Abstract
This study investigated the stability of saponins during the making and simulated digestion of soy and soy–chickpea breads and the bioaccessibility of saponins in digested breads. Recovery of saponins in soy bread exceeded that in soy–chickpea breads, and recovery of type A and B saponins was greater than for type E and DDMP saponins. Simulated digestion of breads resulted in greater relative losses of type A and DDMP saponins than type B and E saponins due in part to conversion of DDMP. Bioaccessibility of type B, E, and DDMP saponins in aqueous fraction of chyme exceeded 50%, but was ~30% for type A saponins. Caco-2 cells accumulated 0.8–2.8% of saponins from apical compartment containing diluted aqueous fraction of chyme. These findings suggest that saponin structure and food matrix affect the stability of saponins during processing and digestion and that uptake of saponins by enterocyte-like cells is poor despite moderate apparent bioaccessibility.
Keywords: saponins, retention during breadmaking, digestive stability, bioaccessibility, Caco-2 cells, soy–chickpea breads
INTRODUCTION
Saponins are a diverse class of glycosides commonly found in plants.1–3 These compounds derive their name from their ability to form stable, soap-like foams in aqueous solutions. Soy and chickpea are two of the richest sources of saponins in the diet, with saponin content of each accounting for 1–5.6 g/100 g seed dry weight.2,3 The structures of soy and chickpea saponins have been described previously.4,5 Briefly, soy contains four different types of saponins that are classified as follows: A, aglycone A and two sugar moieties, one of which is acetylated; B, aglycone B and one sugar moiety; E, oxidized aglycone B and one sugar moiety; and DDMP, aglycone B with one sugar moiety and a single 2,3-dihydro-2,5-dihydroxy-6 methyl-4H-pyranone unit.4,5 Chickpea contains mainly βg saponin (a DDMP type) and lower amounts of Bb and Be saponins.2
Health-promoting effects ascribed to soy saponins are similar to those associated with soy extracts, soy protein, and isoflavonoids in cell and animal models.1–13 These include reduced cholesterol absorption and protection from oxidative damage.1 Chickpea protein isolate, also rich in saponins, has been reported to lower plasma cholesterol in humans and animals.3 The basis for these activities remains unknown as the absorption of ingested saponins is very low. For example, Hu et al.14 reported that urine from adult females fed concentrated soy extract lacked detectable B group saponins or their aglycones. Approximately 9% of ingested saponins appeared in feces as aglycones. Conversion of saponin Bb to sapogenol B during anaerobic fermentation with human stool suggests that the gut microflora metabolizes dietary soy saponins.15 Studies with cultures of Caco-2 human intestinal cells further showed that apical uptake and transepithelial transport of soy saponin Bb during a 4 h incubation were quite limited (<3% from medium with Papp < 4 × 10−6 cm/s).15 Moreover, uptake of the aglycone sapogenol B was lower (<1%) than that of the glycoside. However, the possibility that absorption of limited quantities of saponins or their metabolites may modulate health-promoting processes is supported by the recent report that diosgenin, the aglycone of the most abundant saponin in fenugreek, decreased triglyceride accumulation in cultures of HepG2 cells by inhibiting transactivation of liver-X-receptor-α.16
Inclusion of soy and chickpea products in the U.S. diet remains relatively low. A strategy involving the incorporation of these ingredients into products commonly consumed in a Western diet represents a viable alternative for increasing soy and chickpea consumption in such populations. Legume-based ingredients are being used to develop breads as a potential means of decreasing the risk of cardiovascular disease.17,18 Processing conditions such as heat, pH, and solvents can affect saponin content and profile in breads.19 For example, DDMP type saponins are hydrolyzed to yield B type saponins and maltol during processing.3,20 Thus, the amount of saponins retained in breads is not known.
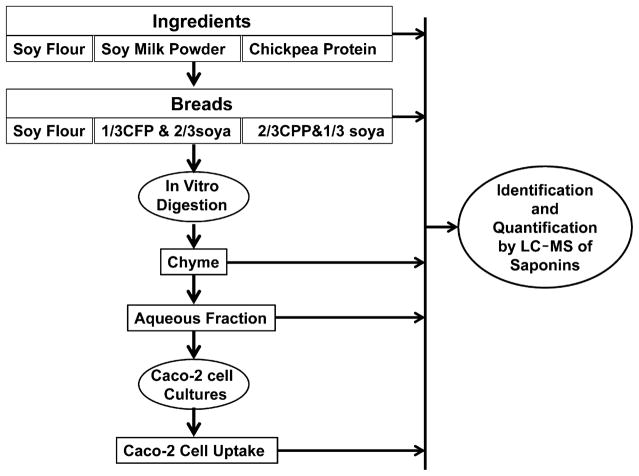
The objectives of the present study were to (1) assess the stability of 17 distinct type A, B, E, and DDMP saponins in soy and chickpea ingredients during the making of breads and the simulated digestion of breads and (2) determine the bioaccessibility of saponins in digested soy and chickpea breads by using a coupled in vitro digestion/Caco-2 human intestinal cell model. The influence of food matrix of saponin stability during these processes also was considered by comparing results for three breads produced with different amounts of soy and chickpea ingredients. The overall design of the study is outlined in Figure 1. The effects of addition of chickpea flour to soy formulation as a means of enriching the saponin content of the product on physicochemical properties and quality of breads will be presented elsewhere (Serventi and Vodovotz, manuscript in preparation).
Figure 1.
Schematic of the study. Parallel digestions with and without bile were performed.
MATERIALS AND METHODS
Materials
Baker’s soy flour was purchased from ADM Protein Specialties Division (Decatur, IL, USA), soy milk powder was purchased from Devansoy Farms (Carrol, IA, USA), and wheat flour (Magnifico Special) was purchased from Conagra Mills (Omaha, NE, USA). Chickpea protein isolate was prepared in Dr. Kerem’s laboratory at The Hebrew University of Jerusalem, Israel. Other ingredients included instant active dry yeast (Red Star; Universal Foods Corp., Milwaukee, WI, USA), salt, sugar, shortening (Crisco, Orrville, OH, USA), and gluten (Bob’s Red Mill, Milwaukee, OR, USA). Porcine pepsin, porcine lipase, porcine pancreatin, porcine bile extract, and porcine amylase were purchased from Sigma Chemical Co. (St. Louis, MO, USA). HPLC grade reagents used for ultrapressure liquid chromatography (UPLC) were purchased from Fisher Scientific (Fair Lawn, NJ, USA).
Preparation of Soy and Soy–Chickpea Breads
Soy bread was prepared using a modified version of the patented soy bread process.21 Briefly, HealthyHearth Baking Blend, consisting of two-thirds soy flour (Baker’s Soy Flour, ADM Protein Specialties Division) and one-third soy milk powder (Devansoy Farms) was incorporated into bread formula replacing 60% of wheat flour. Soy blend contained 50% protein, 28% carbohydrate, 9% fat, 5.5% moisture, 5% fiber, and 2.5% ash. Modifications included dough weight (120 g per loaf) and baking time (20 min).
An isolate of chickpea protein (50% protein, 37% carbohydrate, 5% moisture, 3% fat, 3% fiber, 2% ash) was introduced into the soy bread formulation to increase saponin content and to study the effect of matrix on saponin stability during breadmaking and digestion. Two additional breads were produced by replacing one-third and two-thirds of the soy blend with chickpea protein isolate. The breads produced with these mixed formulations are referred to as ⅓ CCP and ⅔ soy and ⅔ CCP and ⅓ soy, respectively.
In Vitro Digestion of Breads
Bread (1.0 g) containing either soy alone, one-third CCP and two-thirds soy, or two-thirds CCP and one-third soy was homogenized (Ultra Turrax 339619, Tekmar Co.) in 7 mL of phosphate-buffered saline (PBS) and transferred to 50 mL polypropylene tubes. The sample was subjected to simulated oral, gastric, and small intestinal digestion as described elsewhere.22,23 Upon completion of the small intestinal phase of digestion, an aliquot of chyme was centrifuged at 12000g and 4 °C for 45 min (Avanti J-E, Beckman, Palo Alto, CA, USA) to separate the aqueous fraction from undigested materials. Supernatant was passed through a syringe filter (0.2 μm pores) to collect the aqueous, that is, the bioaccessible, fraction. In a separate experiment, bile extract was deleted during the small intestinal phase of digestion to assess the role of mixed micelles in the partitioning of saponins in the aqueous fraction. Aliquots of chyme and aqueous fraction were stored under nitrogen gas at −80 °C until analysis.
Uptake of Micellar Saponins by Caco-2 Human Intestinal Cells
Caco-2 cells (HTB37, ATCC) were maintained in T75 cm3 flasks as previously described.22 Differentiated cultures of Caco-2 cells (11–14 days postconfluency; passages 26–28) were used for experiments. Aliquots of aqueous fractions from simulated digestion were diluted 1:4 with basal Dulbecco’s minimum essential medium (DMEM) and added to washed monolayers of Caco-2 cells. Cultures were incubated in a humidified atmosphere of 95% air and 5% CO2 at 37 °C for 4 h to examine cellular uptake of saponins. Medium was aspirated and monolayers were washed with ice-cold PBS containing 2 g albumin/L, followed by two washes with albumin-free cold PBS. Monolayers were scraped from the surface of culture flasks, collected in cold PBS, and centrifuged for 5 min at 100g and 4 °C. Supernatant was discarded, and cell pellets were stored under nitrogen gas at −80 °C until analysis. The molar quantity of each detected saponin in cell pellets was summed and presented as picomoles of total saponins per milligram of cell protein.
Extraction of Saponins from Samples
Extraction efficiency was determined by quantification of saponin concentration in three replicate samples for each type of bread. Because the majority of saponins were extracted from bread matrices in two steps, extraction efficiency represents the concentration of saponins in both extracts. Extraction efficiency of saponins from the three types of breads and from chyme generated during digestion ranged from 90 to 94%.
Breads
Breads were extracted using a modification of the procedure of Hu et al.14 Entire loaves of breads were processed to a fine paste with a grinder (Oskar Jr. Chopper Plus, Sunbeam, Boca Raton, FL, USA), and aliquots (150 mg) were mixed with 3 mL of 70% aqueous ethanol in 4 mL vials. Mixtures were sonicated (FS30H Fisher Scientific) for 2 min, passed through a nylon filter (0.2 μm, 13 mm, Alltech Associates Inc., Deerfield, IL, USA), and centrifuged (Eppendorf microcentrifuge 5424, Hauppauge, NY, USA) for 2 min prior to injection of supernatant onto the column of the LC-MS system.
Digested Breads
Chyme and filtered aqueous fractions of breads containing soy and soy–chickpea breads were thawed at room temperature and vortexed (model 231, Fisher Scientific) for 1 min. Aliquots of 300 μL were mixed with 700 μL of ethanol, sonicated for 2 min, filtered, and centrifuged prior to LC-MS analysis.
Medium and Caco-2 Cells
Fresh (0 h of incubation) and spent (after 4 h of incubation) media were extracted as described for digested bread samples. Washed cell pellets were resuspended in 500 μL of 70% aqueous ethanol, sonicated for 2 min, filtered, and centrifuged prior to LC-MS injection.
LC-MS Analysis
Analytical Conditions
Soy saponins were separated by reverse phase chromatography using a Waters Acquity UPLC, equipped with a binary pump, autosampler, column oven, and degasser (Acquity, Waters Corp., Milford, MA, USA). An Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm, Waters Corp.) was maintained at 40 °C, and a gradient solvent system of 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B) was delivered at a flow rate of 0.8 mL/min. The gradient elution was applied as follows: 0–2 min, 30.0–43.8% B; 2–2.8 min, 43.8–60.0% B; 2.8–3.8 min, 60.0–95.0% B; 3.8–5 min, re-equilibration to initial conditions. The injection volume was 1 μL for extracts from ingredients and breads, 5 μL for extracts from chyme and aqueous fractions, and 15 μL for extracts from media and cells with the autosampler maintained at 25 °C.
HPLC eluate was split approximately 1:10 and interfaced with a Quattro Ultima triple-quadrupole mass spectrometer (Micromass, Manchester, UK) operated in negative ion electrospray mode. Acquisitions were performed by selected ion recording (SIR). Seventeen channels were set (one for each soy saponin analyzed), and source conditions were as follows: capillary voltage, 2.8 kV; source temperature, 110 °C; desolvation temperature, 480 °C; cone gas flow rate, 102 L/h; desolvation gas flow rate, 801 L/h; and cone voltage, 50 V. High-purity nitrogen was used as desolvation and nebulizing gas and high-purity argon as collision gas (1.87 × 10−4 Pa). All data were acquired and peak areas integrated using Masslynx 4.1 software (Waters Corp., Beverly, MA, USA).
Preparation of Standards
Standards of saponins Ab and Bb were prepared as previously described.24 Saponin βg was prepared by solvent extraction of chickpea flour with a modified version of the method described by Hu et al.14 to achieve higher yield. Briefly, 15 g of chickpea flour was stirred in 100 mL of 70% aqueous ethanol at room temperature for 2.5 h, filtered, centrifuged, and dried by rotoevaporator and lyophilization. The dried powder was redissolved in 80% aqueous methanol as described previously,7,15 although at a higher concentration (100 vs 1 mg/mL). Solubilized extracts (1 mL) were passed through a nylon filter (0.2 μm) and centrifuged for 2 min before injection onto the semipreparatory HPLC column. Two solvents (solvent A, 0.05% trifluoroacetic acid in water; solvent B, 0.05% trifluoroacetic acid in acetonitrile) were used for isocratic elution (50% A, 50% B). The fraction containing saponin βg was identified by specific absorbance at both 205 and 292 nm (absorbance at 292 nm is due to the DDMP moiety) and eluted at 6 min. The obtained sample was immediately dried by applying vacuum in a rotoevaporator with temperature <30 °C and subsequently freeze-dried. Dry powder was collected in 4 mL vials, sealed with parafilm to avoid adsorption of moisture, and stored at −80 °C.
Statistical Analysis of Data
Three replicates of extracted ingredients and breads were analyzed by LC-MS. Five independent in vitro digestions were performed for the soy and soy–chickpea breads, and aliquots of chyme and aqueous fraction from each were analyzed. Four replicate cultures of Caco-2 cells were exposed to diluted aqueous fractions generated during digestion. Test medium, spent medium, and washed cells from each culture were analyzed. Data are expressed as the mean ± SD. Means were compared using one-way analysis of variance followed by Tukey’s post hoc test and independent t test. Statistical software used was SPSS v. 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Stability of Saponins during Making of Soy and Chickpea Breads
The first objective was to identify and quantify saponins in the ingredients before and after breadmaking.
Saponin sources in the breads were soy flour, soy milk powder, and chickpea protein isolate. Seventeen saponins (types A, B, E, and DDMP) were identified and quantified in soy flour and soy milk powder by LC-MS analysis (Table 1; Figure 2). Total saponin content of soy flour was 464 ± 77 μg/g. Type DDMP saponins were most abundant, followed by types A and B and a trace of E. Total saponin content of soy milk powder was twice that of soy flour (1025 ± 24 μg/g, Table 1). βg was the most abundant saponin in both soy flour and soy milk powder. Total saponin content in chickpea protein isolate was comparable to that of soy flour, although only seven saponins were identified and quantified in the chickpea ingredient. Saponins βg and Bb were most abundant in chickpea protein isolate, and type A saponins were not detected. Saponin content for the three types of breads was calculated by considering the formulation. Each of the three breads was expected to have a distinct profile of saponins, but the total quantity of saponins was expected to be similar (541–577 μg/g wet weight) (Table 1). Partial substitution of the soy ingredients with chickpea protein isolate decreased the amount of type A saponins and increased the amounts of type B, E, and DDMP saponins in the formulation for the breads (Table 1).
Table 1.
Saponin Content of Soy–Chickpea Ingredients and Estimated Saponin Content of Soy–Chickpea Breadsa
| saponin | ingredients
|
calculated saponin profile in breads
|
||||
|---|---|---|---|---|---|---|
| soy flour (μg/g) | soy milk powder (μg/g) | chickpea protein (μg/g) | soy (μg/g) | ⅓CPP and ⅔ soy (μg/g) | ⅔ CPP and ⅓ soy(μg/g) | |
| A type | 121 ± 22 | 248 ± 10 | ndb | 145 | 96 | 49 |
| Ab | 61 ± 9 | 136 ± 4 | nd | 76 | 51 | 25 |
| Ac | 14 ± 2 | 26 ± 1 | nd | 16 | 10 | 5.5 |
| Ad | 3.3 ± 0.5 | 6.7 ± 0.3 | nd | 3.9 | 2.6 | 1.4 |
| Af | 33 ± 5 | 61 ± 2 | nd | 38 | 25 | 13 |
| Ah | 9.8 ± 1.5 | 18 ± 0 | nd | 11 | 7.5 | 3.8 |
| B type | 31 ± 6 | 339 ± 8 | 157 ± 1 | 105 | 118 | 138 |
| Ba | 1.2 ± 0.2 | 17 ± 0 | nd | 5.1 | 3.4 | 1.7 |
| Bb | 21 ± 3 | 215 ± 4 | 155 ± 1 | 67 | 92 | 123 |
| Bb′ | 1.3 ± 0.2 | 15 ± 0 | 1.8 ± 0.0 | 4.5 | 3.6 | 2.6 |
| Bc | 7.2 ± 1.2 | 85 ± 2 | 1.6 ± 0.1 | 26 | 18 | 10 |
| Bc′ | 0.62 ± 0.1 | 6.2 ± 0.1 | nd | 1.9 | 1.3 | 0.6 |
| E type | 1.4 ± 0.3 | 25 ± 2 | 19 ± 0 | 7.1 | 10 | 15 |
| Bd E | 0.04 ± 0.03 | 1.8 ± 0.0 | nd | 0.48 | 0.32 | 0.16 |
| Be E | 1.4 ± 0.2 | 23 ± 0 | 19 ± 0 | 6.6 | 10 | 15 |
| DDMP type | 310 ± 65 | 414 ± 9 | 353 ± 9 | 320 | 320 | 340 |
| αg | 9.4 ± 1.9 | 0.22 ± 0.03 | nd | 6.8 | 4.5 | 2.4 |
| βg | 193 ± 31 | 263 ± 5 | 343 ± 8 | 200 | 238 | 293 |
| βa | 79 ± 14 | 116 ± 3 | 5.0 ± 0.4 | 84 | 57 | 31 |
| γa | 16 ± 7 | 26 ± 1 | 5.0 ± 0.2 | 18 | 13 | 9.3 |
| γg | 13 ± 9 | 8.9 ± 0.2 | nd | 11 | 7.5 | 3.8 |
| total | 464 ± 77 | 1025 ± 24 | 529 ± 8 | 577 | 543 | 541 |
Estimations accounted for amount of ingredients in recipes and loss of weight during baking.
nd, not detected.
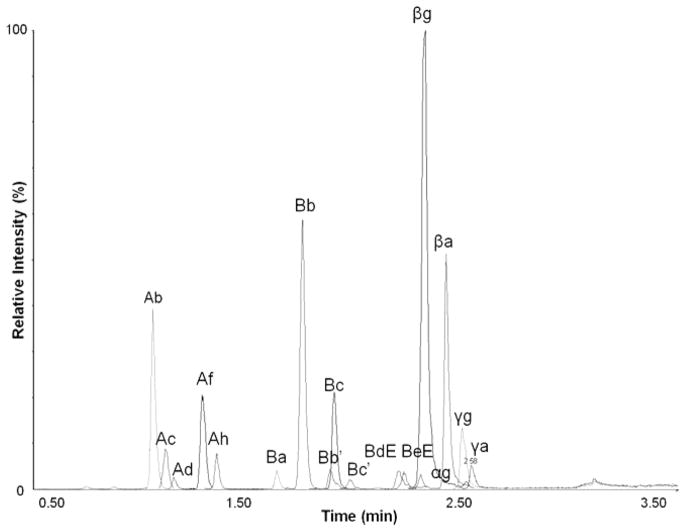
Figure 2.
LC-MS spectrum of saponins in soy flour (50 mg/mL, 1 μL injection). Peak labels represent specific saponins.
Saponin stability during the preparation of bread was determined by analyzing soy and soy–chickpea breads and comparing their saponin profile with the amount calculated for the starting ingredients. Weight loss upon baking was minimal, that is, 2.3, 3.4, and 0.9% for soy, ⅓ CPP and ⅔ soy, and ⅔ CPP and ⅓ soy breads, respectively. Recovery of total saponins was 83% in soy bread, but only 46% in ⅓ CPP and ⅔ soy bread and 42% for ⅔ CPP and ⅓ soy bread (Tables 1 and 2). Recovery of DDMP saponins in soy–chickpea breads was particularly low compared to that in the soy bread (~30 vs 78%, respectively; p = 0.018), as was that for type E saponins (~20 vs 50%, respectively; p = 0.006). The poor recovery of type E saponins in all three breads was likely due to the reactivity of the ketone group at position 22 of the triterpenoid aglycone.16 Loss of DDMP saponins during the preparation of the breads is attributed to the high reactivity of the maltol moiety. Heng et al.20 observed that the maltol moiety of DDMP saponins was readily hydrolyzed by temperatures exceeding 30 °C, slight acidity, and polar solvents such as water, which are all typical conditions applied for making bread. Degradation of DDMP saponins has been proposed to follow two possible pathways. Hydrolysis in media with high dielectric constant such as water containing starch favored generation of an ionized intermediate that undergoes molecular rearrangement with subsequent release of maltol and B saponin.20 The markedly greater loss of DDMP saponins in soy–chickpea breads compared to that in the soy bread suggests that one or more ingredients in the soy blend may have stabilized the acetal linkage during preparation of the soy bread. It is possible that the greater fat content in the soy bread is such a factor as chickpea protein isolate contained 3% fat, whereas soy blend contained 9% fat. The dielectric constant for soybean oil is much lower than that of common food starches (6.3 vs ~50 kV for corn starch),25,26 and this may have decreased formation of the intermediate of DDMP hydrolysis in soy bread. As there was no increase in type B saponins associated with loss of DDMP, degradation appeared to differ from that described by Heng et al.20 Because recovery of type B saponins in breads containing chickpea protein isolate compared to bread with soy alone was similar, degradation appears to have occurred by an alternative process such as reduction of the ketone group that is characteristic for DDMP and E saponins.
Table 2.
Saponin Profile in Baked Breads
| saponin | bread type
|
||
|---|---|---|---|
| soy (μg/g) | ⅓ CPP and ⅔ soy (μg/g) | ⅔ CPP and ⅓ soy (μg/g) | |
| A type | 130 ± 21 | 68 ± 17 | 40 ± 1 |
| Ab | 66 ± 10 | 35 ± 8 | 21 ± 1 |
| Ac | 15 ± 4 | 8.0 ± 2.2 | 4.5 ± 0.1 |
| Ad | 5.1 ± 0.9 | 2.6 ± 0.7 | 1.4 ± 0.0 |
| Af | 32 ± 5 | 16 ± 5 | 9.4 ± 0.1 |
| Ah | 11 ± 2 | 5.7 ± 1.4 | 3.1 ± 0.1 |
| B type | 92 ± 20 | 64 ± 19 | 83 ± 2 |
| Ba | 3.6 ± 0.8 | 1.7 ± 0.5 | 1.2 ± 0.04 |
| Bb | 59 ± 13 | 48 ± 14 | 71 ± 2 |
| Bb′ | 4.0 ± 1.1 | 2.0 ± 0.7 | 1.7 ± 0.2 |
| Bc | 24 ± 5 | 11 ± 3 | 8.0 ± 0.0 |
| Bc′ | 1.8 ± 0.6 | 0.83 ± 0.27 | 0.62 ± 0.01 |
| E type | 3.5 ± 0.7 | 2.4 ± 0.7 | 2.4 ± 0.1 |
| Bd E | 0.18 ± 0.06 | 0.08 ± 0.06 | 0.07 ± 0.02 |
| Be E | 3.3 ± 0.6 | 2.3 ± 0.5 | 2.4 ± 0.0 |
| DDMP type | 255 ± 63 | 116 ± 34 | 100 ± 2 |
| αg | 5.8 ± 3.0 | 2.4 ± 1.7 | 1.8 ± 0.0 |
| βg | 152 ± 37 | 83 ± 25 | 73 ± 2 |
| βa | 75 ± 17 | 21 ± 14 | 20 ± 0 |
| γa | 16 ± 4 | 7.2 ± 2.4 | 4.5 ± 0.0 |
| γg | 6.2 ± 1.7 | 2.8 ± 0.9 | 1.6 ± 0.0 |
| total | 480 ± 106 | 251 ± 69 | 225 ± 5 |
Stability of Saponins during Simulated Digestion of Breads
As information about the stability and bioaccessibility of saponins during transit through the gut is limited, samples of breads were subjected to simulated oral, gastric, and small intestinal digestion using a static model system. Recoveries of total saponins after completion of the small intestinal phase of digestion of samples of breads with soy, ⅓ CPP and ⅔ soy, and ⅔ CPP and ⅓ soy were 71, 88, and 61%, respectively (Tables 2 and 3). The extent of recovery was affected by both the type of saponin and matrix. Recovery of type B (68–119%) and E saponins (88–125%) in all breads exceeded those for type A (53–59%) and DDMP (59–66%) saponins. As recoveries of type B saponins in digested soy bread and ⅓ CPP and ⅔ soy bread were 103 and 119%, respectively, it is likely that there was some conversion of DDMP saponins to these products. Furthermore, it is possible that DDMP hydrolysis and partial oxidation of type B saponins contributed to the apparent stability of type E saponins during digestion. The lower recovery of type B saponins following digestion of the bread containing ⅔ CPP and ⅓ soy compared to the two breads with greater content of soy suggests that the soy ingredients in the bread provided a protective matrix. As type A saponins contain acetylated sugars, their relatively greater susceptibility than type B and E saponins to degradation during digestion may result from the low pH during the gastric phase and pancreatic enzyme activity during the small intestinal phase.16 It is unclear whether the slight increases in type B saponins after ingestion reflect greater stability to the digestive environment compared to type A and DDMP saponins or rather generation due to degradation of the relatively high quantities of DDMP saponins in the breads. Clarification will require simulated digestion of an extract rich in type B saponins.
Table 3.
Saponin Profile in Chyme Generated by Simulated Oral, Gastric, and Small Intestinal Digestion of Breads
| saponin | bread type
|
||
|---|---|---|---|
| soy (μg/g) | ⅓ CPP and ⅔ soy (μg/g) | ⅔ CPP and ⅓ soy (μg/g) | |
| A type | 76 ± 11 | 40 ± 3 | 21 ± 5 |
| Ab | 34 ± 6 | 22 ± 2 | 10 ± 3 |
| Ac | 4.8 ± 0.9 | 2.5 ± 0.2 | 1.6 ± 0.4 |
| Ad | 2.1 ± 0.3 | nda | nd |
| Af | 26 ± 5 | 11 ± 1 | 7.1 ± 2.2 |
| Ah | 8.2 ± 0.9 | 4.9 ± 0.5 | 2.0 ± 0.7 |
| B type | 95 ± 16 | 76 ± 7 | 56 ± 13 |
| Ba | 4.0 ± 0.8 | 2.3 ± 0.3 | 0.99 ± 0.28 |
| Bb | 59 ± 10 | 53 ± 5 | 46 ± 12 |
| Bb′ | 4.4 ± 0.9 | 3.0 ± 0.3 | 1.5 ± 0.4 |
| Bc | 26 ± 5 | 16 ± 2 | 7.1 ± 2.0 |
| Bc′ | 2.2 ± 0.4 | 1.3 ± 0.2 | 0.45 ± 0.29 |
| E type | 3.9 ± 0.8 | 3.0 ± 0.2 | 2.1 ± 0.6 |
| Bd E | 0.25 ± 0.06 | 0.15 ± 0.01 | 0.09 ± 0.01 |
| Be E | 3.6 ± 0.8 | 2.9 ± 0.3 | 2.3 ± 0.3 |
| DDMP type | 167 ± 39 | 76 ± 7 | 59 ± 17 |
| αg | 5.9 ± 1.6 | nd | 1.3 ± 0.4 |
| βg | 113 ± 29 | 70 ± 7 | 44 ± 14 |
| βa | 42 ± 11 | 28 ± 3 | 11 ± 4 |
| γa | 3.3 ± 0.8 | 1.9 ± 0.9 | nd |
| γg | 7.6 ± 2.3 | 3.6 ± 0.7 | 1.9 ± 0.7 |
| total | 342 ± 74 | 222 ± 21 | 138 ± 40 |
nd, not detected.
Bioaccessibility of Saponins
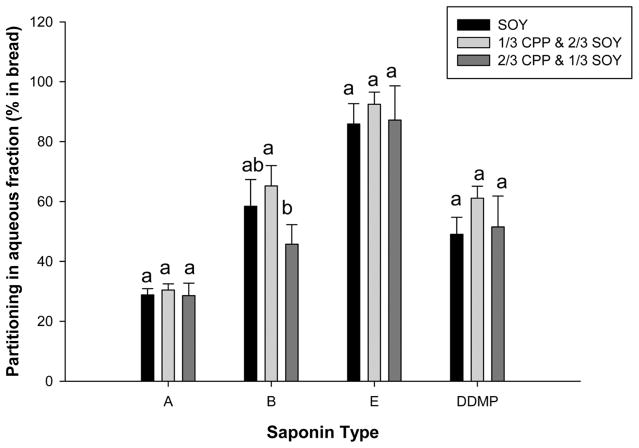
Bioaccessibility refers to the amount of constituent in a food or supplement that is released from the matrix during digestion to a potentially absorbable form.27,28 Centrifugation of the chyme after termination of the small intestinal phase of simulated digestion separated the aqueous or bioaccessible fraction from undigested material. The percentages of type A, B, E, and DDMP saponins transferred to the bioaccessible fraction of the digested breads were 30, 45–65, 86–91, and 51–61%, respectively, and relatively independent of the specific formulation of bread except for type B saponins (Figure 3). Partitioning of type DDMP saponins in the absence of bile was almost negligible (~2%), whereas deletion of bile extract during the small intestinal phase of digestion decreased the amount of type B and E saponins in the aqueous fraction by approximately 50%. These data suggest that type B and E saponins in the aqueous phase are located both external to and within mixed micelles. Unexpectedly, partitioning of type A saponins in the aqueous fraction of chyme lacking bile extract was more efficient than in the presence of bile salts during small intestinal digestion (50–60 vs ~30%, respectively) for the three breads. Because type A saponins contain two hydrophilic tails (monosaccharides) and an additional hydroxyl group, they are more hydrophilic than the other types of saponins.16,24 It is possible that the bidesmosidic structure of type A saponins facilitated aggregation with bile acids followed by formation of salts and precipitation as indicated by others.29 These data show that saponin structure influenced transfer to the aqueous fraction, whereas the composition of the bread minimally affected bioaccessibility.
Figure 3.
Partitioning (% in bread) of A, B, and DDMP saponins in the aqueous fraction of chyme generated during small intestinal digestion of breads in the presence of bile extract. Data are the mean ± SD, n = 5. Different letters above error bars indicate that recovery was significantly (α < 0.05) different in the different matrices.
Uptake of Bioaccessible Saponins from Digested Breads by Caco-2 Cells
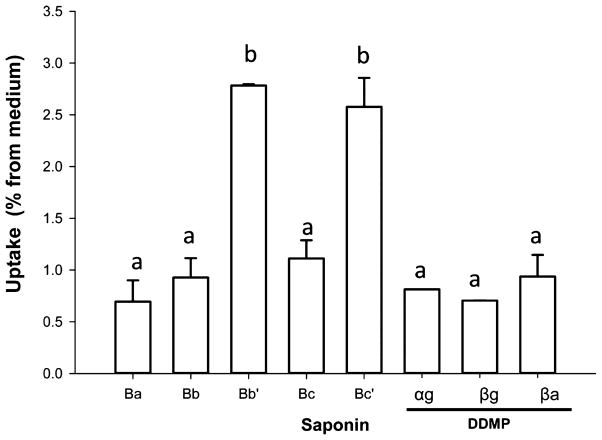
Because Caco-2 human intestinal cells spontaneously differentiate to an enterocyte-like pheno-type, this cell line is widely used to screen the uptake, metabolism, and transepithelial transport of dietary compounds and their metabolites.22,30,31 Relative uptake (%) of specific saponins from medium containing aqueous fraction generated during digestion of the three different breads was not significantly different (p > 0.05). Therefore, data for each saponin were pooled to simplify the presentation. Transfer of saponins from medium to cells was low (<3%) (Figure 4). Approximately 1% of type B and DDMP saponins in medium were detected in washed monolayers of cells and represented 35 and 60% of saponins, respectively, associated with cells. Relative uptake of Bb′ and Bc′ (2.8 and 2.6%, respectively) exceeded that of the other saponins. Bb′ and Bc′ saponins are the product of hydrolysis of rhamnose from Bb and Bc. Type A saponins were not detected in cells. As many phytochemicals rapidly and spontaneously degrade in cell culture medium,32,33 the stability of the saponins in DMEM was examined to assess the possibility that the apparently inefficient uptake might be due to degradation. Comparison of medium containing the diluted aqueous fraction of chyme generated during digestion of the breads at 0 h and after 4 h of incubation in the absence of cells revealed that DDMP and type B saponins were stable in the cell culture environment (~100% recovery). This inefficient uptake of the various saponins by Caco-2 cells in this study is similar to that previously reported by Hu et al. for saponin Bb.15 These investigators hypothesized that the low extent of uptake of saponins in the intestine may be due to their degradation to other compounds. A possible route of degradation occurring during intestinal uptake is the hydrolysis of sugar moieties to generate sapogenols A, B, and E and their subsequent modification into molecules of unknown structure as a result of chemical or microbial degradation.14,15,19 Moreover, it is unclear if the low amounts of the saponins associated with the monolayer may reflect nonspecific trapping or binding to the cell surface, incorporation and/or transfer into the brush border membrane, or some combination of these possibilities. Further investigation is required to address these possibilities and potential effects on absorptive cell functions.
Figure 4.
Uptake (% from medium) of type B and DDMP saponins by Caco-2 cells. Cultures of Caco-2 cells were exposed to the aqueous fraction generated during simulated digestion of soy and soy–chickpea breads. Data are the mean ± SD for pooled results for indicated saponin in medium containing 25% aqueous fraction after digestion of the three breads (n = 5 independent cultures for each digested bread; n = 15 total cultures). Different letters above bars indicate significant differences in the extent of uptake of the various saponins (α < 0.05).
Acknowledgments
Funding: This research was funded by Binational Agricultural Research and Development Fund (BARD) Grant IS-4072-07, the Ohio Agricultural Research and Development Center, and the OSU Center for Innovative Food Technology. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
ABBREVIATIONS USED
- DDMP
2,3-dihydro-2,5-dihydroxy-6 methyl-4H-pyranone
- DMEM
Dulbecco’s minimum essential medium
- PBS
phosphate-buffered saline
- LC-MS
liquid chromatography–mass spectrometry
Footnotes
The authors declare no competing financial interest.
References
- 1.Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 2.Kerem Z, German-Shashoua H, Yarden O. Microwave-assisted extraction of bioactive saponins from chickpea (Cicer arietinum L.) J Sci Food Agric. 2005;85:406–412. [Google Scholar]
- 3.Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y. Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food. 2004;7:67–78. doi: 10.1089/109662004322984734. [DOI] [PubMed] [Google Scholar]
- 4.Price KR, Fenwick GR, Jurzysta M. Soysapogenols – separation, analysis and interconversions. J Sci Food Agric. 1986;37:1027–1034. [Google Scholar]
- 5.Yoshiki Y, Kudou S, Okubo K. Relationship between chemical structures and biological activities of triterpenoids saponins from soybean (review) Biosci, Biotechnol Biochem. 1998;62:2291–2299. doi: 10.1271/bbb.62.2291. [DOI] [PubMed] [Google Scholar]
- 6.Kensil CR. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carrier Syst. 1996;13:1–55. [PubMed] [Google Scholar]
- 7.Berhow MA, Cantrell CL, Duval SM, Dobbins TA, Maynes J, Vaughn SF. Analysis and quantitative determination of group B saponins in processed soybean products. Phytochem Anal. 2002;13:343–348. doi: 10.1002/pca.664. [DOI] [PubMed] [Google Scholar]
- 8.Price KR, Johnson IT, Fenwick GR. The chemistry and biological significance of saponins in foods and feeding stuffs. Crit Rev Food Sci Nutr. 1987;26:27–135. doi: 10.1080/10408398709527461. [DOI] [PubMed] [Google Scholar]
- 9.Shiraiwa M, Harada K, Okubo K. Composition and structure of “group B saponin” in soy seed. Agric Biol Chem. 1991;55:911– 917. [PubMed] [Google Scholar]
- 10.Shiraiwa M, Kudo S, Shimoyamada M, Harada K, Okubo K. Composition and structure of “group A saponin” in soybean seed. Agric Biol Chem. 1991;55:315–322. [PubMed] [Google Scholar]
- 11.Campos-Vega R, Loarca-Piña G, Oomah D. Minor components of pulses and their potential impact on human health. Food Res Int. 2010;43:461–482. [Google Scholar]
- 12.Kerwin SM. Soy saponins and the anticancer effects of soybean and soy-based foods. Curr Med Chem. 2004;4:263–272. doi: 10.2174/1568011043352993. [DOI] [PubMed] [Google Scholar]
- 13.Rao AV, Sung MK. Saponins as anticarcinogens. J Nutr. 1995;125:717S–724S. doi: 10.1093/jn/125.3_Suppl.717S. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Zheng YL, Hyde W, Hendrich S, Murphy P. Human fecal metabolism of soysaponin I. J Agric Food Chem. 2004;52:2689–2696. doi: 10.1021/jf035290s. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Reddy MB, Hendrich S, Murphy PA. Soysaponin I and sapongenol B have limited absorption by Caco-2 intestinal cells and limited bioavailability in women. J Nutr. 2004;134:1867–1873. doi: 10.1093/jn/134.8.1867. [DOI] [PubMed] [Google Scholar]
- 16.Uemura T, Goto T, Kang MS, Mizoguchi N, Hirai S, Lee JY, Nakano Y, Shono J, Hoshino S, Taketani K, Tsuge N, Narukami T, Makishima M, Takahashi N, Kawada T. Diosgenin, the main aglycone of fenugreek, inhibits LXRα activity in HepG2 cells and decreases plasma and hepatic triglycerides in obese diabetic mice. J Nutr. 2011;141:17–23. doi: 10.3945/jn.110.125591. [DOI] [PubMed] [Google Scholar]
- 17.Nilufer D, Boyacioglu D, Vodovotz Y. Functionality of soymilk powder and its components in fresh soy bread. J Food Sci. 2008;73:275–281. doi: 10.1111/j.1750-3841.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 18.Vittadini E, Vodovotz Y. Changes in the physicochemical properties of wheat- and soy-containing breads during storage as studied by thermal analyses. J Food Sci. 2003;68:2022–2027. [Google Scholar]
- 19.Güçlü-Üstündağ Ö, Mazza G. Saponins: properties applications and processing. Crit Rev Food Sci Nutr. 2007;47:231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- 20.Heng L, Vincken JP, Hoppe K, van Konigsveld GA, Decroos K, Gruppen H, van Boekel MAJS, Voragen AGJ. Stability of pea DDMP saponin and the mechanism of its decomposition. Food Chem. 2006;99:326–334. [Google Scholar]
- 21.Vodovotz Y, Ballard C. Composition and process for making high soy protein-containing bakery products. 7,592,028 B2. US Patent. 2009
- 22.Chitchumroonchokchai C, Schwartz SJ, Failla ML. Assessment of lutein bioavailability from meals and a supplement using simulate digestion and Caco-2 human intestinal cells. J Nutr. 2004;134:2280–2286. doi: 10.1093/jn/134.9.2280. [DOI] [PubMed] [Google Scholar]
- 23.Thakkar S, Maziya-Dixon B, Dixon AGO, Failla ML. β-Carotene micellarization during in vitro digestion and uptake by Caco-2 cells is directly proportional to β-carotene content in different genotypes of cassava. J Nutr. 2007;137:2229–2233. doi: 10.1093/jn/137.10.2229. [DOI] [PubMed] [Google Scholar]
- 24.Berhow MA, Kong SB, Vermillion KE, Duval SM. Complete quantification of group A and B soysaponins in soybeans. J Agric Food Chem. 2006;54:2035–2044. doi: 10.1021/jf053072o. [DOI] [PubMed] [Google Scholar]
- 25.Cannon GS, Honary LAT. Soybean based transformer oil and transmission line fluid. 5,958,851. US Patent. 1999 Sep 28;
- 26.Miller LA, Gordon J, Davis EA. Dielectric and thermal transition properties of chemically modified starches during heating. Cereal Chem. 1991;68:441–448. [Google Scholar]
- 27.Failla ML, Chitchumroonchokchai C. In vitro models as tools for screening the relative, bioavailabilities of provitamin A carotenoids in foods. HarvestPlus Technical Monograph Series. 2005;3:32. www.harvestplus.org/pubs.html. [Google Scholar]
- 28.Fernandez-Garcia E, Carvajal-Lerida I, Perez-Galvez A. In vitro bioaccessibilty assessment as a prediction tool of nutritional efficiency. Nutr Res (NY) 2009;29:751–760. doi: 10.1016/j.nutres.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 29.Hwang D, Damodaran S. Selective precipitation of fat globule membranes of cheese whey by saponin and bile salt. J Agric Food Chem. 1994;42:1872–1878. [Google Scholar]
- 30.Delie F, Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit Rev Ther Drug Carrier Syst. 1997;14:221–286. [PubMed] [Google Scholar]
- 31.Tremblay E, Auclair J, Delvin E, Levy E, Menard D, Pshezhetsky AV, Rivard N, Seidman EG, Sinnett D, Vachon PH, Beaulieu JF. Gene expression profiles of normal proliferating and differentiating human intestinal cells: a comparison with the Caco-2 cell model. J Cell Biochem. 2006;99:1175–1186. doi: 10.1002/jcb.21015. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys. 2008;476:107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 33.Long LH, Hoi A, Halliwell B. Instability of, and generation of hydrogen peroxide by, phenolic compounds in cell culture media. Arch Biochem Biophys. 2010;501:162–169. doi: 10.1016/j.abb.2010.06.012. [DOI] [PubMed] [Google Scholar]