Abstract
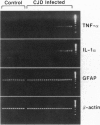
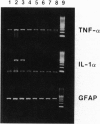
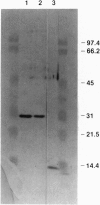
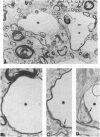
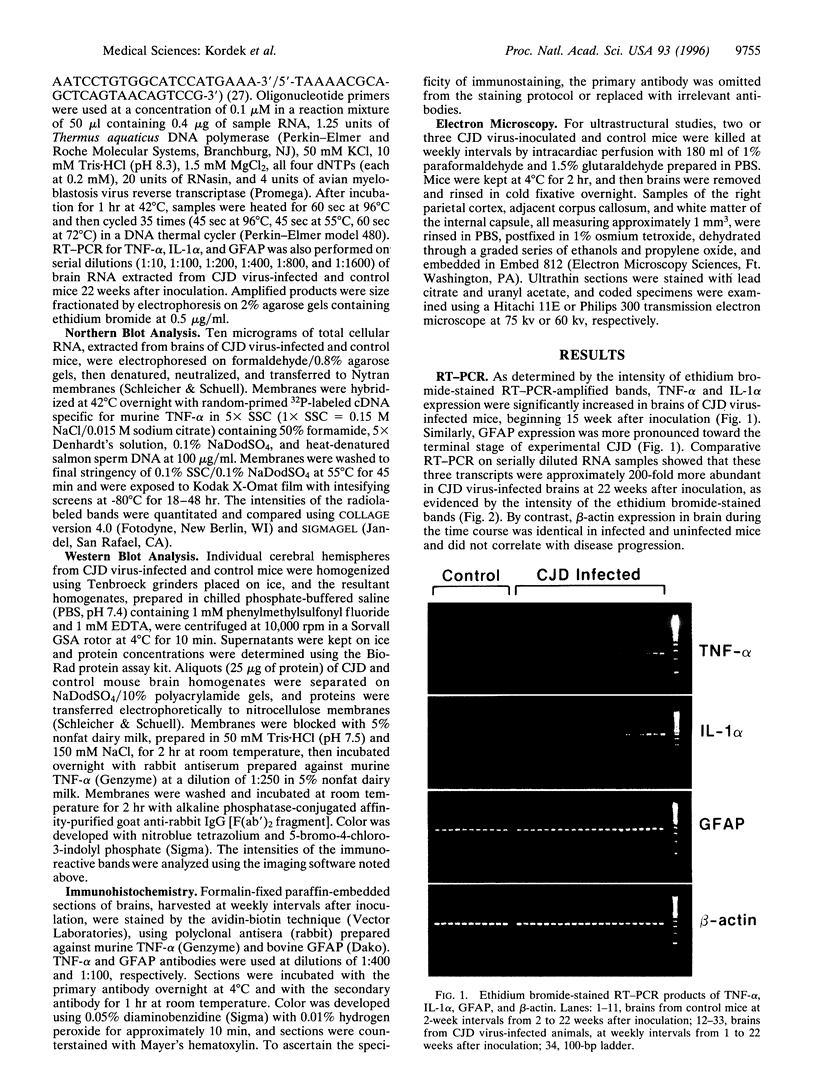
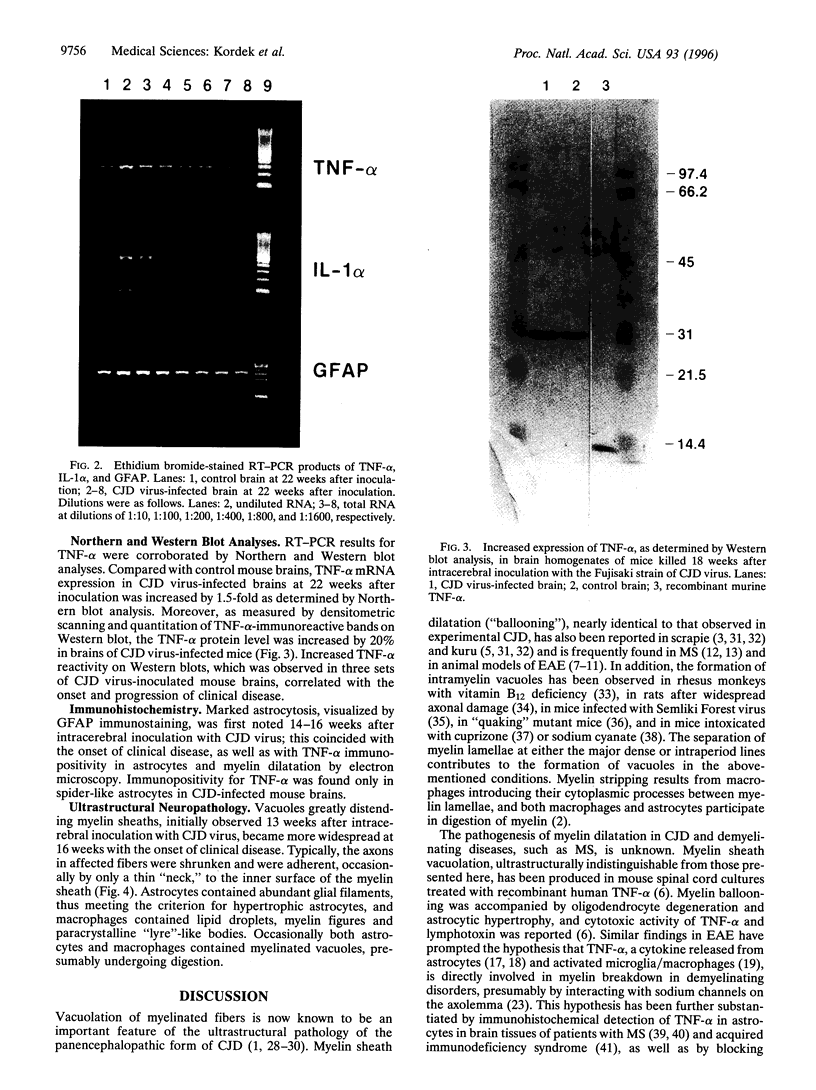
The ultrastructural pathology of myelinated axons in mice infected experimentally with the Fujisaki strain of Creutzfeldt-Jakob disease (CJD) virus is characterized by myelin sheath vacuolation that closely resembles that induced in murine spinal cord organotypic cultures by tumor necrosis factor alpha (TNF-alpha), a cytokine produced by astrocytes and macrophages. To clarify the role of TNF-alpha in experimental CJD, we investigated the expression of TNF-alpha in brain tissues from CJD virus-infected mice at weekly intervals after inoculation by reverse transcription-coupled PCR, Northern and Western blot analyses, and immunocytochemical staining. Neuropathological findings by electron microscopy, as well as expression of interleukin 1 alpha and glial fibrillary acidic protein, were concurrently monitored. As determined by reverse transcription-coupled PCR, the expression of TNF-alpha, interleukin 1 alpha, and glial fibrillary acidic protein was increased by approximately 200-fold in the brains of CJD virus-inoculated mice during the course of disease. By contrast, beta-actin expression remained unchanged. Progressively increased expression of TNF-alpha in CJD virus-infected brain tissues was verified by Northern and Western blot analyses, and astrocytes in areas with striking myelin sheath vacuolation were intensely stained with an antibody against murine TNF-alpha. The collective findings of TNF-alpha overexpression during the course of clinical disease suggest that TNF-alpha may mediate the myelin sheath vacuolation observed in experimental CJD.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agamanolis D. P., Victor M., Harris J. W., Hines J. D., Chester E. M., Kark J. A. An ultrastructural study of subacute combined degeneration of the spinal cord in vitamin B12-deficient rhesus monkeys. J Neuropathol Exp Neurol. 1978 May;37(3):273–299. doi: 10.1097/00005072-197805000-00006. [DOI] [PubMed] [Google Scholar]
- Balcarek J. M., Cowan N. J. Structure of the mouse glial fibrillary acidic protein gene: implications for the evolution of the intermediate filament multigene family. Nucleic Acids Res. 1985 Aug 12;13(15):5527–5543. doi: 10.1093/nar/13.15.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna B. P., Estes M. L., Jacobs B. S., Hudson S., Ransohoff R. M. Human astrocytes proliferate in response to tumor necrosis factor alpha. J Neuroimmunol. 1990 Dec;30(2-3):239–243. doi: 10.1016/0165-5728(90)90108-y. [DOI] [PubMed] [Google Scholar]
- Beck E., Daniel P. M., Asher D. M., Gajdusek D. C., Gibbs C. J., Jr Experimental kuru in the chimpanzee. A neuropathological study. Brain. 1973 Sep;96(3):441–462. doi: 10.1093/brain/96.3.441. [DOI] [PubMed] [Google Scholar]
- Beck E., Daniel P. M., Matthews W. B., Stevens D. L., Alpers M. P., Asher D. M., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease. The neuropathology of a transmission experiment. Brain. 1969;92(4):699–716. doi: 10.1093/brain/92.4.699. [DOI] [PubMed] [Google Scholar]
- Brosnan C. F., Selmaj K., Raine C. S. Hypothesis: a role for tumor necrosis factor in immune-mediated demyelination and its relevance to multiple sclerosis. J Neuroimmunol. 1988 Apr;18(1):87–94. doi: 10.1016/0165-5728(88)90137-3. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Eddleston M., Kemper P., Oldstone M. B., Hobbs M. V. Activation of cerebral cytokine gene expression and its correlation with onset of reactive astrocyte and acute-phase response gene expression in scrapie. J Virol. 1994 Apr;68(4):2383–2387. doi: 10.1128/jvi.68.4.2383-2387.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I. Y., Benveniste E. N. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990 Apr 15;144(8):2999–3007. [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Genis P., Jett M., Bernton E. W., Boyle T., Gelbard H. A., Dzenko K., Keane R. W., Resnick L., Mizrachi Y., Volsky D. J. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992 Dec 1;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Baker T. J., Shih L. C., Lachman L. B. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986 Aug 1;164(2):594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Lachman L. B. Interleukin-1 stimulation of astroglial proliferation after brain injury. Science. 1985 Apr 26;228(4698):497–499. doi: 10.1126/science.3872478. [DOI] [PubMed] [Google Scholar]
- Giulian D., Woodward J., Young D. G., Krebs J. F., Lachman L. B. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988 Jul;8(7):2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goban Y., Saida T., Saida K., Nishitani H., Kameyama M. Ultrastructural study of central nervous system demyelination in galactocerebroside sensitized rabbits. Lab Invest. 1986 Jul;55(1):86–90. [PubMed] [Google Scholar]
- Hemm R. D., Carlton W. W., Welser J. R. Ultrastructural changes of cuprizone encephalopathy in mice. Toxicol Appl Pharmacol. 1971 Apr;18(4):869–882. doi: 10.1016/0041-008x(71)90235-3. [DOI] [PubMed] [Google Scholar]
- Hetier Emmanuelle, Ayala Jésus, Bousseau Anne, Denèfle Patrice, Prochiantz Alain. Amoeboid Microglial Cells and not Astrocytes Synthesize TNF-alpha in Swiss Mouse Brain Cell Cultures. Eur J Neurosci. 1990;2(9):762–768. doi: 10.1111/j.1460-9568.1990.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Cressman M. R. Fine-structural changes of myelin sheaths after axonal degeneration in the spinal cord of rats. Am J Pathol. 1966 Dec;49(6):1139–1155. [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W., Earle K. M., Gibbs C. J., Jr, Gajdusek D. C. Experimentak kuru encephalopathy in chimpanzees and spider monkeysElectron microscopic studies. J Neuropathol Exp Neurol. 1969 Jul;28(3):353–370. doi: 10.1097/00005072-196907000-00001. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Gajdusek D. C., Gibbs C. J., Jr Subacute spongiform virus encephalopathies. Scrapie, Kuru and Creutzfeldt-Jakob disease: a review. Am J Pathol. 1972 Sep;68(3):626–652. [PMC free article] [PubMed] [Google Scholar]
- Lampert P. Electron microscopic studies on ordinary and hyperacute experimental allergic encephalomyelitis. Acta Neuropathol. 1967 Oct 20;9(2):99–126. doi: 10.1007/BF00691436. [DOI] [PubMed] [Google Scholar]
- Lampert P., Hooks J., Gibbs C. J., Jr, Gajdusek D. C. Altered plasma membranes in experimental scrapie. Acta Neuropathol. 1971;19(2):81–93. doi: 10.1007/BF00688486. [DOI] [PubMed] [Google Scholar]
- Liberski P. P., Asher D. M., Yanagihara R., Gibbs C. J., Jr, Gajdusek D. C. Serial ultrastructural studies of scrapie in hamsters. J Comp Pathol. 1989 Nov;101(4):429–442. doi: 10.1016/0021-9975(89)90026-1. [DOI] [PubMed] [Google Scholar]
- Liberski P. P., Yanagihara R., Gibbs C. J., Jr, Gajdusek D. C. White matter ultrastructural pathology of experimental Creutzfeldt-Jakob disease in mice. Acta Neuropathol. 1989;79(1):1–9. doi: 10.1007/BF00308949. [DOI] [PubMed] [Google Scholar]
- Liberski P. P., Yanagihara R., Wells G. A., Gibbs C. J., Jr, Gajdusek D. C. Comparative ultrastructural neuropathology of naturally occurring bovine spongiform encephalopathy and experimentally induced scrapie and Creutzfeldt-Jakob disease. J Comp Pathol. 1992 May;106(4):361–381. doi: 10.1016/0021-9975(92)90022-m. [DOI] [PubMed] [Google Scholar]
- Liberski P. P., Yanagihara R., Wells G. A., Gibbs C. J., Jr, Gajdusek D. C. Ultrastructural pathology of axons and myelin in experimental scrapie in hamsters and bovine spongiform encephalopathy in cattle and a comparison with the panencephalopathic type of Creutzfeldt-Jakob disease. J Comp Pathol. 1992 May;106(4):383–398. doi: 10.1016/0021-9975(92)90023-n. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Okumura A., Oda M., Shiraki H. Panencephalopathic type of Creutzfeldt-Jakob disease: primary involvement of the cerebral white matter. J Neurol Neurosurg Psychiatry. 1981 Feb;44(2):103–115. doi: 10.1136/jnnp.44.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataf S., Louboutin J. P., Chabannes D., Fève J. R., Muller J. Y. Pentoxifylline inhibits experimental allergic encephalomyelitis. Acta Neurol Scand. 1993 Aug;88(2):97–99. doi: 10.1111/j.1600-0404.1993.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Park T. S., Kleinman G. M., Richardson E. P. Creutzfeldt-Jakob disease with extensive degeneration of white matter. Acta Neuropathol. 1980;52(3):239–242. doi: 10.1007/BF00705813. [DOI] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Prineas J., Raine C. S., Wísniewski H. An ultrastructural study of experimental demyelination and remyelination. 3. Chronic experimental allergic encephalomyelitis in the central nervous system. Lab Invest. 1969 Dec;21(6):472–483. [PubMed] [Google Scholar]
- Raine C. S. Biology of disease. Analysis of autoimmune demyelination: its impact upon multiple sclerosis. Lab Invest. 1984 Jun;50(6):608–635. [PubMed] [Google Scholar]
- Raine C. S., Bornstein M. B. Experimental allergic encephalomyelitis: an ultrastructural study of experimental demyelination in vitro. J Neuropathol Exp Neurol. 1970 Apr;29(2):177–191. [PubMed] [Google Scholar]
- Robbins D. S., Shirazi Y., Drysdale B. E., Lieberman A., Shin H. S., Shin M. L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987 Oct 15;139(8):2593–2597. [PubMed] [Google Scholar]
- Sato Y., Ohta M., Tateishi J. Experimental transmission of human subacute spongiform encephalopathy to small rodents. II. Ultrastructural study of spongy state in the gray and white matter. Acta Neuropathol. 1980;51(2):135–140. doi: 10.1007/BF00690455. [DOI] [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cannella B., Brosnan C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991 Mar;87(3):949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K., Raine C. S., Cross A. H. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991 Nov;30(5):694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- Sheahan B. J., Barrett P. N., Atkins G. J. Demyelination in mice resulting from infection with a mutant of Semliki Forest virus. Acta Neuropathol. 1981;53(2):129–136. doi: 10.1007/BF00689993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Andrews J. M., Waltz J. M., Terry R. D. Ultrastructural studies of multiple sclerosis. Lab Invest. 1969 May;20(5):444–454. [PubMed] [Google Scholar]
- Tateishi J., Ohta M., Koga M., Sato Y., Kuroiwa Y. Transmission of chronic spongiform encephalopathy with kuru plaques from humans to small rodents. Ann Neurol. 1979 Jun;5(6):581–584. doi: 10.1002/ana.410050616. [DOI] [PubMed] [Google Scholar]
- Tchelingerian J. L., Quinonero J., Booss J., Jacque C. Localization of TNF alpha and IL-1 alpha immunoreactivities in striatal neurons after surgical injury to the hippocampus. Neuron. 1993 Feb;10(2):213–224. doi: 10.1016/0896-6273(93)90312-f. [DOI] [PubMed] [Google Scholar]
- Tellez-Nagel I., Korthals J. K., Vlassara H. V., Cerami A. An ultrastructural study of chronic sodium cyanate-indiuced neuropathy. J Neuropathol Exp Neurol. 1977 Mar-Apr;36(2):352–363. [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallat J. M., Dumas M., Corvisier N., Leboutet M. J., Loubet A., Dumas P., Cathala F. Familial Creutzfeldt-Jakob disease with extensive degeneration of white matter. Ultrastructure of peripheral nerve. J Neurol Sci. 1983 Oct;61(2):261–275. doi: 10.1016/0022-510x(83)90010-2. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Bingle G. J. Dysmyelination in "quaking" mouse. Electron microscopic study. J Neuropathol Exp Neurol. 1972 Apr;31(2):352–369. doi: 10.1097/00005072-197204000-00010. [DOI] [PubMed] [Google Scholar]
- Williams A. E., van Dam A. M., Man-A-Hing W. K., Berkenbosch F., Eikelenboom P., Fraser H. Cytokines, prostaglandins and lipocortin-1 are present in the brains of scrapie-infected mice. Brain Res. 1994 Aug 22;654(2):200–206. doi: 10.1016/0006-8993(94)90480-4. [DOI] [PubMed] [Google Scholar]