Abstract
This article covers what is currently known about the role of the enzyme indoleamine 2,3-dioxygenase (IDO) in cancer-related immunosuppression and the clinical research on IDO inhibitors. A PUBMED search was performed using the terms IDO, indoleamine 2,3-dioxygenase, 1-MT. IDO is an inducible enzyme that catalyzes the rate-limiting first step in tryptophan catabolism. This enzyme is overexpressed in response to IFNγ in a variety of different malignancies. IDO causes immunosuppression through breakdown of tryptophan in the tumor microenvironment and tumor-draining lymph nodes. The depletion of tryptophan and toxic catabolites renders effector T cells inactive and dendritic cells immunosuppressive. Preclinical data suggest that IDO inhibition can delay tumor growth, enhance dendritic cell vaccines, and synergize with chemotherapy through immune-mediated mechanisms. The lead IDO inhibitor, D-1-methyl-tryptophan (D-1-MT), was selected for phase I trials and seems to have immune modulating activity. Subsequently, another isoform of IDO, IDO2, was discovered and found to be the target of D-1-MT. Multiple single-nucleotide polymorphisms in IDO2 affecting its catalytic activity may serve as a pharmacogenetic predictive biomarker for D-1-MT. The IDO pathway is an important mechanism of tumor-related immunosuppression and blocking it could improve cancer immunotherapy outcomes. Clinical development of D-1-MT and other IDO inhibitors as systemic immunomodulators to be combined with other immune modulators, vaccines, and chemotherapy are ongoing.
Keywords: immunotherapy; indoleamine-pyrrole 2,3-dioxygenase; 1-methyl-tryptophan
The complex relationship between the immune system and cancer has been the subject of great interest for hundreds of years. Spontaneous regressions of tumors after various infections resulting in fevers have been written about in the literature. This led Dr. William Coley to treat cancer patients with bacterial extracts based on observations that cancer patients who survived postoperative sepsis had lower relapse rates than those who did not.1,2 The promise of a cancer-specific immune response lies in its hypothetical ability to precisely target transformed cells throughout the body while sparing healthy tissues. Achieving the true therapeutic potential of cancer immunotherapy has been limited by the realization that established tumors employ a number of immunosuppressive pathways that blunt objective responses to cancer vaccines and cytokines. Understanding the role of these various immune modulating pathways in influencing antitumor responses is paramount in improving cancer immunotherapy outcomes. One such pathway that has garnered a significant amount of attention is an enzyme involved in the metabolism of the essential amino acid tryptophan called indoleamine 2,3-dioxygenase (IDO, EC 1.13.11.52). This article will summarize the pertinent data surrounding its role in cancer immunotherapy.
BACKGROUND
Kotake and Masayama described an enzyme called tryptophan oxygenase constitutively expressed in the liver of mammals in 1936, and this enzyme was initially thought to be the sole enzyme responsible for the breakdown of L-tryptophan to the catabolite L-kynurenine. This enzyme was later renamed tryptophan dioxygenase (TDO, EC 1.13.11.11). It was known that TDO was specific for the L-tryptophan stereoisomer, so scientists were puzzled that mice fed with D-tryptophan could break it down as well.3 It was not until 1967 that Yamamoto and Hayaishi4 could explain this phenomenon by discovering another enzyme, IDO, in rabbit intestine homogenates, which was able to break down both D- and L-tryptophan. The observations that tryptophan metabolism was altered in certain pathologic states such as cancer and infectious diseases were published by multiple investigators in the 1950s.5,6 Further investigation on IDO not only found that this was the enzyme present in various tissues but also found that its expression could be induced by certain infectious agents (ie, influenza), lipopolysaccharides, and cytokines such as interferon gamma (IFNγ).7–9 This information led many to conclude that IDO was likely responsible for the variations in tryptophan metabolism seen during periods of illness. The role of IDO in regulating the immune system was elucidated in a seminal article by Munn et al. Immunocompetent mice pregnant with allogeneic or syngenic concepti were fed an inhibitor of IDO known as 1-methyl-tryptophan (1-MT). The mice with allogeneic concepti spontaneously aborted their fetuses whereas those with syngenic concepti were not affected.10 This illustrated that placental IDO was critical in preventing the maternal immune system from mounting an attack against paternal antigens expressed in fetal tissues during pregnancy. This discovery has led to intense research into IDO’s role in various autoimmune diseases, transplantation medicine, and cancer-related immunosuppression.11–14
MECHANISMS OF ACTION FOR IDO
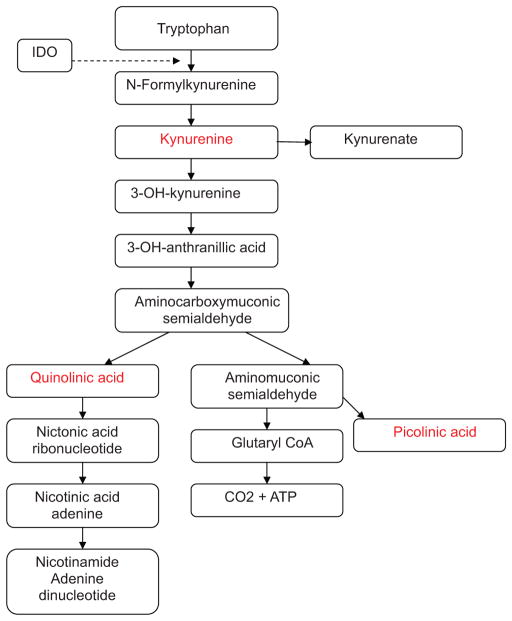
Indoleamine 2,3-dioxygenase is coded by the INDO (or IDO-1) gene located on chromosome 8p12 in humans. The enzyme is a 407 amino acid heme-containing cytoplasmic protein responsible for the first step in the catabolism of tryptophan into N-formyl-kynurenine via cleavage of tryptophan’s pyrrole ring and addition of an oxygen molecule (Fig. 1). From an evolutionary perspective, the current form of IDO predominates in placental and marsupial mammals, whereas only less active prototypical IDO variants have been identified in chicken and fish genomes.15 This observation would bolster the importance of IDO in the maintenance of placental pregnancy. The gene is regulated by upstream IFNγ-responsive elements that bind activated STAT1, interferon regulatory factor-1 (IRF-1), and NF-kβ.16 IDO expression can be induced in the brain, lungs, gut, kidneys, multiple malignancies, and plasmacytoid dendritic cells (pDCs) within draining lymph nodes and the spleen.17–22 In its normal physiologic role, IDO is important in modulating immune activation to antigenic challenges at mucosal surfaces in the digestive tract and lungs.23–25 The induction of IDO and the subsequent depletion of tryptophan in the tissue microenvironment exerts an antiproliferative effect on cancer cells and infectious pathogens such as toxoplasmosis, trypanosomes, and Chlamydia.26 –28 It does not seem to be essential in maintaining self-tolerance because IDO-knockout mice did not develop fulminant autoimmunity.29 IDO may be protective in the cornea of the eye because kynurenine also functions as a natural ultraviolet filter.30
FIGURE 1.
Metabolism of tryptophan. The catabolites in red are directly toxic to T lymphocytes.
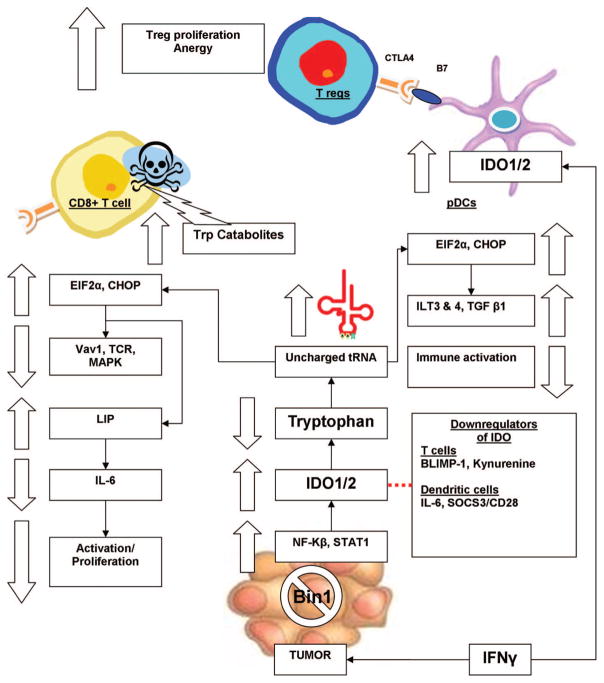
IDO activation leads to many complex changes within the affected cells resulting in suppression of tumor-specific immune response (Fig. 2). Many malignancies can overexpress IDO-1 when exposed to IFNγ through mutation of the tumor suppressor gene Bin1, which leads to increased intracellular levels of STAT1 and NF-kβ.31 The downstream effects of IDO activity are primarily due to depletion of tryptophan and the direct effects of its catabolites in the tissues. Tryptophan depletion retards proliferation of susceptible pathogens and transformed cells and also does the same to tumor-infiltrating lymphocytes (TILs). Low tryptophan in TILs and dendritic cells causes uncharged tryptophan tRNA levels to rise and triggers an integrated stress response pathway mediated through GCN2 signaling and phosphorylation of the transcription factor EIF2α.32 As a result, there is increased expression of NF-kβ, CHOP, and IFNγ receptor and decreased production of IL-6 through up-regulation of the transcription factor LIP.33 IDO also suppresses the activation of Vav1, a guanine nucleotide exchange factor, required for the downstream signaling of the activated T-cell receptor through the MAPK/ERK pathways.34 Effector T cells starved of tryptophan are unable to proliferate and go into G1 cell cycle arrest.32 These cells are more sensitive to Fas-mediated apoptosis as well.35 IDO causes naive T cells to differentiate into CD4+CD25+FoxP3+ T regulatory cells (Treg cells) that propagate systemic anergy toward the presented antigens.20 Metabolites of tryptophan including kynurenine, quinolinic acid, and picolinic acid are directly toxic to CD8+ TILs and CD4+ TH1 cells.36 These catabolites do not have the same effect on TH2 cells, so increased IDO activity seems to skew helper T-cell polarization toward a TH2 phenotype.25,37 Two negative feedback loops that may partially counteract IDO activity include kynurenine increasing IL-6 expression through the Ah receptor and EIF2α leading to increased B-lymphocyte-induced maturation protein 1 (BLIMP-1) levels, which repress the INDO promoter region.38,39 This allows for fine tuning of IDO activity to maintain a balance between immune activation and suppression as necessary.
FIGURE 2.
The mechanism of action of IDO. IDO causes decreased cytotoxic T-cell activity and systemic anergy via tryptophan depletion and toxic tryptophan catabolites. Treg indicates T regulatory cell; Trp, tryptophan; pDCs, plasmacytoid dendritic cells.
Antigen-presenting cells (APCs) such as dendritic cells take up tumor antigens and present portions of them on MHC II to naive T cells in adjacent tumor-draining lymph nodes. Dendritic cells respond to low tryptophan by increasing expression of the inhibitory receptors ILT3 and ILT4 and TGF-β1 rendering them immunosuppressive antigen-presenting cells.40 Tolerogenic CD19+ IDO expressing pDCs were detected in murine and human tumor-draining lymph nodes that induced T-cell anergy toward specific tumor antigens. The B7 receptors on these pDCs bind to CTLA-4 on Treg cells causing them to proliferate and induce antigen-specific anergy throughout the host.41 There are more data that IDO activity is a key mediator in dendritic cell immune regulation through other pathways. Sun et al42 linked the immunosuppressive effects of histone deacetylase inhibitors on dendritic cells through acetylation and activation of STAT3, which in turn increases IDO expression. Reversal of the immunosuppressive phenotype in dendritic cells by IL-6 and CD28 Ig is mediated through IDO binding with suppressor of cytokine signaling 3 (SOCS3) followed by proteosomal degredation of the IDO/SOCS3 complexes.43
Data from animal and human experiments demonstrate that IDO induction postvaccination serves as a negative feedback that dampens the immune response to dendritic cell-based cancer vaccines.44,45 Increased IDO expression has been linked to inferior outcomes in multiple malignancies such as endometrial, ovarian, cervical, leukemia, and colorectal carcinomas.46 –51 In summary, tumors upregulate IDO in response to the IFNγ secreted by the first wave of attacking TILs. The subsequent tryptophan depletion and toxic tryptophan catabolites provide an effective immunosuppressive cloak that renders APCs and cytotoxic T cells impotent against established tumors.52 Based on the available data, a recent National Cancer Institute immunotherapy workshop ranked clinical development of IDO inhibitors very high on its priority list.53
CLINICAL DEVELOPMENT OF IDO INHIBITORS
The indole-containing compound 1-methyl-DL-tryptophan (1-MT) was identified as a competitive inhibitor of IDO in 1991 by Cady et al.54 Qian et al showed that L-1-MT reversed the IDO-mediated arrest on T-cell proliferation in various in vitro models. In addition, higher efficiency was observed when L-1-MT or D/L-1-MT was used in restoring T-cell proliferation arrest by abrogating tryptophan depletion. Furthermore, they showed that D-1-MT was less efficient in inhibiting kynurenine production and was not able to restore tryptophan levels.55 Even though this provides information, evidence of L-1-MT being more efficient than D-1-MT, published articles from 2007 till now show an opposing view. Hou et al showed that even though L-1-MT inhibited kynurenine more efficiently than D-1-MT in vitro, D-1-MT was as effective in the presence of human monocytes-derived dendritic cell when they were expressing IDO. In addition, higher T-cell proliferation and activation was observed in human and murine assays using D-1-MT. In vivo studies, using the melanoma cell line B16F10, showed that the combination of D-1-MT with cyclophosphamide induced a growth delay, having higher efficacy than L-1-MT or D/L-1-MT. In combination with B78H1-GM-CSF, a more immunologic tumor, D-1-MT alone produces a modest but significant effect on growth delay. In addition, only D-1-MT prolonged the survival of mice in the orthotopic 41-luc tumor model in the presence of cyclophosphamide. Finally, in the autochthonous MMTV-Neu breast tumor model, the combination of D-1-MT and paclitaxel illustrated a decrease in tumor volume when compared with L-1-MT and paclitaxel.56 The exact mechanism of this synergy is not clear, but there is evidence that chemotherapy triggers multiple changes in cancer cells that renders them immunogenic.57 Combining 1-MT with chemotherapy likely enhances this cytotoxic immune reaction, and this hypothesis was bolstered by a lack of this synergy in nude athymic mice. It was on the basis of superior antitumor activity that D-1-MT was selected as the lead IDO inhibitor compound, and the phase I CTEP-sponsored trial for D-1-MT was activated in the fall of 2007.
Shortly afterward, data from Peter Terness’s group demonstrated that L-1-MT inhibited IDO in various human cancer tissue and dendritic cells whereas D-1-MT did not.58 The lack of IDO inhibition by D-1-MT was difficult to explain in light of preclinical experiments showing that the immunomodulating effect of D-1-MT was abrogated in IDO-knockout mice.56 Also, some of the initial patients on the phase I D-1-MT trial presented at the 2009 American Society of Clinical Oncology immunotherapy session experienced autoimmune hypophysitis, surges in C-reactive protein levels, and declines in circulating Treg cells.59 The patients who developed de novo hypophysitis on the phase I study received prior experimental immunotherapy treatments months before enrolling on the 1-MT study with anti-CTLA-4 monoclonal antibodies or CD-40L agents. It seems that these patients were primed with the prior treatment and then experienced hypophysitis once they were treated with D-1-MT. It was not clear what the mechanism of action of D-1-MT was until the discovery of another IDO isoform with tryptophan catabolizing activity, which was dubbed IDO2. This larger sized protein coded by the INDOL1 gene upstream of the INDO gene that codes IDO (later termed IDO1 to distinguish it from IDO2) is expressed in dendritic cells and tumor cells.60 – 62 Further studies showed that IDO2 was the target of D-1-MT.62 Lob et al also found that D-1-MT did not inhibit tryptophan metabolism in the tumor specimens they tested, leading them to conclude while IDO2 was expressed in tumors only IDO1 was responsible for tryptophan breakdown. Their assertion was that D-1-MT must be acting through other mechanisms, but that would not explain the IDO1 mouse knockout data.
A possible explanation is that IDO1 activation is an upstream prerequisite event for IDO2 activity. An interesting difference between the 2 enzymes that may serve an important physiologic role is that IDO1 activity is decreased by increased tryptophan levels whereas IDO2 is not. A possible reason for this is dendritic cells and tumor cells would switch on both IDO1 and IDO2 in response to IFNγ while in the tryptophan-poor tumor microenvironment. However, when dendritic cells migrate out of the tumor into the tumor-draining lymph nodes with higher tryptophan levels, they can use IDO2 to maintain and propagate the tumor-induced immunosuppression. What further complicates this model is the identification of low-activity IDO2 allele single-nuclear polymorphisms (SNPs) in approximately 40% of pancreatic cancer samples tested.63 Another public SNP database search revealed these low-activity polymorphisms were found in 50% of whites, 50% of Asians, and 25% in those of African descent may mean that those with low-activity IDO2 alleles may not derive the same benefit from D-1-MT.62 This could explain the lack of tryptophan metabolism inhibition in some tumor samples tested with D-1-MT by Lob et al. This is being evaluated on the current phase I trial to see whether IDO2 SNP analysis is a possible pharmacogenetic marker for D-1-MT. Other polymorphisms in related genes such as IFNγ may also impact the therapeutic benefit of IDO1 and IDO2 inhibition requiring a multigene signature to create an effective predictive biomarker.64 The analysis of kynurenine and tryptophan levels in serum samples by mass spectroscopy is being explored as a means to measure IDO activity in vivo during treatment with IDO inhibitors. This is desirable because IDO enzymes undergo post-translational changes that affect their activity, so measurement of IDO in tumors by immunohistochemistry or mRNA may not completely reflect the ability of the sampled tumors to break down tryptophan.65,66 Although measuring kynurenine/tryptophan ratios in the serum or urine has been used to measure IDO activity in certain settings,19,51,67 it is unclear whether this approach can be used to reliably measure changes in tryptophan metabolism in cancer patients treated with IDO inhibitors. A third approach is using C11-α-methyl-tryptophan (AMT) positron emission tomography as a functional imaging modality to study tryptophan metabolism in tumors.17,68 A trial is underway to see whether changes in AMT localization can be seen when tumors are treated with D-1-MT.
It is not known to what extent each isoform contributes to tumor-related immunosuppression and how much clinical benefit (or autoimmune toxicity) targeting one isoform over another confers. Another unknown is whether IDO inhibitors influence other pathways not directly linked to IDO. These questions will be answered by looking at the results of the proposed clinical trials using D-1-MT with vaccines and chemotherapy agents versus the newer IDO1-specific inhibitors being developed for clinical use. These agents include INCB024360 (a hydroxyamidine), ebselen, and 1-methyl-tryptophan (1-MT)-tira-pazamine.69 –72 At least in preclinical models, compounds that can effectively inhibit both isoforms such as methyl-thiohydantoin or racemic 1-MT exhibit greater antitumor effect.31 It remains to be seen whether a combination of IDO1 and IDO2 inhibitors individually titrated for maximal clinical benefit or a single novel agent that inhibits both isoforms is the best way to move forward. Equally important is the development of effective predictive and therapeutic monitoring biomarkers to maximize the clinical benefit of these agents in cancer immunotherapy.
DISCUSSION
It seems that tryptophan metabolism by IDO acts as a negative feedback mechanism to prevent a cytotoxic immune response from amplifying out of control causing excessive tissue damage. Another effect of IDO is to deprive certain pathogens and parasites of the tryptophan they require to proliferate. The ability of IDO to provide tumors with an immunosuppressive cloak is another example of tumors hijacking physiologic processes to provide them with a survival advantage. So although low tryptophan levels may retard the growth of cancer cells initially, they are able to cope with this better than TILs. This immunosuppressive tumor microenvironment ultimately allows transformed cells to seek shelter from the host immune response until systemic anergy toward tumor antigens is established. Rendering the immune response impotent is an important step in facilitating metastasis and is considered by many to be another hallmark of cancer in addition to the 6 processes initially described by Hanahan and Weinberg.73 Restoring the ability of cytotoxic T cells to attack tumor cells may help curb the metastatic process and improve the eradication of residual disease after curative treatments.
The recent discovery of the 2 different IDO isoforms has further complicated the clinical development of IDO inhibitors. The lead compound, D-1-MT, seems to target IDO2 primarily. As other IDO inhibitors are developed, the importance of IDO1 and IDO2 in mediating tumor immunosuppression will be better characterized. Equally important will be discovering the possible autoimmune toxicities and risk of latent infection reactivations with IDO1-specific inhibitors. There is also much work that remains in understanding the various pathways affected by IDO activation, and the ongoing correlative science will hopefully generate useful in vivo biomarkers for studying the effects of these agents. It is likely that other immune modulators such as anti-CTLA-4 monoclonal antibodies will eventually be combined with IDO inhibitors to simultaneously block multiple immunosuppressive pathways. Current trials are exploring combining D-1-MT with a p53 dendritic cell vaccine and chemotherapy agents such as docetaxel.
The field of cancer immunotherapy has changed significantly over the past few decades, but the clinical benefit for patients has been modest at best. Many investigators in the field recognize that we must overcome the various immunosuppressive mechanisms used by established cancers to make any progress. Our understanding of these mechanisms provides us with an opportunity to better use the immune system either alone or in combination with other standard cytotoxic treatments to improve cancer patient outcomes with less toxicity.
References
- 1.Bickels J, Kollender Y, Merinsky O, et al. Coley’s toxin: historical perspective. Isr Med Assoc J. 2002;4:471– 472. [PubMed] [Google Scholar]
- 2.Hoption Cann SA, van Netten JP, van Netten C, et al. Spontaneous regression: a hidden treasure buried in time. Med Hypotheses. 2002;58:115–119. doi: 10.1054/mehy.2001.1469. [DOI] [PubMed] [Google Scholar]
- 3.Higuchi K, Hayaishi O. Enzymic formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys. 1967;120:397– 403. doi: 10.1016/0003-9861(67)90256-1. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto S, Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967;242:5260–5266. [PubMed] [Google Scholar]
- 5.Spacek M. Kynurenine in disease, with particular reference to cancer. Can Med Assoc J. 1955;73:198–201. [PMC free article] [PubMed] [Google Scholar]
- 6.Musajo L, Benassi CA, Parpajola A. Isolation of Kynurenine and 3-hydroxykynurenine from human pathological urine. Nature. 1955;175:855– 856. doi: 10.1038/175855a0. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida R, Hayaishi O. Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc Natl Acad Sci USA. 1978;75:3998– 4000. doi: 10.1073/pnas.75.8.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida R, Imanishi J, Oku T, et al. Induction of pulmonary indoleamine 2,3-dioxygenase by interferon. Proc Natl Acad Sci USA. 1981;78:129–132. doi: 10.1073/pnas.78.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida R, Urade Y, Tokuda M, et al. Induction of indoleamine 2,3-dioxygenase in mouse lung during virus infection. Proc Natl Acad Sci USA. 1979;76:4084– 4086. doi: 10.1073/pnas.76.8.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 11.Brown RR, Ozaki Y, Datta SP, et al. Implications of interferon-induced tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv Exp Med Biol. 1991;294:425– 435. doi: 10.1007/978-1-4684-5952-4_39. [DOI] [PubMed] [Google Scholar]
- 12.Penberthy WT. Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease. Curr Drug Metab. 2007;8:245–266. doi: 10.2174/138920007780362545. [DOI] [PubMed] [Google Scholar]
- 13.Lob S, Konigsrainer A. Role of IDO in organ transplantation: promises and difficulties. Int Rev Immunol. 2009;28:185–206. doi: 10.1080/08830180902989119. [DOI] [PubMed] [Google Scholar]
- 14.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, et al. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114:5062–5070. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuasa HJ, Takubo M, Takahashi A, et al. Evolution of vertebrate indoleamine 2,3-dioxygenases. J Mol Evol. 2007;65:705–714. doi: 10.1007/s00239-007-9049-1. [DOI] [PubMed] [Google Scholar]
- 16.Chon SY, Hassanain HH, Gupta SL. Cooperative role of interferon regulatory factor 1 and p91 (STAT1) response elements in interferon-gamma-inducible expression of human indoleamine 2,3-dioxygenase gene. J Biol Chem. 1996;271:17247–17252. doi: 10.1074/jbc.271.29.17247. [DOI] [PubMed] [Google Scholar]
- 17.Batista CE, Juhász C, Muzik O, et al. Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol Imaging Biol. 2009;11:460– 466. doi: 10.1007/s11307-009-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao YF, Peng RQ, Li J. The paradoxical patterns of expression of indoleamine 2,3-dioxygenase in colon cancer. J Transl Med. 2009;7:71. doi: 10.1186/1479-5876-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandacher G, Cakar F, Winkler C, et al. Non-invasive monitoring of kidney allograft rejection through IDO metabolism evaluation. Kidney Int. 2007;71:60–67. doi: 10.1038/sj.ki.5002023. [DOI] [PubMed] [Google Scholar]
- 20.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uyttenhove C, Pilotte L, Théate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 22.Paveglio SA, Allard J, Foster Hodgkins SR, et al. Airway epithelial indoleamine 2,3-dioxygenase inhibits CD4+ T cells during Aspergillus fumigatus antigen exposure. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2009-0167OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciorba MA, Bettonville EE, McDonald KG, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol. 2010;184:3907–3916. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maneechotesuwan K, Wamanuttajinda V, Kasetsinsombat K, et al. Der p 1 suppresses indoleamine 2, 3-dioxygenase in dendritic cells from house dust mite-sensitive patients with asthma. J Allergy Clin Immunol. 2009;123:239–248. doi: 10.1016/j.jaci.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Oriss TB, Fei M, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci USA. 2008;105:6690– 6695. doi: 10.1073/pnas.0708809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burke F, Knowles RG, East N, et al. The role of indoleamine 2,3-dioxygenase in the anti-tumour activity of human interferon-gamma in vivo. Int J Cancer. 1995;60:115–122. doi: 10.1002/ijc.2910600117. [DOI] [PubMed] [Google Scholar]
- 27.Daubener W, MacKenzie CR. IFN-gamma activated indoleamine 2,3-dioxygenase activity in human cells is an antiparasitic and an antibacterial effector mechanism. Adv Exp Med Biol. 1999;467:517–524. doi: 10.1007/978-1-4615-4709-9_64. [DOI] [PubMed] [Google Scholar]
- 28.Knubel CP, Martínez FF, Fretes RE, et al. Indoleamine 2,3-dioxigenase (IDO) is critical for host resistance against Trypanosoma cruzi. FASEB J. doi: 10.1096/fj.09-150920. In press. [DOI] [PubMed] [Google Scholar]
- 29.Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:220–225. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Serbecic N, Beutelspacher SC. Indoleamine 2,3-dioxygenase protects corneal endothelial cells from UV mediated damage. Exp Eye Res. 2006;82:416– 426. doi: 10.1016/j.exer.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Muller AJ, DuHadaway JB, Donover PS, et al. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 32.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633– 642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Sharma MD, Hou DY, Liu Y, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102– 6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R, Wei F, Yu J. IDO inhibits T-cell function through suppressing Vav1 expression and activation. Cancer Biol Ther. 2009;8:1402–1408. doi: 10.4161/cbt.8.14.8882. [DOI] [PubMed] [Google Scholar]
- 35.Lee GK, Park HJ, Macleod M, et al. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frumento G, Rotondo R, Tonetti M, et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459– 468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, Zhang GX, Ciric B, et al. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett. 2008;121:1– 6. doi: 10.1016/j.imlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dinatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces inter-leukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes NA, Stephenson SJ, Tooze RM, et al. Amino acid deprivation links BLIMP-1 to the immunomodulatory enzyme indoleamine 2,3-dioxygenase. J Immunol. 2009;183:5768–5777. doi: 10.4049/jimmunol.0803480. [DOI] [PubMed] [Google Scholar]
- 40.Brenk M, Scheler M, Koch S, et al. Tryptophan deprivation induces inhibitory receptors ILT3 and ILT4 on dendritic cells favoring the induction of human CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2009;183:145–154. doi: 10.4049/jimmunol.0803277. [DOI] [PubMed] [Google Scholar]
- 41.Baban B, Hansen AM, Chandler PR, et al. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Chin YE, Weisiger E, et al. Cutting edge: negative regulation of dendritic cells through acetylation of the nonhistone protein STAT-3. J Immunol. 2009;182:5899–5903. doi: 10.4049/jimmunol.0804388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orabona C, Pallotta MT, Volpi C, et al. SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Proc Natl Acad Sci USA. 2008;105:20828–20833. doi: 10.1073/pnas.0810278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ou X, Cai S, Liu P, et al. Enhancement of dendritic cell-tumor fusion vaccine potency by indoleamine-pyrrole 2,3-dioxygenase inhibitor, 1-MT. J Cancer Res Clin Oncol. 2008;134:525–533. doi: 10.1007/s00432-007-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wobser M, Voigt H, Houben R, et al. Dendritic cell based antitumor vaccination: impact of functional indoleamine 2,3-dioxygenase expression. Cancer Immunol Immunother. 2007;56:1017–1024. doi: 10.1007/s00262-006-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 47.Inaba T, Ino K, Kajiyama H, et al. Indoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol Oncol. 2010;117:423– 428. doi: 10.1016/j.ygyno.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 48.Inaba T, Ino K, Kajiyama H, et al. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol. 2009;115:185–192. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Ino K, Yamamoto E, Shibata K, et al. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res. 2008;14:2310–2317. doi: 10.1158/1078-0432.CCR-07-4144. [DOI] [PubMed] [Google Scholar]
- 50.Ino K, Yoshida N, Kajiyama H, et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006;95:1555–1561. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corm S, Berthon C, Imbenotte M, et al. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gamma. Leuk Res. 2009;33:490– 494. doi: 10.1016/j.leukres.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 52.Muller AJ, Sharma MD, Chandler PR, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008;105:17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 54.Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 55.Qian F, Villella J, Wallace PK, et al. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 2009;69:5498–5504. doi: 10.1158/0008-5472.CAN-08-2106. [DOI] [PubMed] [Google Scholar]
- 56.Hou DY, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792– 801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 57.Muller AJ, Prendergast GC. Marrying immunotherapy with chemotherapy: why say IDO? Cancer Res. 2005;65:8065– 8068. doi: 10.1158/0008-5472.CAN-05-2213. [DOI] [PubMed] [Google Scholar]
- 58.Lob S, Konigsrainer A, Schafer R, et al. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;111:2152–2154. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 59.Soliman HH, Antonia S, Sullivan D, et al. Overcoming tumor antigen anergy in human malignancies using the novel indeolamine 2,3-dioxygenase (IDO) enzyme inhibitor, 1-methyl-D-tryptophan (1MT) J Clin Oncol. 2009;27(15s):Abstract 3004. [Google Scholar]
- 60.Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Lob S, Konigsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunol Immunother. 2009;58:153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Metz R, Duhadaway JB, Kamasani U, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 63.Witkiewicz AK, Costantino CL, Metz R, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg. 2009;208:781–787. doi: 10.1016/j.jamcollsurg.2008.12.018. discussion 787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raitala A, Pertovaara M, Karjalainen J, et al. Association of interferon-gamma +874(T/A) single nucleotide polymorphism with the rate of tryptophan catabolism in healthy individuals. Scand J Immunol. 2005;61:387–390. doi: 10.1111/j.1365-3083.2005.01586.x. [DOI] [PubMed] [Google Scholar]
- 65.Thomas SR, Salahifar H, Mashima R, et al. Antioxidants inhibit indoleamine 2,3-dioxygenase in IFN-gamma-activated human macrophages: posttranslational regulation by pyrrolidine dithiocarbamate. J Immunol. 2001;166:6332–6340. doi: 10.4049/jimmunol.166.10.6332. [DOI] [PubMed] [Google Scholar]
- 66.Thomas SR, Terentis AC, Cai H, et al. Post-translational regulation of human indoleamine 2,3-dioxygenase activity by nitric oxide. J Biol Chem. 2007;282:23778–23787. doi: 10.1074/jbc.M700669200. [DOI] [PubMed] [Google Scholar]
- 67.Meloni F, Giuliano S, Solari N, et al. Indoleamine 2,3-dioxygenase in lung allograft tolerance. J Heart Lung Transplant. 2009;28:1185–1192. doi: 10.1016/j.healun.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 68.Juhasz C, Muzik O, Lu X, et al. Quantification of tryptophan transport and metabolism in lung tumors using PET. J Nucl Med. 2009;50:356–363. doi: 10.2967/jnumed.108.058776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koblish HK, Hansbury MJ, Bowman KJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010;9:489–498. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 70.Liu X, Shin N, Koblish HK, et al. Selective inhibition of indoleamine 2,3-dioxygenase (IDO1) effectively regulates mediators of anti-tumor immunity. Blood. 2010;115:3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 71.Terentis AC, Freewan M, Sempértegui Plaza TS, et al. The selenazal drug ebselen potently inhibits indoleamine 2,3-dioxygenase by targeting enzyme cysteine residues. Biochemistry. 2010;49:591– 600. doi: 10.1021/bi901546e. [DOI] [PubMed] [Google Scholar]
- 72.Nakashima H, Uto Y, Nakata E, et al. Synthesis and biological activity of 1-methyl-tryptophan-tirapazamine hybrids as hypoxia-targeting indoleamine 2,3-dioxygenase inhibitors. Bioorg Med Chem. 2008;16:8661– 8669. doi: 10.1016/j.bmc.2008.07.087. [DOI] [PubMed] [Google Scholar]
- 73.Tesniere A, Zitvogel L, Kroemer G. The immune system: taming and unleashing cancer. Discov Med. 2006;6:211–216. [PubMed] [Google Scholar]