Abstract
A study was conducted to characterize the storage stability of lycopene in hot-break tomato juice prepared from two different cultivars and processed by various pressure–heat combinations. Samples were subjected to pressure assisted thermal processing (PATP; 600 MPa, 100 °C, 10 min), high pressure processing (HPP; 700 MPa, 45 °C, 10 min), and thermal processing (TP; 0.1 MPa, 100 °C, 35 min). Processed samples were stored at 4, 25, and 37 °C for upto 52 weeks. HPP and PATP treatments significantly improved the extractability of lycopene over TP and control. All-trans lycopene was found to be fairly stable to isomerization during processing, and the cis isomer content of the control and processed juice did not differ significantly. During storage, lycopene degradation varied as a function of the cultivar, processing method, storage temperature, and time. This study shows that combined pressure–temperature treatments could be an attractive alternative to thermal sterilization for preserving tomato juice quality.
Keywords: High pressure processing, pressure assisted thermal processing, thermal processing, tomato, lycopene, storage stability
INTRODUCTION
A large portion of lycopene in the North American and European diet comes from the consumption of tomato and tomato products (1). Many epidemiological studies suggest the consumption of carotenoid rich foods such as tomato products reduces the risk of developing diseases such as cancer and cardiovascular diseases (2–5). With increased consumer demand for healthy foods, studying the fate of lycopene subjected to various preservation methods has gained interest (6–8).
Lycopene (C40H56) is a carotenoid that occurs in the form of all-trans and cis isomers in red tomatoes. Red tomatoes normally contain 94–96% all-trans-lycopene, which is thermodynamically the most stable form (9). However, lycopene from heat-induced cis-isomer- rich tomato sauce is reportedly more bioavailable than from all-trans-rich tomato sauce (10). Also, human plasma and tissues have been shown to contain 40–80% cis isomers (all-trans, 5-cis, 9-cis, 13-cis, and 15-cis being the most common isomers) (11).
Lycopene in tomato and tomato products exposed to traditional thermal treatments is fairly stable during processing (12). However, the magnitude of lycopene degradation during storage reported in the literature varies greatly and is influenced by temperature, water activity, and the presence or absence of light and oxygen (13, 14). Interconversions between all-trans and cis forms of lycopene in thermally treated tomato juice have also been reported (15).
Because of consumer interest in health, wellness, flavor, and freshness, the food industry is exploring alternative minimal processing approaches such as high pressure processing (16). High pressure studies on tomato juice and/or tomato puree show an increase in the amount of extractable lycopene over untreated or thermally processed juices (17–19). Pressure treated tomato puree samples better retain color over thermally pasteurized and sterilized samples (17, 18). Hsu et al. (19) studied the fate of lycopene in hot break tomato juice extracted from red daydream tomatoes. The hot break tomato juice was processed using high pressure processing (HPP) (300–500 MPa, 25 °C, and 10 min) and heat treatment (98 °C and 15 min), and stored at 4 °C for 28 days. Pressure treatment increased the lycopene extractability from tomato juice relative to that of the control or thermally treated juice and improved its storage stability. In another study, Qiu et al. (20) pressure treated tomato puree at 100–600 MPa, 20 ± 1 °C, for 12 min and studied the degradation and isomerization of lycopene at 4±1 °C and 24± 1 °C for a period of 28 days. Pressure treatment increased the extractable lycopene content and retained its stability during storage. However, microbiological stability of the product was not reported. Most of the current storage studies are limited to less than 30 days in duration, and the stability of pressure treated tomato juice during an extended storage period is not known. Also, the storage stability of lycopene in pressure assisted thermally processed (500–700 MPa, 90–120 °C) tomato juice has not been studied.
The objective of this study was to investigate the storage stability of lycopene in tomato juice stored up to 52 weeks. Juice from two tomato cultivars that differed in lycopene content was processed using thermal processing, pressure assisted thermal processing, and high pressure processing. Samples were subsequently stored at temperatures of 4, 25, and 37 °C. The product was evaluated on the basis of color, lycopene isomer profile, and microbial stability.
MATERIALS AND METHODS
Plant Materials
Two fully ripe tomatoes (Solanum lycopersicum L.) cultivars, high lycopene FG99-218 (old gold crimson ogc homozygous; dark green (dg) homozygous) and commercial variety OX325 (old gold crimson ogc homozygous; alcabaca (alc) heterozgygous), were freshly harvested from the North Central Agricultural Research Center, Fremont, OH and transported to the processing pilot plant in Columbus, OH (~2 h travel). The initial quality attributes of the raw tomato product are summarized in Table 1. It is worth noting that FG99-218 (high lycopene) had nearly twice the amount of total lycopene than that ofOX325 and had higher a values on the CIE color scale.
Table 1.
Selected Attributes of Raw Tomato Flesh and the Hot Break Tomato Juice Used in the Study
| cultivar | °Brix
|
pH | colora
|
lycopene contenta mg/100 g juice
|
total lycopenea mg/100 g juice
|
||||
|---|---|---|---|---|---|---|---|---|---|
| % TSSb | L | a | b | all-trans | cis | all-trans + cis | |||
| FG99-218 | Raw tomato | 6.3 | 4.52 | 26.02 ± 0.21 | 19.86 ± 0.16 | 14.89 ± 0.09 | 15.02 ± 0.26 | 1.02 ± 0.05 | 16.04 ± 0.3 |
| Hot break juice | 6.5 | 4.50 | 26.59 ± 0.13 | 18.02 ± 0.08 | 14.46 ± 0.14 | 14.65 ± 0.66 | 1.4 ± 0.01 | 16.05 ± 0.7 | |
| HPPc | 6.6 | 4.51 | 25.54 ± 0.47 | 17.08 ± 0.26 | 12.14 ± 0.25 | 16.51 ± 0.6 | 1.44 ± 0.01 | 17.95 ± 0.6 | |
| PATPd | 6.5 | 4.50 | 24.99 ± 0.51 | 16.75 ± 0.17 | 12.77 ± 0.20 | 15.74 ± 0.37 | 1.38 ± 0.02 | 17.12 ± 0.4 | |
| TPe | 6.5 | 4.50 | 28.92 ± 0.91 | 15.41 ± 0.38 | 11.89 ± 0.41 | 14.1 ± 0.51 | 1.4 ± 0.03 | 15.50 ± 0.5 | |
| OX 325 | Raw tomato | 5.4 | 4.38 | 25.89 ± 0.11 | 14.52 ± 0.19 | 12.66 ± 0.25 | 8.96 ± 0.15 | 0.88 ± 0.06 | 9.84 ± 0.2 |
| Hot break juice | 5.6 | 4.40 | 25.92 ± 0.09 | 13.99 ± 0.04 | 12.43 ± 0.09 | 8.84 ± 0.22 | 1.38 ± 0.01 | 10.22 ± 0.2 | |
| HPPc | 5.7 | 4.40 | 26.48 ± 0.55 | 14.40 ± 0.60 | 12.64 ± 1.19 | 9.57 ± 0.37 | 1.35 ± 0.01 | 10.88 ± 0.4 | |
| PATPd | 5.7 | 4.41 | 23.75 ± 0.22 | 15.3 ± 1.22 | 15.08 ± 0.59 | 8.93 ± 0.30 | 1.36 ± 0.03 | 10.29 ± 0.3 | |
| TPe | 5.6 | 4.40 | 28.52 ± 0.55 | 11.18 ± 0.14 | 10.39 ± 0.27 | 7.13 ± 0.32 | 1.36 ± 0.01 | 8.49 ± 0.3 | |
Values are the mean ± SD of three replications.
Percent total soluble solids present in the juice.
High pressure processing at 700 MPa, 45 °C, and 10 min.
Pressure assisted thermal processing at 600 MPa, 100 °C, and 10 min.
Thermal processing at 0.1 MPa, 100 °C, and 35 min.
Tomato Juice Processing
The tomatoes were washed, and the juice was extracted within 48 h of harvesting the tomatoes. The juice was extracted using the hot break process (91 ± 2 °C) employing a pilot scale tomato juice extraction system consisting of a tomato crusher (W. J. Fitzpatrick Co. Chicago, IL), concentric tube heat exchanger, and a screw type tomato juice extractor (Chisholm Ryder Co., CJE-350 D-28). The juice was collected and cooled to 20 ± 1 °C. The juice was immediately vacuum packaged (Spiromac vacuum sealer, model 450 T, Québec, Canada) in polypropylene pouches (Thomson Equipment and Supply, Cincinnati, OH). Each pouch contained 50 ± 3 g of tomato juice. The pouches were stored in a walk-in refrigerator (4 ± 0.5 °C) in the dark. The stored tomato juice samples were subjected to various pressure–heat treatments (see the following sections for details) within 2 days of juice extraction. Unprocessed hot break juice was also used as a control to compare the effect of various processing conditions on juice quality attributes during storage. Selected quality attributes of the hot break juice are summarized in Table 1.
High Pressure Processing
The tomato juice samples were high pressure processed using a 5 L sample holding capacity pilot scale high pressure machine (Iso-lab high pressure food processor, Stansted Fluid Power Ltd., Essex, UK). Pressurization and depressurization rates of ~5.3 MPa/s and ~8.3 MPa/s were used. One hundred percent propylene glycol (Brenntag Midsouth Inc., Henderson, KY) was used as the pressure transmitting fluid. The samples (preconditioned at an initial temperature of 20 °C) were high pressure processed (700 MPa, 45 °C for 10 min). Sample temperature under high pressure conditions were monitored using T-type thermocouples (Omega Engineering, Stamford CT). The thermocouple was fed through the pouch using a C-5.2 stuffing box (Ecklund-Harrison Technologies, Fort Myers, FL). After processing, the samples were immediately withdrawn and stored at different temperatures.
Pressure-Assisted Thermal Processing
For processing tomato juice using pressure-assisted thermal processing, the tomato juice samples were first preheated to an initial temperature of 80 °C in a water bath (Fisher Scientific, Pittsburgh, PA). Similarly, the initial temperature of glycol was also adjusted to 80 °C. The preheated samples were loaded into a stainless steel sample holder and then loaded inside a pressure chamber preheated to 105 °C. Samples were pressure treated at 600 MPa, 100 °C, for 10 min. The equipment had a pressurization time of <140 s. More details of the experimental techniques used are provided elsewhere (21). After processing, the samples were depressurized (<30 s), withdrawn from the pressure chamber, and immediately cooled in an ice–water mixture and analyzed.
Thermal Sterilization
Boiling water in a steam jacketed kettle was used for the thermal sterilization (100 °C, 35 min) of vacuum packaged tomato juice (22). The temperature was monitored using a K-type thermocouple (Omega Engineering) and recorded using a data logger (IOtech, Cleveland, OH). The thermocouple was located at the geometric center of the pouch containing the tomato juice. After processing, the samples were immediately cooled in an ice–water bath and transferred for storage at the 3 respective temperatures.
Storage of Processed and Control Samples
The processed samples and control were immediately stored in the dark at three different temperatures (4 ± 0.5 °C, 24 ± 1 °C, and 36 ± 1 °C) and analyzed for lycopene content, color, pH, total soluble solids, and microbial stability after 0, 2, 5, 15, 35, and 52 weeks of storage.
Analysis
Lycopene Extraction
Lycopene was extracted from tomato juice using a method reported by Ferruzzi et al. (23). Briefly, tomato juice (5 g) was mixed with 4 g of Celite and 1 g of calcium carbonate. Fifty milliliters of methanol was added, and the mixture was homogenized at 10,000 rpm for 1 min. Carotenoids were extracted three times with 25 mL of HPLC grade hexane/acetone (1:1 v/v) (Fisher Scientific, USA). The combined hexane layer was collected quantitatively after filtering through anhydrous sodium sulfate, and the volume was made to 100 mL using HPLC grade hexane. Two milliliter aliquots were dried under nitrogen, reconstituted in methyl tert-butyl ether (MTBE)/methanol (1:1 v/v) (HPLC grade, Fisher scientific, USA), and filtered through a 0.2 μm 13 mm nylon syringe filter, and 50 μL was injected into the HPLC (Agilent technologies, Model HP 1050) equipped with a Waters 996 Photodiode-array (PDA) detector. The mixture was separated on a Waters YMC C30 HPLC column (4.5 mm × 150 mm, 5 μm particle size). Separations were achieved using gradient elution with different concentrations of methanol: MTBE, 2% aq. ammonium acetate in reservoirs A (88:5:7) and B (20:78:2). The following gradient was used: at 0 min 0% B, linear gradient to 85% B over 20 min, followed by a linear gradient to 100% B over 10 min, returning to 0% B, and holding for 5 min.
Isomerization of the lycopene standard was performed in hexane by adding an iodine catalyst at a concentration of about 1% (w/w) of the lycopene weight and allowing the mixture to sit for 15 min in fluorescent light of luminance 320 lx (lumens/m2). HPLC analysis was then performed on the isomerized sample (Figure 1). Lycopene isomers in tomato juice were identified by comparing chromatograms of tomato juice with the chromatograms of isomerized lycopene standard.
Figure 1.
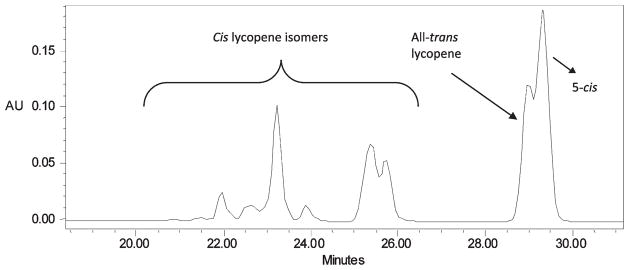
HPLC chromatogram of lycopene isomers obtained by adding an iodine catalyst in hexane at a concentration of about 1% (w/w) of the lycopene weight and allowing the mixture to sit for 15 min in fluorescent light of luminance 320 lx (lumens/m2).
To quantify lycopene in tomato juice samples, a calibration curve was generated using authentic all-trans lycopene standard. Levels of cis-lycopene isomers are given in all-trans lycopene equivalents.
Colorimetry
The color of tomato juice samples was measured using a tristimulus colorimeter (CR-300, Minolta, Osaka, Japan). A standard white tile (Y = 92.6, X = 0.3161, y = 0.3321) was used to calibrate the instrument. The juice samples were placed in a glass Petri dish on top of the light source (15 mm aperture), and L, a, and b values were directly obtained from the colorimeter. Each measurement reported represents the average of 3 readings. L represents lightness, +a represents redness, −a represents greenness, +b represents yellowness, and −b represents blueness. The overall change in color (ΔE) was calculated by the following equation (24):
| (1) |
Aerobic Plate Count
Aerobic plate counts of the processed and control tomato juice samples were taken after 0, 2, 5, 15, 35, and 52 weeks of storage at 4, 24, and 37 °C. Tomato juice (10 g) from the vacuum sealed pouches was aseptically transferred in stomacher bags, and 90 mL of sterile peptone water was aseptically added to each bag. The samples were homogenized in a stomacher (Seward Lab Stomacher, Norfolk, UK) for 2 min. One milliliter of the homogenized sample was then aseptically transferred to the tubes containing 9 mL of sterile peptone water, and serial dilutions were spread plated on trypticase soy agar (TSA) plates. After incubation at 37 °C for 48 and 72 h, the viable count of surviving microorganisms was enumerated. Colonies were counted with a dark-field Quebec colony counter (Leica Microsystems, Richmond Hill, Canada). The detection limit for the enumeration procedure was 100 CFU per mL.
Total Soluble Solids (°Brix) and pH
Total soluble solids (%) and pH of the raw tomatoes and the juice were measured using Atago Digital Hand-Held Pocket Refractometer (Cole-Parmer Instrument Company, Vernon Hills, IL) and portable hand-held pH meter (Omega Engineering), respectively.
Statistical Data Analysis
Data was analyzed with Minitab software, version 14.1 (Minitab, State College, PA). The influence of storage temperature (T = 4, 25, and 37 °C), storage time (t = 0, 2, 5, 15, 35, and 52 weeks), cultivar (FG99-218 or OX325), treatment (HPP, PATP, or TP) as well as their interactions on the concentration of lycopene (Y) in tomato juice were analyzed. Pairwise comparisons for the means of treatment and storage variables (factors) were evaluated with Tukey’s test at 5% significance level (P < 0.05).
Kinetic Modeling
The degradation of lycopene as influenced by storage temperature, time, and processing methods was modeled using two first order rate equations (25).
| (2) |
where Ct is the lycopene concentration in mg/100 g tomato juice at time t weeks, C0 is the initial concentration of lycopene in the respective sample at week 0, S1 and S2 are constants for each process proportional to the lycopene degradation under respective processing and storage conditions, and k1 and k2 are the rate constants for each of the two first-order processes involved in lycopene degradation.
RESULTS AND DISCUSSION
Effect of Hot Break Juice Extraction on Lycopene
The hot break extraction process at 91 ± 2 °C was used to extract tomato juice in order to minimize the undesirable effects of pectin degrading enzymes on the consistency of tomato juice and extractability of lycopene. Hot break treatment of crushed tomatoes is sufficient to completely inactivate pectinmethyl esterase and retains approximately 4% polygalacturonase activity (26). Extraction of juice from both FG99-218 and OX325 did not significantly affect the total lycopene (all-trans + cis-lycopene) content of the juice. However, a significant increase in the cis-isomer of lycopene was observed in juices extracted from both cultivars. This was accompanied by a slight decrease in the redness of the juice as shown by the a color value (Table 1). cis-Isomers of lycopene have been reported to have different physio-chemical characteristics than the all-trans isomers including decrease in the color intensity of the tomato juice (27). Although lycopene has been shown to be fairly stable to isomerization reactions in tomato products exposed to conventional thermal processing (12), the presence of heat, light, oxygen, and/or their combinations during processing does influence the fate of lycopene isomers (27).
Combined Pressure–Heat Treatment Effects on Lycopene Stability
The initial temperature of HPP processed samples was approximately 20 °C. The maximum and average process temperatures at target pressure (700 MPa) over 10 min pressure holding time were 47.0 and 45.7 ± 0.56 °C, respectively. The maximum and average process temperatures of the preheated juice (~80 °C) during 10 min of PATP holding time were 104.0 °C and 101 ± 0.87 °C, respectively. Thermally processed samples were maintained at 100 °C, 0.1 MPa, for 35 min.
HPP (700 MPa, 45 °C, 10 min) and PATP (600 MPa, 100 °C, 10 min) increased the extractable all-trans-lycopene in the high lycopene cultivar FG99-218 by 12% and 7%, respectively, over the unprocessed hot break juice that served as the control. However, the all-trans-lycopene in thermally sterilized samples of FG99-218 was approximately the same as that of the control (Table 1). The cis-lycopene isomer content in HPP, PATP, and TP treated samples as well as the hot break juice (control) did not show significant differences (p < 0.05) and constituted approximately 8% of the total lycopene in processed and control samples (Table 1).
Similarly, the extractable all-trans-lycopene in OX325 increased by approximately 8% for HPP samples (700 MPa, 45 °C, 10 min) but showed insignificant change for PATP (600 MPa, 100 °C, 10 min) samples. Thermally sterilized samples had a 19% decrease in all-trans extractable lycopene (Table 1). The cis-isomers of HPP, PATP, and TP treated samples and the control varied between 12 and 16% of the total lycopene (Table 1). It was also worth noting thatOX325 had 40% less of the all-trans isomer thanFG99-218, the high lycopene variety. The differences in lycopene extractability from tomato juice exposed to similar process treatments could be attributed to cultivar specific differences. Earlier, researchers have also reported an increase in the lycopene extractability from pressure treated tomato products (17–19). However, the extent of reported lycopene extractability differed greatly among various studies. For example, Krebbers et al. (18) applied various pressure–temperature– time combinations (300–500 MPa, 20 °C, 2 min and 700 MPa, 80 and 90 °C for 30 s) and reported that tomato puree processed at 500 MPa at 20 °C for 2 min had a significant increase in lycopene extractability (~27%) than raw juice samples. Similarly, Sanchez-Moreno et al. (17) processed tomato puree at 400 MPa, 25 °C for 15 min and reported ~48% increase in lycopene extractability as compared to the raw unprocessed puree. Hsu et al. (19) processed tomato juice at 300–500 MPa, 25 °C for 10 min and reported a progressive increase in lycopene extractability with increase in pressure. A maximum 60% lycopene extractability was reported in samples processed at 500 MPa, 25 °C for 10 min. The differences in magnitude of lycopene extractability among various studies may be due to the differences in the type of tomato cultivars used, sample preparation method, type of sample used (juice, pulp, puree, etc.), hot break versus cold break juice used for combined pressure–heat treatments, and high pressure process parameters including pressurization and depressurization rates. Since pressure– temperature treatments are known to affect membranes in vegetable cells (13) and macromolecular structures such as proteins and carbohydrates (28), such treatments may increase lycopene extractability. We hypothesize that increased extractability could lead to the increased bioavailability of lycopene. Research has shown that all-trans lycopene in tomato products is fairly stable during traditional thermal processing (12, 27, 15). It has been further reported that cis-lycopene is fairly stable to pressure treatments (20). Since isomerization is a structurally and thermodynamically specific phenomenon, studies on the thermodynamic effects of combined pressure–heat treatments on lycopene might be able to explain its stability to isomerization under such processing conditions. Also, the stability of the all-trans form to degradation and or isomerization is governed by its structural specificity. The differences in shape of the carotenoid molecule influence hydrophobicity, crystalline state and ease of crystal formation, solubility, and other such properties which in turn might influence its stability (29). Lycopene is a linear molecule and has been shown to form multilayers or aggregates (30), and it is proposed that once in the aggregated form, lycopene molecules might be able to resist further structural changes (29).
Microbial Stability of the Processed Samples
All samples processed using HPP, PATP, and TP showed microbial stability over a storage period of 52 weeks at all three storage temperatures (4, 25, and 37 °C). Thus, the pH and total soluble solids of the stored samples did not show a significant change over the storage period (p<0.05). Control samples stored at 25 and 37 °C showed spoilage within 2 weeks (data not shown).
Storage Effects on Lycopene Stability
Figures 2 and 3 present the storage stability of lycopene in tomato juice subjected to HPP, PATP, and TP treatments and stored at three different storage temperatures (4, 25, 37 °C) up to 52 weeks. In general, lycopene from both cultivars was fairly stable to degradation and isomerization over the course of the storage period. For cultivar FG99-218 (high lycopene, 14.65 ± 0.66 mg lycopene/100 g tomato juice), both storage temperature and storage time significantly influenced lycopene degradation (P<0.05). Incultivar OX325with lower lycopene content (8.84±0.22 mglycopene/100 g tomato juice), lycopene degradation was primarily influenced by storage time, but storage temperature did show a statistically significant effect on lycopene degradation during storage (P < 0.05). Decrease in the concentration of cis-isomers was more pronounced at 25 °C storage for both cultivars. The present study did not consider the influence of barrier properties of the packaging material on storage stability of the treated juice, and further research is needed to understand the role of packaging on the stability of processed samples.
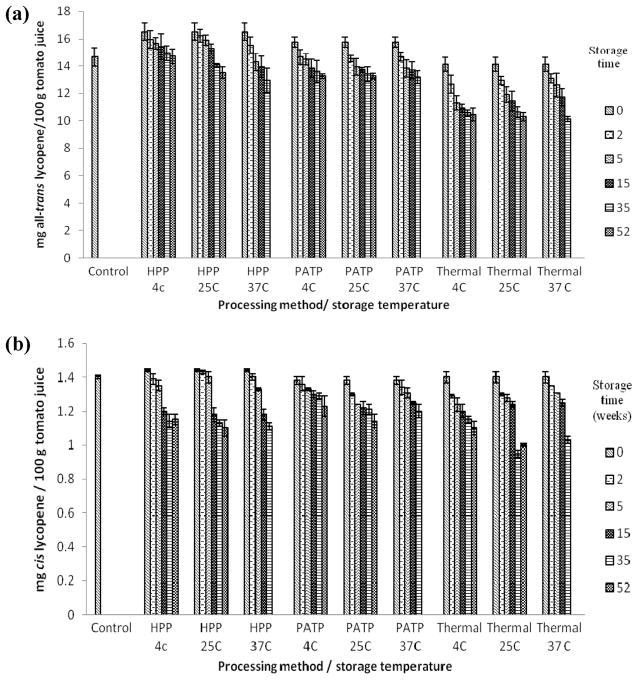
Figure 2.
(a) All-trans lycopene concentrations in high lycopene (FG99-218) tomato juice processed using HPP (700 MPa, 45 °C, 10 min), thermal sterilization (0.1 MPa, 100 °C, 35 min) PATP (600 MPa, 100 °C, 10 min), and stored in the dark at different storage temperatures for 0, 2, 5, 15, 35, and 52 weeks. The untreated control had 14.65±0.66 mg lycopene/100 g juice. Values are the mean±SD of three replications. (b) cis-Lycopene concentrations in high lycopene (FG99-218) tomato juice processed using HPP (700 MPa, 45 °C, 10 min), thermal sterilization (0.1 MPa, 100 °C, 35 min) PATP (600 MPa, 100 °C, 10 min), and stored in the dark at different storage temperatures for 0, 2, 5, 15, 35, and 52 weeks. The untreated control had 1.4±0.01 mg lycopene/ 100 g juice. Values are the mean ± SD of three replications.
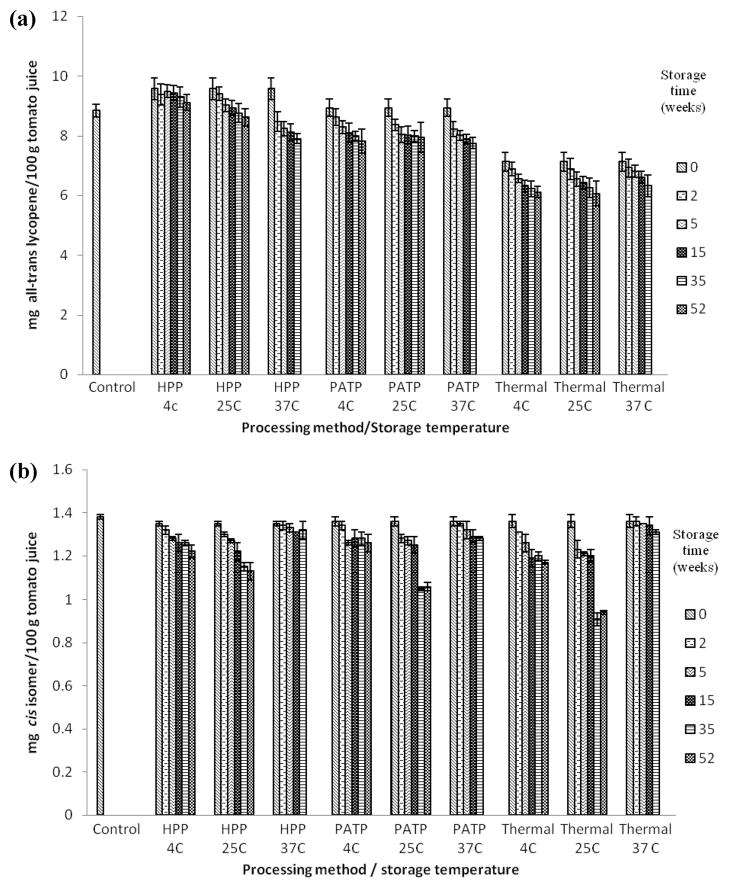
Figure 3.
(a) All-trans lycopene concentrations in OX325 tomato juice processed using HPP (700 MPa, 45 °C, 10 min), thermal sterilization (0.1 MPa, 100 °C, 35 min) PATP (600 MPa, 100 °C, 10 min), and stored in the dark at different storage temperatures for 0, 2, 5, 15, 35, and 52 weeks. The untreated control had 8.84±0.22 mg all-trans lycopene/100 g tomato juice. Values are the mean± SD of three replications. (b) cis-Lycopene concentrations in OX325 tomato juice processed using HPP (700 MPa, 45 °C, 10 min), thermal sterilization (0.1 MPa, 100 °C, 35 min) PATP (600 MPa, 100 °C, 10 min), and stored in the dark at different storage temperatures for 0, 2, 5, 15, 35, and 52 weeks. The untreated control had 1.38±0.01 mg all-trans lycopene/100 g tomato juice. Values are the mean±SD of three replications.
Changes in Tomato Juice Color During Storage
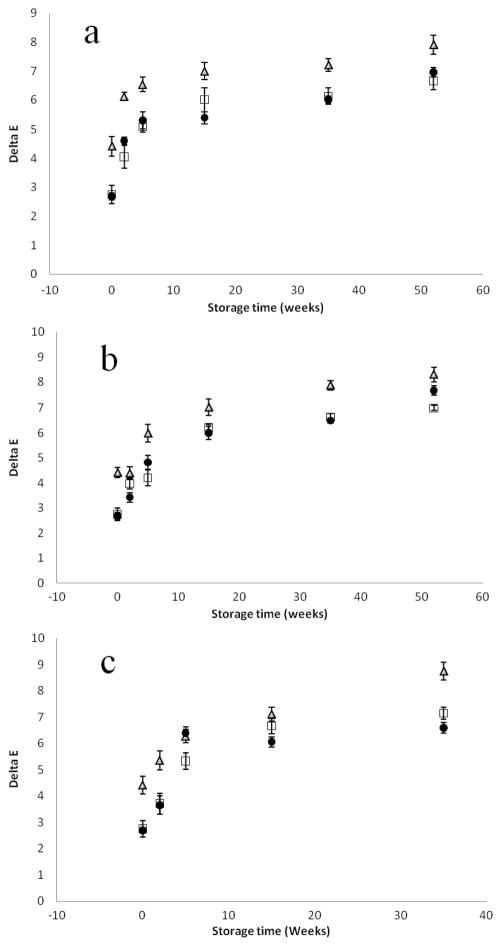
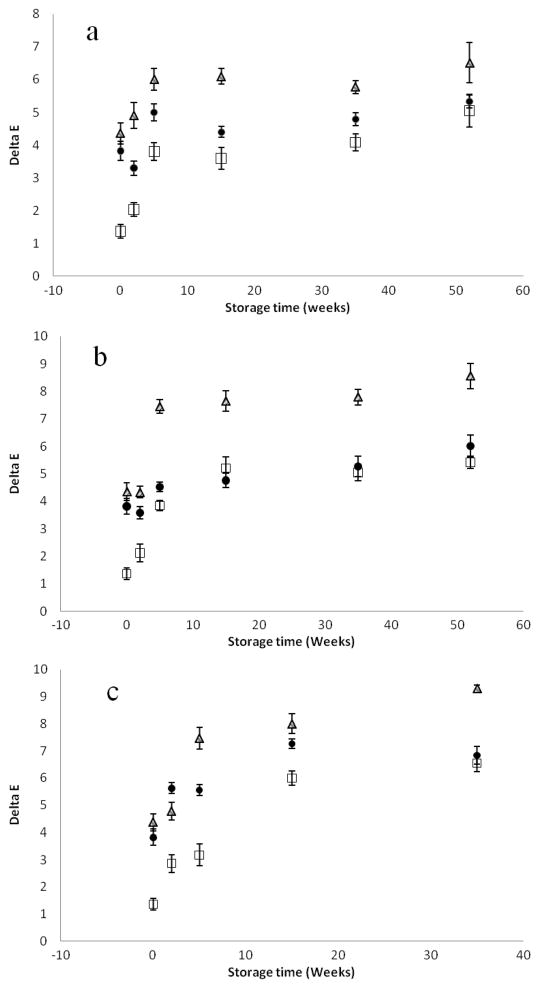
Changes in color values (ΔE) of the processed and control samples over the course of 52 weeks of storage at 4, 25, and 37 °C storage temperatures are shown in Figures 4 and 5. In comparison to the control sample at day zero, all of the processed (TP, HPP, and PATP) samples changed color as a function of storage temperature and time. Among the treatments, HPP samples had better color retention, while TP samples had the most color changes. PATP samples had intermediate color loss, and the degradation was also influenced by the cultivar type. In addition to increased lycopene extractability, better retention of natural color may be an added benefit of PATP over conventional thermal sterilization.
Figure 4.
Differential color values (ΔE) of high lycopene (FG99-218) tomato juice processed using HPP (□), TP (Δ), and PATP (●) and stored in the dark at (a) 4 °C, (b) 25 °C, and (c) 37 °C for 0, 2, 5, 15, and 35 weeks. Values are the mean ± SD of three replications.
Figure 5.
Differential color values (ΔE) of OX325 tomato juice processed using HPP (□), TP (Δ), and PATP (●) and stored in the dark at (a) 4 °C, (b) 25 °C, and (c) 37 °C for 0, 2, 5, 15, and 35 weeks. Values are the mean ± SD of three replications.
Since the conversion of trans to cis involves a decrease in the intensity of red color (31) and lycopene degradation might influence color changes, an attempt was made to correlate color changes to changes in lycopene. With a few exceptions, the changes in color values (ΔE) as a function of lycopene degradation were fairly linear with regression coefficient values greater than 0.85 (R2 > 0.85). Earlier studies (18) reported little dependence on changes in lycopene concentration and L, a, and b color values in samples subjected to thermal and combined pressure–temperature treatments.
Kinetic Modeling of Lycopene Degradation
The plots of lycopene content versus storage time show that lycopene degraded rapidly during the first few weeks of storage (weeks 0–5) and then approached a more linear asymptotic form during the subsequent storage period (Figures 2 and 3). The kinetic data for the degradation of lycopene during storage could not be fitted with zero, first, or second order rate equations. However, for tomato juice exposed to each of the variables, a two-step first order kinetic model could predict the degradation of lycopene within experimental limits. The presence of 2 first order decay processes does suggest multiple (2 or more) pathways of degradation caused by the effects of various treatments, storage temperature and time, and/or their interactions. Figure 6 provides experimental versus predicted lycopene concentrations (using kinetic constants presented in Table 2) in selected tomato juice samples from FG99-218. Each line in Figure 6 was generated by its corresponding equation (eq 2) and offered a reasonable fit to the experimental data within experimental limits.
Figure 6.
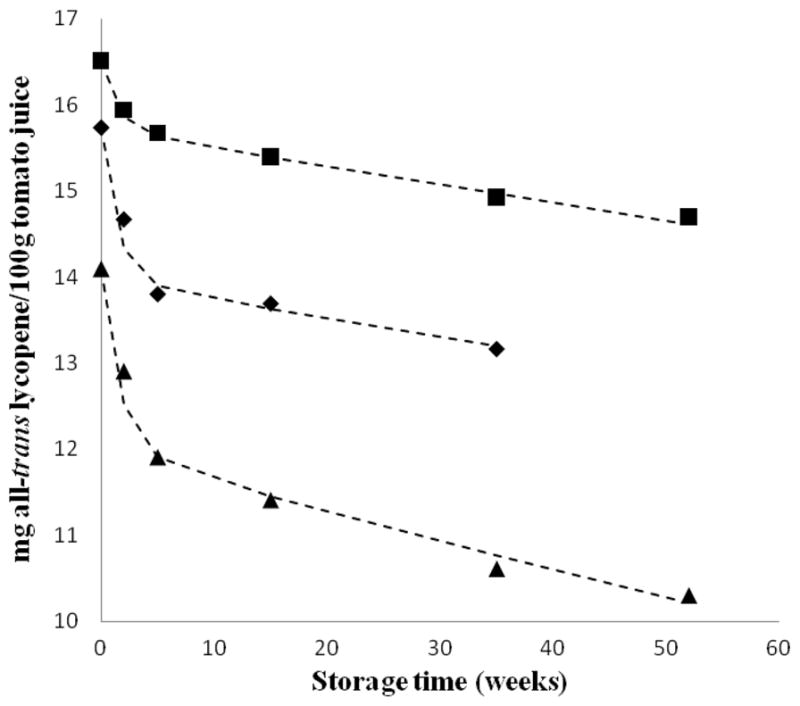
Representative experimental vs predicted values of lycopene concentration in tomato juice (FG99-218) processed using combined pressure–temperature treatments and stored at various temperatures. (■) 700 MPa, 45 °C, and 10 min treatment followed by 4 °C storage; (----) predicted. (▲) 0.1 MPa, 100 °C, and 35 min treatment followed by storage at 25 °C; (----) predicted. (◆) 600 MPa, 100 °C, and 10 min treatment followed by storage at 37 °C; (----) predicted.
Table 2.
Reaction Rate Constants and Correlation Coefficients of Lycopene Degradation in High Lycopene (FG99-218) and OX325 Tomato Juice Treated Using HPP, PATP, and Thermal Sterilization
| cultivar/ storage temperature
|
K1 (rate constant for lycopene degradation during 0 to 5 week storage)
|
K2 (rate constant for lycopene degradation during 5 to 52 week storage)
|
||
|---|---|---|---|---|
| cultivar FG99-218 | K1 (1/s) | correlation coefficient | K2 (1/s) | correlation coefficient |
| HPP | ||||
| 4 °C | 0.5124 | 0.99 | 0.0014 | 0.98 |
| 25 °C | 0.3283 | 0.99 | 0.0036 | 0.98 |
| 37 °C | 0.8086 | 0.93 | 0.0034 | 0.99 |
| Thermal | ||||
| 4 °C | 0.6322 | 0.96 | 0.0015 | 0.89 |
| 25 °C | 0.637 | 0.97 | 0.0031 | 0.97 |
| 37 °C | 1.3158 | 0.97 | 0.0072 | 0.99 |
| PATP | ||||
| 4 °C | 0.3631 | 0.83 | 0.0017 | 0.88 |
| 25 °C | 0.7548 | 0.98 | 0.0011 | 0.97 |
| 37 °C | 0.7342 | 0.97 | 0.0016 | 0.97 |
| cultivar OX325 |
K1 (rate constant for lycopene degradation during 0 to 5 week storage)
|
K2 (rate constant for lycopene degradation during 5 to 52 week storage)
|
||
|---|---|---|---|---|
| K1 (1/s) | correlation coefficient | K2 (1/s) | correlation coefficient | |
| HPP | ||||
| 4 °C | 2.6714 | 0.99 | 0.0009 | 0.98 |
| 25 °C | 1.3016 | 0.88 | 0.001 | 0.99 |
| 37 °C | 0.7717 | 0.90 | 0.0014 | 0.99 |
| Thermal | ||||
| 4 °C | 0.4348 | 0.97 | 0.0014 | 0.89 |
| 25 °C | 0.9019 | 0.91 | 0.0016 | 0.99 |
| 37 °C | 0.545 | 0.99 | 0.0025 | 0.99 |
| PATP | ||||
| 4 °C | 0.5417 | 0.97 | 0.0012 | 0.95 |
| 25 °C | 1.3124 | 0.94 | 0.0002 | 0.99 |
| 37 °C | 0.7648 | 0.99 | 0.0011 | 0.97 |
The rate constants of lycopene degradation during storage are summarized in Table 2. The initial phase of storage (0, 2, and 5 weeks) had larger reaction constant values (k1) (ranging from 0.36/s to 2.67/s) than reaction constant values (k2) estimated (ranging from 0.0009 to 0.0072/s) for subsequent stages of storage (5, 15, 35, and 52 weeks). This shows that rapid lycopene degradation occurred during the first few weeks of storage and then gradually subsided. With few exceptions, samples stored at 4 °C showed the lowest rate of degradation during both first order degradation steps. This biphasic degradation behavior of lycopene was also observed in watermelon samples stored for one year at −20 and −80 °C (25). However, the rate constants (k1 and k2) of lycopene degradation in watermelon tissue under frozen storage were approximately one-tenth of the values reported in this study. This could be attributed to a difference in lycopene level in the studied food matrixes, process treatment, and storage temperature.
Processing methods and cultivars were found to influence the lycopene stability over 52 weeks of storage. For example, with FG99-218, in comparison to pressure treated samples at day 0, pressure treated samples stored for 52 weeks showed 11, 18, and 21% decrease in all-trans-lycopene at 4, 25, and 37 °C, respectively (Figure 2a). Similarly, thermally processed samples after 52 weeks of storage showed 26, 27, and 28% decrease as compared to day 0 samples analyzed immediately after thermal processing. PATP samples showed ~16% decrease at all three storage temperatures (Figure 2a). HPP and PATP treatments resulted in up to 23% and 17% degradation in cis-isomers, respectively, whereas TP resulted in up to 26% cis-lycopene degradation during storage. The cis-isomers of both cultivars were better retained in HPP and PATP processed samples at the end of 52 weeks as compared to the thermally processed samples (Figure 2b). In OX325 over a 52 week period, the change in all-trans-isomers of lycopene in HPP, PATP, and thermally treated samples stored at 4, 25, and 37 °C was 5, 10, 8% (HPP); 12, 11, 13%(PATP) and 14, 15, 11%(TP), respectively (Figure 3a). The cis-isomers in this cultivar did not show a clear dependency on storage temperature (Figure 3b), and samples stored at 25 °C showed more degradation than samples stored at 4 or 37 °C.
Studies that report the fate of lycopene in stored tomato juice processed using combined pressure–temperature are scarce and conducted for less than 4 weeks on samples processed at ambient temperatures (19, 20). These studies conducted over a range of pressures up to 600 MPa and 25 °C show that lycopene is fairly stable in tomato juice/puree exposed to HPP treatments degrading between 1 and 10% over a storage period between 2 and 4 weeks, stored at 25 °C. However, studies on the fate of lycopene in thermally processed tomato products are readily available, though large differences in lycopene stability have been reported. One study reported that commercially canned tomato juice stored for a period of 12 months does not show significant lycopene degradation (32). Additionally, Nguyen et al. (29) reported that extended storage of various tomato products over a 18 month period did not change the isomer distribution of lycopene.
Conclusions
Within the range of experimental conditions used in the study, combined pressure–temperature treatments improved the extractability of lycopene over conventional thermal sterilization. All-trans-lycopene is fairly stable to isomerization during processing, and the cis-isomer content of the control and processed juice did not differ significantly. During storage, the stability of lycopene in tomato juice is influenced by a complex interaction of method of processing, type of cultivar, storage temperature, and storage time. HPP and PATP processed samples better retained both total lycopene and cis-isomers during 52 weeks of storage. During storage, HPP and PATP samples also showed better color retention as compared to the thermally processed samples. Tomato juice samples processed using various treatments (HPP, PATP, and thermal) were microbiologically stable over 52 weeks of storage. A two-step first order equation could be effectively used to predict the changes in lycopene concentration over the course of storage. This study suggests that combined pressure–temperature treatments pose an attractive alternative to thermal sterilization for delivering improved quality and a more functional tomato juice to the consumer.
Acknowledgments
Financial support for this research was provided by USDA-CSREES-NRICGP Grant 2006-35503-17571, by the Ohio Agricultural Research and Development Center (OARDC), and by The Ohio State University. References to commercial products or trade names are made with the understanding that no endorsement or discrimination by The Ohio State University is implied.
LITERATURE CITED
- 1.Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56:35. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 2.Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, Stampfer MJ. Lower prostate cancer risk in men with elevated plasma lycopene levels: Results of a prospective analysis. Cancer Res. 1999;59:1225–1230. [PubMed] [Google Scholar]
- 3.Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in men. Am J Clin Nutr. 2005;81:990–997. doi: 10.1093/ajcn/81.5.990. [DOI] [PubMed] [Google Scholar]
- 4.Sesso HD, Buring JE, Norkus EP, Gaziano JM. Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. Am J Clin Nutr. 2003;79:47–53. doi: 10.1093/ajcn/79.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Bruno RS, Wildman REC, Schwartz SJ. Lycopene: Food Sources, Properties and Health. In: Wildman REC, editor. Handbook of Nutraceuticals and Functional Foods. CRC Press; Boca Raton, FL: 2007. pp. 55–72. [Google Scholar]
- 6.Henry LK, Puspitasari-Nienaber NL, Jaren-Galan M, van Breemen RB, Catignani GL, Schwartz SJ. Effects of ozone and oxygen on the degradation of carotenoids in an aqueous model system. J Agric Food Chem. 2000;48:5008–5013. doi: 10.1021/jf000503o. [DOI] [PubMed] [Google Scholar]
- 7.Oms-Oliua G, Odriozola-Serranoa I, Soliva-Fortunya R, Martín-Belloso O. Effects of high-intensity pulsed electric field processing conditions on lycopene, vitamin C and antioxidant capacity of watermelon juice. Food Chem. 2009;115:1312. [Google Scholar]
- 8.Odriozola-Serranoa I, Soliva-Fortunya R, Hernández-Jovera T, Martín-Belloso O. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chem. 2008;112:258. [Google Scholar]
- 9.Porrini M, Riso P, Testolin G. Absorption of lycopene from single or daily portions of raw and processed tomato. Br J Nutr. 1998;80:353. doi: 10.1079/096582198388300. [DOI] [PubMed] [Google Scholar]
- 10.Unlu NZ, Bohn T, Francis DMN, HN, Clinton SK, Schwartz SJ. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br J Nutr. 2007;98:140–146. doi: 10.1017/S0007114507685201. [DOI] [PubMed] [Google Scholar]
- 11.Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AWM, BJ, Erdman JWJ. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823. [PubMed] [Google Scholar]
- 12.Nguyen ML, Schwartz SJ. Lycopene stability during food processing. Proc Soc Exp Biol Med. 1998;218:101. doi: 10.3181/00379727-218-44274. [DOI] [PubMed] [Google Scholar]
- 13.Shi J, Le Maguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Biotechnol. 2000;20:293. doi: 10.1080/07388550091144212. [DOI] [PubMed] [Google Scholar]
- 14.Xianquan S, Shi J, Kakuda Y, Yueming J. Stability of lycopene during food processing and storage. J Med Food. 2005;8:413. doi: 10.1089/jmf.2005.8.413. [DOI] [PubMed] [Google Scholar]
- 15.Lin CH, Chen BH. Stability of carotenoids in tomato juice during processing. Eur Food Res Technol. 2005;221:274. [Google Scholar]
- 16.Balasubramaniam VM, Farkas D. High-pressure food processing. Food Sci Technol Int. 2008;14:413–418. [Google Scholar]
- 17.Sánchez-Moreno C, Plaza L, de Ancos B, Cano MP. Impact of high-pressure and traditional thermal processing of tomato puree on carotenoids, vitamin C and antioxidant activity. J Sci Food Agric. 2006;86:171. [Google Scholar]
- 18.Krebbers B, Matser AM, Hoogerwerf SW, Moezelaar R, Tomassen MMM, van den Berg RW. Combined high-pressure and thermal treatments for processing of tomato puree: evaluation of microbial inactivation and quality parameters. Innovative Food Sci Emerging Technol. 2003;4:377–385. [Google Scholar]
- 19.Hsu K, Tan F, Chi H. Evaluation of microbial inactivation and physicochemical properties of pressurized tomato juice during refrigerated storage. LWT Food Sci Technol. 2008;41:367–375. [Google Scholar]
- 20.Qiu W, Jiang H, Wang H, Gao Y. Effect of high hydrostatic pressure on lycopene stability. Food Chem. 2006;97:516. [Google Scholar]
- 21.Nguyen LT, Tay A, Balasubramaniam VM, Legan JD, Turek EJ, Gupta R. Evaluating the impact of thermal and pressure treatment in preserving textural quality of selected foods. LWT Food Sci Technol. 2010;43:525–534. [Google Scholar]
- 22.Barringer SA. Vegetables: Tomato Processing. In: Smith JS, Hui YH, editors. Food Processing: Principles and Applications. Blackwell Publishing; Ames, IA: 2004. pp. 273–291. [Google Scholar]
- 23.Ferruzzi MG, Sander LC, Rock CL, Schwartz SJ. Carotenoid determination in biological microsamples using liquid chromatography with a coulometric electrochemical array detector. Anal Biochem. 1998;256:74–81. doi: 10.1006/abio.1997.2484. [DOI] [PubMed] [Google Scholar]
- 24.Ávila IMLB, Silva CLM. Modelling kinetics of thermal degradation of color in peach puree. J Food Eng. 1999;39:161–166. [Google Scholar]
- 25.Fish WW, Davis AR. The effects of frozen storage conditions on lycopene stability in watermelon tissue. J Agric Food Chem. 2003;51:3585. doi: 10.1021/jf030022f. [DOI] [PubMed] [Google Scholar]
- 26.Luh BS, Daoud HN. Effect of break temperature and holding time on pectin and pectic enzymes in tomato pulp. J Food Sci. 1971;36:1039. [Google Scholar]
- 27.Nguyen ML, Schwartz SJ. Lycopene: chemical and biological properties. Food Technol (Chicago) 1999;53:38. [Google Scholar]
- 28.Butz P, Tauscher B. Emerging technologies: chemical aspects. Food Res Int. 2002;35:279. [Google Scholar]
- 29.Nguyen M, Francis D, Schwartz SJ. Thermal isomerization susceptibility of carotenoids in different tomato varieties. J Sci Food Agric. 2001;81:910. [Google Scholar]
- 30.Ray K, Misra TN. Photophysical properties of lycopene organized in Langmuir-Blodgett films: formation of aggregates. J Photochem Photobiol, A. 1997;107:201. [Google Scholar]
- 31.Zechmeister L, Polgár A. Cis—trans isomerization and cis-peak effect in the α-carotene set and in some other stereoisomeric sets. J Am Chem Soc. 1944;66:137–144. [Google Scholar]
- 32.Agarwal A, Shen H, Agarwal S, Rao AV. Lycopene content of tomato products. Its stability, bioavailability, and in vivo anti-oxidant properties. J Med Food. 2001;4:9. doi: 10.1089/10966200152053668. [DOI] [PubMed] [Google Scholar]