent to Mayo Medical (Rochester, MN). Determination of Cu2+-mediated oxidation of LDL+VLDL-C was carried out as described earlier (32). Concentrations of LDL+VLDL-C were also determined during this analysis, using a commercial cholesterol enzymatic assay (Cholesterol SL-Assay, Diagnostic Chemicals, Ltd., Oxford, CT).
Total plasma antioxidant capacity and that of a lipid plasma extract were measured similar as described by Popov et al. (33). To obtain the lipid extract, 150 μL plasma was mixed with 150 μL ethanol and 300 μL water. After extraction with 2 times 2 mL hexane/acetone (3:1, v/v), the extract was dried under nitrogen and resolubilized in 200 μL hexane. A 15 μL aliquot was then analyzed in a Photochem detector (Analytic Jena AG, Jena, Germany). The luminescence intensity was compared to a standard compound (Trolox). For total antioxidant capacity of plasma analysis, 200 μL plasma was diluted with 800 μL water and the mixture directly measured by the Photochem instrument and compared to ascorbic acid.
Statistical analysis
Data were analyzed by SPSS 14.0 (SPSS, Inc., Chicago, IL), using a general linear multivariate model, with observed parameters (such as isoflavone and carotenoid concentrations in the respective biological fluids, biometrical data and biological markers) as dependent variables, and treatment time and subject as independent fixed factors. The influence of gender, equol producer status, initial cholesterol status (either >200 mg/dL or <200 mg/dL), initial LDL-C, age, BMI, and area under curve (AUC) response of lycopene and isoflavones (determined by trapezoidal approximation) were studied by including them into the model as either fixed factors (non-scaled factors) or covariates (scaled factors). Fisher F tests were followed by post hoc tests (Tukey’s) for treatment time to compare effects for different durations (−1, 0, 4, 8 wk). Assumption of normality was verified by Q-Q plots and Kolmogorov-Smirnoff tests, and homogeneity of variance by box plots and Levene’s test. Nonnormal distributed data was log transformed. A P value of <0.05 was considered statistically significant (2-sided). Unless otherwise stated, all presented results are arithmetic means ± SD, and correlation coefficients are Pearson.
RESULTS
Compliance and Tolerance
All subjects completed the 9-wk study. Compliance questionnaires obtained after the washout period indicated excellent adherence to the dietary restrictions. The participants reported no obstacles for juice consumption at the requested rate during the intervention trial. The daily compliance questionnaires indicated that 97.7 ± 3.5% of the juice cans provided were consumed. The remaining cans were returned by the subjects for counting and were in agreement with daily records. No adverse effects using standard NIH-NCI toxicology criteria (34) were reported while consuming the juice for 8 wk.
Isoflavone and Lycopene Kinetics and Metabolism Following a Single Meal
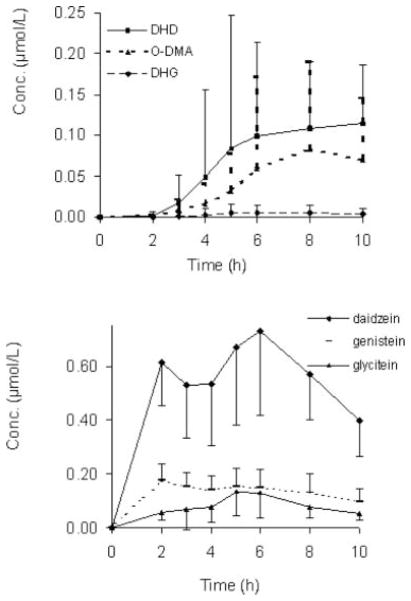
All major isoflavones (daidzein, genistein, and glycitein), except for their bacterial metabolites, were detected in plasma within 2 h of test meal consumption and remained elevated compared to time zero throughout the 10-h sampling period (Fig. 1). The metabolites were detected at 3 h post-intake and remained elevated throughout the next 7-h sampling period. DHD was the most abundant metabolite in plasma and urine, while little DHG was detected (Fig. 1, Tables 2 and 3). When adjusted for the amount of isoflavones ingested, genistein and daidzein showed considerably higher plasma responses, 0.053 ± 0.018 and 0.048 ± 0.011 μmol.h/(L.μmol) compared to glycitein, 0.010 ± 0.005 μmol.h/(L.μmol).
FIG. 1.
Plasma appearance curves for daidzein, genistein, glycitein, dihydrodaidzein (DHD), dihydrogenistein (DHG), and O-desmethylangolensin (O-DMA) after consuming 255 mL soy-fortified tomato juice containing 210.2 ± 12.4 μmol isoflavones at breakfast (8 a.m.), followed by a standardized lunch (12:30 p.m.). Error bars represent SD (n = 18).
TABLE 2.
Mean peak concentrations (Cmax) and baseline corrected area under the curve (AUC) responses after consuming soy germ-fortified tomato juice for breakfast (8 a.m.)*
| Compound | AUC (0–10 h) (nmol.h/L) | Cmax (nmol/L) | Absorption† or urinary recovery‡ (% intake) |
|---|---|---|---|
| Lycopene | |||
| All-trans-lycopene | 34.4 ± 23.0 | 10.0 ± 6.1 | N/A§ |
| Total cis-lycopenes | 49.8 ± 33.7 | 15.0 ± 7.6 | N/A |
| Total lycopene | 84.2 ± 56.3 | 27.7 ± 13.6 | 3.1 ± 2.3 (range 0.4–8.1) |
| Soy isoflavones | |||
| Daidzein | 5290 ± 1260 | 730 ± 310 | 69.5 ± 18.1|| |
| Genistein | 1300 ± 440 | 180 ± 60 | 14.2 ± 6.2 |
| Glycitein | 770 ± 370 | 130 ± 90 | 31.2 ± 14.7 |
| Dihydrodaidzein | 630 ± 620 | 115 ± 72 | N/A |
| Dihydrogenistein | 30 ± 50 | 5.0 ± 8.6 | N/A |
| O-desmethylangolensin | 380 ± 510 | 82 ± 108 | N/A |
| Total isoflavones | 8406 ± 2134 | 1180 ± 520 | 49.3 ± 12.1 |
Values are based on plasma triacylglycerol-rich lipoprotein fraction for lycopene and its isomers, and based on plasma for isoflavones and its metabolites.
255 mL soy-fortified tomato juice containing 210.2 ± 12.4 μmol isoflavones, and 34.6 ± 2.1 μmol lycopene. All results represent mean ± SD.
For lycopene and isomers.
For isoflavones.
Not applicable.
Includes also daidzein metabolites O-desmethylangolensin (O-DMA) and dihydrodaidzein (DHD).
TABLE 3.
Changes in isoflavone concentration in 24-h urine pools and plasma carotenoids in 18 subjects at enrollment, at Day 1 after the 7-day washout and first day of feeding, and at 28 days (4 wk) and 56 days (8 wk) after initiation of the daily intake of juice*
| Compound(s) | Day −1† | Day +1† | Day 28 | Day 56 |
|---|---|---|---|---|
| Soy isoflavones | ||||
| Total isoflavones (μmol) | 9.0 ± 23.2A‡ | 103.7 ± 26.7B | 123.2 ± 36.2BC | 132.7 ± 38.1C |
| Daidzein (μmol) | 5.0 ± 12.9A | 53.4 ± 13.7B | 52.4 ± 18.5B | 59.0 ± 26.0B |
| Genistein (μmol) | 1.2 ± 3.2A | 3.4 ± 1.4B | 4.0 ± 1.7B | 4.4 ± 2.6B |
| Glycitein (μmol) | 0.5 ± 1.3A | 23.6 ± 11.2B | 20.8 ± 7.6B | 21.1 ± 5.4B |
| Equol (μmol) | nd§ | nd | 6.2 ± 12.3A | 13.7 ± 19.5A |
| Dihydrodaidzein (μmol) | 1.5 ± 5.1A | 20.4 ± 15.5B | 27.1 ± 14.7B | 23.2 ± 16.2B |
| O-DMA|| (μmol) | 0.8 ± 2.3A | 2.9 ± 6.3A | 12.7 ± 11.2B | 11.3 ± 8.8B |
| Tomato carotenoids | Day 7 | Day 0** | Day 28 | Day 56 |
| Total lycopene (μmol/L) | 0.60 ± 0.22A | 0.44 ± 0.16B | 1.28 ± 0.27C | 1.24 ± 0.30C |
| 5-cis lycopene (μmol/L) | 0.27 ± 0.10A | 0.17 ± 0.07B | 0.57 ± 0.12C | 0.54 ± 0.14C |
| All-trans-lycopene (μmol/L) | 0.15 ± 0.06A | 0.09 ± 0.04B | 0.23 ± 0.06C | 0.23 ± 0.07C |
| Beta-carotene (μmol/L) | 0.30 ± 0.17A | 0.22 ± 0.13B | 0.43 ± 0.16C | 0.39 ± 0.16C |
294 mL soy-fortified tomato juice/d containing 242 μmol isoflavones and 40 μmol lycopene. All values given as mean ± SD.
Urinary isoflavone values obtained from a 24-h urine collection for the day prior to starting the study, for the day following the test meal, and at 4 and 8 wk.
The values for carotenoids are obtained after the 7 day washout prior to test meal intake.
Values in a row not sharing the same superscript are statistically significant different (P < 0.05, Tukey’s).
nd indicates not detected.
O-desmethylangolensin.
Similarly, lycopene isomers in the TRL fraction showed a significant increase over baseline in the 2- and 3-h samples following juice intake, with a downward trend at 4-h postprandial, followed by a second peak an hour after the lycopene-free midday meal (Fig. 2). Mean fractional absorption of lycopene (Table 2) from baseline corrected AUC (nmol.h/L) was calculated using an equation published by O’Neill and Thurnham (35):
with being the half-life of carotenoids in chylomicrons (0.192 h), plasma volume (mL) calculated as 927 + 31.47 × mean body weight (kg), oral dose the amount lycopene (nmol) in the test meal, and the molecular weight of lycopene being 536.9 g/mol.
FIG. 2.
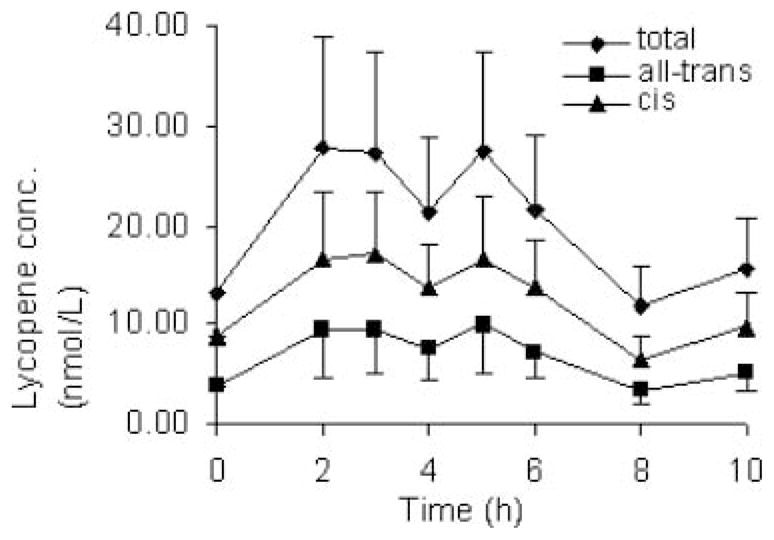
Appearance of lycopene in plasma-TRL fractions after consumption of 255 mL soy-fortified tomato juice containing 34.6 ± 2.1 μmol lycopene at breakfast (8 a.m.), followed by a standardized lunch (12:30 p.m.). Error bars represent SD.
Even though 89% of lycopene in the soy-fortified tomato juice was present in the all-trans form, the plasma TRL fractions indicated that 59.3 ± 4.4% lycopene present were cis-isomers (with 51.0 ± 5.5% 5-cis lycopene of total lycopene). The fraction of the sum of cis-isomers of total lycopene in plasma TRL dropped immediately after test meal consumption, from 69.2 ± 4.1% (Hour 0) to 63.9 ± 3.3% (Hour 2, P < 0.001, Student paired, 2-tailed t-test), and slightly increased thereafter until hour 10 (66.6 ± 2.6%).
Isoflavone and Carotenoid Concentrations in Biological Samples
The total and individual isoflavone concentrations measured in 24 h urine changed significantly over the 4 sampling points (Table 3). Urine isoflavones after 7 days on a soy-free diet (the 24 h prior to the initial exposure to juice) were detected in only 4 out of 18 subjects, whereas all participants showed detectable isoflavones in the urine during the subsequent 24 h period following consumption of juice on Day 1. The serum and urinary isoflavones reflected the pattern found in the juice (Tables 1 and 3). The major metabolite detected throughout the study and in all subjects was DHD. At the 4-wk interval, we observed that 5 (3 women, 2 men) of the 18 subjects (28%) were equol producers, defined by the presence of equol in the urine at a concentration exceeding >1,000 μg/L (36). By 8 wk, 2 additional subjects (1 man, 1 women) became equol producers (39%). There were no significant differences between equol producers and nonequol producers at the onset and during the study for any observed parameters, except that equol producers had significantly higher plasma lycopene concentrations (P = 0.019, Fisher F test) and tended to absorb more lycopene (P = 0.064, Fisher F test). The highest O-DMA concentrations in urine (up to 38 μmoL/24 h urine) were found in subjects not producing equol. No isoflavones or isoflavone metabolites were detected in the red blood cells at any time point. No significant correlation was found between isoflavone concentrations in 24-h pools and the observed blood lipid and oxidative stress markers, with the exception of weak, negative correlations of genistein and total-C (R = −0.282, P = 0.016) and genistein and LDL-C (R = −0.259, P = 0.028).
There was a significant decrease in total lycopene, all-trans lycopene, and 5-cis lycopene plasma concentrations during the 7-day washout, and a nearly 3-fold increase in serum concentrations between Week 0 to Weeks 4–8 (Table 3). The predominant lycopene isomer in plasma at both Weeks 4 and 8 was 5-cis lycopene (45 ± 2 and 43 ± 3% of total plasma lycopene, respectively), and its relative abundance did not change significantly during the study, with exception to a slight decrease after washout [Week 0, where it was 39 ± 2% (P < 0.001, Tukey’s), compared to Week −1 (45 ± 3%)]. The percentage of all-trans lycopene of total lycopene did not change significantly during the study, with exception to Week -1, where it was higher [25 ± 5% versus 18 ± 2% (Weeks 4 and 8), P < 0.001, Tukey’s)]. Interestingly, a nearly 2-fold increase in β-carotene was also observed following juice consumption (P < 0.05).
Biomarkers
Age, gender, BMI, equol producing status, and initial total-C and LDL-C did not interact significantly in the statistical model with time. This suggests that there was no effect on the outcomes reported in Table 4, with the exception that the increase in urinary daidzein and total isoflavones were less in subjects with higher BMI (P = 0.007 and 0.035, respectively, Fisher F test), and that the decrease in LDL-C during washout and the first 4 wk of juice consumption combined was more pronounced in men than in women (P = 0.049, Fisher F test). When results were stratified for gender, only males (P = 0.042, Fisher F test) showed reduced plasma LDL-C concentrations during this period. However, the sum of VLDL and LDL-C measured in parallel dropped significantly (Table 4). In addition, Cu2+-mediated oxidation of LDL and VLDL-C improved significantly from Week -1 to Week 8, consistent with an antioxidant effect of juice consumption.
TABLE 4.
Observed anthropometric parameters and blood lipids in 18 subjects measured during the 9-wk study, including a 1-wk washout and an 8-wk consumption period*
| Parameter | t = −1 wk | t = 0 wk | t = 4 wk | t = 8 wk |
|---|---|---|---|---|
| Weight (kg) | 78.9 ± 19.1A† | 78.9 ± 19.0A | 78.8 ± 19.1A | 79.4 ± 19.5A |
| Pulse (beats/min) | 67.2 ± 10.7A | 67.2 ± 10.8A | 68.5 ± 11.6A | 67.4 ± 10.7 A |
| Blood pressure | ||||
| Systole (mmHg) | 112.7 ± 14.4A | 116.7 ± 12.6A | 115.8 ± 12.3A | 115.5 ± 10.9A |
| Diastole (mmHg) | 72.5 ± 8.8A | 69.4 ± 10.0AB | 66.9 ± 8.8B | 69.3 ± 7.9AB |
| Triacylglycerols (mg/dL)‡ | 110.3 ± 57.4A | 104.2 ± 57.5A | 110.1 ± 70.6A | 102.8 ± 55.2A |
| Total-C§ (mg/dL) | 181.0 ± 30.9A | 163.9 ± 23.6B | 176.7 ± 30.2A | 175.7 ± 30.9AB |
| LDL-C (mg/dL) | 111.6 ± 27.0A | 100.8 ± 25.3B | 106.2 ± 25.9AB | 103.5 ± 26.6AB |
| HDL-C (mg/dL) | 47.3 ± 15.8A | 43.1 ± 15.9B | 48.3 ± 13.6AC | 51.7 ± 14.9C |
| Total-C/HDL-C | 4.25 ± 1.59A | 4.35 ± 1.86A | 3.93 ± 1.31AB | 3.63 ± 1.16B |
| VLDL+LDL-C (mg/dL) | 126.8 ± 33.7A | nm$ | 111.8 ± 34.9AB | 110.5 ± 33.4B |
| C-reactive protein (mg/L) | 2.17 ± .2.18A | nm | 1.85 ± 2.11A | 2.60 ± 3.53A |
| Cu2+-mediated oxidation of LDL+VLDL-C (min) | 138.5 ± 18.2A | nm | 145.0 ± 19.3AB | 146.3 ± 14.7B |
| Apolipoprotein A–I (mg/dL) | 154.9 ± 38.1A | nm | nm | 148.6 ± 45.9A |
| Apolipoprotein B (mg/dL) | 76.6 ± 16.4A | nm | nm | 71.8 ± 17.2A |
| 8-iso-PGF2α (ng/mg creatinine)* | 0.359 ± 0.303A | nm | 0.387 ± 0.414A | 0.405 ± 0.324A |
| PLAC|| (nmol/L) | 25.4 ± 11.5A | nm | 22.6 ± 10.1A | 22.7 ± 7.0A |
| PWAC** (nmol/L) | 200.1 ± 54.0A | nm | 200.4 ± 55.8A | 199.3 ± 54.7A |
Including 294 mL soy-fortified tomato juice/d containing 242 μmol isoflavones and 40 μmol lycopene. All values given as mean ± SD.
Values in a row not sharing the same superscript are statistically significant different (P < 0.05, Tukey’s).
Triacylglycerols.
C = plasma cholesterol.
Plasma antioxidant capacity in lipid extract in trolox equivalent units.
Plasma antioxidant capacity in total plasma in ascorbic acid equivalent units.
nm indicates not measured due to limited amount of blood plasma or resources.
DISCUSSION
In the present study, a tomato variety rich in phytochemicals, with growth characteristics suitable for Ohio agriculture, was combined with soy germ to result in a final product with a well-characterized dose of phytochemicals. Tomato profiles can be influenced by both horticultural conditions such as cultivars, soil, growth conditions, and further by food processing techniques, selectively enhancing phytochemical rich fractions or altering their chemistry to favor absorption and bioactivity (37,38). Thus, from a horticultural, food science, and consumer perspective, tomatoes provided an ideal platform for developing a product enriched with anticancer food components.
The initial human study showed that the soy-fortified tomato juice, consumed twice daily (total of 300 ml) for 8 wk, was easily incorporated into a daily diet, allowed excellent compliance, and was very well tolerated by healthy men and women without any documented adverse effects. In general, regulatory agencies consider the consumption of soy isoflavones (39) and tomato carotenoids (40) to be safe over a large range, with few, if any acute side effects. However, a careful documentation of compliance and toxicity is a prerequisite for proposing longer-term studies.
Carotenoids were readily absorbed from the soy-tomato juice. Lycopene was the major carotenoid in the soy-rich tomato juice and was detected in the plasma-TRL fraction within 2 h following a single-dose test meal, which is in agreement with earlier observations with other tomato products (10). Soy-tomato juice provided higher plasma-TRL lycopene concentrations than tomato salsa with avocado (40 mg lycopene) (10) but was comparable to lycopene capsules (38 mg) ingested with a complex meal (35). It has been suggested that the efficiency of lycopene absorption declines with total dose but increases with concomitant fat intake (41). Lycopene showed 2 absorption maxima, one in the immediate hours after the test meal consumption and another one following the subsequent lycopene-free lunch, possibly as lycopene from the first meal remained in the enterocytes until long-chain fatty acids from a subsequent meal enables lycopene packaging into chylomicrons and movement to the venous circulation via lymphatic flow through the thoracic duct.
During the course of the study, concentrations of lycopene and β-carotene in plasma increased approximately 3-fold and 2-fold, respectively, at 4 and 8 wk compared to baseline. Even though baseline values (both prior and following washout) in the present study were comparable to other intervention studies using tomato juice, the resulting increase in plasma lycopene was somewhat higher than reported previously, typically being in the range of 0.5–0.8 μmol/L (42–44). In some (42,43), but not all studies (32,44), these results were attributable to lower lycopene intake. This indicates that the juice provided consistently elevated and relatively high plasma lycopene concentration such as those reported to be correlated with reduced risk of certain cancers (4,5).
The detected presence of carotenoid cis-isomers in vivo, the factors that influence their formation and the biological relevance remain a source of debate. Although 5-cis lycopene was not detectable in the juice, this isomer was the predominant one in the baseline corrected TRL-AUC response. This indicates that conversion must have occurred at some step following ingestion and entry into the circulation. The finding that 41% of total lycopene was in the trans form in the baseline corrected TRL-AUC response following test meal ingestion but was only 18% of total lycopene in whole plasma during the later part of the study (Weeks 4 and 8), supports the hypothesis that lycopene is continuously isomerized in vivo to cis-isomers.
For the native isoflavones daidzein and genistein (or their glycosides), a similar absorption pattern following test meal consumption with 2 maxima was observed, perhaps due to enterohepatic recycling (45), or absorption from the large intestine (46). Alternatively, fat or other components provided by the second meal could have fostered absorption of isoflavones, as suggested earlier (47,48). Contrary to earlier reports indicating highest plasma appearance for genistein (49), we observed that daidzein and genistein were absorbed to a similar extent, while glycitein appearance in plasma was lower, being in line with previous reports (45,50). However, urinary excretion showed a higher recovery of glycitein compared to genistein, also noted by others (45). It is possible that genistein was more rapidly metabolized to undetected compounds such as p-ethylphenol.
Isoflavone concentrations in urine appeared to remain relatively constant at wk 4 and 8. We observed an excretion level which is approximately 2 times higher than typically reported for Asian populations consuming soy containing foods, i.e., ingesting around 20–100 mg isoflavones/d (17). Thus, the soy-tomato juice product achieved excretion profiles similar to populations experiencing lower risks of specific cancers and may provide a convenient way to achieve a similar phytochemical exposure during long-term clinical trials.
The metabolism of soy isoflavones by humans and how it is modulated by genetics, dietary patterns, and host factors remains an active and complex area of investigation. We observed that 5 of the 18 subjects were equol producers after 4 wk of juice consumption, increasing to 7 subjects during 8 wk of consumption. This demonstrates that subjects do change their isoflavone metabolic status, also suggested in other studies (51,52). As equol may exhibit relatively strong estrogen activity compared with other isoflavonoids (36), it has been speculated that equol producers may have unique biological effects—some potentially beneficial (bone health in women), while others detrimental, such as the increased tumorigenesis and breast cancer formation (16), albeit this is controversially discussed (53). Additional work, such as more long-term studies in this area, is needed to judge on the potential health benefits vs. adverse effects.
Although the main goals of this initial study with soy-tomato juice were compliance, safety, and metabolism, we also assessed several biological responses. Following consumption of soy tomato juice, biological changes observed included increased HDL-C, decreased ratio of total-C/HDL-C and improved VLDL+LDL-C resistance to Cu2+-mediated oxidation. The observed mean increase in plasma HDL-C (9%) in the present study after 8 wk of intervention was in a similar range to that reported in a meta-analysis summarizing soy feeding trials (14). It was also found that LDL-C decreased to a greater extent in men than in women. It has been reported that the cholesterol reducing effect of soy would be more pronounced in subjects with hypercholesterolemia (14,54). One possible explanation is that the slightly higher cholesterol concentrations in men at the onset of the study responded stronger to the intervention.
Both lycopene (9) and isoflavones (47) have reported antioxidant properties in chemical assays and some data suggests the potential to exhibit similar properties in vivo (55,56). It thus remains speculative which compounds in the soy-fortified tomato juice were responsible for the improvements in the blood lipid profile and the antioxidant status as measured by the LDL+VLDL-C oxidation. Consumption of tomato products has been shown to reduce LDL-C oxidation ex-vivo (32). Supplementing soy appeared to have a similar benefit, although isolated isoflavones typically failed to be effective (57,58). It has been shown that incorporation of isoflavones into LDL-C particles is relatively modest (58) in contrast to lycopene, a very hydrophobic compound (1). In other studies, isolated soy isoflavones failed to exhibit beneficial effects on plasma cholesterol concentrations (14,57). It has been suggested that both the presence of soy protein and isoflavones in soy products are required to be effective (14). In our study, soy protein intake was only about 4 g/day, suggesting that other factors were responsible for the observed effects on blood lipids. However, the fact that isolated compounds often failed to be effective highlights the importance of consuming whole foods containing a diverse array of components rather than supplementing individual compounds alone (59).
In conclusion, this study demonstrates that a newly developed functional food, a soy germ-fortified tomato juice, was palatable, convenient, safe to consume, and well tolerated with no observed adverse effects. Furthermore, we demonstrated that lycopene and isoflavones were readily absorbed, remained at relatively high levels in biological fluids similar to that found in epidemiologic studies associated with lower cancer risk, and significantly improved blood lipid and antioxidant status during an 8-wk feeding trial in healthy subjects. This novel approach demonstrates that tomato juice is an excellent vehicle for the development of defined food products containing active phytochemicals. Future studies using new food products to deliver defined amounts and patterns of bioactive phytochemicals for long-term clinical trials targeting specific cancers are warranted.
Acknowledgments
The work was carried out at the Ohio State University, Columbus, Ohio, both at the General Clinical Research Center and at the Department of Food Science and Technology. We thank Paul Keida for assisting in the development and manufacturing the food product, Dr. Elizabeth Miller Grainger for guidance regarding the clinical trial implementation, and Ms. Valerie DeGroff for technical and administrative assistance.
The work was supported by USDA IFAFS Grant No. 2001-52102-11333; the General Clinical Research Center (GCRC) at Ohio State University (OSU); Grant M01-RR00034 from the National Center of Research Resources, National Institutes of Health, National Cancer Institute R01-CA112632; the Comprehensive Cancer Center, OSU Grant P30-CA16058; and Ohio Agricultural Research and Development Center (OARDC)–AgBiosciences Initiative Grant (2005–2009, for Schwartz, S.J., Clinton, S.K., Failla, M.L., Ralston, R.A.): Center for Advanced Functional Food Research and Entrepreneurship. We appreciate the financial support of H.J. Heinz & Co. to the Prostate Cancer Prevention fund of the James Cancer Hospital and Solove Research Institute.
Contributor Information
Torsten Bohn, Department of Food Science and Technology, The Ohio State University, Columbus, Ohio, USA.
Michelle Blackwood, Division of Hematology and Medical Oncology, Department of Internal Medicine, The Ohio State University, Columbus, Ohio, USA.
David Francis, Department of Horticulture and Crop Science, The Ohio State University, Columbus, Ohio, USA.
Qingguo Tian, Department of Food Science and Technology, The Ohio State University, Columbus, Ohio, USA.
Steven J. Schwartz, Department of Food Science and Technology, The Ohio State University, Columbus, Ohio, USA
Steven K. Clinton, Division of Hematology and Medical Oncology, Department of Internal Medicine, The Ohio State University, Columbus, Ohio, USA
References
- 1.Willcox JK, Catignani GL, Lazarus S. Tomatoes and cardiovascular health. Crit Rev Food Sci Nutr. 2003;43:1–18. doi: 10.1080/10408690390826437. [DOI] [PubMed] [Google Scholar]
- 2.Palozza P, Parrone N, Catalano A, Simone R. Tomato lycopene and inflammatory cascade: basic interactions and clinical implications. Curr Med Chem. 2010;17:2547–2563. doi: 10.2174/092986710791556041. [DOI] [PubMed] [Google Scholar]
- 3.Clinton SK. Tomatoes or lycopene: a role in prostate carcinogenesis? J Nutr. 2005;135:2057S–2059S. doi: 10.1093/jn/135.8.2057S. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Tomato products, lycopene, and prostate cancer: a review of the epidemiological literature. J Nutr. 2005;135:2030S–2031S. doi: 10.1093/jn/135.8.2030S. [DOI] [PubMed] [Google Scholar]
- 5.Fleshner N, Zlotta AR. Prostate cancer prevention: past, present, and future. Cancer. 2007;110:1889–1899. doi: 10.1002/cncr.23009. [DOI] [PubMed] [Google Scholar]
- 6.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, et al. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 7.Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW., Jr The tomato as a functional food. J Nutr. 2005;135:1226–1230. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 8.McDaniel MR, Chan N. Masking of soy protein flavor by tomato sauce. J Food Sci. 1988;53:93–98. [Google Scholar]
- 9.Bohn T. Bioavailability of non-provitamin A carotenoids. Curr Nutr Food Sci. 2008;4:240–258. [Google Scholar]
- 10.Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005;135:431–436. doi: 10.1093/jn/135.3.431. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, DeGroff VL, Clinton SK. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J Nutr. 2003;133:2367–2376. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- 12.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer. 2009;61:598–606. doi: 10.1080/01635580902825639. [DOI] [PubMed] [Google Scholar]
- 13.Jian L. Soy, isoflavones, and prostate cancer. Mol Nutr Food Res. 2009;53:217–226. doi: 10.1002/mnfr.200800167. [DOI] [PubMed] [Google Scholar]
- 14.Zhan S, Ho SC. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am J Clin Nutr. 2005;81:397–408. doi: 10.1093/ajcn.81.2.397. [DOI] [PubMed] [Google Scholar]
- 15.Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr. 2008;138:1244S–1249S. doi: 10.1093/jn/138.6.1244S. [DOI] [PubMed] [Google Scholar]
- 16.Andres S, Abraham K, Appel KE, Lampen A. Risks and benefits of dietary isoflavones for cancer. Crit Rev Toxicol. 2011;41:463–506. doi: 10.3109/10408444.2010.541900. [DOI] [PubMed] [Google Scholar]
- 17.van Erp-Baart MAJ, Brants HAM, Kiely M, Mulligan A, Turrini A, et al. Isoflavone intake in four different European countries: the VENUS approach. Br J Nutr. 2003;89:S25–S30. doi: 10.1079/BJN2002793. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YC, Albrecht D, Bomser J, Schwartz SJ, Vodovotz Y. Isoflavone profile and biological activity of soy bread. J Agric Food Chem. 2003;51:7611–7616. doi: 10.1021/jf034679c. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg FM. Soybeans or soymilk: does it make a difference for cardiovascular protection? Does it even matter? Am J Clin Nutr. 2007;85:927–928. doi: 10.1093/ajcn/85.4.927. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalezgalan A, Wang SH, Sgarbieri VC, Moraes MAC. Sensory and nutritional properties of cookies based on wheat-rice-soybean flours baked in a microwave-oven. J Food Sci. 1991;56:1699–1703. [Google Scholar]
- 21.Schultz S. Pass the tofu tacos. Soy-based foods are disease fighters, but they can taste pretty weird. US News World Rep. 1999;127:77–78. [PubMed] [Google Scholar]
- 22.Terhaar Sive Droste JS, Tuynman JB, Van Dullemen HM, Mulder CJ. Chemoprevention for colon cancer: new opportunities, fact or fiction? Scand J Gastroenterol. 2006;(Suppl 243):158–164. doi: 10.1080/00365520600664284. [DOI] [PubMed] [Google Scholar]
- 23.Katdare M, Kopelovich L, Telang N. Efficacy of chemopreventive agents for growth inhibition of Apc [+/−] 1638NCOL colonic epithelial cells. Int J Mol Med. 2002;10:427–432. [PubMed] [Google Scholar]
- 24.Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TW, et al. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60:145–154. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- 25.FDA. CFR 170.3: Code of Federal Regulations, Title 21. Joint Committee on Printing of the United States Congress; Washington DC. 2003. [Google Scholar]
- 26.Tiziani S, Vodovotz Y. Rheological characterization of a novel functional food: tomato juice with soy germ. J Agric Food Chem. 2005;53:7267–7273. doi: 10.1021/jf0511087. [DOI] [PubMed] [Google Scholar]
- 27.Xu SY, Shoemaker CF, Luh BS. Effect of break temperature on rheological properties and microstructure of tomato juices and pastes. J Food Sci. 1986;51:399–402. [Google Scholar]
- 28.Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, et al. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr. 2004;80:396–403. doi: 10.1093/ajcn/80.2.396. [DOI] [PubMed] [Google Scholar]
- 29.Franke AA, Custer LJ, Tanaka Y, Messina M, Erdman JW. Isoflavones in human breast milk and other biological fluids. Am J Clin Nutr. 1998;68:1466S–1473S. doi: 10.1093/ajcn/68.6.1466S. [DOI] [PubMed] [Google Scholar]
- 30.Ferruzzi MG, Nguyen ML, Sander LC, Rock CL, Schwartz SJ. Analysis of lycopene geometrical isomers in biological microsamples by liquid chromatography with coulometric array detection. J Chromatogr B. 2001;760:289–299. doi: 10.1016/s0378-4347(01)00288-2. [DOI] [PubMed] [Google Scholar]
- 31.van den Berg H, van Vliet T. Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr. 1998;68:82–89. doi: 10.1093/ajcn/68.1.82. [DOI] [PubMed] [Google Scholar]
- 32.Hadley CW, Clinton SK, Schwartz SJ. The consumption of processed tomato products enhances plasma lycopene concentrations in association with a reduced lipoprotein sensitivity to oxidative damage. J Nutr. 2003;133:727–732. doi: 10.1093/jn/133.3.727. [DOI] [PubMed] [Google Scholar]
- 33.Popov IN, Lewin G. Photochemiluminescent detection of antiradical activity .4. Testing of lipid-soluble antioxidants. J Biochem Biophys Methods. 1996;31:1–8. doi: 10.1016/0165-022x(95)00021-i. [DOI] [PubMed] [Google Scholar]
- 34.National Cancer Institute. Common Terminology Criteria for Adverse Events, Version 3.0. U.S. Department of Health and Human Services, National Institutes of Health; Bethesda, MD: 2003. [Google Scholar]
- 35.O’Neill ME, Thurnham DI. Intestinal absorption of beta-carotene, lycopene and lutein in men and women following a standard meal: response curves in the triacylglycerol-rich lipoprotein fraction. Br J Nutr. 1998;79:149–159. doi: 10.1079/bjn19980026. [DOI] [PubMed] [Google Scholar]
- 36.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol—a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 37.Unlu NZ, Bohn T, Francis D, Clinton SK, Schwartz SJ. Carotenoid absorption in humans consuming tomato sauces obtained from tangerine or high-beta-carotene varieties of tomatoes. J Agric Food Chem. 2007;55:1597–1603. doi: 10.1021/jf062337b. [DOI] [PubMed] [Google Scholar]
- 38.Unlu NZ, Bohn T, Francis DM, Nagaraja HN, Clinton SK, et al. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br J Nutr. 2007;98:1–7. doi: 10.1017/S0007114507685201. [DOI] [PubMed] [Google Scholar]
- 39.Munro IC, Harwood M, Hlywka JJ, Stephen AM, Doull J, et al. Soy isoflavones: a safety review. Nutr Rev. 2003;61:1–33. doi: 10.1301/nr.2003.janr.1-33. [DOI] [PubMed] [Google Scholar]
- 40.Shao A, Hathcock JN. Risk assessment for the carotenoids lutein and lycopene. Regul Toxicol Pharmacol. 2006;45:289–298. doi: 10.1016/j.yrtph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Gustin DM, Rodvold KA, Sosman JA, Diwadkar-Navsariwala V, Stacewicz-Sapuntzakis M, et al. Single-dose pharmacokinetic study of lycopene delivered in a well-defined food-based lycopene delivery system (tomato paste-oil mixture) in healthy adult male subjects. Cancer Epidemiol Biomarkers Prev. 2004;13:850–860. [PubMed] [Google Scholar]
- 42.Frohlich K, Kaufmann K, Bitsch R, Bohm V. Effects of ingestion of tomatoes, tomato juice and tomato puree on contents of lycopene isomers, tocopherols and ascorbic acid in human plasma as well as on lycopene isomer pattern. Br J Nutr. 2006;95:734–741. doi: 10.1079/bjn20051657. [DOI] [PubMed] [Google Scholar]
- 43.Riso P, Brusamolino A, Martinetti A, Porrini M. Effect of a tomato drink intervention on insulin-like growth factor (IGF)-1 serum levels in healthy subjects. Nutr Cancer. 2006;55:157–162. doi: 10.1207/s15327914nc5502_6. [DOI] [PubMed] [Google Scholar]
- 44.Cohn W, Thurmann P, Tenter U, Aebischer C, Schierle J, et al. Comparative multiple dose plasma kinetics of lycopene administered in tomato juice, tomato soup or lycopene tablets. Eur J Nutr. 2004;43:304–312. doi: 10.1007/s00394-004-0476-0. [DOI] [PubMed] [Google Scholar]
- 45.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by beta-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002;132:2587–2592. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 46.Franke AA, Custer LJ, Hundahl SA. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutr Cancer. 2004;50:141–154. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
- 47.Bohn T. Isoflavonoid bioavailability from foods and supplements: dietary factors impacting utilization. Agro Food Industry Hi-Tech. 2010;21:59–63. [Google Scholar]
- 48.Walsh KR, Zhang YC, Vodovotz Y, Schwartz SJ, Failla ML. Stability and bioaccessibility of isoflavones from soy bread during in vitro digestion. J Agric Food Chem. 2003;51:4603–4609. doi: 10.1021/jf0342627. [DOI] [PubMed] [Google Scholar]
- 49.Setchell KD, Faughnan MS, Avades T, Zimmer-Nechemias L, Brown NM, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003;77:411–419. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 50.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 51.Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean, and American residents. Jpn J Clin Oncol. 2004;34:86–89. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 52.Frankenfeld CL, Atkinson C, Thomas WK, Gonzalez A, Jokela T, et al. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br J Nutr. 2005;94:873–876. doi: 10.1079/bjn20051565. [DOI] [PubMed] [Google Scholar]
- 53.Caan BJ, Natarajan L, Parker B, Gold EB, Thomson C, et al. Soy food consumption and breast cancer prognosis. Cancer Epidemiol Biomarkers Prev. 2011;20:854–858. doi: 10.1158/1055-9965.EPI-10-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das S, Otani H, Maulik N, Das DK. Lycopene, tomatoes, and coronary heart disease. Free Radic Res. 2005;39:449–455. doi: 10.1080/10715760500053685. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Chang SK, Wiesenborn D. Antioxidant properties of soybean isoflavone extract and tofu in vitro and in vivo. J Agric Food Chem. 2005;53:2333–2340. doi: 10.1021/jf048552e. [DOI] [PubMed] [Google Scholar]
- 56.Mackinnon ES, Rao AV, Josse RG, Rao LG. Supplementation with the antioxidant lycopene significantly decreases oxidative stress parameters and the bone resorption marker N-telopeptide of type I collagen in postmenopausal women. Osteoporos Int. 2011;22:1091–1101. doi: 10.1007/s00198-010-1308-0. [DOI] [PubMed] [Google Scholar]
- 57.Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005;37:423–438. doi: 10.1080/07853890510044586. [DOI] [PubMed] [Google Scholar]
- 58.Meng QH, Lewis P, Wahala K, Adlercreutz H, Tikkanen MJ. Incorporation of esterified soybean isoflavones with antioxidant activity into low density lipoprotein. Biochim Biophys Acta. 1999;1438:369–376. doi: 10.1016/s1388-1981(99)00062-1. [DOI] [PubMed] [Google Scholar]
- 59.Bouayed J, Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Ox Med Cell Longev. 2010;3:228–237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]