Abstract
Because many complex physiological processes are controlled by multiple biomolecules, comprehensive treatment of certain disease conditions may be more effectively achieved by administration of more than one type of drug. Thus, the objective of the present research was to develop a multilayered, polymer-based system for sequential delivery of multiple drugs. The polymers used were cellulose acetate phthalate (CAP) complexed with Pluronic F-127 (P). After evaluating morphology of the resulting CAPP system, in vitro release of small molecule drugs and a model protein was studied from both single and multilayered devices. Drug release from single-layered CAPP films followed zero-order kinetics related to surface erosion of the association polymer. Release studies from multilayered CAPP devices showed the possibility of achieving intermittent release of one type of drug as well as sequential release of more than one type of drug. Mathematical modeling accurately predicted the release profiles for both single layer and multilayered devices. The present CAPP association polymer-based multilayer devices can be used for localized, sequential delivery of multiple drugs for the possible treatment of complex disease conditions, and perhaps for tissue engineering applications, that require delivery of more than one type of biomolecule.
Keywords: multiple drug delivery, sequential drug release, zero-order release, cellulose acetate phthalate, Pluronic F-127
1. INTRODUCTION
Several conditions, such as severe bacterial infection,[1] periodontitis,[2] and traumatic bone loss along with infection,[3] require repeated administration of a drug or administration of more than one drug for efficacious treatment. As reviewed by Chen et al., delivery of multiple growth factors is also important for tissue engineering.[4] Thus, this research was directed at developing a bioerodible system capable of delivering one or more types of drug in a predetermined temporal sequence, which could be helpful for treatment of different stages of complex diseases and also in tissue engineering.
Polyanhydrides and poly(ortho esters) are two common classes of surface-eroding polymers employed for controlled delivery of drugs for a variety of purposes, including antimicrobial, anti-inflammatory, analgesic, cancer, and ocular applications.[5-8] Surface-eroding polymers provide a constant rate of drug release that is directly proportional to polymer erosion.[6] As such, they provide highly controllable and reproducible drug release profiles[5] that would be useful for designing multiple drug delivery systems. Ease of processing is an important consideration for designing and developing a versatile drug delivery system capable of delivering more than one type of drug, but the high processing temperature and poor solubility in organic solvents cause difficulty in fabrication of some polyanhydrides and polyorthoesters into dosage forms.[8, 9]
An alternative, but less well known, surface-eroding system is composed of cellulose acetate phthalate (CAP) and Pluronic F-127 (P). When mixed in an aprotic solvent, the polymers associate via hydrogen bonds.[10] The properties of the CAPP system, such as ease in fabrication and drug loading, make it a suitable candidate for designing a surface-eroding multiple drug delivery system. The CAPP association polymer system has already been studied for the release of a single drug.[10, 11] For example, Xu et al. demonstrated the effect of the CAP to Pluronic ratio on erosion rate and drug release.[10] The CAPP association polymer has also been used in the form of consolidated microspheres for the release of protein.[12] Jeon et al. fabricated CAPP microsphere-based devices for intermittent release of simvastatin and showed positive results for osteoblast responses and bone formation in vitro and in vivo, respectively.[13, 14] The same group also studied intermittent release of two different drugs using CAPP microsphere-based devices.[15] There is less research, however, toward delivery of more than two drugs or biomolecules in a predetermined temporal sequence.
In the present studies, different small molecule drugs, such as metronidazole (antibiotic), doxycycline (antibiotic/anti-resorptive), ketoprofen (anti-inflammatory) and simvastatin (hypolipidemic/osteogenic) along with a model protein (lysozyme) were loaded in CAPP films. After evaluating individual layers, the morphology of multilayered devices and subsequent intermittent and sequential release profiles were measured. To determine effects of encapsulation and release on bioactivity, enzymatic activity of the released model protein was determined.
2. MATERIAL AND METHODS
2.1 Fabrication of CAPP films
CAPP films were fabricated by solvent casting. CAP (MW 2,534 Da; Sigma, St. Louis, MO) and Pluronic F-127 (Sigma) were mixed in the weight ratio of 70:30, respectively, and dissolved in acetone to obtain an 8% polymer solution. Either 2.5 or 5 wt% of drug was added to the acetone-polymer (CAPP) solution and mixed thoroughly until the drug was completely dissolved, except for the case of the model protein, which did not completely dissolve. The drug-polymer solution was poured in a Teflon dish and stored at 4°C for 24 hours to slow solvent evaporation and avoid bubbles. Afterward, films were dried and stored at room temperature before use. Blank CAPP films were prepared in the same way but without the addition of drugs. For the present study, CAPP films were loaded with metronidazole (MW 171 Da; Sigma), doxycycline (MW 444 Da; Sigma), ketoprofen (MW 254 Da; Sigma), lysozyme (MW 14,304 Da; Sigma), or simvastatin (MW 419 Da; Haorui Pharma-Chem, Inc., Edison, NJ). Samples with 6 mm diameter and 0.5 mm thickness were punched from the CAPP films for further study.
Multilayered CAPP films were fabricated to obtain intermittent release of the same drug or sequential release of more than one drug. Figure 1 shows a schematic representation of the fabrication process. The drug-loaded and blank CAPP films were arranged in the desired sequence and then bonded together by compressing them after 5 μL of acetone were applied between the layers. For intermittent release of drugs, four-layered CAPP devices were prepared with alternating layers of blank and metronidazole-loaded films. For achieving sequential release of more than one type of drug, three-layered CAPP devices were fabricated using metronidazole- and ketoprofen-loaded CAPP films with blank films between the drug-containing layers. The stacked CAPP films were inserted into a 6 mm diameter polystyrene well, which acted as in impermeable backing to enable unidirectional polymer erosion and drug release. A similar procedure was followed to fabricate multilayered devices loaded with simvastatin and doxycycline.
Figure 1.
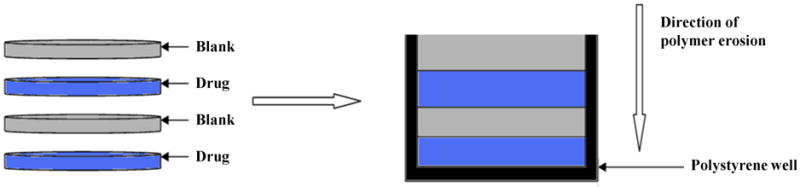
Schematic representation of the process for fabricating multilayered CAPP devices.
2.2. Morphological characterization
2.2.1. Scanning electron microscopy (SEM)
SEM imaging was used to study the overall morphology and interfaces of the blank and drug-loaded CAPP films that form the device. For this purpose, four-layered devices were fabricated with alternating metronidazole-loaded and blank films. The CAPP films were freeze-fractured, and the cross-section was sputter-coated with platinum and observed using an S-3200-N Hitachi instrument.
2.2.2. Fluorescence imaging
To analyze the spatial distribution of drug between loaded and unloaded layers following device fabrication, a fluorescent molecule was incorporated into multilayered films. Fluorescein (Sigma) was loaded in CAPP films at 0.16 wt%, and multilayered CAPP films were fabricated with alternating layers of fluorescein-loaded and blank films. Thin (5 μm) cross-sections of the multilayered CAPP films were cut with a microtome and observed under epifluorescence (Olympus IX51). To determine the effect of aging on interlayer diffusion of fluorescein, samples were incubated at 37°C for 6 days followed by sectioning and microscopic analysis. Line profiling of the fluorescent microscopic images was conducted using ImageJ software.
2.3. Drug release and mass loss
In vitro release studies were conducted for single-layered CAPP films by eroding the materials in 4 mL of phosphate-buffered saline (PBS), pH 7.4, at 37°C on an orbital shaker. Release supernatant was collected every hour and replaced with fresh PBS until samples were completely eroded. Blank CAPP films of the same dimensions were used as controls. Multilayered devices were eroded in either 2 or 4 mL of PBS to study the effect of sink volume on device erosion and drug release. For multilayered devices, release supernatants were collected approximately every 8-10 hours and replaced with fresh PBS.
The total amount of the drug present in the films was determined two ways. Initially, theoretical loading was calculated based on the mass of a CAPP films and the weight percentage of drug used during fabrication. Subsequent studies were conducted with CAPP samples randomly cut cast films, which were then completely dissolved in PBS and the amount of drug measured as described below. The theoretical and actual amounts were similar.
Mass loss was also measured during the release studies. After removal of the supernatant, residual PBS was wicked off the samples, and the remaining mass was recorded at each time point during the course of erosion in 2 ml of PBS. These data were used to construct the mass loss profiles of the multilayered CAPP films.
Three-layered blank devices were used as controls for the release and erosion studies. Because lysozyme loaded in the films did not dissolve completely, protein particles were distributed in the CAPP films. To determine whether the heterogeneous distribution affected release, the lysozyme-loaded films were tested in two orientations (protein side up and protein side down) within the polystyrene well.
Supernatants were analyzed using UV spectroscopy (Powerwave HT, Biotek) to determine the concentration of metronidazole (318 nm) and doxycycline (350 nm). High performance liquid chromatography (HPLC; Shimadu Prominence) was used to measure the concentration of ketoprofen (mobile phase of acetonitrile (60):trifluroacetic acid (TFA) buffer (40); UV detection at 260 nm) and simvastatin (mobile phase of acetonitrile (70):TFA buffer (30); UV detection at 240 nm). The BCA protein assay (Pierce, Rockford, IL) was used to quantify the concentration of lysozyme.
2.4. Mathematical modeling
Release profiles for drugs released from the CAPP system were evaluated using Hopfenberg’s model for controlled release from erodible slabs (Equation 1):
| (1) |
where Mt is the amount of drug released (mg) at time t (hours), M∞ the total amount of drug released from the device (mg), ko the erosion constant (mg/hr/mm2), Co initial concentration of the drug in the device (mg/mm3), a the half thickness of the slab, and n=1 for a slab.[16] Based on the conditions provided for the model, CAPP devices were considered erodible slabs. Furthermore, only one side of the slab was exposed for polymer erosion and drug release due to the presence of the polystyrene well. To accommodate this condition of unidirectional erosion and release, the term a (half the thickness of the slab) was replaced with 2a (total thickness of the slab in mm) in equation (1). The predicted release profiles were compared with the experimentally determined cumulative release profiles.
2.5. Bioactivity of the released protein
Lysozyme bioactivity was measured by its ability to lyse cell walls of Micrococcus lysodeikticus (Sigma).[17, 18] Lysozyme release supernatant or standard dilutions of lysozyme in PBS were added to 0.5 mg/mL of M. lysodeikticus, and the absorbance at 450 nm was measured at 0 and 10 minutes. The observed and expected (obtained from the standard curve) absorbances were compared to determine the relative bioactivity of released lysozyme.
2.6. Statistical analysis
Experimental data were analyzed for statistical significance by the Student’s t-test using InStat (GraphPad Software, Inc., La Jolla, CA). Slopes of the release profiles for different drugs as well as those obtained from mathematical modeling were analyzed by linear regression using Graphpad Prism software.
3. RESULTS
3.1. Morphological characterization
SEM showed clear demarcation between the alternating layers of drug-loaded and blank CAPP films along with some interlayer voids and defects that were likely created during multilayered fabrication (Figure 2A). Figure 2B shows the cross-section of a multilayered CAPP film with alternating layers of fluorescein-loaded and blank CAPP films visualized by fluorescence microscopy. Heterogeneity in distribution was observed on the top/side of the CAPP layer where it was attached to another CAPP layer. Figure 2C shows a cross-section of a sample after 6 days of incubation at 37°C. Both fluorescence images showed that layers loaded with fluorescein were distinctly separate from the blank CAPP layers. Line profiling quantitatively confirmed the distinction between the fluorescein-loaded and blank CAPP films. Line profiles obtained at three different sections of the multilayered device showed clear separation in brightness between layers
Figure 2.
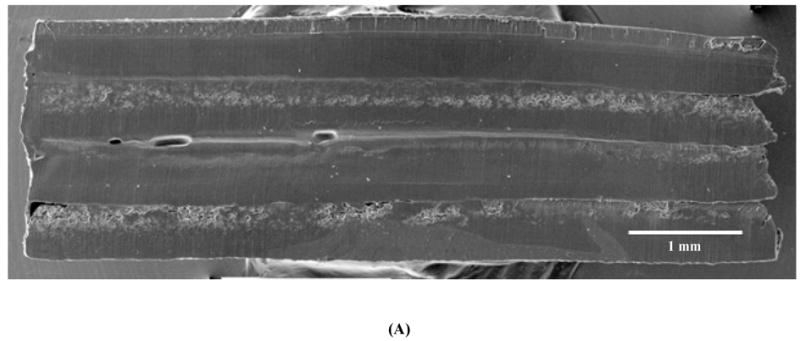
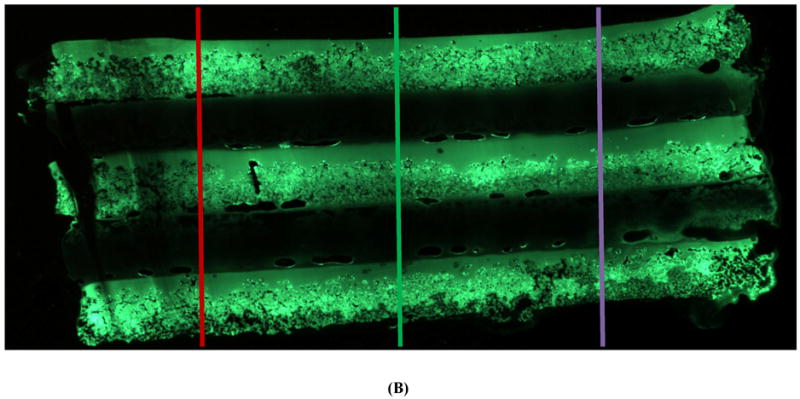
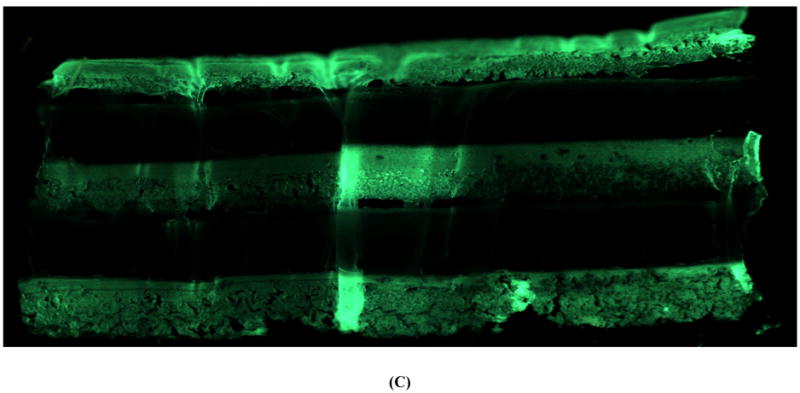
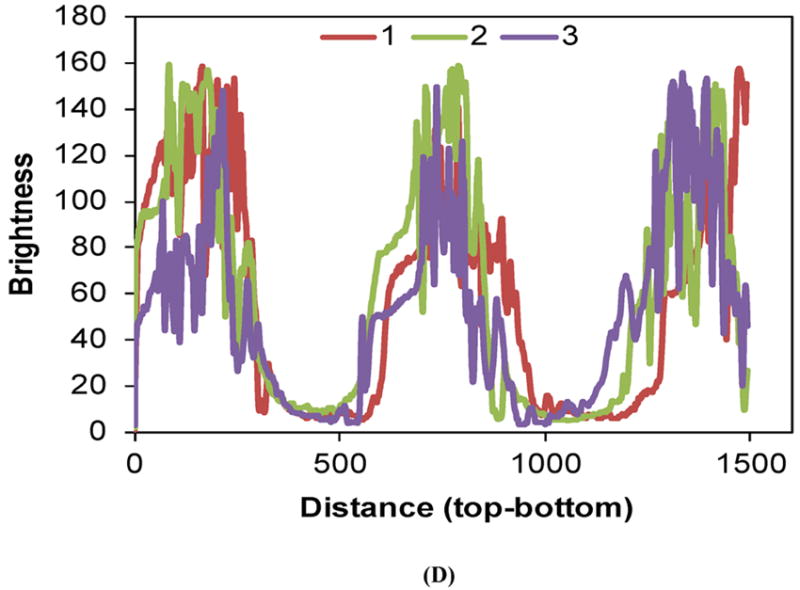
Morphology of multilayered CAPP devices. (A) SEM image of the cross-section showing four CAPP films attached to each other. Fluorescent images of multilayered CAPP devices with alternating fluorescein-loaded and blank films obtained (B) one day after fabrication and (C) after 6 days of incubation at 37°C. (D) Line profiles showing the distinct difference between the fluorescein-loaded and blank layers at different locations (shown in Figure 2B).
3.2. Single layer drug release profiles
Release of individual drugs (metronidazole, ketoprofen, simvastatin, doxycycline, and lysozyme) from a single layer of CAPP showed sustained release of the drug during the course of erosion (8-10 hours) reflecting near zero-order kinetics (Figure 3A and 3B). The total amount of drug released from the CAPP films corresponded to the amount of drug loaded in the CAPP films. For example, 5 wt% of metronidazole and ketoprofen was loaded in the CAPP films, which resulted in 1 mg of drug in each sample. The cumulative release profile showed that, on average, 97% of the metronidazole and 89% of ketoprofen loaded in the CAPP films were detected (Figure 3A). The loading of simvastatin and doxycycline was 2.5 wt%, resulting in 0.5 mg of drug present in each sample. The average percentages of simvastatin and doxycycline released were 100% and 98%, respectively (Figure 3B). Slopes of the release profiles for different small molecule drugs released from single-layered CAPP films were statistically similar. The release profiles of lysozyme-loaded CAPP films, however, differed from those of the other drugs (Figure 3C). Approximately 60% of the protein was released either during the first 4 hours of film erosion or during the final 4 hours, depending on which surface of lysozyme films was exposed to PBS.
Figure 3.
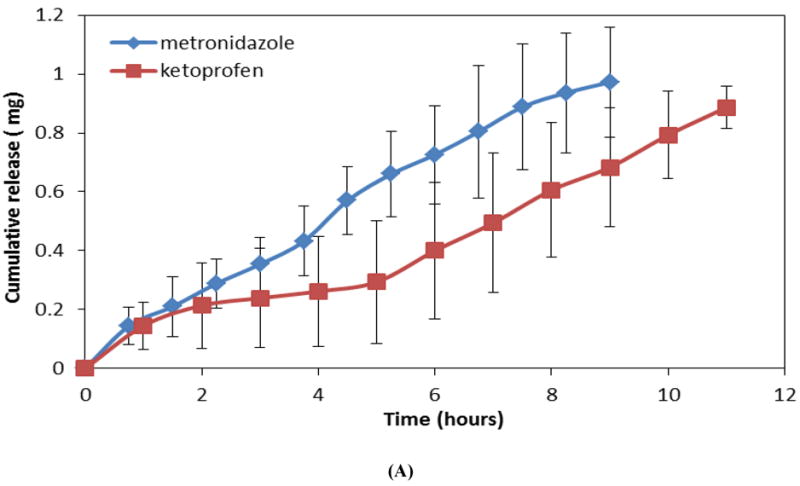
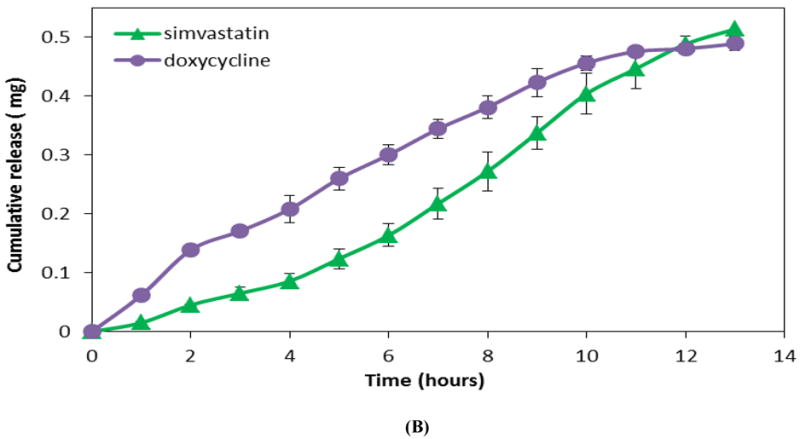
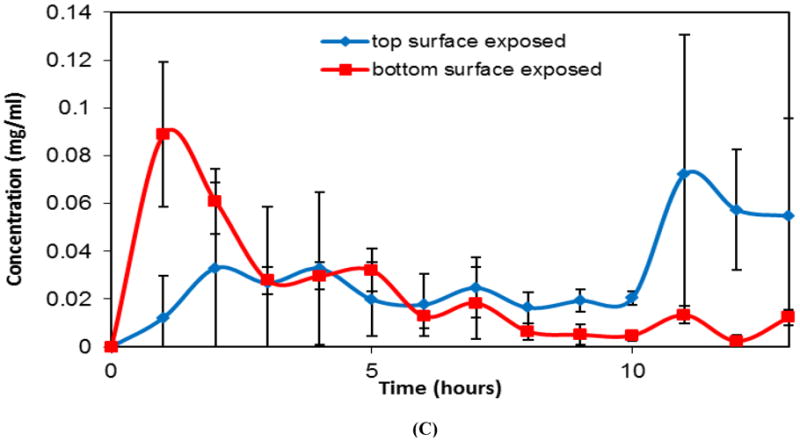
Profiles showing release of drugs from CAPP films. (A) Cumulative release of metronidazole and ketoprofen (5 wt% loading; 1 mg/sample). (B) Cumulative release of doxycycline and simvastatin (2.5 wt% loading; 0.5 mg/sample). (C) Instantaneous release of lysozyme from CAPP films with top or bottom surface exposed to PBS. Data are mean ± standard deviation (n=3).
3.3. Intermittent and sequential drug release profiles
Polymer erosion of multilayered (four-layered) CAPP devices with alternating metronidazole-loaded and blank layers resulted in intermittent release of the same drug (Figure 4A). This release profile showed no release of drug during the initial stages of erosion (first 10 hours) due to the presence of blank layer on top, followed by release of metronidazole from the second CAPP layer (approximately 10-40 hours). The third (blank) layer delayed release of metronidazole from the fourth layer, while metronidazole from the final (bottom) layer of the device was released during the last stages (last 40 hours) of erosion.
Figure 4.
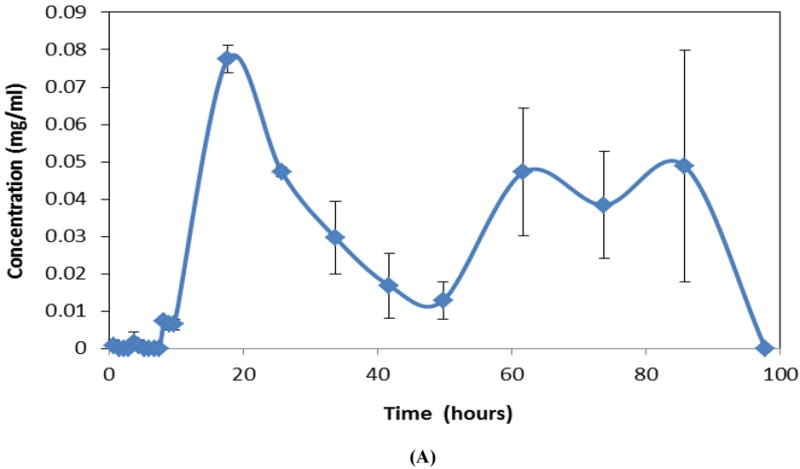
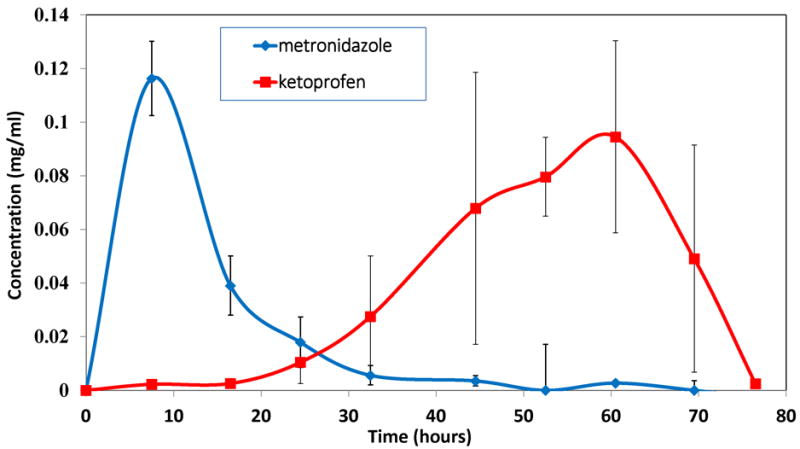
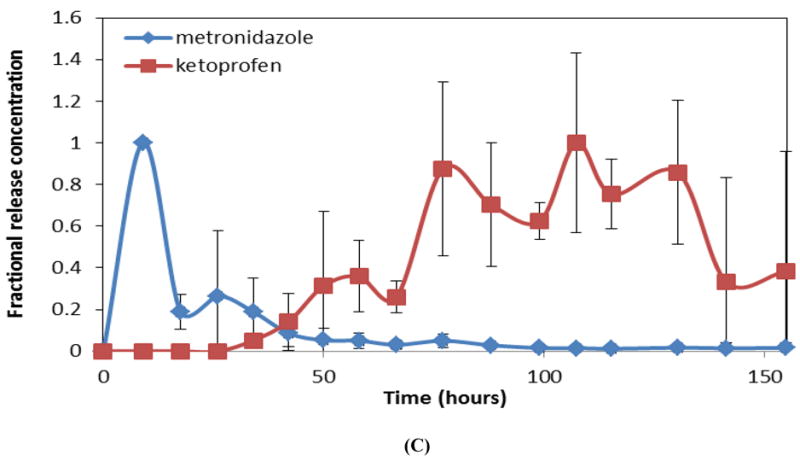
Instantaneous drug release profiles for multilayered CAPP devices. (A) Intermittent release of metronidazole (blank-metronidazole-blank-metronidazole) during erosion in 4 mL of PBS. Sequential release of metronidazole followed by ketoprofen (metronidazole-blank-ketoprofen) during erosion in (B) 4 mL or (C) 2 mL of PBS. Data are mean ± standard deviation (n=3).
Figure 4B shows release profiles for three-layered devices with metronidazole- and ketoprofen-loaded layers separated by an intermediate blank film eroded in 4 mL of PBS. Based on design of the device, metronidazole was released during the first 20-25 hours of device erosion. The blank layer delayed the next phase of release, which involved release of ketoprofen during the final stages of device erosion (last 40-50 hours). When the same type of device was eroded in 2 mL of PBS, the total erosion time was around 155 hours compared to only 77 hours observed for 4 mL PBS (Figure 4C). Even though the device eroded more slowly when the amount of medium (PBS) was reduced, sequential drug release was still achieved. Metronidazole was released during the first 40 hours of device erosion followed by a small delay in the release of ketoprofen due to the presence of the blank layer; ketoprofen was again released during the last 100 hours of the device erosion.
Figure 5A shows both empirical and predicted release of one drug (metronidazole) from a single layer CAPP film. As for films containing metronidazole and ketoprofen, multilayered devices with simvastatin and doxycycline eroded in 2 mL of PBS also followed the same sequential release pattern, with a total erosion time of approximately 160 hours (Figure 5B). Simvastatin was released significantly faster during the first 50 hours of device erosion than doxycycline was released during the last 100 hours (p<0.05), but there were no significant differences between the experimentally measured slopes and those predicted by mathematical modeling.
Figure 5.
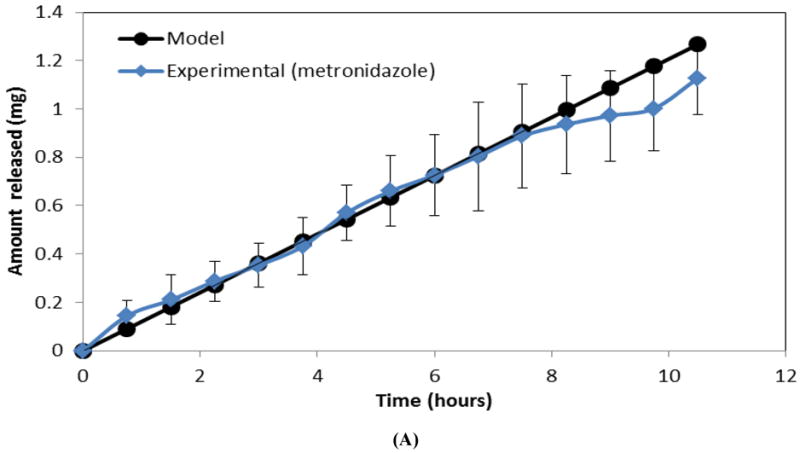
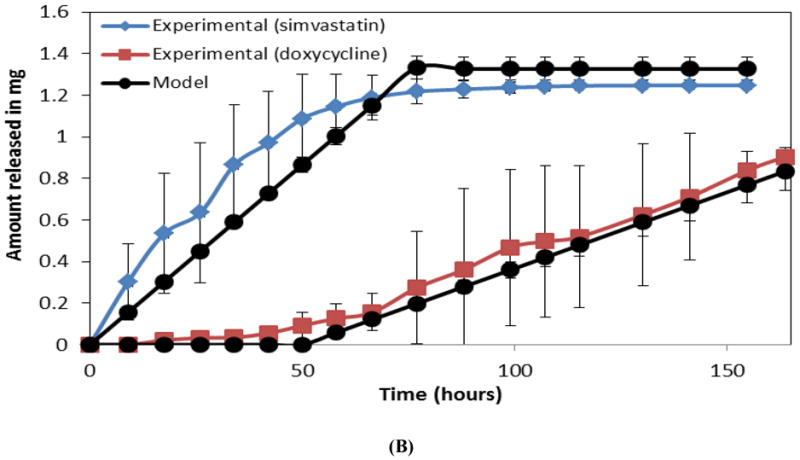
Comparison of observed and mathematically predicted cumulative release profiles for (A) metronidazole in a single layer CAPP film and (B) simvastatin followed by doxycycline in a three-layered film. Data are mean ± standard deviation (n=3).
3.4. Loading and release efficiency
In general, 96.5% of the small molecule drugs loaded into CAPP films was released, irrespective of the type or wt% of the drug (Figure 6A). There was no statistically significant difference between the observed and expected amount of metronidazole and doxycycline loaded and released from the CAPP multilayer devices. In the case of ketoprofen, 83% of the expected amount was released, and in the case of simvastatin 90% of the expected amount was released.
Figure 6.
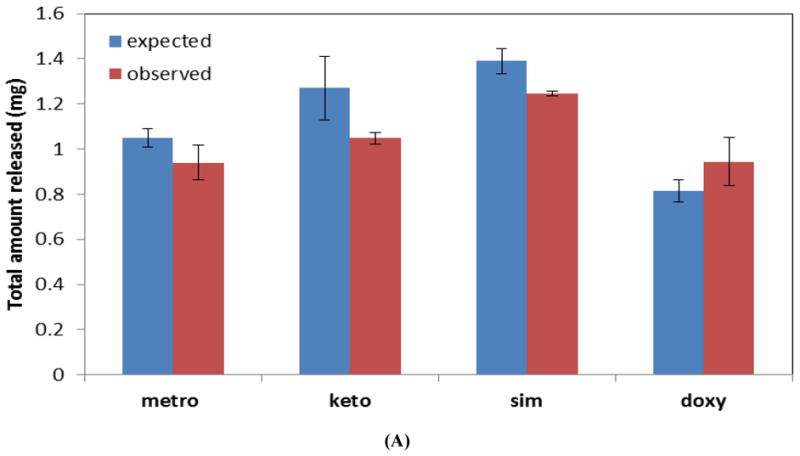
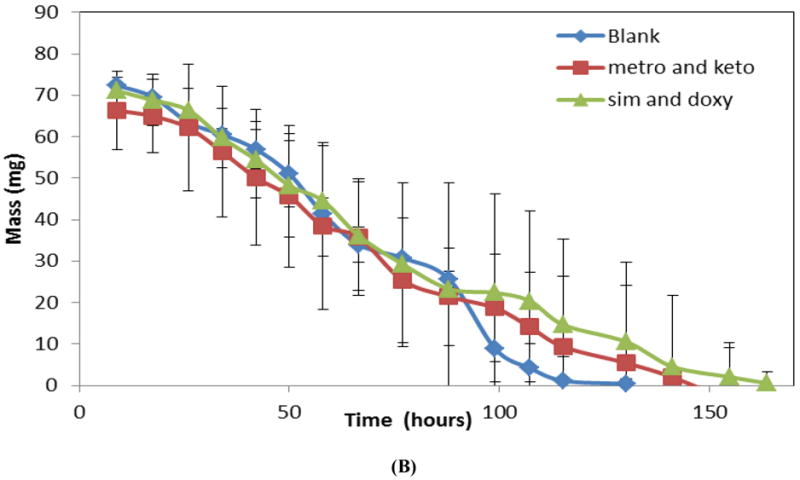
(A) Observed and expected amounts of drugs released from multilayered CAPP devices. (B) Mass loss profiles for multilayered devices with blank layers (blank), layers loaded with metronidazole and ketoprofen (metro and keto), or layers loaded with simvastatin and doxycycline (sim and doxy) degraded in 2 mL of PBS.
3.5. Mass loss profiles
Figure 6B shows mass loss profiles for three-layered devices eroded in 2 mL of PBS. The profiles presented are for blank, sequential metronidazole and ketoprofen, and sequential simvastatin and doxycycline films. Both the blank devices and the drug-loaded devices eroded with linear mass loss profile characteristic of a zero-order system. Neither loading nor the type of drug incorporated into the films had a significant effect on the erosion rate.
3.6. Bioactivity of released protein
Figure 7 shows the bioactivity of lysozyme in release supernatants during the final 6 hours (time points when lysozyme release occurred) of film erosion. Results showed that, on an average, lysozyme released from the CAPP films retained 57% of the expected bioactivity.
Figure 7.
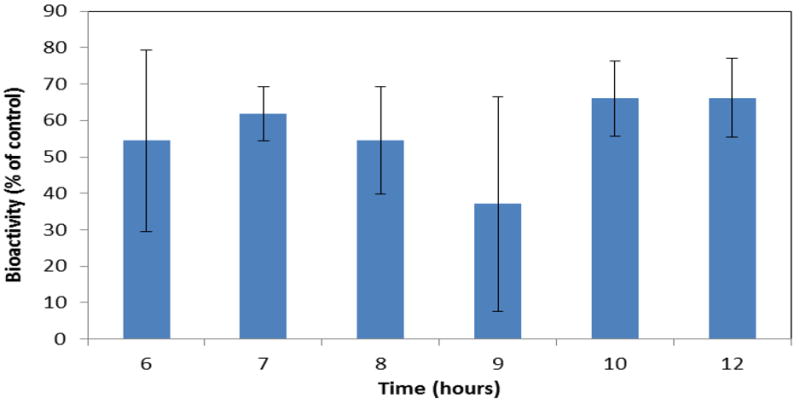
Retention of lysozyme bioactivity following release from CAPP films. Data are mean ± standard deviation (n=3).
4. DISCUSSION
The main aim of this research was to develop a CAPP film-based system that will serve as a platform for delivery of different types of drugs, suitable for treatment of a broad range of disease conditions. These multilayered devices may also be adapted for delivery of more than one type of biomolecule for tissue engineering applications. The drugs used and release profile needed for efficacy will depend on, among other factors, the particular condition or tissue defect being treated.
The two polymers used for the association polymer, CAP and Pluronic F-127, form intermolecular hydrogen bonds in aprotic solvents, such as acetone, used during fabrication. In this case, carboxylic acid groups in CAP act as proton donors, and the ether sites in the non-ionic surfactant Pluronic F127 form the proton acceptors.[11] When the CAPP system is exposed to physiological conditions, deprotonation occurs and leads to dissolution of the CAPP into its CAP and Pluronic components. This type of mechanism results in erosion-based, sustained release of drugs.[11-13] CAP is commonly used as an enteric coating on tablets[19], and Pluronic, an amphiphilic triblock copolymer, has been widely used for drug delivery purposes and as a surfactant. [20] In vivo studies have been performed with the CAPP association polymer system without any adverse effects.[14]
The CAPP polymer system has been used in the form of microspheres[13] and single-layered films[10] for zero-order release of different drugs, but the present research focused on its use in multilayered devices for delivery more than one type of drug. CAPP in the form of films is more appropriate for this application, as it suits the design of multilayered devices for sequential delivery of multiple drugs. The fabrication of the CAPP films involves a relatively simple solvent evaporation technique compared with other fabrication processes, such as melt processing and injection molding, needed for other surface-eroding polymers. Fabrication of the multilayered devices involved adhering individual CAPP films using acetone as a solvent for plasticizing the surface of the CAPP films without altering the bulk properties of the film. This simple technique without the use of potentially harsh processing conditions, such high temperature and pressure, reduces the chances of small molecule drugs losing bioactivity during the fabrication process. Limitations of these CAPP films include the non-uniform distribution of hydrophilic molecules, such as proteins and lack of mechanical flexibility of the films which is being addressed by inclusion of a plasticizing agent during the fabrication process.
4.1. Morphological characterization
SEM and fluorescence microscopy were used to characterize the morphological properties of the multilayered CAPP device. SEM images of the cross-section of a four-layered device showed demarcation between the CAPP layers, indicating the separation between the drug-loaded and blank layers. This type of separation between the layers was necessary to maintain the layer-based design of the device and enable sequential drug release. Cross-sectional images also showed that the small volume of solvent applied to bond layers dissolved only the surface of the films and did not affect the internal portions of the CAPP films.
Further characterization using fluorescence imaging showed clear distinction between the fluorescent and blank layers. Both qualitatively and quantitatively, fluorescence was not observed in the blank layers, which confirmed that there was no diffusion of fluorescein from loaded to unloaded layers during fabrication. Some non-uniformity within the loaded layers likely resulted from the solvent evaporation process used to cast the films. An absence of interlayer diffusion even during incubation (“aging”) at 37°C for six days was confirmed by the distinct separation of fluorescein-loaded and blank layers.
4.2. Single layer drug release profiles
As indicated previously, the primary aim of this research was to fabricate a drug delivery device that serves as a platform for delivery of wide spectrum of drugs in a specified sequential order. For this purpose, some of the most commonly used drug types, such as antibiotics (metronidazole and doxycycline), an anti-inflammatory agent (ketoprofen), and a potentially osteogenic small molecule drug (simvastatin), along with a model protein (lysozyme) were chosen, and their loading and release were studied.[21-24] All the CAPP films with drugs were inserted into a polystyrene well to aid unidirectional polymer erosion. Results for the drugs investigated in this study showcase the ability of the CAPP system to serve as delivery platform for a variety of biomolecules.
Except for lysozyme, the other four drugs that were loaded and released are currently used for treatment in patients. Metronidazole is effective against most Gram-negative and Gram-positive anaerobic bacteria and a wide variety of protozoans.[25] Doxycycline is one of the commonly prescribed tetracycline antibiotics that are effective against variety of infectious agents.[26] In addition, it also possesses bone anti-resorptive properties.[27] Ketoprofen, which is commonly used for treatment of arthritis[28] and in dentistry,[29] is a phenylproprionic acid derivative with analgesic, anti-inflammatory, and antipyretic properties. The fourth small molecule drug used in this study was simvastatin, which is widely used for controlling high cholesterol level.[30] Importantly, however, simvastatin has the ability to stimulate bone formation via enhanced expression of bone morphogenetic protein 2 (BMP-2).[31]
All four drugs were released in a sustained manner from the CAPP films and followed zero-order release kinetics. In vitro release results for the single layer CAPP films were statistically comparable to predictions from Hopfenberg’s model developed to predict drug release from a slab.[16] Irrespective of the type of drug or the amount of drug that was loaded, similar release rates were measured, and nearly all of the drug was accounted for during the experiments. As a nonionic surfactant, the Pluronic F-127 component of this association polymer increases the solubilizing power of the system.[10] This property allows a wide range of dosages to be achieved.
The model protein that was loaded into and released from CAPP films was lysozyme. Being hydrophilic, lysozyme did not dissolve completely in acetone during the fabrication process. To determine the effect of the non-uniformly distributed lysozyme particles on the release profiles, lysozyme-loaded CAPP films were eroded in two different orientations. When the film surface that was in contact with the Teflon dish (bottom surface, where the undissolved lysozyme settled during fabrication) was attached face down in the impermeable well, the top surface (with fewer lysozyme particles) eroded initially, and protein was predominantly released during the final stages of the film erosion. When the film surface that was exposed to the atmosphere (top surface) during film fabrication faced the polystyrene, release of lysozyme occurred during the initial stages of films erosion. These release profiles further confirm that release of lysozyme occurred by surface erosion of polymer, because lysozyme was released only when the part of the polymer film with high concentration of lysozyme was exposed.
4.3. Intermittent and sequential release of drugs
Erosion of multilayered devices in vitro resulted in successful release of drugs in both intermittent and sequential manners. These multilayered devices also had a polystyrene backing layer for unidirectional polymer erosion and drug release. Further research is being conducted to replace the non-degradable polystyrene with a biodegradable backing material suitable for in vivo implantation.
Intermittent release of the antibiotic metronidazole was achieved using an intermediate blank CAPP layer. Similarly, sequential release of an antibiotic, metronidazole, and an anti-inflammatory agent, ketoprofen, was also demonstrated by placing a blank CAPP layer between drug-loaded layers. When incubated in a smaller volume of PBS, the same devices with metronidazole and ketoprofen eroded at a slower rate. These findings show the effect that the sink has on device erosion and the consequent release profile. In both release studies, the interval at which the samples were collected was the same (every 8-10 hours). The release byproducts generated during erosion of CAPP might have saturated the smaller volume of release medium and thereby prevented (slowed) further polymer erosion. When a larger volume of release medium was used, saturation with erosion byproducts would have occurred relatively slower, thereby resulting in faster erosion. In spite of the change in erosion rate, sequential release of metronidazole followed by the release of ketoprofen was not altered. In the multilayered devices, release of the second drug occurred in a relatively more sustained manner when compared to the first drug. When multilayered devices were eroded in 4 mL of PBS, the first drug was released within 20 hours, and the second drug was released over the last 50 hours. The slower release at later stages of erosion can be explained by the cylindrical polystyrene wells in which the layers were inserted. As CAPP eroded, PBS was retained more deeply within the well, and the reduced circulation of buffer near the final layer resulted in relatively slower erosion and drug release. With only 2 mL of PBS, the first drug was released within 40 hours, and the second drug was released over the last 100 hours. In this case, the combined effects of the reduced sink conditions and reduced mixing within the wells further slowed erosion, but the total amount of drug released was not significantly affected. Mass balance calculations again showed comparable expected and observed drug amounts loaded and released. Hopfenberg’s equation was also suitable for modeling sequential release of more than one drug from the multilayer CAPP device. The predicted profiles also showed that the rate of release of the first drug was approximately twice as fast as that for the second drug. The predicted and experimentally measured release profiles were statistically similar for both the single layer and multilayered devices, indicating the suitability of this particular model for the present delivery system. This model for predicting the release profiles from CAPP devices would be helpful for further design of advanced multilayered devices.
Mass loss profiles showed that CAPP devices, irrespective of the type of drugs loaded, followed a surface-erosion pattern. As such, the duration of the drug release as well as the time interval between the release peaks of the same or different drugs can be increased or decreased by altering the thickness of the blank CAPP films. Overall, the type and amount of drug loaded in the CAPP films can be altered to achieve a desired intermittent or sequential release using this multilayered system.
4.4. Bioactivity of released protein
Because proteins are more unstable compared to small molecule drugs, initial bioactivity testing was conducted for only the protein that was released from the CAPP films. Results showed that lysozyme lost approximately 40% of its activity during loading and delivery. Although protein may have interacted with the polymers, exposure of lysozyme to acetone during the fabrication process resulted in aggregation and precipitation, which reduced bioactivity. This was reflected in the two-sided nature of the lysozyme-loaded films. Other commonly used bioerodible/biodegradable delivery systems, such as poly(lactic-co-glycolic acid) (PLGA) and poly(ethylene glycol) (PEG), also result in loss of protein activity by aggregation, hydrolytic degradation, and chemical modification during the necessary manufacturing process, which can involve heating, pH changes, shear forces, organic solvents, drying and others.[32] With the CAPP delivery system, however, the amount of protein loaded can be easily altered, and excipients may enhance preservation of bioactivity.
The present studies have shown that CAPP film-based devices can be used to deliver a wide variety of drugs and can be used to achieve sequential delivery of multiple drugs. These advantages will be helpful for customizing devices different applications. For example, some bacterial infections, which might involve more than one type of microorganism, require combination of antibiotics for treatment[33] to eliminate the infection and reduce the potential for developing antibiotic resistance.[1] The ability of this CAPP system to sequentially deliver anti-inflammatory agents with other drugs creates the possibility of inflammation control and pain management during wound healing. The system may also be useful for delivery of multiple growth factors for tissue engineering. A growing body of research suggests the importance of more than one growth factor for regeneration of tissues, such as bone,[15][34][35] blood vessels,[36] and cartilage.[37][38]
There have been several attempts towards the delivery of multiple growth factors.[4] Most of these approaches, however, have been successful for simultaneous delivery.[39-50] Temporally controlled release has been obtained by other strategies, including fabrication scaffolds and/or microspheres consisting of one or more polymers each loaded with a different drug, e.g., PLGA in combination with gelatin hydrogels, poly(propylene fumarate), poly(4-vinyl pyridine), alginic acid, cellulose acetate.[51-55] The system presented in this paper was composed of a single association polymer system in the form of multilayered films, which simplifies the fabrication process and eases loading of wide variety of drugs to obtain localized delivery of multiple drugs in a required temporal sequence. Furthermore, the system can be modified to adjust the duration of drug release based on a particular application, such as by changing the ratio of CAP to Pluronic and/or inclusion of intermediate polymeric barriers between erodible layers.
5. CONCLUSION
The easy to fabricate CAPP association polymer can be used to achieve zero-order release of a wide variety of drugs. As such, this system can serve as a general platform for localized, controlled drug delivery to treat several disease conditions. Different release profiles can be designed, including sustained release of one drug, intermittent release of a drug, or sequential release of multiple drugs. This system could be used for applications that require delivery of more than one type drug in a predetermined temporal sequence.
Acknowledgments
This research was supported by NIH (DE019645 and AR060964) and Kentucky NASA EPSCoR (NNX08BA13A). We dedicate this work in memory of Dr. Mark V. Thomas, who passed away as the studies were being conducted.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dowling HF, Finland M, Hamburger M, Jawetz E, Knight V, Lepper MH, et al. The Clinical Use of Antibiotics in Combination. AMA Arch Intern Med. 1957;99:536–8. doi: 10.1001/archinte.1957.00260040036003. [DOI] [PubMed] [Google Scholar]
- 2.Rosen PS. Treatment of Plaque-Induced Gingivitis, Chronic Periodontitis, and Other Clinical Conditions. Journal of Periodontology. 2001;72:1790–800. doi: 10.1902/jop.2001.72.12.1790. [DOI] [PubMed] [Google Scholar]
- 3.Younger ASE, Duncan CP, Masri BA. Treatment of Infection Associated with Segmental Bone Loss in the Proximal Part of the Femur in Two Stages with Use of an Antibiotic-Loaded Interval Prosthesis. The Journal of Bone & Joint Surgery. 1998;80:60–9. doi: 10.2106/00004623-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Chen F-M, Zhang M, Wu Z-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 5.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric Systems for Controlled Drug Release. Chemical Reviews. 1999;99:3181–98. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 6.Jain JP, Modi S, Domb AJ, Kumar N. Role of polyanhydrides as localized drug carriers. Journal of Controlled Release. 2005;103:541–63. doi: 10.1016/j.jconrel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Kumar N, Langer RS, Domb AJ. Polyanhydrides: an overview. Advanced Drug Delivery Reviews. 2002;54:889–910. doi: 10.1016/s0169-409x(02)00050-9. [DOI] [PubMed] [Google Scholar]
- 8.Heller J, Barr J, Ng SY, Abdellauoi KS, Gurny R. Poly(ortho esters): synthesis, characterization, properties and uses. Advanced Drug Delivery Reviews. 2002;54:1015–39. doi: 10.1016/s0169-409x(02)00055-8. [DOI] [PubMed] [Google Scholar]
- 9.Göpferich A, Tessmar J. Polyanhydride degradation and erosion. Advanced Drug Delivery Reviews. 2002;54:911–31. doi: 10.1016/s0169-409x(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Lee PI. Programmable Drug Delivery from an Erodible Association Polymer System. Pharmaceutical Research. 1993;10:1144–52. doi: 10.1023/a:1018960016756. [DOI] [PubMed] [Google Scholar]
- 11.Gates KA, Grad H, Birek P, Lee PI. A New Bioerodible Polymer Insert for the Controlled Release of Metronidazole. Pharmaceutical Research. 1994;11:1605–9. doi: 10.1023/a:1018913921956. [DOI] [PubMed] [Google Scholar]
- 12.Raiche AT, Puleo DA. Association polymers for modulated release of bioactive proteins. IEEE engineering in medicine and biology magazine. 2003;22:35–41. doi: 10.1109/memb.2003.1256270. [DOI] [PubMed] [Google Scholar]
- 13.Jeon JH, Thomas MV, Puleo DA. Bioerodible devices for intermittent release of simvastatin acid. International Journal of Pharmaceutics. 2007;340:6–12. doi: 10.1016/j.ijpharm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon JH, Piepgrass WT, Lin Y-L, Thomas MV, Puleo DA. Localized Intermittent Delivery of Simvastatin Hydroxyacid Stimulates Bone Formation in Rats. Journal of Periodontology. 2008;79:1457–64. doi: 10.1902/jop.2008.080004. [DOI] [PubMed] [Google Scholar]
- 15.Jeon JH, Puleo DA. Alternating release of different bioactive molecules from a complexation polymer system. Biomaterials. 2008;29:3591–8. doi: 10.1016/j.biomaterials.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopfenberg HB. Controlled Release from Erodible Slabs, Cylinders, and Spheres. Controlled Release Polymeric Formulations: Americal Chemical Society. 1976:26–32. [Google Scholar]
- 17.Jiang H, Hu Y, Li Y, Zhao P, Zhu K, Chen W. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. Journal of Controlled Release. 2005;108:237–43. doi: 10.1016/j.jconrel.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Ghaderi R, Carlfors J. Biological Activity of Lysozyme After Entrapment in Poly (d,l-lactide-co-glycolide)-Microspheres. Pharmaceutical Research. 1997;14:1556–62. doi: 10.1023/a:1012122200381. [DOI] [PubMed] [Google Scholar]
- 19.Roxin P, Karlsson A, Singh SK. Characterization of Cellulose Acetate Phthalate (CAP) Drug Development and Industrial Pharmacy. 1998;24:1025–41. doi: 10.3109/03639049809089946. [DOI] [PubMed] [Google Scholar]
- 20.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. Journal of Controlled Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 21.Ponchel G, Touchard F, Wouessidjewe D, Duchêne D, Peppas NA. Bioadhesive analysis of controlled-release systems. III. Bioadhesive and release behavior of metronidazole-containing poly(acrylic acid)-hydroxypropyl methylcellulose systems. International Journal of Pharmaceutics. 1987;38:65–70. [Google Scholar]
- 22.Bounaceur A, Rodier E, Fages J. Maturation of a ketoprofen-cyclodextrin mixture with supercritical carbon dioxide. The Journal of Supercritical Fluids. 2007;41:429–39. [Google Scholar]
- 23.Garcia-Ruiz C, Marina ML. Recent advances in the analysis of antibiotics by capillary electrophoresis. Electrophoresis. 2006;27:266–82. doi: 10.1002/elps.200500430. [DOI] [PubMed] [Google Scholar]
- 24.Barrett B, Huclová J, Borek-Dohalský V, Nemec B, Jelínek I. Validated HPLC-MS/MS method for simultaneous determination of simvastatin and simvastatin hydroxy acid in human plasma. Journal of Pharmaceutical and Biomedical Analysis. 2006;41:517–26. doi: 10.1016/j.jpba.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Freeman CD, Klutman NE, Lamp KC. Metronidazole. A therapeutic review and update. Drugs. 1997;54:679–708. doi: 10.2165/00003495-199754050-00003. [DOI] [PubMed] [Google Scholar]
- 26.Cunha BA, Domenico P, Cunha CB. Pharmacodynamics of doxycycline. Clinical Microbiology and Infection. 2000;6:270–3. doi: 10.1046/j.1469-0691.2000.00058-2.x. [DOI] [PubMed] [Google Scholar]
- 27.Vernillo AT, Rifkin BR. Effects of Tetracyclines on Bone Metabolism. Advances in Dental Research. 1998;12:56–62. doi: 10.1177/08959374980120012101. [DOI] [PubMed] [Google Scholar]
- 28.Veys EM. 20 Years’ Experience with Ketoprofen. Scandinavian Journal of Rheumatology. 1991;19:3–44. [PubMed] [Google Scholar]
- 29.Johnny GC. Ketoprofen in dentistry: A pharmacologic review. Oral Surgery, Oral Medicine, Oral Pathology. 1988;66:620–4. doi: 10.1016/0030-4220(88)90386-6. [DOI] [PubMed] [Google Scholar]
- 30.Todd PA, Goa KL. Simvastatin. A review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs. 1990;40:583–607. doi: 10.2165/00003495-199040040-00007. [DOI] [PubMed] [Google Scholar]
- 31.Chen P-Y, Sun J-S, Tsuang Y-H, Chen M-H, Weng P-W, Lin F-H. Simvastatin promotes osteoblast viability and differentiation via Ras/Smad/Erk/BMP-2 signaling pathway. Nutrition Research. 2010;30:191–9. doi: 10.1016/j.nutres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Gombotz WR, Pettit DK. Biodegradable Polymers for Protein and Peptide Drug Delivery. Bioconjugate Chemistry. 1995;6:332–51. doi: 10.1021/bc00034a002. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths GS, Ayob R, Guerrero A, Nibali L, Suvan J, Moles DR, et al. Amoxicillin and metronidazole as an adjunctive treatment in generalized aggressive periodontitis at initial therapy or re-treatment: a randomized controlled clinical trial. Journal of Clinical Periodontology. 38:43–9. doi: 10.1111/j.1600-051X.2010.01632.x. [DOI] [PubMed] [Google Scholar]
- 34.Raschke M, Wildemann B, Inden P, Bail H, Flyvbjerg A, Hoffmann J, et al. Insulin-like growth factor-1 and transforming growth factor-I accelerates osteotomy healing using polylactide-coated implants as a delivery system: a biomechanical and histological study in minipigs. Bone. 2002;30:144–51. doi: 10.1016/s8756-3282(01)00640-8. [DOI] [PubMed] [Google Scholar]
- 35.Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials. 2009;30:3551–9. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotech. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 37.Elisseeff J, McIntosh W, Fu K, Blunk T, Langer R. Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. Journal of Orthopaedic Research. 2001;19:1098–104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 38.Fukumoto T, Sperling JW, Sanyal A, Fitzsimmons JS, Reinholz GG, Conover CA, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis and Cartilage. 2003;11:55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 39.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562–9. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 40.Schmidmaier G, Wildemann B, Heeger J, Gäbelein T, Flyvbjerg A, Bail HJ, et al. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone. 2002;31:165–72. doi: 10.1016/s8756-3282(02)00798-6. [DOI] [PubMed] [Google Scholar]
- 41.Lynch SE, Castilla GR, Williams RC, Kiritsy CP, Howell TH, Reddy MS, et al. The Effects of Short-Term Application of a Combination of Platelet-Derived and Insulin-Like Growth Factors on Periodontal Wound Healing. Journal of Periodontology. 1991;62:458–67. doi: 10.1902/jop.1991.62.7.458. [DOI] [PubMed] [Google Scholar]
- 42.Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal Regeneration in Humans Using Recombinant Human Platelet- Derived Growth Factor-BB (rhPDGF-BB) and Allogenic Bone. Journal of Periodontology. 2003;74:1282–92. doi: 10.1902/jop.2003.74.9.1282. [DOI] [PubMed] [Google Scholar]
- 43.Chen F-M, Chen R, Wang X-J, Sun H-H, Wu Z-F. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials. 2009;30:5215–24. doi: 10.1016/j.biomaterials.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Riley CM, Fuegy PW, Firpo MA, ZhengShu X, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials. 2006;27:5935–43. doi: 10.1016/j.biomaterials.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–40. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peattie RA, Rieke ER, Hewett EM, Fisher RJ, Shu XZ, Prestwich GD. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials. 2006;27:1868–75. doi: 10.1016/j.biomaterials.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 47.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue engineering Part A. 2009;15:2347–62. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doğan AK, Gümüşderelioğlu M, Aksöz E. Controlled release of EGF and bFGF from dextran hydrogels in vitro and in vivo. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2005;74B:504–10. doi: 10.1002/jbm.b.30231. [DOI] [PubMed] [Google Scholar]
- 49.Nillesen STM, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–31. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 50.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proceedings of the National Academy of Sciences. 107:3287–92. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. Sequential release of bioactive IGF-I and TGF-beta 1 from PLGA microsphere-based scaffolds. Biomaterials. 2008;29:1518–25. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Buket Basmanav F, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29:4195–204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Kempen DHR, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–25. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 54.Chen R, Silva E, Yuen W, Mooney D. Spatio–temporal VEGF and PDGF Delivery Patterns Blood Vessel Formation and Maturation. Pharmaceutical Research. 2007;24:258–64. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 55.Tengood JE, Kovach KM, Vescovi PE, Russell AJ, Little SR. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials. 2010;31:7805–12. doi: 10.1016/j.biomaterials.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


