Abstract
Ghrelin is a stomach-derived peptide hormone that acts in the brain to regulate many important physiological functions. Ghrelin receptor, named the growth hormone secretagogue receptor (GHSR), is present in many brain areas with or without obvious direct access to ghrelin circulating in the bloodstream. Ghrelin is also present in the cerebrospinal fluid (CSF) but the brain targets of CSF ghrelin are unclear. Here, we studied which brain areas are accessible to ghrelin present in the CSF. For this purpose, we centrally injected mice with fluorescein-labeled ghrelin (F-ghrelin) peptide tracer and then systematically mapped the distribution of F-ghrelin signal trough the brain. Our results indicated that centrally injected F-ghrelin labels neurons in most of the brain areas where GHSR is present. Also, we detected F-ghrelin uptake in the ependymal cells of both wild type and GHSR-null mice. We conclude that CSF ghrelin is able to reach most of brain areas expressing GHSR. Also, we propose that the accessibility of CSF ghrelin to the brain parenchyma occurs through the ependymal cells in a GHSR-independent manner.
Keywords: GHSR, ependymal cells, hypothalamus, acyl-ghrelin
INTRODUCTION
Ghrelin is an octanoylated peptide hormone, synthesized mainly by endocrine cells located within the gastric oxyntic mucosa (Kojima et al., 1999). Ghrelin acts in the brain to regulate growth hormone secretion, food intake, energy expenditure, glucose homeostasis, anxiety/depression-related behaviors and stress responses (Kojima and Kangawa, 2005). Two subtypes of ghrelin receptors have been described, the growth hormone secretagogue receptor 1a (GHSR-1a) isoform, which is activated by ghrelin, and the splice variant isoform GHSR-1b, which is a functionally inactive truncated form of GHSR (Howard et al., 1996). Analyses of the distribution of GHSR-1a within the rodent brain using many different techniques have shown that GHSR-1a is present in several brain sites (Guan et al., 1997, Mitchell et al., 2001, Zigman et al., 2006, Perello et al., 2012). For instance, GHSR-1a mRNA is highly expressed in brain areas that presumably have direct access to circulating ghrelin, such as the hypothalamic arcuate nucleus (ARC) and the dorsal vagal complex (DVC) of the medulla (Guan et al., 1997, Willesen et al., 1999, Zigman et al., 2006). However, GHSR-1a is also present in some brain areas distantly situated from circumventricular organs and without immediate access to ghrelin circulating in the bloodstream (Guan et al., 1997, Mitchell et al., 2001, Zigman et al., 2006, Perello et al., 2012, Scott et al., 2012). The physiological relevance of GHSR in many of these brain areas is unclear. Since ghrelin is also present in the cerebrospinal fluid (CSF) (Tritos et al., 2003, Grouselle et al., 2008), we decided to study which brain areas have ghrelin targets accessible to CSF ghrelin. For this purpose, we injected mice with fluorescein-labeled ghrelin (F-ghrelin) peptide tracer via an intra-cerebro-ventricular cannula and then systematically mapped the distribution of F-ghrelin signal trough the whole brain. Our results indicated that CSF ghrelin is able to access to most of the areas where GHSR-1a is present. Interestingly, we also detected F-ghrelin uptake in the ependymal cells of both wild type and GHSR-null mice, suggesting that ghrelin can interact with these specialized cells in a GHSR-independent manner.
MATERIALS AND METHODS
Animals
Mice were generated in the animal facility of the IMBICE and housed in a 12-h light/dark cycle with regular chow and water available ad lib. In this study, we used adult (8-10 weeks old) C57BL6/J wild type and GHSR-null mice. GHSR-null mice were derived from crosses between heterozygous animals back-crossed for more than 10 generations onto a C57BL6/J genetic background (Zigman et al., 2005). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and all efforts were made to minimize suffering. All experimentation received approval from the Institutional Animal Care and Use Committees located in Multidisciplinary Institute of Cell Biology (approval ID 10-0112).
Reagents
Octanoylated ghrelin was purchased from Global Peptide (cat# PI-G-03). F-ghrelin (2741 Da) is an 18-amino acid analog of ghrelin conjugated to fluorescein isothiocyanate through the addition of a lysine at its C terminus ([Dpr octanoyl)3, Lys(fluorescein)19]-ghrelin(1–19)). Fluorescein-ghrelin was synthesized, as previously detailed (McGirr et al., 2011), and provided by Dr. Leonard Luyt from the Department of Chemistry, The University of Western Ontario, Canada. All the reagents were purchased from Biopack (Argentina), except as indicated.
Experimental procedures
Mice were stereotaxically implanted with a single indwelling guide cannula (4 mm long, 22 gauge, Plastics One) into the lateral ventricle (intra-cerebroventricular, ICV). The placement coordinates for the lateral ventricle were: AP:−0.34 mm; L:+1 mm and V: −2.3 mm. A 28-gauge obturator was inserted into each cannula. After surgery, animals were individually housed and allowed to recover for at least 5 days. On the morning of the experimental day, animals were ICV injected with 3 μL of vehicle (artificial CSF) alone or containing 16.6 pmol of ghrelin or F-ghrelin. To determine the specificity of the method, an additional set of mice were ICV injected with 3 μL of vehicle containing 16.6 pmol of F-ghrelin plus 166 pg of ghrelin. All ICV injections were made over 2 min through a 30 gauge needle that extend 0.5 mm below the guide cannula and that was connected by polyethylene tubing to a Hamilton syringe. The needle was left in place for 2 min, following the injection, to prevent back flow of the injected solution. Between injections and sacrifices, mice were exposed to a pre-weighed amount of rodent ’s food to determine food intake. Then mice were anesthetized 30 min after treatment and perfused with formalin as previously described (Cabral et al., 2012). Each experimental group contained 4 mice. Brains were removed, post-fixed, immersed in 20% sucrose, and cut coronally at 25 μm into three equal series on a sliding cryostat. Correct placement of the cannula was confirmed by histological observation at the end of the experiment.
Immunohistochemistry
Brain sections were used for visualization in a fluorescent microscope and either fluorescent or cromogenic immunostaining. For fluorescent immunohistochemistry, brain sections were treated with blocking solution (3% normal donkey serum and 0.25% TritonX in PBS) and incubated with goat anti-fluorescein antibody conjugate to Alexa Fluor 488 (Molecular Probes, cat# A-11096, 1:100) for two days at 4 °C. For cromogenic immunohistochemistry, brain sections were pretreated with 0.5 % H2O2, treated with blocking solution and incubated with goat anti-fluorescein antibody (Molecular Probes, cat# A-11095, 1:1,500) for two days at 4 °C. Then, sections were overnight treated with biotinylated donkey anti-goat antibody (Vector Laboratories, cat# BA-1000, 1:1,000) and with Vectastain Elite ABC kit (Vector Laboratories, cat# PK-6200) for 1 h, according to manufacturer ’s protocols. Then, visible signal was developed with 3-3′-diaminobenzidine (DAB)/Nickel solution, giving a black/purple precipitate. Immunostaining of negative control, which did not show any antiserum immunolabeling, included sequential elimination of either the primary or secondary antibody from the staining procedure. Sections were sequentially mounted on glass slides, and cover slipped with mounting media. Fluorescent images were acquired with a Leica TSC SP5 confocal laser scanning microscope. Fluorescein and Alexa 488 were excited at 488 nm and detected in the 496–532 nm range. Bright-field images were acquired with a Nikon Eclipse 50i and a DS-Ri1 Nikon digital camera. An image editing software program, Adobe Photoshop CS2, was used to adjust contrast and brightness of microphotographs.
Neuroanatomical Analysis
The mouse brain atlas of Paxinos and Franklin (2001) was used to identify coronal brain sections and to describe the mouse brain nuclei (Paxinos and Franklin, 2001). The location of fluorescein-immunoreactive (IR) signal was determined in 1-in-3 series of 25 μm thick immunostained sections from the level of the olfactory bulbs down to the cervical spinal cord. Semi-qualitative estimates of fluorescein-IR for the different brain sites were made by considering both signal strength and number of labeled cells as compared to signal observed in negative control samples. Series were used to make projection drawings upon which the IR cells were plotted.
Statistical analyses
The food intake data were expressed as the mean ± SEM and analyzed by two-ways ANOVA test for comparison of different mean values. Significant differences were considered when P<0.05.
RESULTS
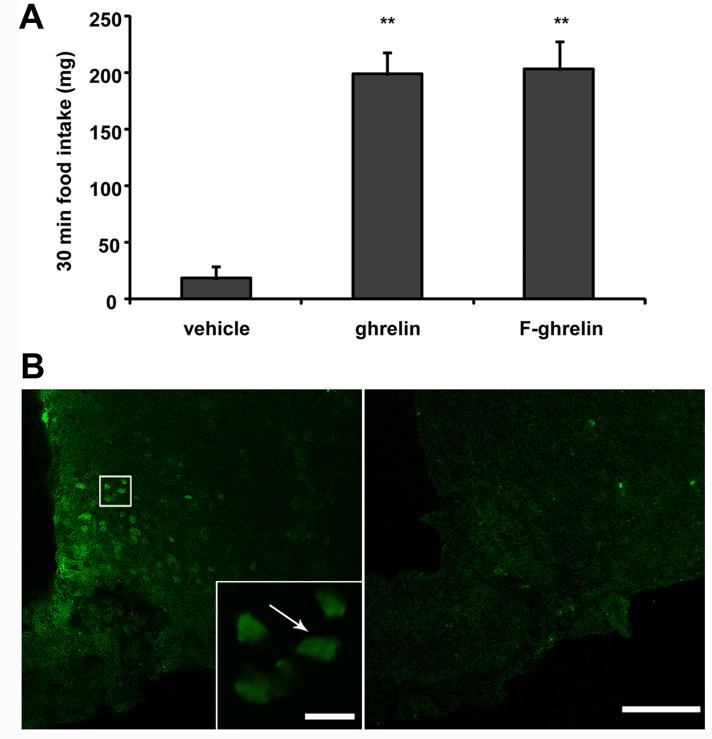
Mice ICV-injected with either ghrelin or F-ghrelin significantly increased food intake (199 ±19 and 203 ±30 mg, respectively), as compared to vehicle-treated mice (18 ±11 mg, Fig. 1A). Of note, the magnitude of F-ghrelin- and ghrelin-induced food intake was not statistically different. Initially, coronal brain slices from the three experimental groups were mounted for examination in the fluorescence microscope. Under this condition, strong cell body-shaped green fluorescent signal was observed exclusively in F-ghrelin-injected mice and no signal was observed in vehicle- or ghrelin-treated mice. Even though samples were covered with commercial mounting media to prevent photobleaching, endogenous F-ghrelin fluorescence lasted only a brief time and disappeared when brain slices were exposed to light. In order perform a detailed neuroanatomical characterization of F-ghrelin positive sites, immunostaining against fluorescein were performed. We used either a fluorescent anti-fluorescein antibody or further amplification of the signal achieved by a biotynilated secondary antibody followed by streptavidin-peroxidase, the presence of which was visualized with a cromogenic reaction. Both fluorescent and cromogenic immunostainings for fluorescein showed specificity as no signal was observed when primary or secondary antibodies were omitted or when brain samples from vehicle- or ghrelin-treated mice were used. In addition, F-ghrelin presence in the different brain sites showed specificity since fluorescein-IR signal was significantly reduced by an excess of unlabeled ghrelin (Fig. 1B). Both fluorescent and cromogenic immunostainings against fluorescein showed very similar and consistent patterns of signal. However, cromogenic immunostaining was more sensitive due to the extra amplification steps of the reaction. Thus, cromogenic fluorescein-IR signal in brain samples of mice ICV-injected with F-ghrelin was used for the detailed neuroanatomical analysis of CSF ghrelin accessible sites. The observed fluorescein-IR patterns are outlined in Table 1 and visually summarized in representative schematic diagrams contained in Figure 2. The abbreviations used in the figures and throughout the text are denoted in Table 1.
Figure 1. F-ghrelin increases food intake and specifically binds to brain areas in mice.
Panel A shows 30 min food intake in mice ICV injected with vehicle alone or containing 16.6 pmol of ghrelin or F-ghrelin. Data represent the mean ±SEM. **, p ≤ 0.01. Panel B shows confocal representative photomicrographs of fluorescein-IR signal in ARC coronal sections of mice that were ICV injected with 16.6 pmol of F-ghrelin alone (left) or plus 166 pmol of ghrelin (right). Insert shows high magnification of the area marked in the low magnification image. Arrows point fluorescein-IR cells. Scale bars, 100 μm (low magnification), 10 μm (high magnification).
TABLE 1.
Relative density of fluorescein-IR in the central nervous system of F-ghrelin injected mice1
| Hippocampus and septum | |
| Ammon’s horn, CA1 | + |
| Ammon’s horn, CA2 | + |
| Ammon’s horn, CA3 | + |
| Lateral septal nucleus -LS | + |
| Thalamus | |
| Paraventricular thalamic nucleus –PV | ++ |
| Hypothalamus | |
| Anterior hypothalamic area –LHA | + |
| Arcuate hypothalamic nucleus –ARC | +++ |
| Dorsomedial nucleus –DMH | + |
| Paraventricular nucleus –PVH | ++ |
| Periventricular hypothalamic nucleus –Pe | ++ |
| Premamillary nucleus, ventral –PMV | ++ |
| Supramammillary nucleus –SuM | + |
| Suprachiasmatic nucleus –SCh | +/− |
| Ventromedial nucleus –VMH | +++ |
| Ventromedial preoptic nucleus –VMPO | +++ |
| Subfornical organ –SFO | ++ |
| Midbrain, pons, and medulla oblongata | |
| Area postrema –AP | ++ |
| Dorsal motor nucleus of the vagus –DMNV | + |
| Dorsal raphe nucleus –DR | ++ |
| Dorsal tegmental nucleus –DTg | ++ |
| Edinger Westphal nucleus –EW | +/− |
| Laterodorsal tegmental nucleus –LDTg | ++ |
| Locus coeruleus –LoC | ++ |
| Magnocellular nucleus of the posterior commissure –MCPC | +/− |
| Nucleus ambiguous –Amb | +/− |
| Nucleus of the solitary tract –NTS | ++ |
| Substantia nigra –SN | +/− |
| Ventral tegmental area –VTA | + |
Qualitative estimates of fluorescein-IR were made by considering both signal strength and the number of labeled cells: +++, high density; ++, moderate density; +, low density; +/−, inconsistent visualization.
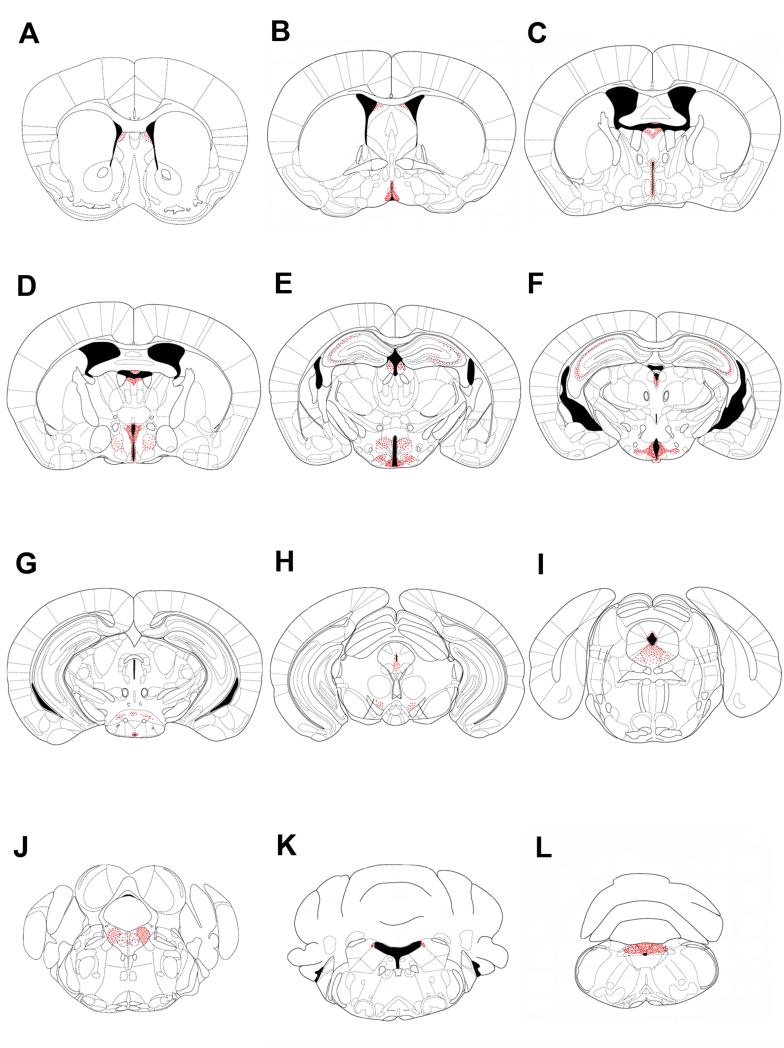
Figure 2. Profile of fluorescein-IR cells in mice ICV-injected with F-ghrelin.
A series of line drawings illustrating the fluorescein-IR sites in mice ICV-injected with F-ghrelin. Sections are arranged in a rostralto-caudal manner. Panels A to L illustrate the following bregma levels: 1.10, 0.26, −0.34, −0.58, −1.82, −2.30, −2.80, −3.52, −4.60, −5.20, −5.80, −7.48 mm, respectively. Each dot represents a positive cell.
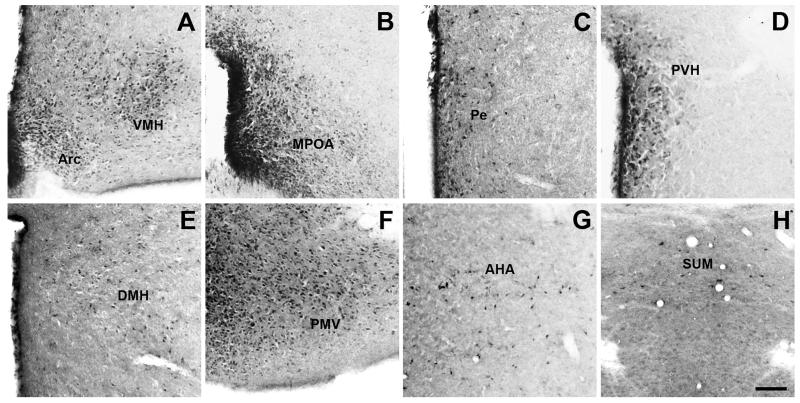
In general, the pattern of fluorescein-IR in F-ghrelin-injected mice was consistent with previous reports of the distribution of GHSR-1a mRNA (Zigman et al., 2006, Perello et al., 2012). The higher number and strongest intensity of fluorescein-IR cells were observed in the hypothalamus. The ARC was one of the areas with the highest amount of fluorescein-IR cells, which were located across the entire rostral-caudal axis and more concentrated in its medial part (Fig. 3A). The VMH also contained abundant fluorescein-IR signal, which were found throughout the entire nuclei (Fig. 3A). The MPOA contained dense fluorescein-IR signal, which also spanned the rostral-caudal expanse of the nucleus (Fig. 3B). The Pe showed fluorescein-IR cells thought the whole area surrounding the third ventricle (Fig. 3C). Fluorescein-IR signal within the PVH was moderate and enriched in the parvocellular part of the nucleus (Fig. 3D). The DMH also showed fluorescein-IR signal, with most positive cells located in the caudal part of the nucleus (Fig. 3E). The PMV, AHA and SUM also contained a significant amount of fluorescein-IR cells of moderate intensity (Fig. 3F-H). The Sch contained low presence of fluorescein-IR cells.
Figure 3. Fluorescein-IR cells are found in several hypothalamic nuclei of F-ghrelin-injected mice.
Panels show a series of representative microphotographs of coronal sections of different hypothalamic regions after cromogenic immunohistochemistry for fluorescein (black/purple signal). Panels A to G show the ARC and VMH (A, bregma −1.82 mm), MPOA (B, bregma 0.26 mm), Pe (C, bregma −0.34 mm), PVH (D, bregma −0.58 mm), DMH (E, bregma −1.82 mm), PMV (F, bregma −2.30 mm), AHA (G, bregma −0.58 mm) and SUM (H, bregma −2.80 mm) regions. Scale bar: 100 μm.
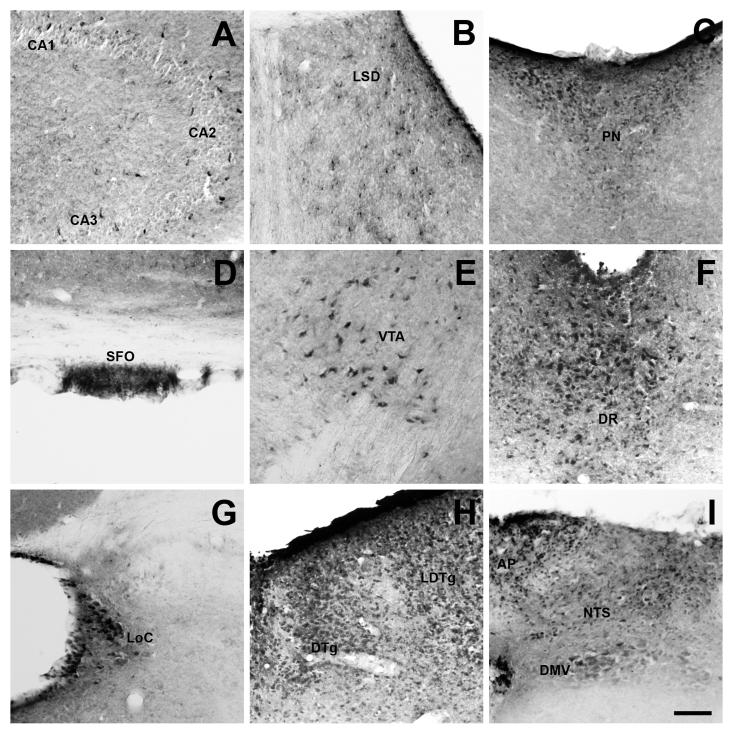
Many extra-hypothalamic areas contained fluorescein-IR signal. Positive cells were found in Ammon’s horns of the hippocampus (Fig. 4A), LSD of the septum (Fig. 4B), PN of the thalamus (Fig. 4C) and SFO (Fig. 4D). In the midbrain, fluorescein-IR cells were observed in the VTA (Fig. 4E) and DR (Fig. 4F). Among these regions, the most abundant number of fluorescein-IR cells was found in the DR, and less abundantly in the VTA. In the pons, fluorescein-IR cells were present in the LoC (Fig. 4G). The LDTg and DTg of the brainstem also showed a significant amount of fluorescein-IR cells (Fig. 4H). In the medulla oblongata, fluorescein-IR cells were observed in all three components of the DVC: the AP, the NTS and the DMV (Fig. 4I). Of note, we found little or inconsistent fluorescein-IR cells in the SN, EW, Amb and MCPC, where GHSR-1a mRNA expression has been previously reported (Zigman et al., 2006, Perello et al., 2012, Scott et al., 2012).
Figure 4. Fluorescein-IR cells are found in several extra-hypothalamic nuclei of F-ghrelin-injected mice.
Panels show a series of representative microphotographs of coronal sections of different extrahypothalamic regions after cromogenic immunohistochemistry for fluorescein (black/purple signal). Panels A to G show the Ammon’s horns of the hippocampus (A, bregma −1.82 mm), LSD of the septum (B, bregma 1.10 mm), PN of the thalamus (C, bregma −0.58 mm), SFO (D, bregma −0.58 mm), VTA (E, bregma −3.52 mm), DR (F, bregma −4.60 mm) and LoC (G, bregma −5.80 mm). Panel H shows the LDTg and DTg (bregma −5.20 mm), and panel I shows the AP, NTS and DMV (bregma −7.48 mm). Scale bar: 100 μm.
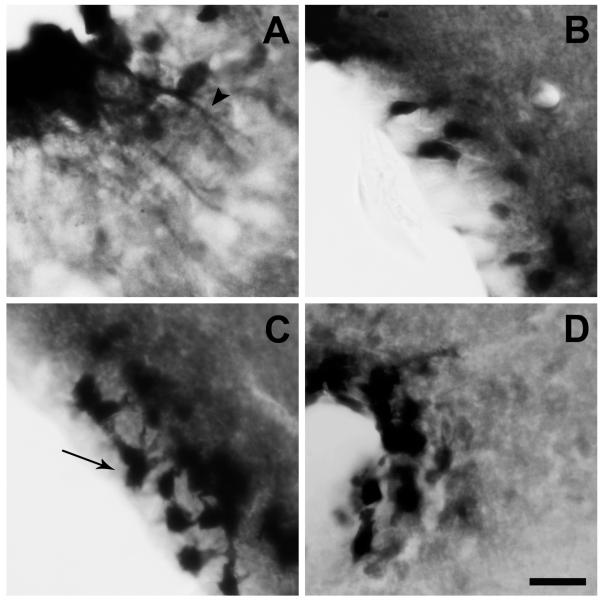
As it can be observed in Figures 3 and 4, fluorescein-IR signal also was consistently detected in the ependymal-like cells lining the ventricular system of the brain (Zigman et al., 2006, Perello et al., 2012). Fluorescein-IR cells were observed in the ependymal-like cells of the third ventricle, lateral ventricle, forth ventricle, aqueduct of Sylvius and central canal. In the bottom part of the third ventricle, most of ependymal cells lining the floor of the infundibular recess, named tanycytes, were fluorescein-IR (Fig. 5A). Within tanycytes, fluorescein-IR signal was found in the cell body as well as in their cellular processes that extend deep into the median eminence (ME) (Fig. 5A). It has been recently shown that tanycytic-like cells are also present in other circumventricular organs (Langlet et al., 2013b); however, fluorescein-IR signal in cellular processes was exclusively found at the ME level in our experimental conditions. In contrast, distribution of fluorescein-IR signal within the rest of the ependymal layer, including those of the upper part of the third ventricle, showed a patchy profile with positive signal present exclusively in the cell body of some, but not all, ependymal cells (Fig. 5B-D). Fluorescein-IR signal in the ependymal layer was specific as it was significantly reduced by an excess of unlabeled ghrelin (Fig. 6). Interestingly, fluorescein-IR signal was detected exclusively in the ependymal-like cells lining the ventricular system of the brain of GHSR-null mice ICV-injected with F-ghrelin (Fig. 6).
Figure 5. Fluorescein-IR signal is observed in the ependymal cells of F-ghrelin-injected mice.
Panels show a series of representative microphotographs of coronal sections of different regions of the ventricular system of the brain after immunohistochemistry for fluorescein (black/purple signal). Panels A to D show the ependymal cells lining the bottom (A) or upper (B) part of the third ventricle, the aqueduct of Sylvius (C) and the central canal (D). Arrowheads point fluorescein-IR cellular processes and arrows point fluorescein-IR cell bodies. Scale bar: 20 μm.
Figure 6. Fluorescein-IR signal in the ependymal layer of F-ghrelin-injected mice is specific and independent of GHSR expression.
Left (A and D) and center (B and E) panels show representative photomicrographs of fluorescein-IR signal in ependymal layer of wild-type mice ICV treated with F-ghrelin alone or with a combination of F-ghrelin and ghrelin, respectively. Right (C and F) panels show representative photomicrographs of fluorescein-IR signal in ependymal layer of GHSR-null mice ICV treated with F-ghrelin. Upper (A-C) and bottom (D-F) horizontal panels show the upper and bottom part of the third ventricle, respectively. Scale bars, 100 μm.
DISCUSSION
The current study provides the first characterization of brain nuclei accessible to ghrelin present in the CSF. For this purpose, we extensively mapped the brain distribution of F-ghrelin in mice ICV injected with this tracer. Our results indicated that CSF ghrelin is able to reach most of the forebrain, midbrain and hindbrain areas where GHSR-1a gene expression has been reported (Zigman et al., 2006, Perello et al., 2012). Interestingly, we also detected F-ghrelin uptake in the ependymal cells of the ventricular system of the brain, where GHSR-1a is presumably not present.
Here we show that F-ghrelin is an excellent fluorescent ghrelin analog to imaging ghrelin binding sites. This peptide tracer is an 18-amino acid analog of ghrelin conjugated to a fluorescent molecule at its C terminus (McGirr et al., 2011). Of note, the receptor-binding region of ghrelin resides within the first five N-terminal amino-acids, which are sufficient for activation of GHSR-1a (Bednarek et al., 2000). Thus, modifications to the C terminus preserve the bioactive side chain of ghrelin. McGirr and colleagues have shown that F-ghrelin behaves similarly to native full-length ghrelin in terms of serum stability, receptor binding and agonist activity (McGirr et al., 2011). Here, we expanded the characterization of this tracer and showed that it increases food intake in the same extent as ghrelin, suggesting that F-ghrelin is fully bioactive in vivo. In addition, we showed that F-ghrelin fails to label brain nuclei of GHSR-null mice, confirming that this molecule specifically binds to GHSR. Of note, fluorescence lifetime of F-ghrelin was short in our experimental conditions, which is expected for this compound (Saylor, 1995). Thus, we used anti-fluorescein antibodies in order to stabilize and also increase the sensitivity and intensity of the signal. Overall, this experimental strategy allowed us to fully characterize the brain nuclei accessible CSF ghrelin.
The pattern of F-ghrelin labeling was in general agreement with previous studies using in situ hybridization immunohistochemistry for visualization of GHSR-1a mRNA in the mouse brain (Zigman et al., 2006). The only nucleus in which we found fluorescein-IR signal that had not previously been recognized as containing GHSR-1a is the LoC (see below). Also, we observed fluorescein-IR in the SFO, which was not listed as a GHSR-expressing brain region by Zigman and colleagues (Zigman et al., 2006, Perello et al., 2012). However, Pulman et al. have shown that SFO neurons express GHSR and dose-dependently respond to ghrelin in rats (Pulman et al., 2006). In contrast, we observed low or no fluorescein-IR signal in some brain regions that had previously been recognized as containing GHSR-1a mRNA (Zigman et al., 2006, Perello et al., 2012, Scott et al., 2012). These brain nuclei include the SCh in the hypothalamus as well as the Amb, EW, MCPC and SN. Thus, these brain regions appear to be inaccessible to CSF ghrelin. Alternatively, GHSR-1a mRNA may be expressed in low levels in these nuclei and, therefore, only detected by a sensitive method such as the radioactive in situ hybridization immunohistochemistry. It is also possible that GHSR-1a mRNA is expressed in these brain areas but the resulting mRNA is not translated to protein, or that GHSR-1a protein is transported to the synaptic terminals of these neurons and not located in their cell body. In fact, a presynaptic mode of action of ghrelin in some brain areas has been suggested (Cowley et al., 2003, Wiedmer et al., 2011). Despite minor inconsistencies, our data provide strong evidence that CSF ghrelin is able to interact in vivo with the majority of the areas where GHSR-1a is expressed.
To our knowledge, this is the first in vivo analysis of brain nuclei accessible to CSF ghrelin. Previously, incubation of biotin-labeled ghrelin on coronal brain slices has been used to determine ghrelin binding sites. These in vitro binding studies have shown that ghrelin binds to several hypothalamic areas (including the ARC, DMH, AHA, MPOA and PVH), the VTA and the CA regions of the hippocampus (Cowley et al., 2003, Abizaid et al., 2006, Diano et al., 2006, Wiedmer et al., 2011). Similar pattern of ghrelin binding sites within the hypothalamus was detected by performing in vitro labeling of brain sections with either [125I-his9]-ghrelin followed by quantitative autoradiography or native ghrelin followed by immunostaining against ghrelin (Harrold et al., 2008, Cabral et al., 2012). Here, we have not only confirmed previous studies showing the presence of ghrelin binding sites in these brain areas but also shown that CSF ghrelin is able to access them. Current observations are in agreement with previous reports showing that the marker of cellular activation c-fos can be detected in most of these brain areas after ICV-injections of ghrelin (Nakazato et al., 2001). Of note, a recent study have used in vivo multi-photon microscopy together with peripheral administration of fluorescently labeled ghrelin to show that circulating ghrelin is rapidly transported through the fenestrated capillaries of the ME and binds to neurons of the ARC (Schaeffer et al., 2013). Thus, at least some brain ghrelin binding sites seems to be accessible to both bloodstream and CSF ghrelin.
In order to reach its brain targets, CSF ghrelin needs to cross the CSF–brain barrier constituted by the ependymal cells lining the ventricles. Here we report that fluorescein-IR signal is found in ependymal cells, including tanycytes lining the floor of the third ventricle, which show no detectable GHSR-1a mRNA (Zigman et al., 2006, Perello et al., 2012). Additionally, we found F-ghrelin signal in ependymal cells of GHSR-deficient mice. These findings can be interpreted as ependymal cells accumulate and transport CSF ghrelin to the brain in a GHSR-independent manner. The mechanisms transporting CSF molecules through the ependymal layer are currently unclear; however, tracing studies indicate that they are very selective. For instance, ICV-injected wheat-germ lectin is accumulated in ependymal cells, including tanycytes, and not transported to brain parenchyma (Cheunsuang et al., 2006). In contrast, ICV-injections of a FluoroGold equivalent or horseradish peroxidase result in uptake of the tracers in ependymal cells but tanycytes lining the floor of the infundibular recess (Cheunsuang et al., 2006, Rodriguez et al., 2010). Additionally, ICV-injected cholera toxin subunit B or Evans blue are accumulated by ependymal cells and also access to neurons of the raphe nuclei or the ARC, respectively (Mikkelsen et al., 1997, Mullier et al., 2010). Studies using ICV administration of digoxigenin-labeled hormones have shown that leptin is accumulated by ependymal cells and serotonergic neurons in the raphe nuclei, while insulin and insulin-like growth factor-I are accumulated in tanycytes and cells of the ARC and ME (Fernandez-Galaz et al., 1996, Fernandez-Galaz et al., 2002). Thus, CSF ghrelin appears to have a higher accessibility, as compared to other hormones, and quickly access to different brain areas. Interestingly, it has been shown that noradrenergic neurons of the avian LoC accumulate CSF components, which are initially endocytosed and transcytosed by ependymal cells (Feng et al., 2011). This uptake of molecules from the CSF is highly selective as internalizes nerve growth factor and urotensin-1, but not other neurotrophic factors (Feng et al., 2011). Here we report that fluorescein-IR cells are present in the LoC, which presumably lacks GHSR-1a (Zigman et al., 2006, Perello et al., 2012). Thus, similar trafficking pathways as shown in avian LoC may take place for CSF ghrelin in mice. Future studies will be needed to elucidate the mechanism by which ependymal cells internalize and traffic CSF ghrelin.
CSF ghrelin concentrations are subjected to a dynamic regulation and respond to energy balance status (Tritos et al., 2003, Grouselle et al., 2008). However, the origin of CSF ghrelin is currently unclear. It has been suggested that peripheral ghrelin can enter to the CSF by crossing the blood–brain barrier. In support of this possibility, it has been shown that CSF ghrelin levels correlates with plasma ghrelin levels and that CSF ghrelin increases around 30 min after a peripheral injection of ghrelin in a ewe model (Grouselle et al., 2008). Also, CSF ghrelin concentration shows a pulsatile profile, and around half of CSF ghrelin peaks are preceded by a peak of plasma ghrelin (Grouselle et al., 2008). Experimental studies assessing the permeability of the blood–brain barrier to ghrelin in mice have showed that human ghrelin can be transported from brain-to-blood and from blood to-brain by saturable systems (Banks et al., 2002). Mouse ghrelin, which differs from human ghrelin by two amino acids, crosses the mouse blood-brain barrier predominantly in the brain-to-blood direction (Banks et al., 2002). In order to reach the CSF, peripheral ghrelin could cross the blood–CSF barrier in the choroids plexus and/or to be transported to the ventricles from the brain, after going across the blood–brain barrier at the cerebral endothelium (Banks et al., 2002). A central origin of CSF ghrelin in mice appears to be unlikely since most studies have failed to confirm the presence of ghrelin-producing cells in the mouse brain (Sakata et al., 2009, Furness et al., 2011). However, future studies will be required in order to determine if significant amounts of brain-derived ghrelin can reach the CSF under particular physiological conditions, such as food deprivation, when hypothalamic ghrelin production is presumably increased (Gahete et al., 2010, Kageyama et al., 2010). In this regard, current data allow us to hypothesize that central ghrelin-IR cells detected in some studies could due to ghrelin uptake, rather that CNS-production (Kojima et al., 1999, Lu et al., 2002, Cowley et al., 2003, Mondal et al., 2005). Of note, studies of CSF ghrelin are difficult to address in mice since the available assays for ghrelin require large amounts of fluid. Thus, future studies employing different experimental systems will be required to get insights about the origin of CSF ghrelin.
Regardless of its origin, the observation that CSF ghrelin can access to most ghrelin responsive brain nuclei may have important physiological implications. In particular, it could offer a role for brain nuclei expressing GHSR-1a without immediate access to circulating ghrelin. In terms of appetite, ghrelin not only increases food intake itself but also regulates hedonic aspects of eating (Perello et al., 2010, Dickson et al., 2011, Perello and Zigman, 2012). Thus, it can be hypothesized that brain areas responsive to plasma and/or CSF ghrelin may have different but complementary roles. Peripheral administration of ghrelin stimulates feeding almost instantly, suggesting that this effect is mainly mediated by brain areas accessible to circulating hormone, such as the ARC and the DVC (Cummings, 2006). In addition, plasma ghrelin may be able to act on VTA neurons, which strongly drives motivation for food (Abizaid et al., 2006, Chuang et al., 2011). However, some aspects of hedonic eating behaviors would also involve actions of ghrelin on hippocampal or meso-limbic regions that are not easily accessible to peripheral ghrelin but would be reachable by CSF ghrelin. Similarly, ghrelin effects on more complex functions such as stress responses, learning, sleep duration, memory consolidation, anxiety and depression symptoms may be also modulated by hormone present in the CSF (Carlini et al., 2002, Diano et al., 2006, Lutter et al., 2008, Cabral et al., 2012). Recent evidences indicate that the permeability of the blood-CSF barrier in the hypothalamus undergoes to dynamic changes in response to the metabolic status (Langlet et al., 2013a). Thus, if the accessibility of CSF ghrelin to brain nuclei can be modulated by different physiological conditions is an interesting possibility that deserves to be specifically investigated.
In summary, we conclude that CSF ghrelin is able to reach most of GHSR-expressing brain areas, and that the accessibility of CSF ghrelin to the brain parenchyma presumably occurs through the ependymal cells in GHSR-independent manner. Future studies will be needed to elucidate the molecular mechanisms mediating the transcellular trafficking of ghrelin through ependymal layer as well as the physiological consequences of the high accessibility of CSF ghrelin to the brain.
Cerebrospinal fluid ghrelin reaches most brain areas expressing ghrelin receptors.
Ependymal cells are able to uptake ghrelin present in the cerebrospinal fluid.
Ghrelin uptake in ependymal cells occurs in a ghrelin receptor-independent manner.
ACKNOWLEDGEMENTS
This work was supported by grants of the National Agency of Scientific and Technological Promotion of Argentina (PICT2010-1954 and PICT2011-2142) and the NIH (R03TW008925-01A1) to MP. We would like to thank Dr. Jeffrey Zigman for critically reading the manuscript, and Anabela Patrone and Mirta Reynaldo for their technical support. AC was supported by CONICET.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A.C., G.F. and M.P. have nothing to declare.
References
- Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschop MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]
- Cabral A, Suescun O, Zigman JM, Perello M. Ghrelin indirectly activates hypophysiotropic CRF neurons in rodents. PLoS One. 2012;7:e31462. doi: 10.1371/journal.pone.0031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini VP, Monzon ME, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299:739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- Cheunsuang O, Stewart AL, Morris R. Differential uptake of molecules from the circulation and CSF reveals regional and cellular specialisation in CNS detection of homeostatic signals. Cell Tissue Res. 2006;325:397–402. doi: 10.1007/s00441-006-0162-z. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, Zigman JM. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340:80–87. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Feng CY, Wiggins LM, von Bartheld CS. The locus ceruleus responds to signaling molecules obtained from the CSF by transfer through tanycytes. J Neurosci. 2011;31:9147–9158. doi: 10.1523/JNEUROSCI.5018-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Diano S, Horvath TL, Garcia-Segura LM. Leptin uptake by serotonergic neurones of the dorsal raphe. J Neuroendocrinol. 2002;14:429–434. doi: 10.1046/j.1365-2826.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Galaz MC, Torres-Aleman I, Garcia-Segura LM. Endocrine-dependent accumulation of IGF-I by hypothalamic glia. Neuroreport. 1996;8:373–377. doi: 10.1097/00001756-199612200-00073. [DOI] [PubMed] [Google Scholar]
- Furness JB, Hunne B, Matsuda N, Yin L, Russo D, Kato I, Fujimiya M, Patterson M, McLeod J, Andrews ZB, Bron R. Investigation of the presence of ghrelin in the central nervous system of the rat and mouse. Neuroscience. 2011;193:1–9. doi: 10.1016/j.neuroscience.2011.07.063. [DOI] [PubMed] [Google Scholar]
- Gahete MD, Cordoba-Chacon J, Salvatori R, Castano JP, Kineman RD, Luque RM. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol Cell Endocrinol. 2010;317:154–160. doi: 10.1016/j.mce.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouselle D, Chaillou E, Caraty A, Bluet-Pajot MT, Zizzari P, Tillet Y, Epelbaum J. Pulsatile cerebrospinal fluid and plasma ghrelin in relation to growth hormone secretion and food intake in the sheep. J Neuroendocrinol. 2008;20:1138–1146. doi: 10.1111/j.1365-2826.2008.01770.x. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Dovey T, Cai XJ, Halford JC, Pinkney J. Autoradiographic analysis of ghrelin receptors in the rat hypothalamus. Brain Res. 2008;1196:59–64. doi: 10.1016/j.brainres.2007.12.055. [DOI] [PubMed] [Google Scholar]
- Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J, Paress PS, Diaz C, Chou M, Liu KK, McKee KK, Pong SS, Chaung LY, Elbrecht A, Dashkevicz M, Heavens R, Rigby M, Sirinathsinghji DJ, Dean DC, Melillo DG, Patchett AA, Nargund R, Griffin PR, DeMartino JA, Gupta SK, Schaeffer JM, Smith RG, Van der Ploeg LH. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Kageyama H, Takenoya F, Shiba K, Shioda S. Neuronal circuits involving ghrelin in the hypothalamus-mediated regulation of feeding. Neuropeptides. 2010;44:133–138. doi: 10.1016/j.npep.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A, Mazur D, Carmeliet P, Bouret SG, Prevot V, Dehouck B. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013a;17:607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol. 2013b doi: 10.1002/cne.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Guan JL, Wang QP, Uehara K, Yamada S, Goto N, Date Y, Nakazato M, Kojima M, Kangawa K, Shioda S. Immunocytochemical observation of ghrelin-containing neurons in the rat arcuate nucleus. Neurosci Lett. 2002;321:157–160. doi: 10.1016/s0304-3940(01)02544-7. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr R, McFarland MS, McTavish J, Luyt LG, Dhanvantari S. Design and characterization of a fluorescent ghrelin analog for imaging the growth hormone secretagogue receptor 1a. Regul Pept. 2011;172:69–76. doi: 10.1016/j.regpep.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Hay-Schmidt A, Larsen PJ. Central innervation of the rat ependyma and subcommissural organ with special reference to ascending serotoninergic projections from the raphe nuclei. J Comp Neurol. 1997;384:556–568. [PubMed] [Google Scholar]
- Mitchell V, Bouret S, Beauvillain JC, Schilling A, Perret M, Kordon C, Epelbaum J. Comparative distribution of mRNA encoding the growth hormone secretagogue-receptor (GHS-R) in Microcebus murinus (Primate, lemurian) and rat forebrain and pituitary. J Comp Neurol. 2001;429:469–489. doi: 10.1002/1096-9861(20010115)429:3<469::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, Nakazato M. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul Pept. 2005;126:55–59. doi: 10.1016/j.regpep.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain-Second Edition. Academic Press; 2001. [Google Scholar]
- Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry. 2010;67:880–886. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Scott MM, Sakata I, Lee CE, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Elmquist JK, Zigman JM. Functional implications of limited leptin receptor and ghrelin receptor coexpression in the brain. J Comp Neurol. 2012;520:281–294. doi: 10.1002/cne.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Zigman JM. The role of ghrelin in reward-based eating. Biol Psychiatry. 2012;72:347–353. doi: 10.1016/j.biopsych.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulman KJ, Fry WM, Cottrell GT, Ferguson AV. The subfornical organ: a central target for circulating feeding signals. J Neurosci. 2006;26:2022–2030. doi: 10.1523/JNEUROSCI.3218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Sakata I, Nakano Y, Osborne-Lawrence S, Rovinsky SA, Lee CE, Perello M, Anderson JG, Coppari R, Xiao G, Lowell BB, Elmquist JK, Zigman JM. Characterization of a novel ghrelin cell reporter mouse. Regul Pept. 2009;155:91–98. doi: 10.1016/j.regpep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor JR. Photobleaching of disodium fluorescein in water. Experiments in Fluids. 1995;18:445–447. [Google Scholar]
- Schaeffer M, Langlet F, Lafont C, Molino F, Hodson DJ, Roux T, Lamarque L, Verdie P, Bourrier E, Dehouck B, Baneres JL, Martinez J, Mery PF, Marie J, Trinquet E, Fehrentz JA, Prevot V, Mollard P. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci U S A. 2013;110:1512–1517. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Perello M, Chuang JC, Sakata I, Gautron L, Lee CE, Lauzon D, Elmquist JK, Zigman JM. Hindbrain ghrelin receptor signaling is sufficient to maintain fasting glucose. PLoS One. 2012;7:e44089. doi: 10.1371/journal.pone.0044089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritos NA, Kokkinos A, Lampadariou E, Alexiou E, Katsilambros N, Maratos-Flier E. Cerebrospinal fluid ghrelin is negatively associated with body mass index. J Clin Endocrinol Metab. 2003;88:2943–2946. doi: 10.1210/jc.2003-030300. [DOI] [PubMed] [Google Scholar]
- Wiedmer P, Strasser F, Horvath TL, Blum D, Dimarchi R, Lutz T, Schurmann A, Joost HG, Tschop MH, Tong J. Ghrelin-induced hypothermia: a physiological basis but no clinical risk. Physiol Behav. 2011;105:43–51. doi: 10.1016/j.physbeh.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]