Abstract
In plants, folate occurs predominantly as 5-methyltetrahydrofolate (5MTHF) polyglutamyl forms. Differences in stability and bioavailability of food folate compared to synthetic folic acid have been attributed to the presence of the polyglutamyl chain. High-pressure processing (HPP) was tested for whether it might shorten polyglutamyl chains of 5MTHF species in fresh vegetables by enabling action of native γ-glutamylhydrolase (GGH). A validated ultrahigh-performance reversed-phase liquid chromatography–tandem mass spectrometry method using stable isotope as internal standard was applied for characterizing 5MTHF polyglutamyl profiles. HPP conditions included 300, 450, and 600 MPa at 30 °C for 0 or 5 min, and vegetables were vacuum-packed before treatment. Investigated vegetables included cauliflower (Brassica oleracea), baby carrots (Daucus carota), and carrot greens (D. carota). HPP treatment caused conversion of polyglutamyl 5MTHF species to short-chain and monoglutamyl forms. Maximal conversion of polyglutamyl folate to monoglutamyl folate occurred at the highest pressure/time combination investigated, 600 MPa/30 °C/5 min. Under this condition, cauliflower monoglutamyl folate increased nearly 4-fold, diglutamyl folate 32-fold, and triglutamyl folate 8-fold; carrot monoglutamyl increased 23-fold and diglutamyl 32-fold; and carrot greens monoglutamyl increased 2.5-fold and the diglutamyl form 19-fold. Although some folate degradation was observed at certain intermediate HPP conditions, total 5MTHF folate was largely preserved at 600 MPa/5 min. Thus, HPP of raw vegetables is a feasible strategy for enhancing vegetable monoglutamate 5MTHF.
Keywords: polyglutamyl 5-methyltetrahydrofolate, deglutamylation, γ-glutamyl hydrolase, high-pressure processing
INTRODUCTION
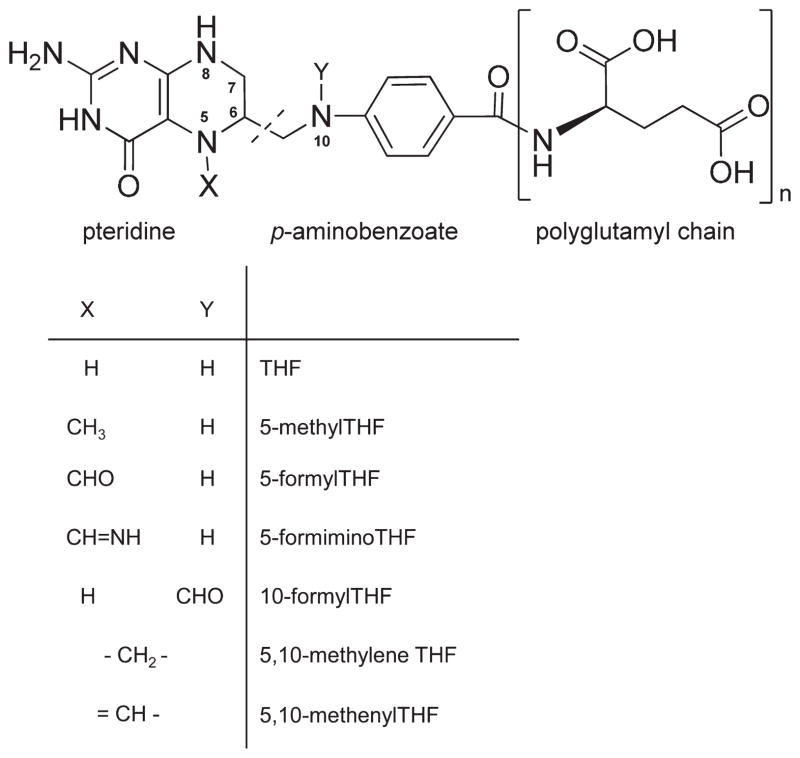
Folate belongs to the water-soluble B group vitamins. Chemically, folate consists of three parts: pteridine, p-aminobenzoate, and a glutamyl chain (from 1 to 14) (Figure 1). It participates in one-carbon metabolism by supplying one-carbon units as essential coenzymes.1 Accumulated evidence from laboratory and human investigations supports that “low” folate status enhances some chronic diseases such as cardiovascular disease, colon cancer, and anemia as well as the risk of neural tube defects.2 Mandating folic acid fortifications in some developed countries has created a rising concern about folic acid fortification for its possible antagonism against anticancer drugs,3 masking of B12 deficiency,4 promotion of existing tumors,5 and exposure to unmetabolized folic acid in the bloodstream.6 As an alternative, 5-methyltetrahydrofolate (5MTHF) has been accepted for folate fortification by the European Union.7 Because vegetables are critical contributors for natural folate intake worldwide, increasing vegetable consumption may still be the best way to optimize folate status without health concerns.
Figure 1.
Chemical structures of folate species and polyglutamyl folate. Folate consists of a pteridine moiety, p-aminobenzoate, and a glutamyl tail of variable length. Folates can differ in the oxidation state of the pteridine and C1 substituent. Oxidation of reduced pteridine can give dihydrofolate (double bond C7–N8) and folic acid (double bonds C7–N8 and C6–N5).
The bioavailability of food folate is usually inferior compared to that of synthetic folic acid, and the polyglutamate conjugate is regarded as an important limiting factor.8,9 Using HPLC methods, it was found that the 5MTHF species were the major folate forms in plants, accounting for 28–90% of total folate in vegetables and biofortified vegetables.10–15 It is well-known that folates are sensitive to oxidative degradation, resulting in spontaneous cleavage of the molecule into biologically inactive forms (pterin and p-aminobenzoate). This makes folate in the reduced state vulnerable to degradation during high-pressure processing (HPP) and thermal treatment.16 Several studies have investigated the effect of polyglutamyl chain length on folate bioavailability in humans. Clinical studies have found conflicting results where monoglutamyl folate has greater bioavailability than polyglutamyl folate17,18 or that there was no difference.19,20 Of these, the study by Melse-Boonstra et al.17 may provide the clearest evidence that the inherent bioavailability of 5MTHF is greater than that of hexaglutamyl 5MTHF. Because the folates were administered in pill form, it was not possible to comment on the potential modulating role of the food matrix in the process. Indeed, other studies suggest that deconjugation of the polyglutamyl chain may not be the limiting factor for folate absorption, and the food matrix, folate entrapment, γ-glutamylhydrolase (GGH) inhibitors, folate instability in the digestive system, and genetic polymorphism are critical variables.20,21 Nevertheless, it may be desirable to apply processes that convert food polyglutamyl folates to their respective monoglutamyl forms to improve folate bioavailability.
GGH is a ubiquitous enzyme existing not only in animals and microorganisms but also in plants.22 Interestingly, polyglutamyl folates colocalize with GGH in the plant cell vacuole yet polyglutamyl folate is maintained, apparently due to protein binding.23 Upon cell decompartmentalization of fresh vegetables polyglutamyl folates are accessible to GGH, and they can be hydrolyzed to short-chain or monoglutamyl folate. Cell disruption can occur by different processes including crushing tissue, freeze–thaw cycling and juicing. However, in commercial production vegetables are often blanched or steamed, which largely inactivates GGH such that long-chain polyglutamyl folates are preserved.
HPP as an alternative food preservation technique has attracted a lot of research attention over the past 20 years as a means of extending food shelf life.24,25 Elevated pressures in combination with heat treatment can achieve the same effect as traditional heat treatment in terms of pasteurization and sterilization for microbial and enzyme inactivation.26 HPP technology has been applied as a pasteurization method for a series of food products, including fruits and vegetables, using pressure–temperature–time combinations of 600 MPa and 20–45 °C for various food products.27 Due to less thermal stress, this technique often better retains nutrients and the general quality of fresh fruits and vegetables compared to traditional thermal treatments.28–30 By virtue of its unique pressure condition, HPP might also be able to effect novel transformations of phytochemicals not possible with current commercial technologies.31
Several researchers have shown that 5MTHF polyglutamates are hydrolyzed under HPP conditions for a variety of vegetables and fruits.32–34 However, these observations may be in part an artifact of sample handling and workup. It has been shown in a previous study15 that if vegetables were not boiled or steamed prior to extraction, a large portion of polyglutamyl folate species was deglutamylated. This is most likely related to the action of GGH. In these previous studies32–34 samples were usually homogenized at room temperature or in liquid nitrogen followed by boiling extraction, and in the time delay for the sample to reach GGH-inactivating temperature, deglutamylation could have occurred. This effect is most prominent for polyglutamyl folate profiles of untreated vegetables in which GGH activity is highest or, in other words, for control vegetable samples in a processing study.
The study described here involved evaluating the impact of high-pressure–temperature–time combinations on the levels and profiles of polyglutamyl 5MTHF chain lengths in cauliflower, carrot, and carrot greens using our recently described HPLC-MS/MS method.15 Three different pressures (300, 450, and 600 MPa) and two holding times (0 and 5 min) were investigated at 30 °C.
MATERIALS AND METHODS
Chemicals
LC-MS grade water and acetonitrile were obtained from Fisher Scientific (Fair Lawn, NJ), formic acid (99%, purity) and glacial acetic acid were from Acros (Morris Plains, NJ); ammonium acetate was from Mallinckrodt Baker, Inc. (Phillipsburg, NJ); ascorbic acid (99%, crystalline) was from Sigma (St. Louis, MO); 2-mercaptoethanol was obtained from Bio-Rad (Hercules, CA); amylase was from Fluka, 1065 (St. Louis, MO); Pronase was from Calbiochem, 53702 (San Diego, CA); rat serum was from Sigma, S9759; and sodium borohydride was from Sigma, S9125.
Standards (6R,S)-5-methyl-5,6,7,8-tetrahydropteroyldi-γ-L-glutamate, (6R,S)-5-methyl-5,6,7,8-tetrahydropteroyltri-γ-L-glutamate, (6R,S)-5- methyl-5,6,7,8-tetrahydropteroyltetra-γ-L-glutamate, (6R,S)-5-methyl-5,6,7,8-tetrahydropteroylpenta-γ-L-glutamate, pteroylhexa-γ-L-glutamic acid, pteroylhepta-γ-L-glutamic acid, ammonium salt, and 10-formylfolic acid were purchased from Schirck’s Laboratories (Jona, Switzerland). Standards (6R,S)-5-methyl-5,6,7,8-tetrahydrofolate, sodium salt; (6S)-5-formyltetrahydrofolate, sodium salt; (6R)-10-formyltetrahydrofolate; (6R)-5,10-methenyltetrahydrofolate, –Cl × HCl; (6R)-5,10-methylenetetrahydrofolate, sodium salt; (6S)-tetrahydrofolate, sodium salt; pteroylglutamic acid, sodium salt; and 7,8-dihydrofolate were gifts from Merck Eprova AG (Schaffhausen, Switzerland). (6S)-5-Methyl-5,6,7,8-tetrahydrofolate-[13C5] Glu, calcium salt, was a gift from Abbott Nutrition (Columbus, OH).
5MTHF hexaglutamyl, 5MTHF heptaglutamyl, and 10-formyldihydrofolate stock solutions were prepared and identified by spectra and MS/MS transition.15 All of the other folate species and 5-MTHF polyglutamyl folates were identified by MS/MS transition and retention time coincident with those of authentic standards.
Vegetable Sample Preparation
The vegetables investigated were cauliflower and baby carrot with greens attached. All vegetables were purchased from Whole Foods, a local supermarket in Columbus, OH, and kept refrigerated until same-day processing. Carrot roots and greens were separated by slicing with a knife prior to processing. The cauliflower floret, carrot, and carrot greens (ca. 5 g) were packaged individually and sealed by a vacuum sealer (Ultravac, UV 250, Koch Supplies Inc., Kansas City, MO) in a clear nylon/EVOH/polyethylene pouch with high barrier properties (Win-Pak Ltd., Winnipeg MB, Canada). Due to the rigidity of carrot roots, the pouch containing carrot could be easily punctured during HPP treatment, and double bagging was conducted to prevent this.
High-Pressure Processing
All of the HPP treatments were carried out using a 5 L capacity, Iso-Lab high-pressure food processor (Stansted Fluid Power Ltd., Essex, U.K.). USP kosher polypropylene glycol (Brenntag Mid-South, Inc., St. Louis, MO) was used as the pressure-transmitting fluid. Three pressures at two different hold times were investigated: 300, 450, and 600 MPa at 30 °C for either 0 or 5 min holding time. The pressure vessel was maintained at ambient temperature (25 °C). When 0 min holding time is indicated, samples were brought to pressure/temperature in the HPP unit, held at that pressure for 1 s, and immediately depressurized. The HPP system is fully programmable, allowing reproducible pressure buildup and decompression times. Pressure come-up time was 75 s, and decompression time was 60 s.
During HPP, the temperature of high moisture content foods generally increases about 3 °C for every 100 MPa of compression.35 To achieve similar final process temperatures (30 °C) under pressure, prior to the treatment the samples were prechilled at 18, 12, and 4 °C for the 300, 450, and 600 MPa treatments, respectively. The samples were then loaded into a stainless steel cylindrical product holder (11 cm × 65.5 cm) insulated with 0.5 cm polytetrafluoroethylene (PTFE). The product holder was then filled with prechilled polypropylene glycol and loaded into the pressure vessel. Time to load and unload the samples into the pressure vessel was standardized to 180 s. The average sample process temperature under pressure was 30–27 °C. Three analytical replicates were performed for each pressure/time condition, and each treatment was conducted in duplicate for a total of six replicates. The sample pouches were withdrawn from the pressure chamber and rinsed with water before they were subjected to steaming to stabilize polyglutamyl folates.
Stabilization of Polyglutamyl Folates with Steaming
To understand the changes occurring to polyglutamyl folates during HPP, polyglutamyl distribution needed to be stabilized immediately after HPP and artifacts could not be introduced through sample handling that would not necessarily be due to HPP. Steam inactivation of GGH was chosen as it was effective in a previous study.15 Because the vegetables were steamed without a vacuum bag in Wang et al.,15 steaming samples in vacuum bags was tested whether it was an effective means of stabilizing the polyglutamyl folates. By keeping the bags sealed during steaming, possible losses of leachate after HPP treatment was avoided.32 For reference and HPP-treated samples, the sample pouches were withdrawn from the pressure chamber, rinsed with water, and steamed for 10 min suspended above boiling water in a covered pot. The samples were then immediately chilled in ice–water and analyzed within 2 h of processing as outlined in the following section.
Folate Extraction and Determination
All folate standards and chemical reagents employed in this study and sample preparation for extracting 5MTHF polyglutamyls in cauliflower are described by Wang et al.15 For carrot and carrot greens, because the polyglutamyl folate profiles of these two vegetables have not been characterized before, presteaming time (0, 10, and 20 min) was optimized before the extraction protocol was carried out. Satisfactory inactivation was recognized when extended steaming time had no effect on the polyglutamyl 5MTHF profile and total 5MTHF content.
To confirm if 5MTHF is the predominant species in processed vegetables, folate species distribution in three nonprocessed vegetables were determined because no papers in the literatures report consistent distribution of folate species in the same vegetable.10,32,36 The distribution of folate species in carrot greens has not been reported previously. 5MTHF, 5MTHF polyglutamates, and other folate species were determined following another paper.15
Data Analysis
Data in the figures and text are expressed as the mean ± standard deviation (n = 6). Significant difference was determined by ANOVA with Tukey’s post hoc test. A significant difference between means was considered to be present when p < 0.05 (Minitab 15.0 Inc., State College, PA).
RESULTS AND DISCUSSION
Folate Species Distributions
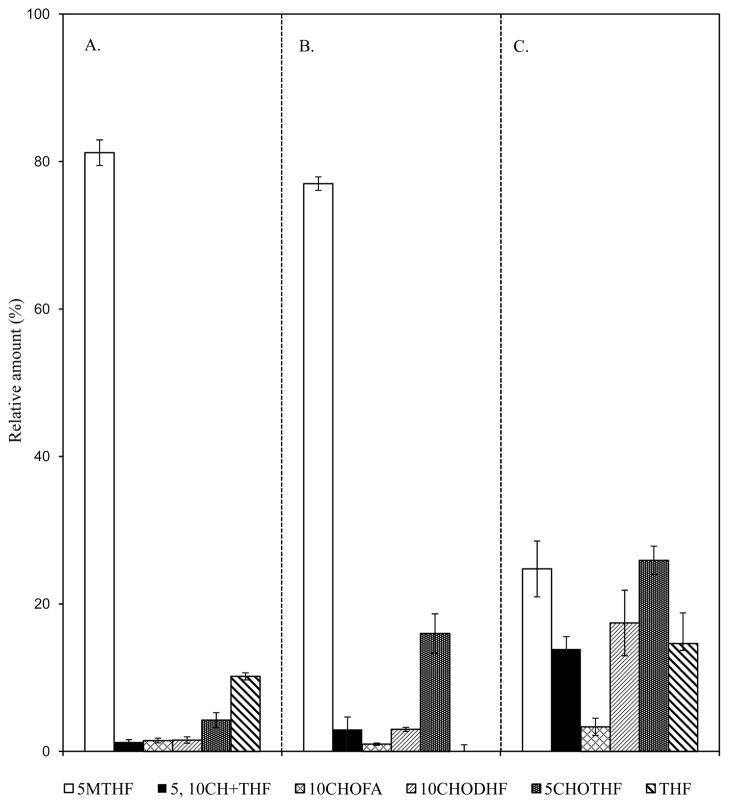
The distributions of folate species in the reference (no HPP) carrot, carrot greens, and cauliflower samples are presented in Figure 2. It was found that folates in cauliflower and carrot consisted of 77–81% 5MTHF and very low concentrations of 5,10-methenyltetrahydrofolate (5,10-CH+THF) (1.3–3%), 10-formylfolic acid (10-CHOFA) (1–1.5%), 10-formyldihydrofolate (10-CHODHF) (1.6–3%), 5-formyltetrahydrofolate (5-CHOTHF) (4.2–16%), and tetrahydrofolate (THF) (0–10%). However, in carrot greens, it was found that folates occurred as a much more diverse set of folate species with 5MTHF (25%), 5-CHOTHF (26%), 10-CHODHF (17%), 5,10-CH+THF (14%), THF (15%), and 10-CHOFA (3%). The observed folate species in carrot and cauliflower were consistent with available literature results,10,11 in which 5MTHF is the predominant form, whereas the distribution in carrot greens had not been reported previously. Because 5MTHF was a major folate species in all three vegetables, this study focused on the effect of HPP on the 5MTHF polyglutamyl profile.
Figure 2.
Distribution of folate species in raw vegetables: (A) carrot (Daucus carota); (B) cauliflower (Brassica oleracea); (C) carrot greens (D. carota). Folate species were determined by external calibration with authentic standards using HPLC-MS/MS. The mobile phase included formic acid, and thus 5,10-CH+THF represents (5,10-CH+THF + 10-formyltetrahydrofolate (10-CHOTHF)) and THF represents (THF + 5,10-methylenetetrahydrofolate (5,10-CH2THF)). Data represent the mean of three replicates.
Stabilizing 5MTHF Polyglutamates after HPP
It is expected that during HPP processing the polyglutamyl 5MTHF profile and folate content would change due to disruption of tissue and action of endogenous GGH. To preserve the folate species, vegetables were steamed immediately after HPP. In previous work it was established that steaming and extraction in reducing buffer are an effective means of inactivating GGH and chemically preserving polyglutamyl species, respectively, for many vegetables.15 This preanalysis steaming treatment was also optimized here for carrots and carrot greens because they were not part of our earlier study. Steaming time was optimized according to achievement of a consistent polyglutamyl profile and level with increasing time for test times of 0, 10, and 20 min.
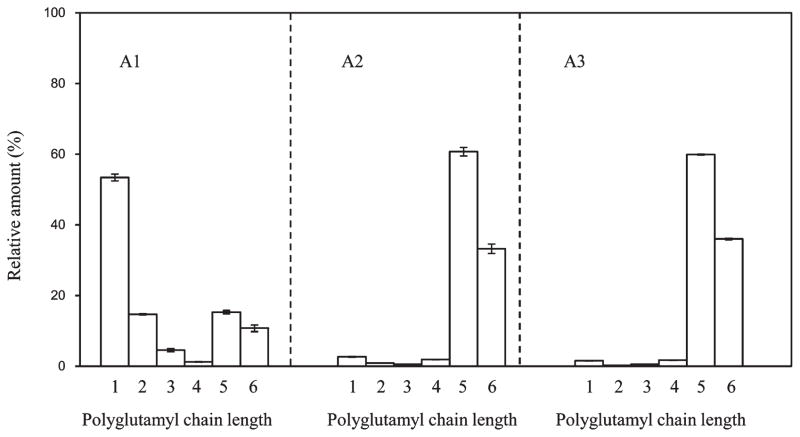
Figure 3 shows that 5MTHF-Glu5 and 5MTHF-Glu6 were enhanced with increased steaming time in carrot. Comparing no steaming to a 10 min steaming showed that the relative contribution of 5MTHF decreased by 95%, 5MTHF-Glu2 by 94%, and 5MTHF-Glu3 by 88%, whereas the percentage of 5MTHF-Glu5 increased 3-fold and 5MTHF-Glu6 2-fold. Steaming for 20 min achieved the same profile as steaming for 10 min. Total 5MTHF increased by 47% when 10 min of steaming was compared to no steaming, and the level was stable through 20 min. For carrot greens no significant change of polyglutamyl folate profiles was observed with steaming, whereas total folate in carrot greens was improved 59% with steaming for 10 min and was the same as steaming for 20 min. Thus, presteaming for 10 min was applied to all of the vegetables after HPP treatments.
Figure 3.
Effect of steaming time on the polyglutamyl 5-methyltetrahydrofolate (5MTHF-Glun) species of carrot (D. carota): (A1) no steaming; (A2) steaming for 10 min; (A3) steaming for 20 min. The folate levels were determined by HPLC-MS/MS based on the methods reported by Wang et al.15 Data represent the mean of three replicates.
As discussed in our recent paper,15 steaming seemed to not only inactivate GGH and thereby stabilize the polyglutamyl profile but also denature folate-binding proteins (FBP), improving recovery. Thus, the apparent increase in total folate for both carrot and carrot greens with steaming is correctly viewed as an increase in folate recovery. In effect, steaming replaces the trienzyme (amylase, protease, (de)conjugase) treatment, includes a deglutamylation step (by serum deconjugase), and does not allow the determination of folate polyglutamates. In fact, steaming here is a replacement of treatment with two enzymes, because deglutamylation is not wanted. The lack of effect of steaming on the 5MTHF polyglutamyl profile of carrot greens suggests that GGH activity was very low, whereas carrot roots contain significant GGH activity.
Influence of HPP on the Polyglutamyl Profile of 5MTHF
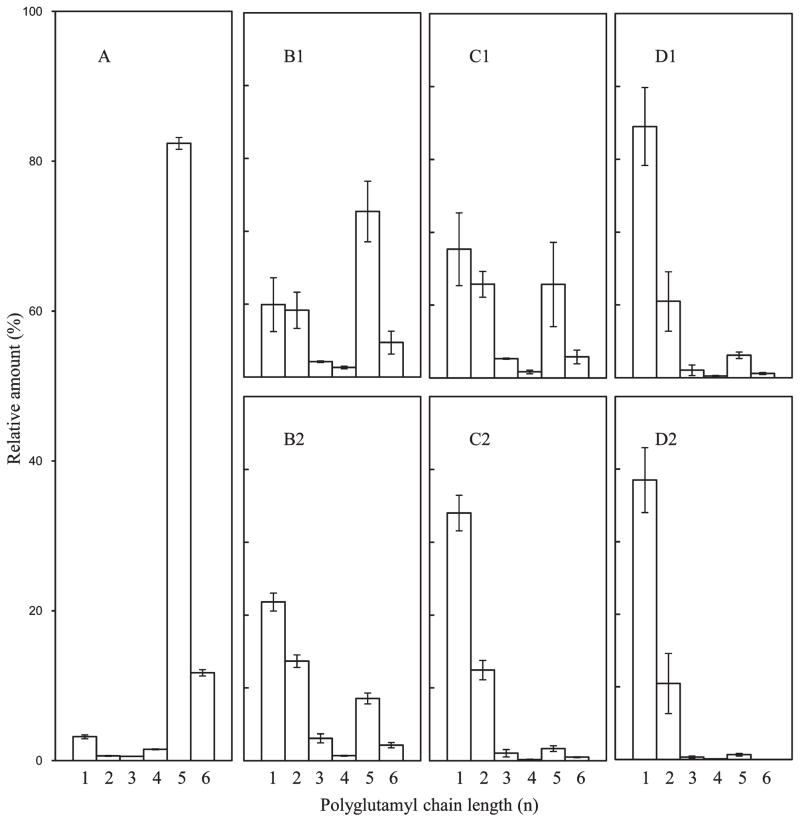
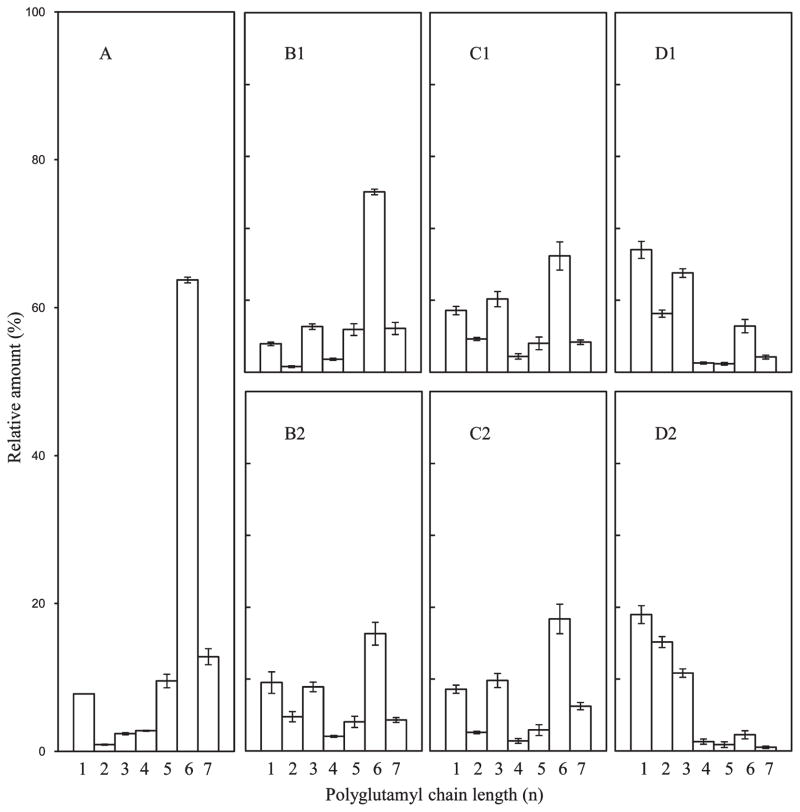
The influence of HPP on the polyglutamyl 5MTHF species present in cauliflower, carrot, and carrot greens was assessed for treatments performed at 30 °C combined with pressures of 300, 450, and 600 MPa (0 and 5 min each). An overview of the results for carrot is visualized in Figure 4. The 5MTHF polyglutamyl folate (5MTHF-Glun) in carrot reference (Figure 4A) consisted of two main forms, 5MTHF-Glu5 (82%) and 5MTHF-Glu6 (12%), accompanied by several minor shorter chain forms, 5MTHF (3%), 5MTHF-Glu2 (0.6%), 5MTHF-Glu3 (0.6%), and 5MTHF-Glu4 (1.5%). Comparing the mildest pressure/time treatment, 300 MPa/0 min (Figure 4B1), to carrot reference (Figure 4A) revealed that significant conversion of penta- and hexaglutamyl 5MTHF to mono- and diglutamyl 5MTHF had already occurred. 5MTHF increased approximately 5-fold and 5MTHF-Glu2 increased 28-fold, whereas 5MTHF-Glu5 decreased >40%. With extended holding time (5 min) at 300 MPa (Figure 4B2) the conversion continued as 5MTHF increased 13-fold, 5MTHF-Glu2 increased 42-fold, and 5MTHF-Glu5 and 5MTHF-Glu6 further declined compared to reference. With increasing pressure (450 and 600 MPa) and time, the change became more dramatic with a nearly complete conversion of the long-chain polyglutamyls to mono- and diglutamyl 5MTHF (Figure 4C1–D2). Comparison of 600 MPa/5 min (Figure 4D2) to unprocessed carrot (Figure 4A) showed that relative to the total 5MTHF, monoglutamyl 5MTHF increased from 3 to 80% and 5MTHF-Glu2 from <1 to 20%, whereas 5MTHF-Glu5 decreased by 95% and 5MTHF-Glu6 by 99%.
Figure 4.
Effect of pressure and holding time on the distribution of polyglutamyl 5MTHF of carrot: (A) raw carrot; (B1) 300 MPa/0 min; (B2) 300 MPa/5 min; (C1) 450 MPa/0 min; (C2) 450 MPa/5 min; (D1) 600 MPa/0 min; (D2) 600 MPa/5 min. The scale for panels B1–D2 is matched to that of panel A, that is, 0–100%. Data represent the mean of six replicates.
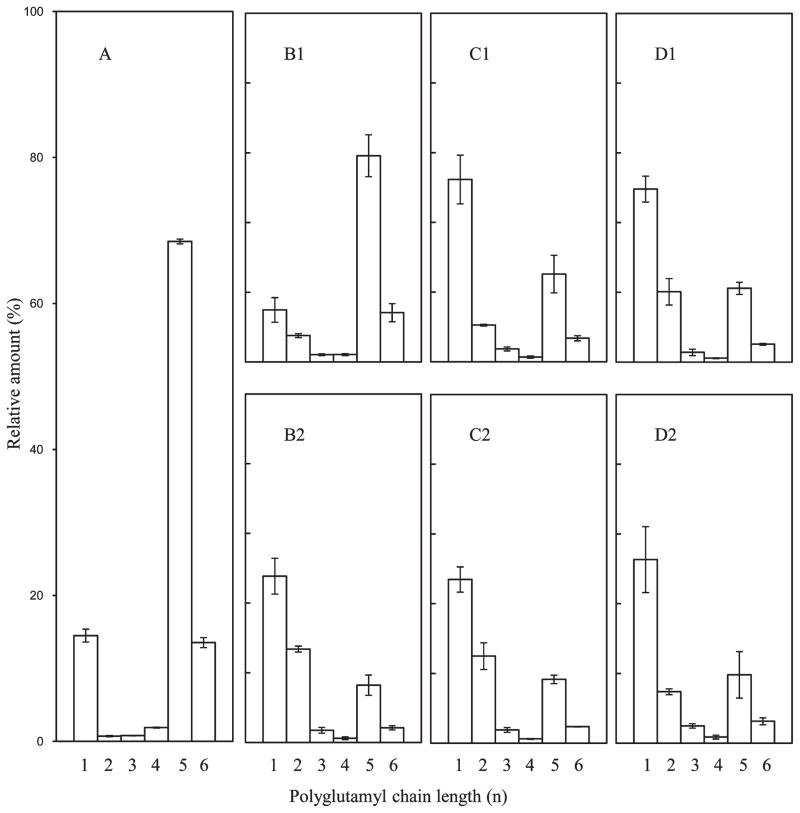
An overview of the HPP results for cauliflower is summarized in Figure 5. The polyglutamyl 5MTHF in cauliflower reference (Figure 5A) consisted mainly of 5MTHF-Glu6 (64%) with lesser amounts of the other chain length species: 5MTHF-Glu7 (13%), 5MTHF-Glu5 (9.5%), 5MTHF (7.8%), 5MTHF-Glu3 (2.4%), 5MTHF-Glu4 (2.8%), and 5MTHF-Glu2 (0.9%). Comparing 300 MPa/0 min (Figure 5B1) to cauliflower reference (Figure 5A) revealed that significant conversion had occurred where 5MTHF-Glu6 decreased with 5MTHF-Glu3 increasing 4-fold. With extended holding time (5 min) at 300 MPa this conversion continued: 5MTHF increased 40%, 5MTHF-Glu2 increased 9-fold, and 5MTHF-Glu3 increased 6-fold, with 5MTHF-Glu5–7 decreasing (Figure 5, panel B1 versus B2). As for carrots, with increasing pressure/time the conversion of polyglutamyls toward monoglutamyl 5MTHF progressed, although triglutamyl remained as a major species once polyglutamyls were consumed (Figure 5D2). Indeed, 5MTHF-Glu3 appears to be a relatively stable intermediate chain length form as it accumulated as a function of pressure as did 5MTHF, whereas 5MTHF-Glu2 increased only at the extreme conditions (600 MPa, 0 or 5 min). A rise in this intermediate 5MTHF-Glu3 form was noted with other Brassica species when GGH was not inactivated,15 suggesting a difference in GGH isoform specificity toward polyglutamyl substrates and reaction products. Relative to total 5MTHF, 5MTHF increased from 8 to 40%, 5MTHF-Glu2 from 2 to 30%, and 5MTHF-Glu3 from 5 to 19%, whereas 5MTHF-Glu6 decreased by 93%, 5MTHF-Glu7 by 92%, and 5MTHF-Glu5 by 81%.
Figure 5.
Effect of pressure and holding time on the distribution of polyglutamyl 5MTHF of cauliflower: (A) raw cauliflower; (B1) 300 MPa/0 min; (B2) 300 MPa/5 min; (C1) 450 MPa/0 min; (C2) 450 MPa/5 min; (D1) 600 MPa/0 min; (D2) 600 MPa/5 min. The scale for panels B1–D2 is matched to that of panel A, that is, 0–100%. Data represent the mean of six replicates.
Carrot greens were chosen as a comparison for carrot root as it was wondered whether the polyglutamyl profile and susceptibility to change under HPP conditions would vary for different organs of the same plant. They also have nutritional significance in that carrot greens are eaten in some cultures. An overview of the HPP results for carrot greens is summarized in Figure 6. The polyglutamyl 5MTHF in the carrot greens reference (Figure 6A) consisted mainly of 5MTHF-Glu5 (69%) with lower levels of 5MTHF-Glu6 (14%), 5MTHF (15%), 5MTHF-Glu4 (1.9%), 5MTHF-Glu3 (0.8%), and 5MTHF-Glu2 (0.7%). Comparison of 300 MPa/0 min (Figure 6B1) to carrot greens reference (Figure 6A) showed that conversion occurred where 5MTHF-Glu5 decreased slightly, with 5MTHF-Glu2 increasing 9-fold. At 300 MPa/5 min more significant conversion was induced as 5MTHF increased from 15 to 48% and 5MTHF-Glu2 increased from <1 to 26%, whereas 5MTHF-Glu5 decreased by 76% and 5MTHF-Glu6 decreased by 69% (Figure 6, panel B2 versus A). When pressure was elevated to 450 or 600 MPa and held for 5 min (Figure 6C2, D2), only slightly more conversion was observed. Thus, HPP appears to have enabled GGH action, and perhaps the GGH was labile at the high-pressure conditions and inactivation limited deglutamylation.
Figure 6.
Effect of pressure and holding time on the distribution of polyglutamyl 5MTHF of carrot greens: (A) raw carrot greens; (B1) 300 MPa/0 min; (B2) 300 MPa/5 min; (C1) 450 MPa/0 min; (C2) 450 MPa/5 min; (D1) 600 MPa/0 min; (D2) 600 MPa/5 min. The scale for panels B1–D2 is matched to that of panel A, that is, 0–100%. Data represent the mean of six replicates.
A few recent investigations32–34 have indicated that the deglutamylation of long-chain polyglutamyl folate in intact tissue during high-pressure treatments may be due to the action of vacuolar GGH. It was observed that different vegetables have different degrees of deglutamylation under extreme HPP condition. Carrot had the largest degree of conversion, cauliflower was second, and carrot greens had the least conversion. Recently this ubiquitous enzyme has been well characterized in soy, tomato, and Arabidopsis.23,37,38 Plants usually have several GGH genes, although animals have only one. Several plant isoforms of GGH have been described with various pH optima and substrate specificities.23,38,39 Leichter et al.40 has compared the GGH activities in different vegetables and found spinach to have the highest activity. Therein cauliflower had 8 times lower GGH activity than spinach, but there are no reports characterizing GGH forms in carrot or carrot greens.
There may be other factors affecting deglutamylation during HPP. Nonenzymatic hydrolysis is not expected here because Verlinde et al.33 did not observe any hydrolysis of 5MTHF polyglutamyl standards under different combinations of pressure and temperature. There are reports that pH values can decrease ~0.3 unit for each pressure increase of 100 MPa.41–43 If so, the pH may have decreased to 4.3 for cauliflower when the pressure was elevated to 600 MPa. Conversion of triglutamyl to monoglutamyl 5MTHF has been reported for cabbage and broccoli leaves at pH near 5.39,44 Cauliflower is in the same vegetable family as cabbage and broccoli, and they may share similar GGH isoforms. During HPP treatment, FBP may have also been denatured. FBP can protect the folate from oxidation and modulate its metabolism. Thus, loss of binding may lead to a decreased net folate intake due to greater susceptibility to oxidative degradation. Because monoglutamyl folate species have less affinity for FBP, they might also be more vulnerable to degradation in HPP-processed vegetables. Further work is required to investigate the stability of vegetable folate during storage post-HPP processing. If the polyglutamyl folate and GGH both exist in the vacuole, as suggested by Orsomando et al.,23 denaturation of FBP might make polyglutamyl folate available for GGH hydrolysis. Although these scenarios are reasonable in the literature context, they are also highly speculative, and the exact mechanisms at work under HPP will require further study.
To the best of our knowledge, there are only a few studies examining the effect of HPP on the polyglutamyl folate in intact vegetable tissues. Melse-Boonstra et al.32 examined the effect of HPP on monoglutamyl folate and polyglutamyl folate under relatively mild HPP conditions (50–200 MPa). These authors found significant increases in monoglutamyl folate in leek and green beans, whereas polyglutamyl folate in cauliflower was mostly unchanged under such conditions. The finding for cauliflower is consistent with our results because slight conversion occurred under our low HPP condition (300 MPa), although it was found that the most dramatic changes occurred at higher pressure/time treatments. Whereas particular polyglutamyl species present were detailed, others32 limited their characterization to mono- versus polyglutamyl folates (with or without conjugase). Verlinde et al.33 conducted comprehensive HPP treatments to study the effects on the polyglutamyl folate profile of broccoli and found significant conversion at certain HPP treatment conditions. Because the authors extracted folates by boiling frozen tissue, they allowed samples to pass through a temperature region optimal for GGH action before being inactivated. In other words, it is not possible to tell what change occurred during HPP versus post-HPP handling. Admittedly, differences found when comparing treatment conditions where much of the change occurred postprocessing could still have an impact on nutritional quality. This paper expands on the potential of HPP to manipulate polyglutamyl 5MTHF profiles in several vegetables. By virtue of stabilizing the 5MTHF pool prior to analysis, we have confidence that changes in folates have been isolated to those that occurred during HPP itself or in the short period between HPP and steaming prior to analysis. Further work is required to elucidate when deglutamylation occurs, but in a practical sense HPP effected polyglutamyl folate deglutamylation.
Influence of HPP on Total 5MTHF
The total 5MTHF levels (sum of various chain length forms of 5MTHF) in each vegetable as a function of pressure/time are given in Table 1. The reader should keep in mind that 5MTHF is not equal to total folate, which would be the sum of all folate forms. 5MTHF is the predominant form in carrot (81%) and cauliflower (77%). In carrot greens, 5MTHF is one of the two major forms and is only of equal abundance to 5-CHOTHF (25%) (Figure 2). According to our total 5MTHF results (Table 1) and considering the folate speciation (Figure 2), the cauliflower and carrot greens qualified as excellent folate sources, whereas the carrots were a good folate source (USDA).45 The impact of HPP on total 5MTHF was investigated in carrot, cauliflower, and carrot greens under 300–600 MPa for 0–5 min at 30 °C. Significant loss of total 5MTHF was observed in carrot at 300 MPa/5 min, 450 MPa/0 min, and 450 MPa/5 min, with a 30% loss compared to reference (Table 1). For cauliflower, 450 MPa/0 min caused 41% loss and 600 MPa/0 min caused 27% loss of total 5MTHF. Also, significant loss happened in carrot greens at 600 MPa/5 min (49% loss) and 450 MPa/0 min (32% loss). Despite the losses observed in various HPP experiments, carrot and cauliflower folate was preserved at the most extreme pressure/time conditions and carrot greens folate was retained under most treatments. Most loss was incurred for the 0 min condition, which involved bringing the sample to target pressure and immediately depressurizing. This treatment could decompartmentalize cells and allow for enzymatic degradation, whereas under conditions when samples were held at pressure for an extended time, these same degradative enzymes may have been inactivated.
Table 1.
Effect of High-Pressure Processing (HPP) Treatment on the Total 5-Methyltetrahydrofolate Content of Carrot, Cauliflower, and Carrot Greensa
| HPP pressure/time | carrot (pmol/g) | cauliflower (pmol/g) | carrot greens (pmol/g) |
|---|---|---|---|
| referenceb | 1316±73 a | 1774±60 a | 1520±157 a |
| 300 MPa/0 min | 1053±136 ab | 1729±109 a | 1510±696 ac |
| 300 MPa/5 min | 918±51 b | 1560±193 a | 1409±85 a |
| 450 MPa/0 min | 959±235 b | 1042±156 c | 1030±114 c |
| 450 MPa/5 min | 882±109 b | 1712±190 a | 1474±68 a |
| 600 MPa/0 min | 1135±106 ab | 1303±71 c | 1251±130 ac |
| 600 MPa/5 min | 1332±238 a | 1726±310 a | 768±35 d |
Data are presented as the mean ± standard deviation from six replicates. Letters after the value indicate which treatment resulted in a significantly different concentration of total 5MTHF compared to the unprocessed vegetables.
Reference: vegetables were steamed for 10 min to inactivate GGH and potential folate degrading enzymes.
Folate loss during HPP has been reported by several investigators. Melse-Boonstra et al.32 investigated cauliflower folate at 200 MPa for 5 min and found 43% loss of total folate. This study found no change for cauliflower at 300 MPa for 0 and 5 min. The loss reported by Melse-Boonstra et al. may have been due in part to leaching because the investigators did not pack the vegetables in vacuum bags. To the best of our knowledge, there are no reports investigating folate stability in intact carrot and carrot greens under HPP conditions. Verlinde et al.33 investigated the total folate in vacuum-packed broccoli and found significant folate loss (48–78%) at 600 MPa/30 min with different processing temperatures. In contrast, Indrawati et al.34 investigated the stability of 5MTHF in vacuum-packed asparagus and found 5MTHF was fairly stable at 500 MPa and 60 °C for 5–100 min but found a great loss (49%) of total 5MTHF in carrot juice under the same treatment. Interestingly, addition of ascorbic acid significantly improved the stability of 5MTHF in carrot juice, suggesting folate was lost to oxidation when carrot juice was processed without fortification. Recently, Verlinde et al.39 reported significant losses of 5MTHF in a water system under 200–800 MPa, different time courses, and temperature. The main degradation products were s-triazine, 5-methyldihydrofolic acid, and p-aminobenzoyl-L-glutamate. However, 5MTHF stability in situ could be different from that in solution, and 5-methyldihydrofolic acid can be easily converted back to 5MTHF with reducing extraction buffer. Another potential route for 5MTHF loss is for it to be converted to other folate species either by enzymes involved in folate metabolism or chemically during HPP. Indeed, further research into folate speciation may help to explain the fate of folate during HPP.
This study investigated the effects of HPP pressure and time at 30 °C on the profile of polyglutamyl 5MTHF in carrot, carrot greens, and cauliflower. Such profiles have not been characterized in these vegetables before. During HPP treatment polyglutamyl 5MTHF was largely deglutamylated. Carrot greens were partially deglutamylated without significant losses in total 5MTHF at 300 MPa/5 min and 450 MPa/5 min. Although losses up to 30% in total 5MTHF were found for carrot and cauliflower during HPP, at the extreme condition (600 MPa/5 min) no significant losses were incurred. Because 600 MPa/5 min is considered to be an HPP pasteurization condition, it may lead to production of vegetables with extended shelf life and enhanced levels of highly bioavailable monoglutamyl folate.
ABBREVIATIONS USED
- HPP
high-pressure processing
- 5MTHF
5-methyltetrahydrofolate
- GGH
γ-glutamyl hydrolase
- 5MTHF-Glun
5-methyltetrahydrofolate polyglutamyl
- 10-CHODHF
10-formyldihydrofolate
- 10-CHOTHF
10-formyltetrahydrofolate
- THF
tetrahydrofolate
- 5-CHOTHF
5-formyltetrahydrofolate
- 5,10-CH+THF
5,10-methenyltetrahydrofolate
- 5,10-CH2THF
5,10-methylenetetrahydrofolate
- 10-CHOFA
10-formylfolic acid
- FBP
folate binding protein
- PTFE
polytetrafluoroethylene
References
- 1.Suh JR, Herbig AK, Stover PJ. New perspectives on folate catabolism. Annu Rev Nutr. 2001;21:255–282. doi: 10.1146/annurev.nutr.21.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Stover PJ. Physiology of folate and vitamin B12 in health and disease. Nutr Rev. 2004;62:s3–s12. doi: 10.1111/j.1753-4887.2004.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 3.Dary O. Nutritional interpretation of folic acid interventions. Nutr Rev. 2009;67:235–244. doi: 10.1111/j.1753-4887.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- 4.Blancquaert D, Storozhenko S, Loizeau K, De Steur H, De Brouwer V, Viaene J, Ravanel S, Rébeillé F, Lambert W, Van Der Straeten D. Folates and folic acid: from fundamental research toward sustainable health. Crit Rev Plant Sci. 2010;29:14–35. [Google Scholar]
- 5.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80:1123–1128. doi: 10.1093/ajcn/80.5.1123. [DOI] [PubMed] [Google Scholar]
- 6.Wright AJA, Dainty JR, Finglas PM. Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. Br J Nutr. 2007;98:667–675. doi: 10.1017/S0007114507777140. [DOI] [PubMed] [Google Scholar]
- 7.Finglas PM, De Meer K, Molloy A, Verhoef P, Pietrzik K, Powers HJ, Van der Straeten D, Jagerstad M, Varela-Moreiras G, Van Vliet T, Havenaar R, Buttriss J. Research goals for folate and related B vitamins in Europe. Eur J Clin Nutr. 2006;60:287–294. doi: 10.1038/sj.ejcn.1602315. [DOI] [PubMed] [Google Scholar]
- 8.Wei MM, Bailey LB, Toth JP, Gregory JF. Bioavailability for humans of deuterium labelled monoglutamyl and polyglutamyl folates is affected by selected foods. J Nutr. 1996;126:3100–3108. doi: 10.1093/jn/126.12.3100. [DOI] [PubMed] [Google Scholar]
- 9.Wei MM, Gregory JF. Organic acids in selected foods inhibit porcine jejunal brush border pteroylpolyglutamate hydrolase: potential mechanism affecting the bioavailability of dietary polyglutamyl folate. J Agric Food Chem. 1998;46:211–219. doi: 10.1021/jf970662g. [DOI] [PubMed] [Google Scholar]
- 10.Konings EJM, Roomans HHS, Dorant E, Goldbohm RA, Saris WHM, van den Brandt PA. Folate intake of the Dutch population according to newly established liquid chromatography data for foods. Am J Clin Nutr. 2001;73:765–776. doi: 10.1093/ajcn/73.4.765. [DOI] [PubMed] [Google Scholar]
- 11.Vahteristo L, Lehikoinen K, Ollilainen V, Varo P. Application of an HPL Cassay for the determination of folate derivatives in some vegetables, fruits and berries consumed in Finland. Food Chem. 1997;59:589–597. [Google Scholar]
- 12.Finglas PM, Wigertz K, Vahteristo L, Witthöft C, Southon S, De Froidmont-Görtz I. Standardisation of HPLC techniques for the determination of naturally-occurring folates in food. Food Chem. 1999;64:245–255. [Google Scholar]
- 13.Müller H. Determination of the folic acid content of vegetables and fruits using high-performance liquid-chromatography (HPLC) Z Lebensm Unters Forsch. 1993a;196:137–141. doi: 10.1007/BF01185573. [DOI] [PubMed] [Google Scholar]
- 14.De La Garza RID, Gregory JF, Hanson AD. Folate biofortification of tomato fruit. Proc Natl Acad Sci US A. 2007;104:4218–4222. doi: 10.1073/pnas.0700409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Riedl KM, Schwartz SJ. A liquid chromatography-tandem mass spectrometric method for quantitative determination of native 5-methyltetrahydrofolate and its polyglutamyl derivatives in raw vegetables. J Chromatogr, B: Anal Technol Biomed Life Sci. 2010;878:2949–2958. doi: 10.1016/j.jchromb.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verlinde PhCJ, Oey I, Deborggraeve WM, Hendrickx ME, Van Loey AM. Mechanism and related kinetics of 5-methyltetrahydrofolic acid degradation during combined high hydrostatic pressure-thermal treatments. J Agric Food Chem. 2009;57:6803–6814. doi: 10.1021/jf900832g. [DOI] [PubMed] [Google Scholar]
- 17.Melse-Boonstra A, Verhoef P, West CE, van Rhijn JA, van Breemen RB, Lasaroms JJP, Garbis SD, Katan MB, Kok FJ. A dual-isotope-labeling method of studying the bioavailability of hexaglutamyl folic acid relative to that of monoglutamyl folic acid in humans by using multiple orally administered low doses. Am J Clin Nutr. 2006;84:1128–1133. doi: 10.1093/ajcn/84.5.1128. [DOI] [PubMed] [Google Scholar]
- 18.Gregory JF, Bhandari SD, Bailey LB, Toth JP, Baumgartner TG, Cerda JJ. Relative bioavailability of deuterium-labeled monoglutamyl and hexaglutamyl folates in human subjects. Am J Clin Nutr. 1991;53:736–740. doi: 10.1093/ajcn/53.3.736. [DOI] [PubMed] [Google Scholar]
- 19.McKillop DJ, McNulty H, Scott JM, McPartlin JM, Strain JJ, Bradbury I, Girvan J, Hoey L, McCreedy R, Alexander J, Patterson BK, Hannon-Fletcher M, Pentieva K. The rate of intestinal absorption of natural food folates is not related to the extent of folate conjugations. Am J Clin Nutr. 2006;84:167–173. doi: 10.1093/ajcn/84.1.167. [DOI] [PubMed] [Google Scholar]
- 20.Konings EJM, Troost FJ, Castenmiller JJM, Roomans HHS, van den Brandt PA, Saris WHM. Intestinal absorption of different types of folate in healthy subjects with an ileostomy. Br J Nutr. 2002;88:235–242. doi: 10.1079/BJN2002613. [DOI] [PubMed] [Google Scholar]
- 21.McNulty H, Pentieva K. Folate bioavailability. Proc Nutr Soc. 2004;63:529–536. doi: 10.1079/pns2004383. [DOI] [PubMed] [Google Scholar]
- 22.Quinlivan EP, Hanson AD, Gregory JF. The analysis of folate and its metabolic precursors in biological samples. Anal Biochem. 2006;348:163–184. doi: 10.1016/j.ab.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Orsomando G, De la Garza RD, Green BJ, Peng M, Rea PA, Ryan TJ, Gregory JF, Hanson AD. Plant γ-glutamyl hydrolases and folate polyglutamates: characterization, compartmentation, and co-occurrence in vacuoles. J Biol Chem. 2005;280:28877–28884. doi: 10.1074/jbc.M504306200. [DOI] [PubMed] [Google Scholar]
- 24.Rastogi NK, Raghavarao KS, Balasubramaniam VM, Niranjan K, Knorr D. Opportunities and challenges in high pressure processing of foods. Crit Rev Food Sci Nutr. 2007;47:69–112. doi: 10.1080/10408390600626420. [DOI] [PubMed] [Google Scholar]
- 25.San Martín MF, Barbosa-Cánovas GV, Swanson BG. Food processing by high hydrostatic pressure. Crit Rev Food Sci Nutr. 2004;42:627–645. doi: 10.1080/20024091054274. [DOI] [PubMed] [Google Scholar]
- 26.Norton T, Sun D. Recent advances in the use of high pressure as an effective processing technique in the food industry. Food Bioprocess Technol. 2008;1:2–34. [Google Scholar]
- 27.Nguyen LT, Tay A, Balasubramaniam VM, Legan JD, Turek EJ, Gupta R. Evaluating the impact of thermal and pressure threatment in preserving textural quality of selected foods. LWT –Food Sci Technol. 2010;43:525–534. [Google Scholar]
- 28.Sánchez-Moreno C, De Ancos B, Plaza L, Elez-Martínez P, Cano MP. Nutritional approaches and health-related properties of plant foods processed by high pressure and plused electric fields. Crit Rev Food Sci Nutr. 2009;49:552–576. doi: 10.1080/10408390802145526. [DOI] [PubMed] [Google Scholar]
- 29.Ludikhuze L, Van Loey A, Indrawati; Smout C, Hendrickx M. Effects of combined pressure and temperature on enzymes related to quality of fruits and vegetables from kinetic information to process engineering aspects. Crit Rev Food Sci Nutri. 2003;43:527–586. doi: 10.1080/10408690390246350. [DOI] [PubMed] [Google Scholar]
- 30.Guerrero-Beltrán JA, Barbosa-Cánovas GV, Swanson BG. High hydrostatic pressure processing of fruit and vegetable products. Food Rev Int. 2005;21:411–425. [Google Scholar]
- 31.van Boekel M, Fogliano V, Pellegrini N, Stanton C, Scholz G, Lalljie S, Somoza V, Knorr D, Jasti PR, Eisenbrand G. A review on the beneficial aspects of food processing. Mol Nutr Food Res. 2010;54:1215–1247. doi: 10.1002/mnfr.200900608. [DOI] [PubMed] [Google Scholar]
- 32.Melse-Boonstra A, Verhoef P, Konings EJM, van Dusseldorp M, Matser A, Hollman PCH, Meyboom S, Kok FJ, West CE. Influence of processing on total, monoglutamate and polyglutamate folate contents of leeks, cauliflower, and green beans. J Agric Food Chem. 2002;50:3473–3478. doi: 10.1021/jf0112318. [DOI] [PubMed] [Google Scholar]
- 33.Verlinde P, Oey I, Hendrickx M, Loey AV. High-pressure treatments induce folate polyglutamate profile changes in intact broccoli (Brassica oleraceae L. cv. italica) tissue. Food Chem. 2008;111:220–229. [Google Scholar]
- 34.Indrawati, Arroqui C, Messagie I, Nguyen MT, Van Loey A, Hendrickx M. Comparative study on pressure and temperature stability of 5-methyltetrahydrofolic acid in model systems and in food products. J Agric Food Chem. 2004;52:485–492. doi: 10.1021/jf0349432. [DOI] [PubMed] [Google Scholar]
- 35.Rasanayagam V, Balasubramaniam VM, Ting E, Sizer CE, Bush C, Anderson C. Compression heating of selected fatty food materials during high pressure processing. J Food Sci. 2003;68:254–259. [Google Scholar]
- 36.Freisleben A, Schieberle P, Rychlik M. Specific and sensitive quantification of folate vitamers in foods by stable isotope dilution assays using high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2003;376:149–156. doi: 10.1007/s00216-003-1844-y. [DOI] [PubMed] [Google Scholar]
- 37.Akhtar TA, McQuinn RP, Naponelli V, Gregory JF, Giovannoni JJ, Hanson AD. Tomato gamma-glutamylhydrolases: expression characterization and evidence for heterodimer formation. Plant Physiol. 2008;148:775–85. doi: 10.1104/pp.108.124479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huangpu J, Pak JH, Graham MC, Rickle SA, Graham JS. Purification and molecular analysis of an extracellular γ-glutamyl hydrolase present in young tissues of the soybean plant. Biochem Biophy Res Commun. 1996;228:1–6. doi: 10.1006/bbrc.1996.1608. [DOI] [PubMed] [Google Scholar]
- 39.Tamura T, Buehring KU, Stokstad EL. Enzymatic hydrolysis of pteroylpolyglutamates in cabbage. Proc Soc Exp Biol Med. 1972;141:1022–1025. doi: 10.3181/00379727-141-36924. [DOI] [PubMed] [Google Scholar]
- 40.Leichter J, Landymore AF, Krumdieck CL. Folate conjugase activity in fresh vegetables and its effect on the determination of free folate content. Am J Clin Nutr. 1979;32:92–95. doi: 10.1093/ajcn/32.1.92. [DOI] [PubMed] [Google Scholar]
- 41.Neuman RC, Kauzmann W, Zipp A. Pressure dependence of weak acid ionization in aqueous buffers. J Phys Chem. 1973;77:2687–2691. [Google Scholar]
- 42.Kitamura Y, Itoh T. Reaction volume of protonic ionization for buffering agents. Prediction of pressure dependence of pH and pOH. J Solution Chem. 1987;16:715–725. [Google Scholar]
- 43.Hayert M, Perrier-Cornet JM, Gervais P. A simple method for measuring the pH of acid solutions under high pressure. J Phys Chem. 1999;103:1785–1789. [Google Scholar]
- 44.Zheng L, Lin Y, Lin S, Cossin EA. The polyglutamate nature of plant folates. Phytochemistry. 1992;31:2227–2282. [Google Scholar]
- 45.USDA National Nutrient Database for Standard Reference – Release 23. http://www.ars.usda.gov/Services/docs.htm?docid=8964.