Abstract
We developed a novel method to spatiotemporally control activity of signaling molecules. A newly synthesized photocaged rapamycin derivative induced rapid dimerization of FKBP (FK-506 binding protein) and FRB (FKBP-rapamycin binding protein) upon UV irradiation. With this system and the spatially confined UV-irradiation, we achieved subcellularly localized activation of Rac, a member of small GTPases. Our technique offers a powerful approach to studies of dynamic intracellular signaling events.
Dynamic regulation of the Rho family of small guanosine triphosphatases (GTPases) with great spatiotemporal precision is essential for various cellular functions such as cell migration, phagocytosis, adhesion and secretion1. In order to drive these events, Rho GTPases become activated at specific intracellular locations to interact with distinctive downstream effector molecules2–5. Their spatiotemporally dynamic nature has been revealed by visualization of the activity and localization in real time6–8. In order to gain deeper understanding of their roles in diverse cellular functions at the molecular level, the next step should be perturbation of protein activities at a precise subcellular location and timing. Unfortunately, few techniques are available for rapidly controlling location and activity of Rho GTPases in living cells. We have previously developed a rapamycin-triggered heterodimerization strategy for activation or inactivation of Rho GTPases9, in which rapamycin induces translocation of FKBP-fused GTPases or its activator to the plasma membrane where FRB is anchored9. We then assumed that such inducible activation of Rho GTPases can be spatially confined to a subcellular level, if administration of rapamycin is localized to the small region of the cells.
The photocaging method has been frequently used to release small molecules in a spatially and temporally controlled manner by means of light10–11. A photo-caged compound is a small molecule whose activity is suppressed with a photocleavable protecting group known as a caging group. Light irradiation at an appropriate wavelength initiates a photochemical reaction that results in removal of the caging group from the mother compound, leading to restoration of the original activity. While there have been two reports on the synthesis of caged rapamycin12–13, neither of these has shown that the caged rapamycin is capable of inducing FKBP-FRB dimerization at the subcellular level. A general principle of photocaging technique requires a caged molecule to be completely inert until UV illumination. It has been reported that the modification of C40 hydroxyl group of rapamycin with a relatively large functional group such as fluorescein (molecular weight: 332) reduces affinity against FKBP by more than 5000 fold14, making this position an ideal link to a caging group. However, even 5000 fold reduction may not be sufficient to completely prevent the dimerization event in cells given the very high concentration of caged molecules (10−5 to 10−3 M) commonly used in the cellular environment to complement generally poor uncaging efficiency11.
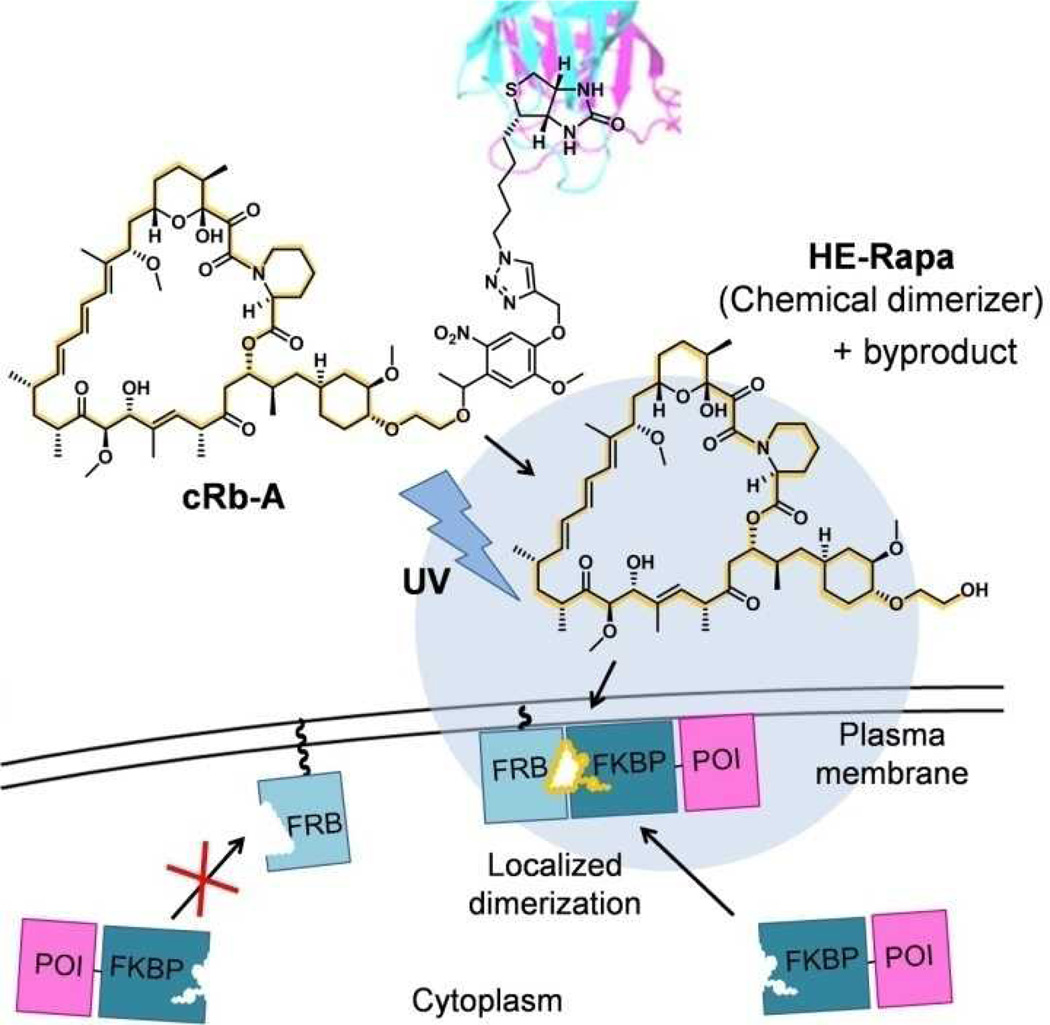
To circumvent this pitfall, we designed a caged rapamycin that is tethered to a macromolecule such that the resulting large complex does not cross the plasma membrane, leading to virtually no background activity as a chemical dimerizer inside cells. However, UV irradiation releases rapamycin that can now permeate cells to induce dimerization events (Figure 1). To connect rapamycin to a macromolecule, we employed avidin and biotin, whose interaction is extremely stable with a dissociation constant in the order of femtomolar. A similar strategy should be achieved by modifying caged rapamycin with hydrophilic groups instead of macromolecules.
Figure 1.
Schematic of spatially confined protein dimerization using caged rapamycin-avidin conjugate (cRb-A) and UV light. UV irradiation cleaves the linker between rapamycin and biotin, resulting in the release of chemical dimerizer (HE-Rapa) and its by-product. The released dimerizer then diffuses into cells and induces dimerization between FKBP-POI (protein of interest) and plasma membrane anchored FRB only in the proximity of the irradiated region (shown as a blue circle).
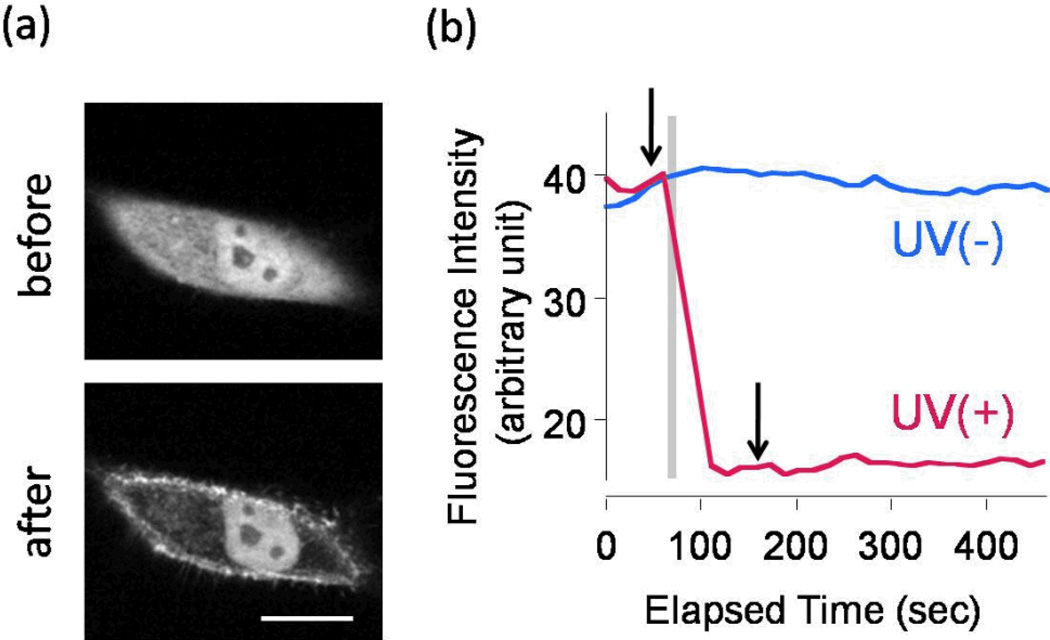
We first synthesized caged rapamycin-biotin conjugate cRb by linking rapamycin to biotin through a caging group (see Supporting Information for the detailed synthetic scheme). As a caging group we used 4,5-dimethoxy α-methyl nitrobenzyl group15. An additional linker was inserted between the nitrobenzyl group and the C40 hydroxyl group of rapamycin, as direct caging of the C40 hydroxyl group was not successful in our hands despite previous reports12,16. The following high-performance liquid chromatography (HPLC) analysis of cRb assured that the molecule releases C40-O-(2-hydroxyethyl) rapamycin (HE-Rapa, also known as Everolimus or Rad001) upon irradiation with UV light (Figure S1), which was confirmed with high-resolution mass spectrometry analysis. Independently, we also confirmed that HE-Rapa is fully functional as a chemical dimerizer (Figure S2, a–c). cRb itself functioned as a weak chemical dimerizer (Figure S2, d), suggesting that the biotin and nitrobenzyl group provide insufficient steric hindrance. cRb and avidin conjugate (cRb-A) was then prepared by incubating cRb in the presence of an excess amount of avidin in phosphate buffered saline at room temperature, followed by purification with a size-exclusion column. To test if rapamycin can be released to function as a chemical dimerizer in cells, we transfected HeLa epithelial cells with DNA constructs encoding YFP-FKBP (YF) and membrane-targeted FRB (LDR)9. With this system we have previously shown that addition of rapamycin or its analog to the bulk media induces rapid dimerization of these two proteins, promoting translocation of YF from cytoplasm to the plasma membrane. Addition of the purified cRb-A to the bulk media did not induce any protein translocation (Figures 2a and 2b). However, the subsequent UV irradiation to a broad region of the transfected cells resulted in a rapid translocation of YF proteins to the plasma membrane (Figures 2a and 2b). We confirmed that neither UV irradiation with avidin (without cRb), nor UV irradiation with avidin and rapamycin mixture that had been purified with a size-exclusion column (which should exclude any free rapamycin), induced translocation of YF (Figure S3, S4). In another control experiment we observed no effect of UV irradiation on translocation of YF that had been induced by bulk rapamycin (Figure S5). These results indicate that cRb-A can release a functional chemical dimerizer upon UV irradiation.
Figure 2.
Translocation of YF in HeLa cells induced by global UV irradiation with cRb-A conjugate. (a) Confocal fluorescence images of YF before and after photo irradiation. (b) Time course of fluorescence intensity in cytosol of the irradiated or non-irradiated cell. Arrows indicate the time of acquiring images in (a). UV was irradiated for 0.5 sec at the period shown as a gray bar. Scale bar: 20 µm.
We then tested if spatially restricted UV irradiation could trigger a dimerization event at the subcellular level. Toward this end, we aimed to achieve localized activation of Rho GTPases. Activation of Rac, a member of Rho family GTPases, induces membrane ruffle formation and plays an important role in cell migration especially at the leading edge of the cells3. We have previously shown that recruitment of Tiam1, a specific guanine exchange factor for Rac, to the plasma membrane using a rapamycin-triggered protein dimerization technique is sufficient to induce Rac activation and subsequent ruffle formation9. In order to achieve localized Rac activation, we transfected NIH3T3 fibroblasts with YFP-FKBP-Tiam1 (YF-Tiam1) and LDR. As a light source for localized UV irradiation, we employed a light-emitting diode (LED) coupled to a microscope through an optical fiber. After adding cRb-A to the bulk media, LED UV light was applied to a small region near a cellular edge. This operation rapidly induced formation of localized ruffles only in the irradiated area (Figure 3a, Supplementary movie 1). To confirm that this was indeed local activity, we then globally irradiated with UV, or added rapamycin to the bulk media, both of which induced global ruffle formation throughout the membrane (Figure S6, Supplementary movie 2). Global UV irradiation after local irradiation induced relatively smaller global ruffle formation, at least partly because the molecular machinery for ruffle formation, such as Rac and WAVE, may have been exploited for the preceding local ruffling. Consistent with this, we observed more striking, global ruffles with global stimulation without any prior operation (Supplementary movie 3). For a quantitative analysis, we divided target cells into four quadrants, and counted the frequency of ruffle formation upon local as well as following global irradiation in each quadrant (Figures 3b and c). While all the cells tested showed ruffle formation in an irradiated quadrant (n=13, Table S1), only a small number of cells showed ruffling in non-irradiated quadrants (p<0.0002). Ruffle formation in non-targeted quadrants may result from rapamycin overspill or lateral diffusion of dimerization complex and/or activated endogenous Rac along the plasma membrane. With global UV irradiation, on the other hand, more than 90% of the quadrants showed ruffle formation upon irradiation (n=12, Table S2). We confirmed that local UV irradiation with or without avidin did not induce any ruffle formation in these cells (Figure S7). These results indicated that spatially restricted uncaging of cRb-A can achieve Rac activation at the subcellular level.
Figure 3.
Spatially confined ruffle formation in a transfected NIH3T3 cell induced by localized UV irradiation with cRb-A conjugate. (a) Confocal fluorescence images of YF-Tiam1 before and after local UV irradiation at 365 nm (yellow circle). (b–c) Frequency of ruffle formation in each quadrant with local or global UV irradiation in the presence of cRb-A. Cells were divided into quadrants with the irradiated spot in the area i (b) and ruffle formation was counted in each quadrant (c). Scale bar: 10 µm.
In conclusion, we demonstrated development of a technique that employs a novel caged compound together with a FKBP-FRB heterodimerization system in order to manipulate Rho GTPase activity at a precise subcellular location on a time scale of seconds. Although it was predicted to be difficult to “cage” the effectiveness of rapamycin using only small functional groups, a large protein such as avidin could achieve this goal by precluding rapamycin’s access into cytosol. This strategy greatly helped to silence the compound activity as a chemical dimerizer until UV irradiation. Upon cleavage of the linker between rapamycin and biotin-avidin with UV irradiation, local release of a chemical dimerizer was achieved, leading to subcellularly localized Rho GTPase activation.
There are two limitations of the present approach. Firstly, a target location needs to be at or near plasma membrane. Secondly, molecular diffusion can affect precise confinement of intended effects particularly at later time points. This effect depends on parameters associated with UV irradiation (such as size, mode –continuous vs. pulsed-, and energy), and diffusion coefficients of chemical dimerizers, the FRB-FKBP dimerization complex as well as activated endogenous signaling molecules such as Rac. Further optimization of these parameters enables more stringent confinement of signal activities. For example, smaller sized, pulsed and higher energy UV irradiation should help minimize the effect of diffusion without compromising uncaging efficiency. It also helps to reduce diffusion of chemical dimerizers by using denser extracellular medium. In order to achieve slower lateral diffusion, a transmembrane motif, instead of lipid modification, could be used to anchor FRB to the plasma membrane.
Recently, Wu et al.17, Levskaya et al.18 and Yazawa et al.19 reported an elegant approach to activate Rac at a subcellular location using light-sensitive plant proteins. Compared to their strategies, our current technique is more feasible to expand the number of target proteins, since we and others have already developed a variety of dimerization probes that can manipulate the activity of diverse signaling molecules at the plasma membrane9,20–23. Our caging strategy is readily applicable to all these molecular probes to achieve subcellularly targeted manipulation of cell signaling, thus making it a powerful tool to probe spatiotemporally dynamic cellular events.
Supplementary Material
Acknowledgement
We thank Prof. T. Furuta and Dr. T. Kobayashi for helpful discussions. This study was supported in part by NIH (MH084691, DK090868 and GM092930 to TI). NU and TU are recipients of a fellowship from the Japan Society for the Promotion of Science.
Footnotes
Supporting Information Available: Synthesis, cell culturing, transfection, confocal microscopy and other experimental details are available free of charge via the Internet at http://pubs.acs.org/.
REFERENCES
- 1.Takai Y, Sasaki T, Matozaki T. Physiol. Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S, Hall A. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 3.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 4.Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR. J. Cell Biol. 2006;174:437–445. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. J. Cell Biol. 1999;147:1009–1021. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M. Mol. Cell. Biol. 2002;22:6582–6591. doi: 10.1128/MCB.22.18.6582-6591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong K, Pertz O, Hahn K, Bourne H. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pertz O, Hodgson L, Klemke RL, Hahn KM. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. Nat. Methods. 2005;2:415–418. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis-Davies GCR. Nat. Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kao JPY. Chapter 6, Unit 6.20. Curr. Protoc. Neurosci. 2006 [Google Scholar]
- 12.Borchardt A, Liberles SD, Biggar SR, Crabtree GR, Schreiber SL. Chem. Biol. 1997;4:961–968. doi: 10.1016/s1074-5521(97)90304-5. [DOI] [PubMed] [Google Scholar]
- 13.Sadovski O, Jaikaran ASI, Samanta S, Fabian MR, Dowling RJO, Sonenberg N, Woolley GA. Bioorg. Med. Chem. 2010 doi: 10.1016/j.bmc.2010.04.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banaszynski LA, Liu CW, Wandless TJ. J. Am. Chem. Soc. 2005;127:4715–4721. doi: 10.1021/ja043277y. [DOI] [PubMed] [Google Scholar]
- 15.Aujard I, Benbrahim C, Gouget M, Ruel O, Baudin JB, Neveu P, Jullien L. Chem. Eur. J. 2006;12:6865–6879. doi: 10.1002/chem.200501393. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Nakanishi K, Merrill D, Eng CP, Molnar-Kimber KL, Failli A, Caggiano TJ. Bioorg. Med. Chem. Lett. 1995;5:1355–1358. [Google Scholar]
- 17.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levskaya A, Weiner OD, Lim WA, Voigt CA. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazawa M, Sadaghiani AM, Hsueh B, Dolmetsch RE. Nat. Biotechnol. 2009;27:941–945. doi: 10.1038/nbt.1569. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber SL, Kapoor TM, Wess G. Chemical biology. Weinheim: Wiley-VCH; 2007. [Google Scholar]
- 21.Suh BC, Inoue T, Meyer T, Hille B. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komatsu T, Kukelyansky I, McCaffery JM, Ueno T, Varela LC, Inoue T. Nat. Methods. 2010;7:206–208. doi: 10.1038/nmeth.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balla T, Szentpetery Z, Kim YJ. Physiology (Bethesda) 2009;24:231–244. doi: 10.1152/physiol.00014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.