Mutations in evolutionarily conserved zinc finger proteins of Arabidopsis thaliana are silent at optimal growth temperature but arrest leaf growth at 10°C, a response which arises from their roles in the maturation of the eukaryotic ribosome.
Abstract
The evolutionarily conserved proteins REI1-LIKE (REIL1) and REIL2 have four conserved zinc finger domains and are Arabidopsis thaliana homologs of the cytosolic 60S ribosomal maturation factor Rei1p (for Required for isotropic bud growth1 protein) from yeast (Saccharomyces cerevisiae) and its paralog Reh1p (for REI1 homologue1 protein). The yeast and A. thaliana paralogs result from independent gene duplications. The A. thaliana REIL paralogs are required specifically in the cold (10°C) but not for growth at optimal temperature (20°C). A reil1-1 reil2-1 double mutant is arrested at 10°C prior to the emergence of the first rosette leaf. Two allelic reil2 mutants, reil2-1 and reil2-2, form small spoon-shaped leaves at 10°C. This phenomenon reverts after emergence of the inflorescence in the cold or upon shift to 20°C. Except for a slightly delayed germination, a reil1-1 mutant shows no further growth phenotype under the currently investigated conditions. A comparative analysis demonstrates conserved coexpression of orthologous genes from yeast and A. thaliana that are coregulated with yeast rei1 or with A. thaliana REIL2, respectively. The conserved correlations point to a role of A. thaliana REIL proteins in the maturation of the eukaryotic ribosomal 60S subunit. We support this conclusion by heterologous complementation of the cold-induced growth defect of the yeast Δrei1 deletion.
Genes coding for zinc finger proteins are abundant features of plant, fungal, and animal genomes (Riechmann et al., 2000). Approximately 0.8% of all proteins from the budding yeast (Saccharomyces cerevisiae) have zinc finger domains (Böhm et al., 1997). Plant genomes harbor similar proportions of zinc finger proteins. The eudicot model plant Arabidopsis thaliana, for example, has 176 zinc finger proteins (Englbrecht et al., 2004; Ciftci-Yilmaz and Mittler, 2008). A family of 189 zinc finger proteins is encoded by the genome of the monocot model Oryza sativa (Agarwal et al., 2007). Most of the members of these large protein families are plant-specific transcriptional regulators. Plant zinc finger proteins contain one or more typical C2H2-type zinc finger domains but may also have variations of this motif (Englbrecht et al., 2004; Agarwal et al., 2007; Ciftci-Yilmaz and Mittler, 2008). Only 32 proteins of A. thaliana contain four zinc finger domains. The four zinc finger proteins of this study, i.e. the REI1-LIKE protein REIL1 encoded by At4g31420 and the REIL2 paralog encoded by At2g24500, represent a small gene family and belong to the few (18.8%) evolutionarily conserved zinc finger proteins (Englbrecht et al., 2004; Ciftci-Yilmaz and Mittler, 2008).
In agreement with the predominant function of zinc finger domains, both A. thaliana REIL genes are currently annotated as putative sequence-specific DNA-binding transcription factors (http://www.arabidopsis.org). Even though most plant zinc finger proteins are transcription factors, the ligand range of the zinc finger motif is known to have diversified during evolution. Zinc fingers that specifically interact with proteins (Gamsjaeger et al., 2007) or with RNA, e.g. ribosomal RNA, mRNA, and poly(A)-RNA, have been described (Hall, 2005; Kelly et al., 2007).
Contraindicative to the current provisional functional annotation of A. thaliana REIL proteins, the zinc finger motifs of these proteins are reported to show similarity to U1-zinc fingers (Englbrecht et al., 2004). These motifs form characteristic domains of the U1 small nuclear ribonucleoproteins, which bind to the 5′ splice site of pre-mRNA in the course of spliceosome assembly. In view of this conflicting evidence, we considered the REIL proteins of A. thaliana at the beginning of our study to represent true orphans without functional assignment.
Attempts to deduce the function of plant REIL genes by previously characterized orthologs of other taxa yield conflicting results. Several clusters of orthologs (COGs) are reported that contain the A. thaliana REIL proteins (Tatusov et al., 2003; O’Brien et al., 2005; Kuzniar et al., 2009; Östlund et al., 2010). The members of these COGs are exclusively of eukaryotic origin and comprise proteins of plants, fungi, nematodes, insects, and mammals. With only few exceptions, REIL orthologs are not functionally characterized. One of these exceptions is a single-copy mammalian protein. This protein is named ZINC FINGER-LIKE PROTEIN9 (ZRP9; Seong et al., 2002, 2003) and is also known as zinc finger protein 622 (ZNF622). The second exception is a set of two functionally characterized paralogs from yeast, namely Rei1p (for Required for isotropic bud growth1 protein) encoded by YBR267W and Reh1p (for REI1 homolog1 protein) encoded by the YLR387C locus (Iwase and Toh-e, 2004; Parnell and Bass, 2009).
The current studies on the mammalian and the yeast orthologs point to apparently different cellular functions and fail so far to provide an unambiguous functional assignment of the plant orthologs. Mammalian ZRP9 appears to be functionally linked to cell proliferation and differentiation in mammals (Seong et al., 2002, 2003). ZRP9 relocates from the cytoplasm to the nucleus dependent on the status of its phosphorylation sites, S276, Y284, S314, or T318, numbered here according to the human ZRP9 protein sequence (http://www.phosphosite.org). ZRP9 is also a positive regulator of the apoptosis signal-regulating kinase1 (ASK1) and is in turn phosphorylated by ASK1 at the positions S314 and T318 (Seong et al., 2011).
The initial study on yeast Rei1p and its paralog Reh1p also points toward an involvement in cell proliferation processes (Iwase and Toh-e, 2004). This study describes Rei1p as a cytoplasmic protein that appears to belong to the mitotic signaling network of yeast. According to a mitotic phenotype of the Δrei1 deletion mutant, Rei1p is named for being required for isotropic bud growth (Iwase and Toh-e, 2004). This line of investigation was, however, not further pursued when two laboratories demonstrated that Rei1p takes part in the maturation process of the 60S preribosomal subunit. In more detail, Rei1p interacts with the cytosolic shuttling factors Associated with ribosomal export complex1 (Arx1p), ARX1 little brother1 (Alb1p), Ribosomal-like protein24 (Rlp24p), ribosomal protein of the large subunit24A (Rpl24Ap), Rpl24Bp, Type III j-protein1 (Jjj1p), and translation initiation factor6 (Tif6p) (Hung and Johnson, 2006; Lebreton et al., 2006; Meyer et al., 2007). Rei1p is thought to be recruited to the cytosolic pre-60S subunit by Rlp24p. Rlp24p needs to be removed before Rei1p can load onto the cytosolic 60S preribosome. Rpl24Ap or the almost-identical Rpl24Bp are thought to replace Rlp24p in the cytosol. Jjj1p is an Hsp40 heat shock chaperone (Demoinet et al., 2007) and cooperates with Rei1p to remove Alb1p from the pre-60S subunit (Meyer et al., 2010). This step initiates Arx1p and Alb1p recycling to the nucleus. Arx1p release, in turn, is thought to be prerequisite of the final releases of Tif6p (Basu et al., 2001) and Nonsense-mediated mRNA decay3 protein (Nmd3p) from the pre-60S subunit (Lo et al., 2010). These processes form the final control point that renders the cytosolic pre-60S ribosomal subunits of yeast mature and translationally active. While Rei1p is central to the mechanism of Arx1p-dependent 60S subunit maturation, the paralog Reh1p appears to stabilize the 60S subunits in the absence of Rei1p and may have a partially redundant function. Reh1p possibly takes part in a basic 60S subunit maturation process, which is thought to be independent of Arx1 recycling (Parnell and Bass, 2009). These processes were discovered in yeast and can currently be considered to represent the mechanistic model of the final steps of eukaryotic 60S ribosomal maturation (Lo et al., 2010; Panse and Johnson, 2010).
Growth phenotypes of the yeast Δrei1, Δreh1, and double deletion mutants link Rei1p function to abiotic stress response mechanisms of this organism. Rei1p is required to maintain growth when yeast is shifted to even moderately suboptimal temperature conditions. Growth of the Δrei1 mutant is reduced 2- to 3-fold at 23°C to 25°C but normal at optimal 30°C conditions (Iwase and Toh-e, 2004; Lebreton et al., 2006). By contrast, Reh1p is not required for growth at low temperature. However, the double deletion mutant is growth arrested already at temperatures below or equal to 30°C and can only be maintained at 37°C (Parnell and Bass, 2009). Gene expression of rei1 but not of reh1 is under temperature control and exhibits almost thermometer-like, reciprocal responses to high- and low-temperature stresses. In detail, rei1 mRNA is early up-regulated when cells are shifted from 28°C to 10°C and likewise early down-regulated upon shift from 28°C to 37°C (Strassburg et al., 2010; Walther et al., 2010).
Based on the evidence summarized above, we investigated whether the function of the REIL genes in A. thaliana is conserved. For this purpose, we isolated transfer DNA (T-DNA) mutants of the A. thaliana REIL genes and tested their growth response to suboptimal temperature. In addition, we demonstrate conserved function by comparative coexpression analysis and functional complementation of the Δrei1 mutant.
RESULTS
The Topology of A. thaliana REIL Proteins
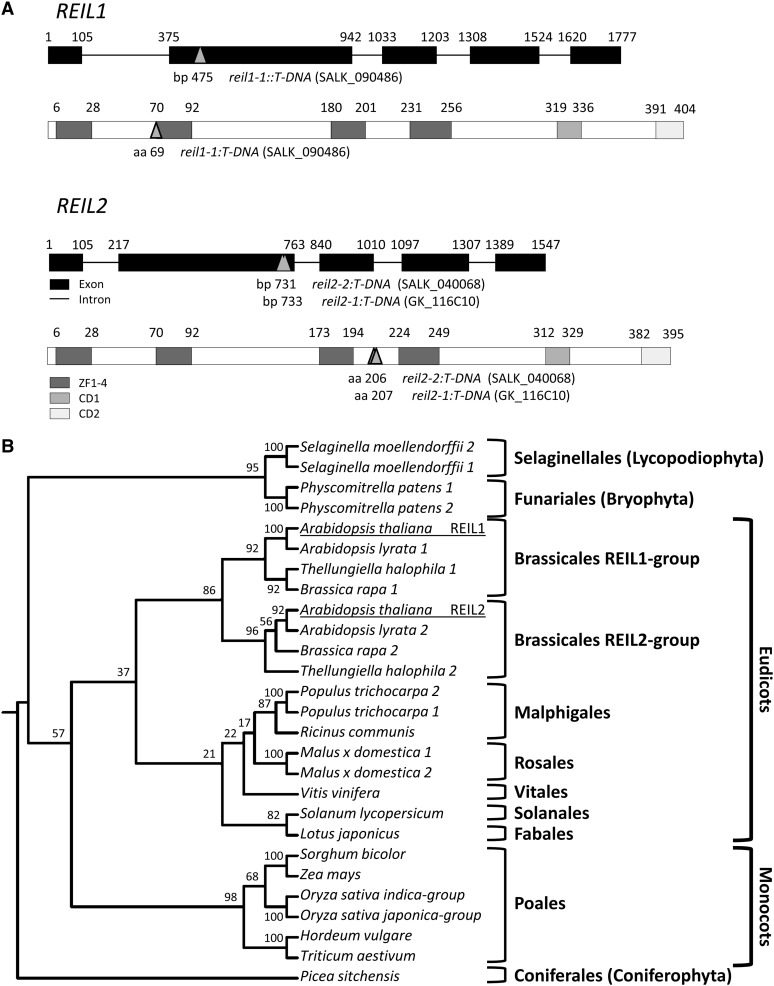
The A. thaliana REIL genes have five exons, which encode a 404-amino acid REIL1 protein, gene model At4g31420.1, and a 395-amino acid REIL2 protein, gene model At2g24500.1 (Fig. 1A). REIL1 has a putative splice site variant, At4g31420.2, which adds an additional Ser at position 281 of REIL1 but leaves the protein otherwise unchanged. The two genes REIL1 and REIL2 are conserved among A. thaliana, Arabidopsis lyrata ssp. lyrata, and Thellungiella halophila (Supplemental Figs. S1 and S2; Supplemental Document S1). An ortholog translated from the genomic sequence of Brassica rapa ssp. pekinensis aligns to REIL1. A partial sequence of the same subspecies that aligns to REIL2 indicates the presence of a second paralog in Brassica rapa ssp. pekinensis (Supplemental Fig. S2). The four zinc finger domains of REIL proteins are positioned in two pairs, ZF1/ZF2 and ZF3/ZF4, at the N terminus and in the center of the sequence. Two additional highly conserved domains, here provisionally termed CD1 and CD2, are located close to and at the C terminus (Fig. 1A). The phosphorylation sites of the human ortholog ZRP9 (Seong et al., 2011) are located within a highly conserved region adjacent to ZF3, sites S276 and Y284 of ZRP9, and within ZF4, sites S314 and T318 of ZRP9. Only one of the phosphorylation sites, Y284, is conserved in the REIL proteins of the Brassicaceae. The eukaryotic orthologs of the A. thaliana REIL proteins are mostly single copy (Supplemental Fig. S3; Supplemental Table S1). Gene duplications occurred independently during evolution of the Saccharomycetales and of the Embryophyta. None of the species with full genome information has more than two paralogs.
Figure 1.
Topology and phylogeny of the REIL proteins from A. thaliana. A, Topology of A. thaliana REIL genes and proteins. The REIL1 and REIL2 genes have five conserved exons. The respective REIL proteins, namely the 404-amino acid REIL1 and the 395-amino acid REIL2 protein, contain four zinc finger domains and two additional conserved domains, CD1 and CD2. The mutants, reil1-1 (SALK_090486), reil2-1 (GK_166C10), and reil2-2 (SALK_040068), carry T-DNA insertions in exon 2 (compare with arrow heads). B, Phylogenetic analysis of the plant REIL proteins. The plant REIL proteins were subject to gene duplications, which occurred independently in several plant phylae. The REIL1 and the REIL2 paralogs of A. thaliana originated from gene duplication during the speciation of the Brassicales.
The origin of the yeast rei1 and reh1 paralogs appears to be linked to a gene duplication following the phylogenetical ancient speciation of the Hemiascomycete Yarrowia lipolytica (Dujon et al., 2004; Dujon, 2006). Y. lipolytica contains only one rei1-like copy, whereas modern yeast species have two (Supplemental Fig. S3). Species of the plant phylum, except for the monocots, tend to have independent gene duplications of the rei1-like genes (Fig. 1B). In addition to the gene duplication, which gave rise to the REIL1 and REIL2 paralogs of the Brassicaceae, gene duplications are present within the Physcomitrella patens and Selaginella moellendorffii genomes, as well as in Populus trichocarpa and Malus domestica.
The A. thaliana reil Mutants
To match the yeast gene deletion mutants, we isolated a reil1-1 mutant and two allelic reil2 mutants of A. thaliana, namely reil2-1 and reil2-2, from public T-DNA insertion mutant collections. The selected T-DNA insertions of reil1-1, reil2-1, and reil2-2 were in exon 2 at base pairs 475, 733, and 731, respectively. All tests of the mutants for the presence of full-length mRNA, both before and after flowering and at 10°C as well as at 20°C (Supplemental Fig. S4), were negative. The homozygous single gene mutants and a homozygous reil1-1 reil2-1 double mutant were fully viable under optimal growth conditions in a 16-h/8-h day/night cycle at 20°C/18°C. The mutant plants propagated under these conditions without obvious defect compared to the ecotype Columbia-0 (Col-0) wild type. To avoid differential priming effects on seed batches, all seed material of this study was from plants that completed a full life cycle under optimum growth conditions. The germination rates of these mutant seed batches were not significantly changed compared with a 90.1% ± 2.8% germination rate of the wild type (Supplemental Table S2).
Characterization of the Mutant Phenotypes: the reil1-1 reil2-1 Double Mutant Has Pointed-Leaves Morphology
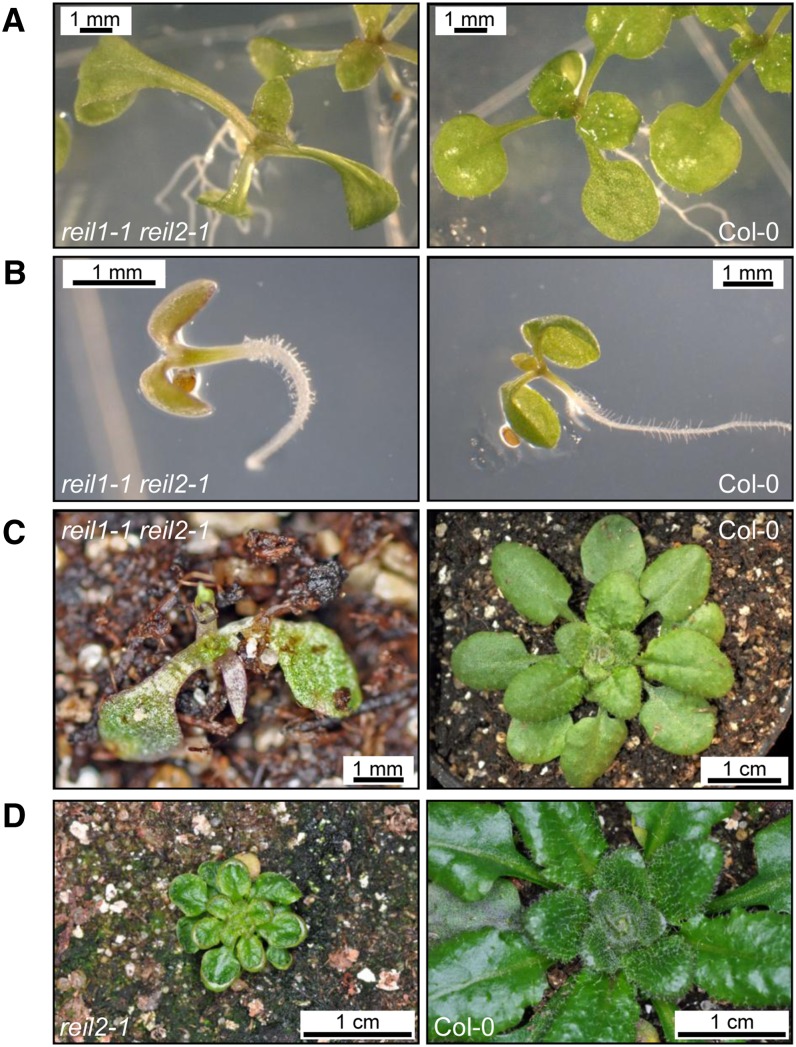
Germination of the reil mutant lines was assayed in vitro under an optimum 20°C temperature regime and staged according to a system for the phenotypic analysis of A. thaliana development (Boyes et al., 2001). The emergence of the first pair of rosette leaves, stage 1.02 (Fig. 2), was delayed in the reil1-1 reil2-1 double mutant but not the emergence of the radicle at stage 0.5 or the emergence of the cotyledons at stage 0.7 (data not shown). The reil1 and reil2 single gene mutants did not differ from the wild type at 20°C (Fig. 2). In contrast to the wild type and the single gene mutants, the leaf morphology of early leaves generated by the reil1-1 reil2-1 double mutant was aberrant (Fig. 3, A and B). These developing leaves were spear shaped, with an acute tip and two basal serrations instead of the typical rounded leaves of the Col-0 wild type. The aberrant leaf morphology was similar to the so-called pointed-leaves mutations (Van Lijsebettens et al., 1994; Berná et al., 1999; Horiguchi et al., 2011). The pointed-leaves phenotype of the reil1-1 reil2-1 double mutant gradually disappeared at later growth stages and was lost after transfer to soil.
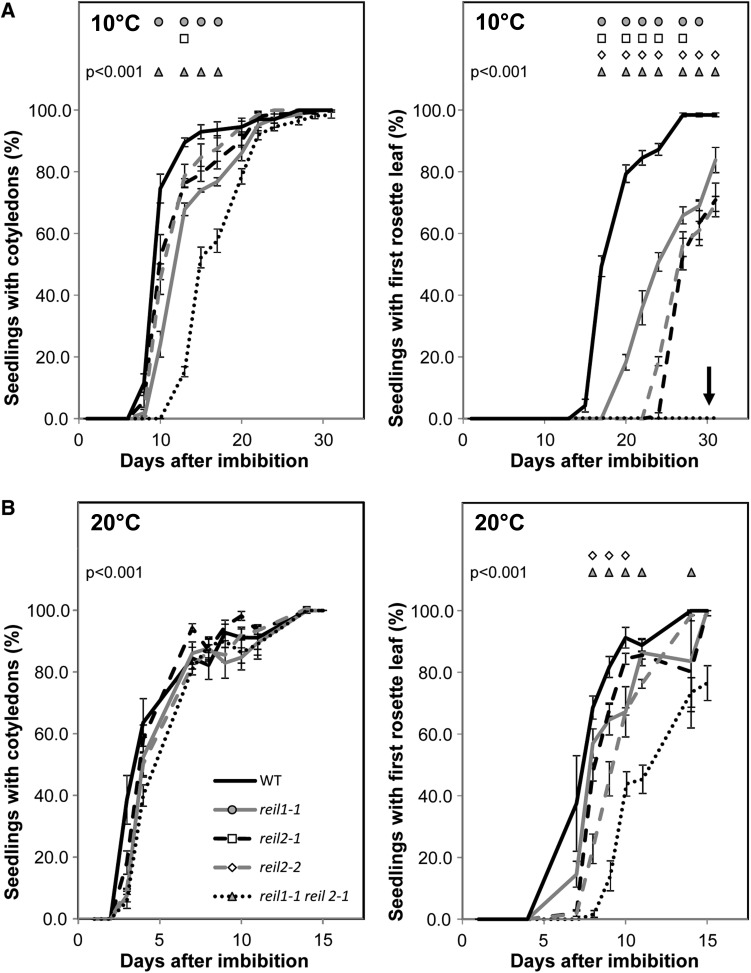
Figure 2.
The germination phenotype of reil mutants. A, Germination of reil mutants in the cold (10°C) compared with the Col-0 wild type. B, Germination of reil mutants at optimal temperature (20°C) compared with the Col-0 wild type. Significant differences relative to the wild type are indicated within the top section of each graph by circles, squares, diamonds, and triangles (P < 0.001, n = 4–5 plates per genotype). Each plate had approximately 80 seeds. The percentage of germinated seeds with cotyledons (left) and of seeds with a first rosette leaf greater than 1 mm (right) was scored per plate. The total number of germinated seeds per plate (100%) was scored at the last time point of each assay. Note that the rosette leaves of the reil1-1 reil2-1 double mutant emerged later at optimal temperature and did not emerge in the cold (arrow).
Figure 3.
The aberrant leaf phenotypes of the reil1-1 reil2-1 double mutant and of the reil2-1 mutant. A, Pointed-leaves morphology of the reil1-1 reil2-1 double mutant compared with Col-0 after 16 d at 20°C. B, Growth arrest of the reil1-1 reil2-1 double mutant at stage 1.0 (Boyes et al., 2001) after 27 d at 10°C compared with Col-0. C, Phenotype of the reil1-1 reil2-1 double mutant germinated at 10°C, transferred to soil, and kept for 10 weeks strictly at 10°C compared with the Col-0 wild type. D, Representative phenotype of the reil2-1 mutant germinated at 10°C, transferred to soil, and kept for 5 to 6 weeks strictly at 10°C compared with the Col-0 wild type.
The reil1-1 reil2-1 Double Mutant Is Growth Arrested in the Cold
Lowering temperatures delayed the germination process of the reil1-1 reil2-1 double mutant further and enhanced the pointed-leaves morphology. A complete growth arrest was reached at 10°C (Fig. 2). At 10°C, the seedlings of the reil1-1 reil2-1 double mutant generated roots and cotyledons with a delay of approximately 3 to 4 d compared with the wild type and did not develop beyond stage 1.0 (Boyes et al., 2001). Upon prolonged exposure to cold, the double mutant bleached and accumulated a violet tinge (Fig. 3B). When transferred to soil, the seedlings stayed arrested, did not generate rosette leaves, and ultimately died in the cold (Fig. 3C). The reil1-1 mutant produced the first rosette leaf with only a slight delay and was otherwise inconspicuous during the germination assays and the subsequent morphological assessments. By contrast, the germination of both reil2 mutants was delayed at 10°C (Fig. 2). Germination and cultivation at 4°C was attempted, as this temperature is the typical choice of cold stress cues applied to A. thaliana. Our experiments failed to produce precise results at 4°C due to slow and variable germination of the reil mutant seedlings. For this reason, we choose 10°C as the standard cold stress condition of this study.
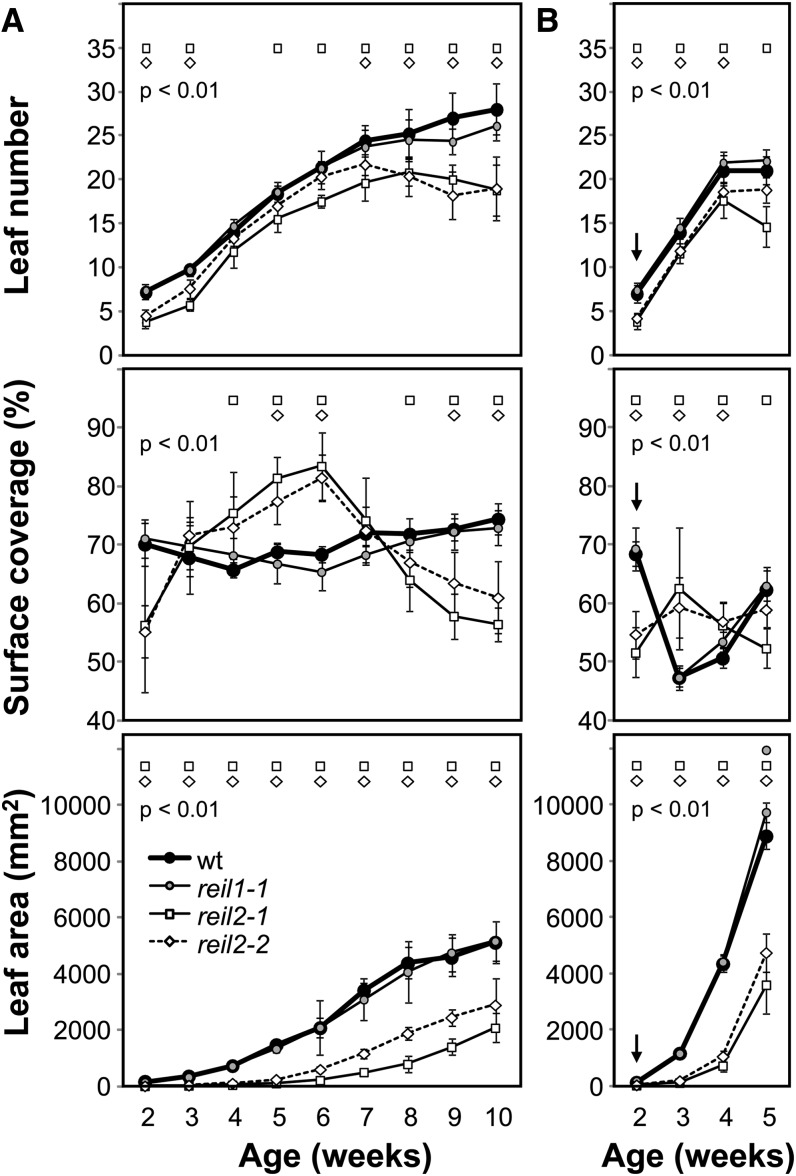
Leaves of the reil2 Mutants Have an Aberrant Shape That Reverts after Continued Cultivation in the Cold
In contrast to the double mutant, the reil2 mutants stayed viable at 10°C. The leaf morphology of both the reil2-1 and the reil2-2 mutants was irregular during cold germination. The leaves had a corrugated adaxial and a smooth abaxial surface and became spoon shaped upon transfer to soil and continued cultivation at 10°C (Fig. 3). However, the aberrant shape of reil2-1 and reil2-2 leaves reverted after continued cultivation at 10°C (Fig. 4, A and B). Morphometric analysis of the reil2 mutants showed a significant growth deficit in the cold compared with the wild type and the inconspicuous reil1-1 mutant (Fig. 5). The growth deficit was apparent in all monitored aspects, namely leaf number, planar rosette area, and surface coverage, i.e. the percentage of the planar leaf area within the convex hull of a rosette (compare with morphometric definitions in “Materials and Methods”). The growth deficit was maintained throughout development in the cold (Fig. 5, left). Between week 5 and 6 on soil in the cold, the leaf shape of the reil2 mutants reverted to a shape that was similar to the wild type. Reversion included longitudinal leaf growth and coincided with the emergence of the first floral buds (Figs. 4B and 5). The transition between the two leaf morphologies was best represented by the surface coverage of the rosette (Fig. 5). The wild type and reil1-1 mutant had almost constant surface coverage throughout development in the cold. By contrast, the reil2 mutants started with lower surface coverage, reached a maximum higher than the wild type at 6 weeks, and then reverted to lower surface coverage. The reil2-1 mutant had a slight but significantly stronger growth deficit compared with the reil2-2 mutant (Fig. 5).
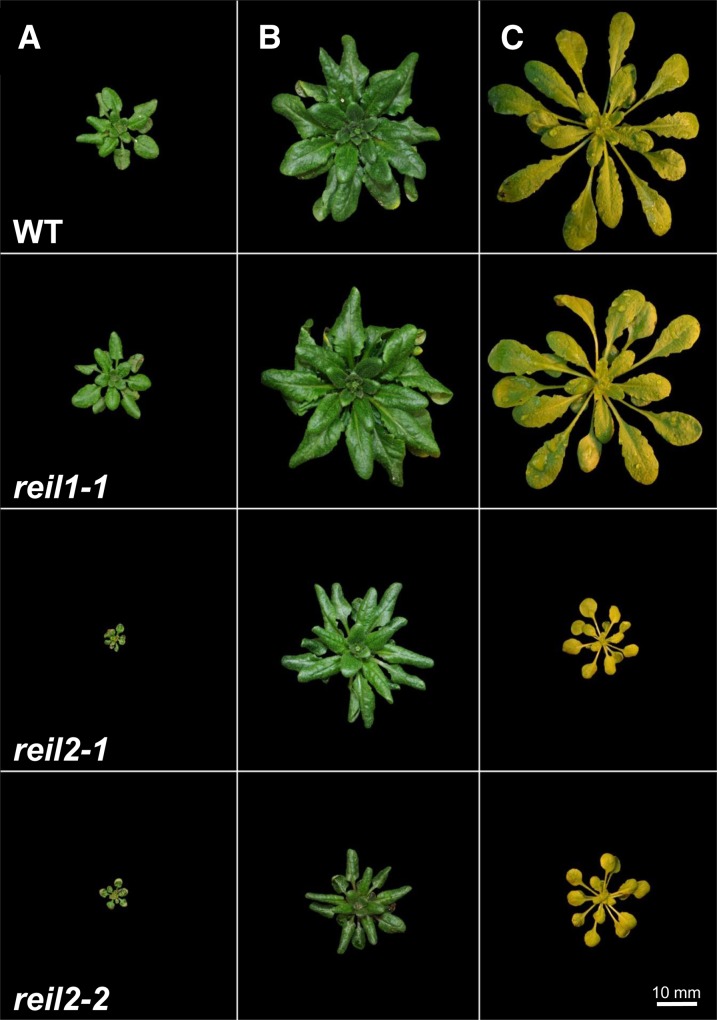
Figure 4.
Phenotype of the reil1-1, reil2-1, and reil2-2 mutants compared with the Col-0 wild type under diverse temperature regimes. A, Phenotype after germination at 10°C, transfer to soil at stage 1.02 to 1.03, and continued growth at 10°C for a period of 4 weeks. B, Phenotype after germination at 10°C, transfer to soil at stage 1.02 to 1.03, and continued growth at 10°C for a period of 8 weeks. Note reversal of the reil2 leaf phenotype and emergence of inflorescences from all mutant and wild-type rosettes. C, Phenotype after germination at 10°C, transfer to soil at stage 1.02 to 1.03, continued growth at 10°C for a period of 2 weeks, and subsequent shift to 2 weeks at 20°C. Note reversal of the reil2 leaf phenotype after shift to optimum temperature. All photographs have equal scale. Vertically aligned photographs were taken with identical illumination.
Figure 5.
Morphometric analyses of the reil1-1, reil2-1, and reil2-2 mutants compared with the Col-0 wild type. A, Plants cultivated strictly at 10°C (compare with Fig. 4, A and B). B, Plants germinated at 10°C, transferred to soil at stage 1.02 to 1.03, kept at 10°C for 2 weeks, and shifted to 20°C (compare with arrow and Fig. 4C). Significant differences relative to the wild type are indicated by circles, squares, and diamonds within the top section of each graph (P < 0.01, n = 5–10).
The Aberrant Shape of reil2 Leaves Is Lost after Shift to Optimal Temperature
The aberrant shape of reil2-1 and reil2-2 leafs reverted also after shift to optimum cultivation conditions at 20°C (Fig. 4C). However, the growth deficit compared with the wild type was maintained. The reil2-1 mutant was again more affected than the reil2-2 mutant (Fig. 5, right). The trends of changes of the reil2 surface coverage were essentially the same as in prolonged cold, but more rapid. Less leaves with longer petioles and lower surface coverage were generated after the shift to 20°C (Figs. 4C and 5). The main difference between the two modes of reversal was the elongation of the leaf lamina in prolonged cold, in contrast to a continually stunted and close-to-circular leaf lamina after shift to 20°C.
The Expression of REIL2 and Yeast rei1 Is Controlled by Temperature
Our own and the other studies had shown that the expression of the yeast rei1 and reh1 genes was under differential temperature control (Gasch et al., 2000; Sahara et al., 2002; Strassburg et al., 2010; Walther et al., 2010). The rei1 gene was up-regulated after cold stress and down-regulated after application of heat; the second yeast paralog, reh1, was not temperature responsive (Supplemental Fig. S5). As a consequence, only the rei1 paralog of yeast met the requirement of coexpression analysis. The A. thaliana REIL paralogs showed a similar differential response to temperature cues. REIL1 transcript levels were only slightly influenced by temperature (Kaplan et al., 2007; Kilian et al., 2007; Caldana et al., 2011). By contrast, the REIL2 transcript levels were clearly up-regulated in the cold but not significantly changed by heat (Supplemental Fig. S5). REIL2 responded 3 h after the cold cue and was delayed compared with the immediate response of yeast rei1 within the first minutes of cold stress. Again, only one paralog, REIL2, was amenable to coexpression analysis. Notably, the paralogs of both species, which were required for normal growth in the cold, namely REIL2 and rei1, were both under transcriptional control.
The Growth Defect of the reil Mutants Is Specific for Cold Stress
To test the specificity of the mutant phenotypes for cold stress, we performed a meta-analysis of the transcriptional responses of REIL1 and REIL2 using public transcriptome compendia (Zimmermann et al., 2004; Kilian et al., 2007; Hruz et al., 2008). This analysis indicated that the gene expression of the A. thaliana REIL genes was modified also by other stress factors, namely by high light (190 µE m–2 s–1) and low light (65 µE m–2 s–1), increased osmolarity by addition of mannitol (150 mm) or NaCl (100 mm), and chemical stresses induced by omission of Suc from the medium or by addition of Glc (1%, w/v), zeatin (1 µm), abscisic acid (10 µm), and paraquat (0.05 and 0.10 µm). We tested growth and development of the reil1-1 reil2-1 double mutant and of the single reil mutants under the respective described in vitro conditions. None of the above conditions caused a growth arrest of the double mutant or a growth defect of either the reil1 or the reil2 single mutants. To test for more subtle changes of growth rate, we choose an in vitro vertical plate assay and scored the length of the primary root of young A. thaliana plantlets. Under control conditions, the single gene mutants and the Col-0 wild type had identical root growth rates, approximately 6.6 mm day–1. Only the root growth of the reil1-1 reil2-1 double mutant was reduced to 4.7 mm day–1. In each case, root growth was linear between 7 and 14 d after germination (Supplemental Fig. S6). We defined the sensitivity to a stress factor as the ratio of the growth rate of each genotype under stress conditions divided by the growth rate of the same genotype under control conditions (Supplemental Fig. S7). The reil mutants and Col-0 were mostly equally sensitive to the tested stress factors. Only minor changes were observed compared with the complete growth arrest of the reil1-1 reil2-1 double mutant at 10°C. Specifically, the reil2 mutants were slightly more sensitive to high light and to oxidative stress induced by paraquat (Supplemental Fig. S7). These results supported the specificity of the growth defect of the reil mutants under cold stress.
A. thaliana REIL2 and Yeast rei1 Have Similar Coexpression Patterns
To test for a conserved function of A. thaliana REIL genes, we compared the coexpression patterns between the temperature-controlled yeast rei1 and A. thaliana REIL2 isoforms. The preceding specificity test of the mutant phenotype allowed us to focus our coexpression analysis on temperature stress experiments only, rather than to perform an unspecific coexpression analysis based on all available diverse stress response data. To take into consideration that yeast rei1 is controlled by cold and heat stress while A. thaliana REIL2 responds only to cold, we performed two parallel analyses. First, we investigated cold-controlled coexpression using only the cold stress data from each of the selected transcript studies. Second, we analyzed the temperature-controlled coexpression modules combining both the cold and the heat stress data from each of the studies mentioned above. Coexpression was analyzed by calculation of rank correlation coefficients. This procedure sorts the numerical expression data of each gene from small to large and assigns ranks, i.e. 1, 2, 3, etc., to each expression value. As a consequence, the rank correlation coefficients are less influenced by outliers, i.e. single very high or very low expression values (Steinhauser et al., 2004). Taken together, we distinguished between four modes of coexpression, namely positive and negative correlation under either cold or temperature control.
Our coexpression analysis demonstrated firstly that REIL2 is positively coregulated with genes that are phylogenetically conserved between A. thaliana and yeast (Supplemental Fig. S8). By contrast, no such enrichment was demonstrated for the negatively coexpressed genes. Secondly, an overrepresentation analysis demonstrated that REIL2 was positively coexpressed with genes involved in translation initiation and protein synthesis. Genes of the eukaryotic ribosomal subunits were highly significant and robustly enriched in all three analyzed transcriptome studies both under cold and temperature control (Tables I and II). Plant photosystem genes, which are obviously not conserved in yeast, were overrepresented among genes that were negatively coexpressed with REIL2. Furthermore, we found that A. thaliana genes with a positive correlation to REIL2 had yeast orthologs that were also positively correlated to rei1. This coexpression pattern was conserved between species both under cold and temperature control, but more significant under temperature control (Supplemental Fig. S9). The pairs of A. thaliana/yeast orthologs with conserved coexpression to REIL2 and rei1, respectively, are listed in Supplemental Table S3. This list contains genes of all major steps in ribosomal maturation, including the cytosolic maturation machinery (compare with “Discussion”).
Table I. Significance values of the overrepresentation analysis of functional annotations among genes that are coexpressed with REIL2 under cold control or under temperature control.
The table shows functional categories (bins) that have a robust and common overrepresentation with P < 0.05 in each of the independent coexpression analyses. Spearman’s rank correlations of REIL2 transcript levels with all other available genes of each transcript data set were calculated independently. Functional categories, so-called bins, of MapMan were applied (Usadel et al., 2005).
| Data Set |
Cold-Controlled Coexpression |
Temperature-Controlled Coexpression |
||||||
|---|---|---|---|---|---|---|---|---|
| Bin | Bin Name | Bin Size | Caldana et al. (2011) | Kaplan et al. (2007) | Kilian et al. (2007) | Caldana et al. (2011) | Kaplan et al. (2007) | Kilian et al. (2007) |
| P Valuea | ||||||||
| Negative coexpression | ||||||||
| 1 | PS | 187 | 4.30E-07 | 1.76E-03 | 1.30E-18 | 1.26E-02 | 1.40E-03 | 2.70E-07 |
| 1.1 | PS.lightreaction | 136 | 3.90E-07 | 4.28E-02 | 5.62E-09 | 2.93E-06 | 8.50E-03 | 7.01E-02 |
| 11 | lipid metabolism | 375 | 4.88E-02 | 7.69E-03 | 1.08E-02 | 1.51E-01 | 2.91E-03 | 1.22E-03 |
| 27.3.5 | RNA.regulation of transcription. ARR | 23 | 8.20E-03 | 2.01E-02 | 4.89E-02 | 6.92E-01 | 4.30E-01 | 1.53E-01 |
| 27.3.6 | RNA.regulation of transcription. bHLH, Basic Helix-Loop-Helix family | 93 | 1.11E-02 | 5.43E-03 | 1.98E-03 | 2.87E-01 | 1.69E-01 | 2.82E-02 |
| Positive coexpression | ||||||||
| 27.1 | RNA.processing | 254 | 3.26E-04 | 3.86E-10 | 1.13E-06 | 5.30E-01 | 1.36E-02 | 1.03E-13 |
| 27.1.2 | RNA.processing.RNA helicase | 29 | 4.56E-03 | 3.28E-04 | 4.89E-02 | 5.82E-02 | 8.26E-03 | 5.72E-02 |
| 29.2 | protein.synthesis | 515 | 7.73E-13 | <1.0E-20 | <1.0E-20 | <1.0E-20 | <1.0E-20 | <1.0E-20 |
| 29.2.1 | 371 | 5.94E-05 | <1.0E-20 | 1.68E-07 | 2.37E-08 | <1.0E-20 | <1.0E-20 | |
| 29.2.1.2 | 236 | <1.0E-20 | <1.0E-20 | <1.0E-20 | <1.0E-20 | <1.0E-20 | <1.0E-20 | |
| 29.2.1.2.1 | protein.synthesis.ribosomal protein.eukaryotic.40S subunit | 88 | 1.80E-04 | 5.57E-09 | 9.74E-08 | 5.20E-07 | <1.0E-20 | <1.0E-20 |
| 29.2.1.2.2 | protein.synthesis.ribosomal protein.eukaryotic.60S subunit | 148 | 2.06E-12 | <1.0E-20 | <1.0E-20 | <1.0E-20 | <1.0E-20 | <1.0E-20 |
| 29.2.2.50 | protein.synthesis.misc ribosomal protein.BRIX | 6 | 4.69E-03 | 7.59E-03 | 2.75E-03 | 3.25E-03 | 2.94E-03 | 1.56E-03 |
| 29.2.3 | protein.synthesis.initiation | 84 | 5.59E-08 | 6.44E-11 | 4.15E-11 | 7.73E-07 | 1.82E-07 | 2.94E-10 |
Significance of a Wilcoxon rank-sum test after Benjamini-Hochberg correction. Bold text indicates P values < 0.001.
Table II. Correlation coefficients of the overrepresentation analysis of functional annotations among genes that are coexpressed with REIL2 under cold control or under temperature control.
Correlation coefficients were analysed independently for overrepresentation of functional categories among the positively and negatively correlated genes. Functional categories, so-called bins, of MapMan were applied (Usadel et al., 2005).
| Data Set |
Cold-Controlled Coexpression |
Temperature-Controlled Coexpression |
||||||
|---|---|---|---|---|---|---|---|---|
| Bin | Bin Name | Bin Size | Caldana et al. (2011) | Kaplan et al. (2007) | Kilian et al. (2007) | Caldana et al. (2011) | Kaplan et al. (2007) | Kilian et al. (2007) |
| Correlation Coefficienta | ||||||||
| Negative coexpression | ||||||||
| 1 | PS | 187 | –0.257 | –0.145 | –0.370 | –0.168 | –0.140 | –0.155 |
| 1.1 | PS.lightreaction | 136 | –0.324 | –0.130 | –0.311 | –0.302 | –0.144 | –0.101 |
| 11 | lipid metabolism | 375 | –0.100 | –0.092 | –0.119 | –0.082 | –0.099 | –0.088 |
| 27.3.5 | RNA.regulation of transcription. ARR | 23 | –0.558 | –0.327 | –0.347 | –0.106 | –0.164 | –0.197 |
| 27.3.6 | RNA.regulation of transcription. bHLH, Basic Helix-Loop-Helix family | 93 | –0.257 | –0.188 | –0.243 | –0.163 | –0.112 | –0.123 |
| Positive coexpression | ||||||||
| 27.1 | RNA.processing | 254 | 0.110 | 0.181 | 0.163 | 0.033 | 0.127 | 0.186 |
| 27.1.2 | RNA.processing.RNA helicase | 29 | 0.261 | 0.398 | 0.285 | 0.222 | 0.335 | 0.198 |
| 29.2 | protein.synthesis | 515 | 0.132 | 0.200 | 0.175 | 0.194 | 0.260 | 0.194 |
| 29.2.1 | 371 | 0.085 | 0.164 | 0.126 | 0.158 | 0.254 | 0.173 | |
| 29.2.1.2 | 236 | 0.202 | 0.294 | 0.333 | 0.329 | 0.433 | 0.307 | |
| 29.2.1.2.1 | protein.synthesis.ribosomal protein.eukaryotic.40S subunit | 88 | 0.185 | 0.272 | 0.302 | 0.295 | 0.413 | 0.291 |
| 29.2.1.2.2 | protein.synthesis.ribosomal protein.eukaryotic.60S subunit | 148 | 0.212 | 0.307 | 0.352 | 0.349 | 0.445 | 0.316 |
| 29.2.2.50 | protein.synthesis.misc ribosomal protein.BRIX | 6 | 0.733 | 0.723 | 0.874 | 0.731 | 0.818 | 0.826 |
| 29.2.3 | protein.synthesis.initiation | 84 | 0.282 | 0.356 | 0.392 | 0.299 | 0.311 | 0.278 |
Average Spearman’s rank correlation to REIL2 of each reported bin. Bold text indicates P values < 0.001.
The A. thaliana Orthologs of the Cytosolic Maturation Machinery of the 60S Ribosomal Subunit Show Conserved Coexpression
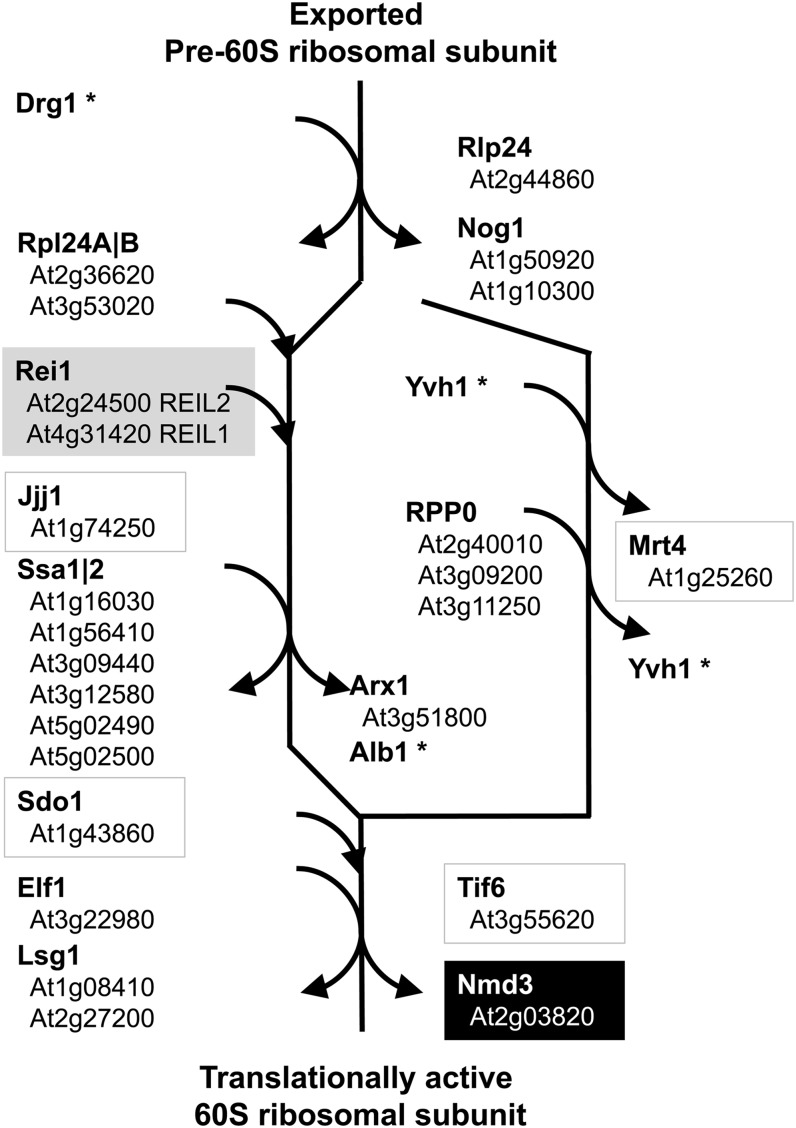
In yeast, rei1, the homolog of A. thaliana REIL, has a well-characterized function as part of the cytosolic maturation machinery of the 60S ribosomal subunit (Lo et al., 2010). Most of the yeast genes involved in this process had A. thaliana orthologs, which were, previous to our study, not annotated. We show the currently suggested scheme of cytosolic 60S maturation in yeast and the results of our homology searches (Fig. 6).
Figure 6.
Mapping of A. thaliana orthologs to the scheme of the currently known cytosolic maturation machinery of the 60S large ribosomal subunit (Lo et al., 2010). Note that the ortholog of nmd3 was robustly coexpressed with REIL2 both under cold and under temperature control (black box). The other boxed orthologs were robustly coexpressed with REIL2 either under cold control or under temperature control. An asterisk indicates yeast genes without currently known A. thaliana orthologs.
The yeast genes ATPase family gene2, nucleolar G-protein gene1, rlp24, arx1, yeast vaccinia virus VH1 homolog, mRNA Turnover4, elongation factor-like1, tif6, large-subunit GTPase1, and the previously mentioned nmd3 were robustly coexpressed with yeast rei1 both under temperature and cold control (Supplemental Table S4). This coexpression pattern was stringently conserved in the A. thaliana nmd3 ortholog At2g03820 (Fig. 6). Moreover, the tif6, Shwachman-Bodian-Diamond syndrome protein ortholog1, and mRNA Turnover4 orthologs of A. thaliana were robustly coexpressed with REIL2 either under cold or under temperature control (Fig. 6; Supplemental Table S4).
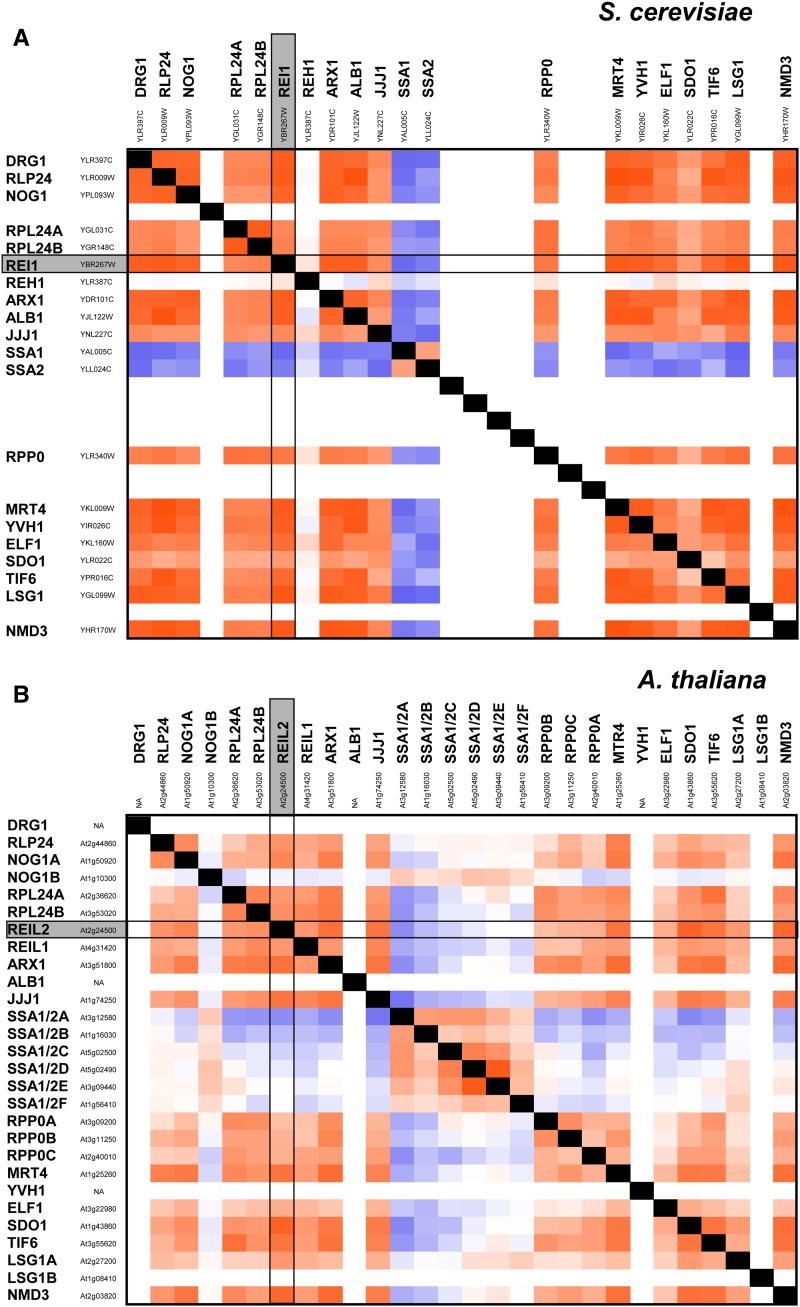
The comparison of the complete correlation matrix of the yeast genes involved in cytosolic 60S ribosomal maturation to the complete correlation matrix of their A. thaliana homologs demonstrated further conservation of the coexpression patterns (Fig. 7). Instead of considering only the top-scoring significant correlations, we compared the averages across the respective independent experiments so as to also evaluate conserved absence of coexpression. The temperature- and cold-controlled correlation matrices of yeast were almost identical (Fig. 7; Supplemental Fig. S10). Most of the yeast genes were positively correlated, except for the negatively correlated stress-seventy subfamily A gene1 (ssa1) and ssa2 and the noncorrelated reh1 gene.
Figure 7.
Comparison of the coexpression matrix of the yeast 60S cytosolic maturation machinery to the coexpression matrix of A. thaliana paralogs. A, Yeast coexpression matrix under temperature control. B, A. thaliana coexpression matrix under temperature control. Note that average Spearman correlation coefficients (r) were calculated using independent transcript profiling experiments. The color scale represents positive coexpression with a maximum of +1.0 coded by red, with a minimal coexpression of –1.0 coded by blue and with white representing 0.0, i.e. the absence of coexpression. Empty cells were introduced in cases of gene families and for missing transcriptome data. Thirty-nine percent of the correlation coefficients match between A. thaliana and yeast with a deviation of r less than 0.250.
The equivalent A. thaliana coexpression matrices were highly similar to the yeast matrices (Fig. 7; Supplemental Fig. S10). Only the coexpression of one of the two A. thaliana nucleolar G-protein gene1 paralogs, namely At1g10300, and four of the six ssa1/ssa2 paralogs, At5g02500, At5g02490, At3g09440, and At1g56410, did not match to their yeast homologs. As judged by the average correlation coefficients, the A. thaliana genes were less tightly intercorrelated than the yeast genes.
The coexpression pattern of A. thaliana REIL2 and REIL1 was similar to the patterns of rei1 and reh1 in yeast (Fig. 7). In yeast, rei1 was strongly coexpressed, whereas reh1 was not. In A. thaliana, REIL1 correlation was attenuated compared with REIL2 and did not pass the stringent criteria of our preceding coexpression analysis. This was expected according to our initial test, which showed the absence of a significant temperature response of REIL1 transcription.
REIL1 Complements the Growth Defect of the Yeast Δrei1 Deletion Mutant
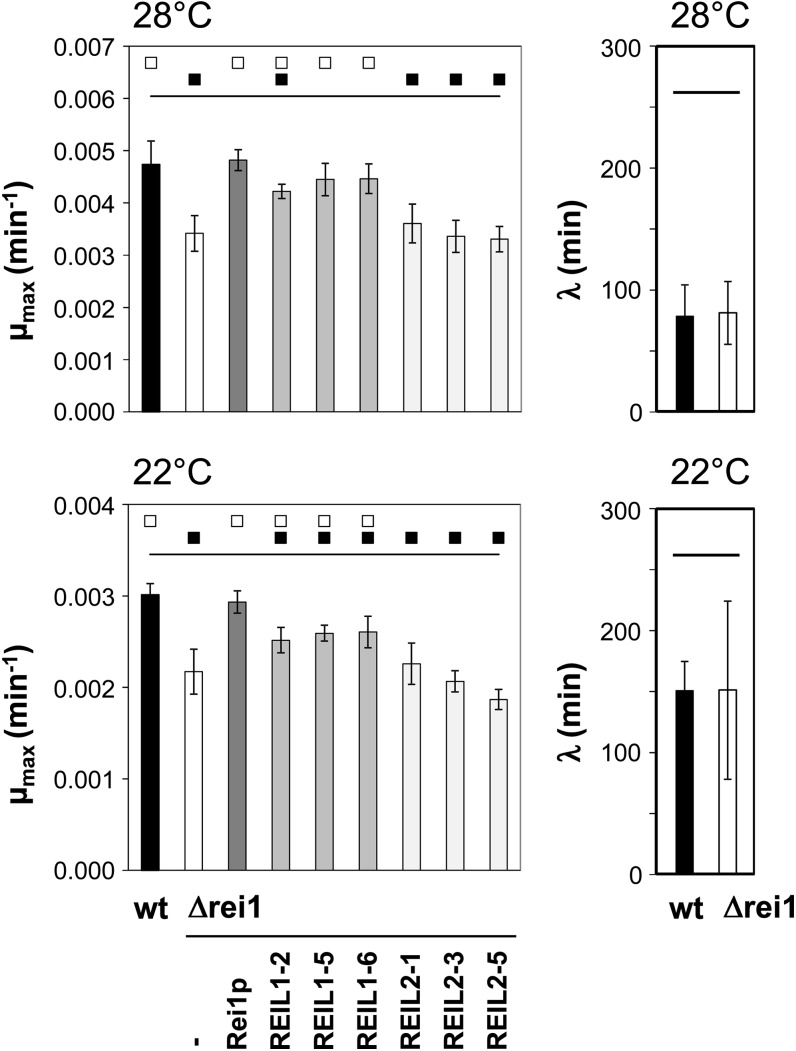
To test complementation, we established a temperature-controlled, liquid culture, microtiter plate reader assay set to 28°C and 22°C. The Δrei1 deletion mutant did not differ significantly from the BY4742 (S288C) wild type in regard to cell size and cell density of exponentially grown cultures adjusted to an optical density at 595 nm (OD595) of 1.0. The respective populations of wild-type cells had 1.4 107 ± 0.1 107 cells mL–1 and an average cell size of 4.9 ± 0.3 µm. The Δrei1 deletion mutant had a cell density of 1.2 107 ± 0.2 107 cells mL–1 and 5.1 ± 0.4 µm average size. As a consequence, we recorded OD595 as a comparable measure for mutant and wild-type growth. We tested the lag phase and the maximal growth rate from high-resolution growth kinetics as potential indicators for complementation of the Δrei1 growth deficit by heterologous expression of the full-length REIL1 or REIL2, compared with homologous expression of yeast Rei1p (Supplemental Fig. S11). The yeast Δrei1 deletion mutant (Iwase and Toh-e, 2004; Lebreton et al., 2006) and the wild type did not differ in regard to the lag phase. The lag phases of the wild type and the mutant were approximately 78 ± 26 min at 28°C and almost doubled to 150 ± 24 min at 22°C (Fig. 8). By contrast, the maximum relative growth rate of the Δrei1 deletion mutant was significantly reduced 0.72-fold compared with the wild type at both temperatures. Upon prolonged cultivation at 22°C or 28°C, the Δrei1 deletion mutant reached identical or even slightly higher cell densities than the wild type. The wild type entered a death phase following the stationary phase. By contrast, the Δrei1 mutant still increased OD595, however, at a low rate (Supplemental Fig. S11). This phenomenon was not reversed to the wild type by expression of yeast Rei1p and rendered simple colony complementation assays on solid media difficult to score. In conclusion, the maximum relative growth rate in liquid culture was the best available measure to test complementation of the Δrei1 mutant phenotype.
Figure 8.
Complementation of the growth phenotype of the yeast Δrei1 mutant by heterologous expression of REIL1 or REIL2. The growth phenotype was characterized by calculations of the maximum relative growth rate (µmax) and the lag phase (λ) from semilogarithmic plots of growth curves (compare with Supplemental Fig. S11). The complementation is compared at suboptimal temperatures, 28°C and 22°C, to the BY4742 wild type (wt, black bars), the yeast Δrei1 mutant (–, white bars), and the yeast Δrei1 mutant complemented by homologous expression of yeast rei1 (Rei1p, dark gray bars). Note that the white squares at the top of each graph indicate significant differences compared with the Δrei1 mutant, i.e. complementation. Black squares indicate fully or partially maintained significant differences compared with the wild type. Three independently transformed strains expressing REIL1 or REIL2 were tested (P < 0.01, n = 16–32).
The expression of yeast Rei1p and of REIL1 but not the expression of REIL2 complemented robustly the Δrei1 growth deficit at suboptimal temperatures (Fig. 8). In detail, expression of REIL1 restored on average 92% of the wild-type maximum relative growth rate at 28°C and 85% at 22°C. Heterologous expression of REIL1 was, thus, almost as efficient compared with the approximately full complementation by expression of the endogenous yeast Rei1p.
DISCUSSION
The Link of A. thaliana REIL Proteins to Cold Stress Physiology
To cope with cold stress, A. thaliana can cold acclimate. Cold acclimation activates metabolic and transcriptional changes (Hannah et al., 2005; Kaplan et al., 2007; Guy et al., 2008), which allow leaves that were generated under optimal temperature conditions to survive and successfully function in suboptimal temperature conditions. Much is known about the molecular basis and transcriptional regulation of plant cold acclimation (Fujita et al., 2006; Thomashow, 2010), while the developmental programming that leads to the production of new leaves with a specific cold morphology only recently moved into the focus of attention (Gorsuch et al., 2010a, 2010b).
We present evidence that the A. thaliana REIL paralogs are required at two checkpoints of leaf development in the cold. First, the reil1-1 reil2-1 double mutant arrests development in the cold prior to the cell proliferation of the nascent leaf (Figs. 2 and 3). The presence of either REIL1 or REIL2 is required for cell proliferation in the cold but not at optimal temperature. The developmental arrest of the reil1-1 reil2-1 double mutant appears to be similar to the growth arrest of the bypass mutant bps1-2 (Van Norman et al., 2011). The bps1-2 mutant arrests at 16°C, also prior to the emergence of the first rosette leaves. The function of BYPASS1, however, appears to be linked to a still unknown root-derived signal, which is required for general leaf development. Other than the reil1-1 reil2-1 double mutant, bps1-2 is affected both at suboptimal and at optimal growth temperatures.
Second, the allelic reil2 mutants indicate the existence of a subsequent developmental checkpoint in the cold during early leaf development (Figs. 3 and 4). REIL2 but not REIL1 is required to pass this checkpoint and to maintain normal leaf growth in the cold. The loss of REIL2 function causes small, spoon-shaped leaves and an arrest of leaf growth. REIL2 is only required during vegetative growth in the cold. A so-far unknown signal, which coincides with the appearance of inflorescences in the cold (Fig. 5), overrides the REIL2-mediated arrest or allows REIL1 to take over the function of REIL2. A similar phenomenon appears to be associated with a shift to optimal temperatures (Figs. 4 and 5).
The Conservation of REIL Function in A. thaliana
The A. thaliana REIL proteins are a striking example of functional conservation between plant and yeast orthologs. Two paralogs with high sequence homology exist in each species. Loss of one paralog, namely yeast Rei1p or A. thaliana REIL2, causes a conditional growth defect in the cold. Loss of the second paralog alone appears to be irrelevant for the cold response. But the loss of both paralogs shows that REIL proteins are essential for the growth at even slightly suboptimal temperatures.
We currently interpret these matching observations as a case of conserved function. We are well aware of the fact that a growth defect of a unicellular organism, such as yeast, and a biphasic arrest of the development and growth of the multicellular leaf organ will likely share basic aspects but will also certainly involve fundamentally different mechanistic details. We also caution that the conserved functional context may not necessarily be the result of a phylogenetic conservation. As we currently lack evidence from other species, the conserved function may also result from convergent evolution. The main indicators of convergence are the clearly independent gene duplications in the course of yeast and A. thaliana phylogeny (Fig. 1B; Supplemental Fig. S3).
The Association between A. thaliana REIL Proteins and Eukaryotic Ribosomal Maturation
In this initial study on the A. thaliana REIL proteins we decided to explore those molecular aspects that are shared between A. thaliana and yeast. So far, the annotation of A. thaliana REIL proteins was based on the presence of zinc finger domains. A. thaliana REIL proteins may have other functions than the initially inferred transcriptional regulation, not least because the small fraction of evolutionary conserved zinc finger proteins to which Rei1p, Reh1p, and the A. thaliana REIL proteins belong comprise a high number of RNA-interacting proteins (Englbrecht et al., 2004).
A first link of A. thaliana REIL proteins to ribosomal function was indicated by the pointed-leaves phenotype and the slight growth retardation of the reil1-1 reil2-1 double mutant at optimal temperature. The first described pointed-leaves mutation pfl1 was an allele of the ribosomal RIBOSOMAL PROTEIN OF THE SMALL SUBUNIT18A (RPS18A) gene. The pfl1 mutation changes the shape of the lamina of juvenile leaves to be pointed (Van Lijsebettens et al., 1994). Subsequently, mutations of several other structural ribosomal genes, e.g. RPS6A, RPS13A, RPS21B, RPS24B, RPL4D, RPL5A, RPL5B, RPL7B, RPL10aB, RPL18C, RPL28A, and RPL39C, were found to cause similar developmental and growth phenotypes. The high number ribosomal genes causing similar effects clearly indicate a link between eukaryotic ribosomes and leaf development (Horiguchi et al., 2011).
Because the yeast Rei1p also had a well-described role in eukaryotic ribosomal maturation, we focused on testing the A. thaliana REIL proteins for functional conservation. Our coexpression analysis led us from the proof of enrichment of the functional categories, translation initiation, and protein synthesis among the coexpressed genes (Tables I and II) to the discovery that REIL2 and rei1 are specifically coexpressed with genes that transiently associate with eukaryotic ribosomes at all main steps of ribosomal maturation (Supplemental Table S3). The genes with conserved coexpression were linked predominantly to the maturation machinery of eukaryotic ribosomes or to structural ribosomal genes, namely RPS2, RPS4, RPL27, and RPL35A (Supplemental Table S3). The list contained six 90S preribosome proteins, U three protein7 (Utp7p), Ribosomal RNA processing protein9 (Rrp9p), Pre-mRNA processing protein4, Nucleolar protein9, Nucleolar complex-associated protein4 (Noc4p), and Utp15p. Specifically, Utp7p is part of the U3 protein complex required for transcription 90S preribosomal assembly complex (Henras et al., 2008). Rrp9p belongs to the U3 small nucleolar ribonucleoprotein. U3 small nucleolar ribonucleoprotein is, next to the t-UTP complex, a second particle required for 90S assembly (Henras et al., 2008). Four other members of the short list, namely Core interacting component1, Nuclear import protein7, Suppressor of ste4(sterile 4) protein1, and Suppressor of ste4 protein2, are associated with the 66S preribosomal subunit that is downstream of the 90S particle and precedes the nuclear pre-60S subunit (Horsey et al., 2004). Furthermore, Utp14p and Utp23p and the previously mentioned Rrp9p, Noc4p, Utp7p, and Utp15p are part of the 40S processome that precedes the nuclear pre-40S particle (Dragon et al., 2002). Noc4p is, in addition, part of the 40S preribosome export complex (Henras et al., 2008). Noc2p belongs to the Noc1p-Noc2p and Noc3p-Noc2p complexes that are required for the intranuclear trafficking of the preribosomes (Milkereit et al., 2001). Ribosome assembly protein4 and Severe depolymerization of actin protein1 of the short list both need to be removed from the pre-60S particle prior to export into the cytosol. The absence of these proteins causes nuclear retention of the pre-60S particles (Henras et al., 2008; Panse and Johnson, 2010). We would like to highlight the fact that REIL2 is coexpressed with the Nmd3 and Tif6 orthologs At2g03820 and At3g55620. The Nmd3p and Tif6p of yeast are specifically involved in the final steps of the cytosolic ribosome maturation process. Nmd3p is required for nuclear export of the 60S preribosome and one of the last factors to be released in the cytosol before the 60S subunit becomes translationally active. Nmd3p is also discussed to perform structural proofreading, a process that is thought to ensure that only correctly assembled subunits are exported from the nucleus and rendered active (Johnson et al., 2002; Lo et al., 2010). Tif6p acts as an antiassociation factor for the yeast ribosome as was recently shown by crystallography (Gartmann et al., 2010; Klinge et al., 2011). As a consequence, Tif6p needs to be released from the pre-60S subunit during the final steps of cytosolic maturation. Based on this knowledge, we finally made the observation that the genes of the cytosolic ribosomal maturation machinery of yeast, including rei1, and of their A. thaliana orthologs, including REIL2 (Fig. 6), have highly similar coexpression matrices (Fig. 7; Supplemental Table S4).
To further support the so-far circumstantial evidence of our coexpression analysis, which indicated that A. thaliana REIL proteins may be essential for ribosomal maturation in the cold, we initially considered tests for changes of the composition of ribosomal RNAs and respective precursors. We so-far rejected this approach because mutations that impair eukaryotic ribosome biogenesis in yeast typically do not lead to a massive accumulation of the corresponding pre-ribosomal RNAs (Henras et al., 2008). Therefore, respective observations in A. thaliana will be difficult to interpret and compare. Instead, we demonstrated functional complementation of the Δrei1 mutant growth phenotype by REIL1, which is the strongest evidence so far for functional conservation. Under the conditions currently tested, A. thaliana REIL1 can still almost completely substitute yeast Rei1p function. By contrast, REIL2 appears to be mutated beyond full complementation, a fact to be expected of an isoform that plays a plant-specific role in leaf development at low temperatures.
CONCLUSION
We demonstrate that A. thaliana activates a conditional developmental program that is required for leaf development and growth in the cold. This program involves REIL proteins that are conserved parts of the maturation machinery of eukaryotic ribosomes. We see our study as a first step toward a deeper understanding as to how a housekeeping process such as eukaryotic ribosomal maturation may be linked to the developmental processes of plant cell differentiation or leaf organ growth and as to how this process may be modified by low temperatures. Even mildly lowered temperatures inherently slow down biological processes. Our germination assays, for example, clearly demonstrate that the speed of germination is about halved by a temperature shift from 20°C to 10°C. Germination is even further delayed when one or both REIL proteins are missing. The deceleration of biological processes is generally explained by the cold-induced reduction of enzyme activities. But those processes that involve the formation or the dissociation of protein complexes, such as the assembly of a ribosome, will also be slower. We currently think that the release of maturation factors, such as Nmd3p and Tif6p, may be severely delayed by low temperatures and that the role of REIL proteins may be the facilitation of these dissociation processes at low temperatures.
Our investigations of REIL function are ongoing and will shed new light on the relationship between housekeeping genes and plant development, an interaction that was found to be more important and wide spread than previously thought (Tsukaya et al., 2013). We hope to contribute novel aspects to the recently reviewed and systematically investigated interaction of the plant ribosome with leaf development (Schippers and Mueller-Roeber, 2010; Horiguchi et al., 2011, 2012).
MATERIALS AND METHODS
Identification of T-DNA Insertion Mutants of REIL1 and REIL2
Seeds of the T-DNA insertion mutants SALK_090487 (reil1-1) and SALK_040068 (reil2-2) were obtained through the Nottingham European Arabidopsis Stock Centre (Scholl et al., 2000). The T-DNA mutant GK_166C10 (reil2-1) was generated through the GABI-Kat program (Rosso et al., 2003) and was provided by Bernd Weisshaar (Max Planck Institute for Plant Breeding Research). Homozygous lines were generated in this study and confirmed by PCR amplification of genomic DNA with reil gene-specific and T-DNA-specific oligonucleotides (Supplemental Table S5). PCR was performed using 36 cycles of 15-s denaturation at 94°C followed by 30-s annealing at 55°C and 2-min elongation at 72°C. The T-DNA-specific oligonucleotides were outward-directed left-border primers LBb1 (SALK) and GKo8409 (GABI-Kat). The REIL2 (At2g24500) and REIL1 (At4g314200) gene-specific primers were positioned 5′ and 3′ relative to the approximate T-DNA insertion site reported by the seed stock providers. The 5′-oligonucleotide primers at2g24500for and at4g314200for were positioned at the start codons, and the 3′-oligonucleotide primers at2g24500rev and at4g314200rev were placed at the stop codons. The exact T-DNA insertion sites of each line were determined by sequencing of the left-border insertion site. In agreement with the genetic background of the mutants, Col-0 was used as wild-type control throughout this study.
Total RNA for reverse transcription PCR (Supplemental Fig. S4) was extracted with the RNeasy Plant Mini Kit (Qiagen) according to manufacturer’s instructions. The complementary DNA (cDNA) was amplified from total RNA using oligo(dT)12–18 primers (Invitrogen Life Technologies) and the SuperScript II Reverse Transcriptase (Invitrogen Life Technologies). The PCR reactions were performed with the primer pairs at2g24500for and at2g24500rev (REIL2) and at4g314200for and at4g314200rev (REIL1). The actin2 transcript was amplified by primers act2_for and act2_rev (Supplemental Table S5).
Sequence Analysis and Comparison Tools
Sequence searches and analyses were performed using the National Center for Biotechnology Information protein and nucleotide sequence repository, the National Center for Biotechnology Information BLAST resources (http://blast.ncbi.nlm.nih.gov/Blast.cgi; Altschul et al., 1997), and the Bioinformatics Toolkit (http://toolkit.tuebingen.mpg.de; Biegert et al., 2006). Protein sequences were either taken from BLASTp results or manually translated from retrieved complementary DNA or genomic sequences. Phylogenetic conservation analysis between Arabidopsis thaliana and yeast (Saccharomyces cerevisiae) was according to the InParanoid (Version 7) annotations of eukaryotic COGs (http://inparanoid.sbc.su.se/cgi-bin/index.cgi; O’Brien et al., 2005; Östlund et al., 2010). Multiple sequence alignments for phylogenic tree analysis were generated by ClustalX (Thompson et al., 1997) with gap-opening penalty set to 15 and gap extension set to 0.3. PHYLIP version 3.68 (http://evolution.genetics.washington.edu/phylip.html) was applied for inference of phylogenic trees using the maximum-likelihood method, bootstrapping, and the display of a rooted version of the resulting consensus tree (Felsenstein, 1989).
Seed Batches, Germination, and in Vitro Growth Assays
At least two independent seed batches of each homozygous genotype were generated using plant populations that completed a full life cycle from germination to seed setting and harvest under optimum temperature conditions. All seed batches were stored at 4°C for at least 1 month prior to the assessments of germination and growth behavior. Seeds were sterilized at room temperature with sodium hypochlorite and germinated without additional stratification using one-half-strength Murashige and Skoog-Agar plates, 0.8% (w/v) agar, and 2% (w/v) Suc (Murashige and Skoog, 1962). Seeds for germination assays were plated equally spaced in batches of approximately 80 per mutant line or the Col-0 wild type. The assays were performed in controlled-environment chambers under sterile conditions with a transparent lid. Germination assays were performed in short days with an 8-h/16-h day/night cycle at 20°C to approximate optimum growth conditions or with an 8-h/16-h day/night cycle at 10°C. Strict maintenance of cold conditions was required for a full suppression of rosette leaf emergence from the reil1-1 reil2-1 double mutant. The germination of A. thaliana seedlings, specifically the appearance of the radicle, cotyledons, and first rosette leaf, as well as the general plant development was staged according to Boyes et al. (2001). Emergence of the first leaf was scored per plate assay and calculated as a percentage relative to the final number of fully germinated seeds, i.e. the number of seeds that had a radicle and cotyledons at the end of the assay, i.e. 15 d at 20°C or 31 d in the cold. Seeds that did not germinate were not scored.
Vertical plate growth assays were performed using one-half-strength Murashige and Skoog-Agar medium containing 2% (w/v) Suc. Germinated seeds were transferred to the vertical plates at 5 d after germination and kept up to 14 d after germination in a 16-h/8-h day/night cycle at 20°C/18°C and 120 µE m–2 s–1 during the day. Parallel to these control conditions plantlets were exposed to high light (190 µE m–2 s–1) or to low light (65 µE m–2 s–1), to increased osmolarity, i.e. mannitol (150 mm) or NaCl (100 mm), to Glc (1%, w/v), to the absence of Suc from the medium, and to the presence of zeatin (1 µm), abscisic acid (10 µm), or the oxidative stress-inducing agent paraquat (0.05 and 0.10 µm). The length of the primary root, i.e. the length from the root/hypocotyl constriction to the tip, was determined manually using scaled microphotographs.
20°C and 10°C Growth Conditions on Soil
Optimal Growth Condition (20°C Condition) for Mutant Line Maintenance
After 2 weeks of sterile cultivation on Murashige and Skoog-Agar plates (compare with paragraph above), seedlings were transferred to soil. Plants were transferred at stage approximately 1.02 to 1.03 into pots of 10-cm diameter with A. thaliana soil (Stender AG). Acclimation to soil was in short days with an 8-h/16-h day/night cycle at 20°C/16°C and 100 to 150 µmol m–2 s–1 light intensity using a controlled-environment chamber. After 2 weeks, the plantlets reached stage 1.05 to 1.06 and were continued until seed harvest in the greenhouse with 16-h light at 20°C to 21°C, approximately 150 µmol m–2 s–1 light intensity, and an 8-h night at 17°C to 18°C.
Constant Growth in the Cold (10°C Condition) and Temperature Shift to 20°C
Plants that were germinated and kept in constant 10°C cold conditions (compare with paragraph above) required 4 weeks of sterile cultivation until transfer to soil at transplanting stage approximately 1.02 to 1.03. Acclimation to soil was 2 weeks in long days with a 16-h/8-h day/night cycle at 10°C/8°C and approximately 100 m–2 s–1 light intensity using a controlled-environment chamber. Wild-type plantlets reached stage 1.05 to 1.06 after 2 weeks. After these 2 weeks, plants were either continued in the controlled environment at 10°C or transferred to the green house with optimum 20°C conditions, namely 16-h light at 20°C to 21°C, approximately 150 m–2 s–1 minimum light intensity, and an 8-h night at 17°C to 18°C.
Morphometric Analyses
The A. thaliana rosettes were digitally photographed at 4,288- × 2,848-pixel resolution using an SLR Nikon D5000 camera, an AF-S DX Nikkor 18- to-55 mm, f/3.5–5.6 VR lens, and a 1.0-mm resolution scale on each photograph. Morphometric analyses were performed in weekly intervals starting at 2 weeks in the cold. Five to 10 replicate plants of each genotype were cultivated in parallel using a Latin square cultivation array. Number of rosette leaves greater than 1 mm, planar rosette leaf area (mm2), convex hull of the rosette (mm2), and surface coverage (%) of the plant rosettes were determined manually supervised using Adobe Photoshop CS4 Extended (Version 11.0) and normalized to the scale within each photograph. We followed previous recommendations for automated morphometric analyses of the A. thaliana rosette (Jansen et al., 2009; Walter et al., 2009), where the surface coverage of a rosette is defined as the proportion (%) of planar leaf area inside the rosette’s convex hull and where the convex hull is defined as the area inside the shortest line around a plant rosette.
Gene Annotations and Functional Overrepresentation Analysis
Yeast gene names, gene identifiers, and functional annotations were from the Saccharomyces Genome Database (http://www.yeastgenome.org/gene_list.shtml). A. thaliana gene names and identifiers were according to The Arabidopsis Information Resource (http://www.arabidopsis.org) with functional classifications obtained from MapMan (Usadel et al., 2005). Overrepresentation analysis using the MapMan functional bin annotations and Benjamini-Hochberg-corrected Wilcoxon rank-sum significance was as described previously (Usadel et al., 2005). Robustly overrepresented functional categories were obtained by intersection applying a common P < 0.05 threshold to independently generated and processed transcript data sets (compare with below).
Analysis of COGs
Phylogenetic conservation was judged according to the InParanoid 7 resource on conserved genes (O’Brien et al., 2005; Östlund et al., 2010). This database annotated 8,046 pairs of orthologs between yeast and A. thaliana that were grouped into 2,045 COGs. Seven thousand two hundred eight orthologous gene pairs were present in the selected yeast and A. thaliana transcript studies. We thus compared the REIL2 coexpression with 5,026 A. thaliana genes to the rei1 coexpression with their respective 2,378 phylogenetically conserved yeast orthologs.
Homologs of the human MATERNAL EMBRYONIC LEUCINE ZIPPER KINASE (MELK), MYELOBLASTOSIS FAMILY TRANSCRIPTION FACTOR-RELATED PROTEIN B (B-MYB), APOPTOSIS SIGNAL-RELATING KINASE1 (ASK1; MAP3K5) genes (Seong et al., 2002, 2003, 2011) were not present among these pairs of A. thaliana and yeast orthologs reported by the InParanoid 7 resource. The human proteins MELK and ASK1 were furthermore not conserved in A. thaliana (O’Brien et al., 2005; Östlund et al., 2010). Only the human B-MYB gene was part of a large and nondifferentiated COG, which comprised three human paralogs and 25 members of the 188 A. thaliana MYB domain proteins and MYB-related transcription factors. Without clear orthologs of MELK, B-MYB, and ASK1 in A. thaliana, we did not attempt a comparative coexpression analysis of REIL and ZRP9/ZNF622.
Gene Expression Data Sets
Gene expression studies for the analysis of cold- and temperature-controlled coexpression modules were selected according to a best match of the respective experimental descriptions. Genome-wide time series analyses to cold-stress cues were searched, starting with previous comparative reports on the A. thaliana cold response (Bieniawska et al., 2008) and general transcriptome archiving resources (Zimmermann et al., 2004; Hruz et al., 2008). Three studies that investigated the transcriptional response of REIL2 to cold and heat in illuminated rosette leaves under similar conditions were selected for coexpression analysis (Kaplan et al., 2007; Kilian et al., 2007; Caldana et al., 2011). These three studies comprised independent time series analyses following a temperature shift from optimal temperature to 4°C or to 32°C to 42°C heat. From these studies, we selected only the experiments that were performed in the light. All experiments used complete rosettes of nonacclimated plants and were controlled by diurnal time series at 20°C to 21°C optimum temperature.
Equivalent transcriptome profiling experiments for the comparative coexpression analysis of yeast, were previously performed and reported by our laboratory (Strassburg et al., 2010; Walther et al., 2010). We selected two similar transcriptome analyses for robustness analysis (Gasch et al., 2000; Sahara et al., 2002).
The analysis of the transcriptional responses of REIL1 and REIL2 to a wide range of abiotic stresses was initiated with a single reference data set (Kilian et al., 2007), confirmed and complemented by independent experiments that were archived by the Genevestigator database for the meta-analysis of transcriptional profiles (Zimmermann et al., 2004; Hruz et al., 2008).
Comparative Coexpression Analysis
Only REIL2 was subjected to a systematic gene-targeted coexpression analysis. REIL1 transcript levels were not significantly changed under cold or heat stress and therefore not deemed suitable for a correlation-based coexpression analysis under the influence of these stress factors.
The normalized, preprocessed, and corrected transcriptome data published by each selected study were used. All time series data selected for this study were corrected by subtraction of logarithmic expression values of the respective temporal controls prior to coexpression analysis. This procedure was necessary to account for the known diurnal effects that are active in a photoautotrophic plant (Bieniawska et al., 2008). We applied the same correction procedure to the yeast gene expression data, which were profiled in batch culture.
The A. thaliana transcriptome data sets were (1) the rosette leaf data from the cold and heat subset of a global stress response compendium (Kilian et al., 2007) with correction by the general control experiment, (2) the data of a study on the cold stress response of A. thaliana rosettes with paralleled heat stress data submitted to the NASCArrays (Kaplan et al., 2007), and (3) part of the data of a combinatorial stress study on the interactions of temperature and light regimes in A. thaliana rosettes (Caldana et al., 2011). Of the latter, only the subset of illuminated samples was used. The 4°C cold stress data obtained at 85 µE m–2 sec–1 were corrected by the 21°C and 75 µE m–2 sec–1 set. The 32°C and 150 µE m–2 sec–1 heat stress data were corrected using the also available 21°C set at identical light intensity.
The transcriptome data of the budding yeast, were the previously published time series analyses following heat or cold stress performed by our laboratory (Strassburg et al., 2010; Walther et al., 2010) and combined from previously published studies on cold stress (Sahara et al., 2002) and a compendium of abiotic environmental stress responses (Gasch et al., 2000). Of the latter data set, we used the temperature shift from 37°C to 25°C and the continuous growth at various temperatures to complement the five time points after cold stress of Sahara et al. (2002) for the cold-controlled coexpression analysis. The general temperature control analysis was performed together with the 37°C heat shock from 25°C, the 37°C heat shock from various temperatures, and the mild 33°C heat shock experiments of Gasch et al. (2000).
Spearman rank correlations were calculated as a robust outlier-insensitive measure for the coexpression analysis (Steinhauser et al., 2004). The correlation coefficient was used throughout this study. This measure allowed the ranking of A. thaliana and yeast genes according to best positive, +1.0, or best negative, –1.0, coexpression with REIL2 and rei1, respectively. The transcriptome data sets were independently analyzed to allow the averaging across experiments or a more stringent robustness testing by intersection analysis (Sanchez et al., 2010).
Yeast Complementation Analysis
The yeast Δrei1 mutant, genotype BY4742, Mat a, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, YBR267w::kanMX4, and the parent strain BY4742, Mat a, his3Δ1, leu2Δ0, lys2Δ0, ura3Δ0, derived from yeast S288C (Winzeler et al., 1999) were obtained from EUROSCARF. Full-length complementary DNAs of rei1, REIL1, and REIL2 were cloned into the low-copy Gateway (Alberti et al., 2007) destination vector pAG425GPD-ccdB or into the integrating destination vector pAG305GPD-ccdB (Addgene plasmid 14154) and transformed into the yeast Δrei1 mutant. Positive transformed colonies were screened by complementation of the Leu autotrophy. The presence of the correct plasmid was confirmed by PCR. The presence of the Δrei1 mutant background was confirmed by resistance to 0.05 mg mL–1 kanamycin. The average cell size and cell density of cultures taken during exponential growth and adjusted to OD595 of 1.0 were analyzed by a Cellometer Auto M10 system (Nexcelom Bioscience) according to manufacturer’s instructions. The growth assays were performed using a temperature-controlled, automated microtiter plate reader system that was set to 28°C and 22°C, respectively. Lower operating temperatures were beyond system specifications. Yeast strains were precultured in liquid yeast peptone dextrose medium. All cultures were adjusted carefully prior to the growth assay to OD595 of 0.4 for the 28°C assay or to OD595 of 0.8 for the assay at 22°C. Kanamycin was omitted so as to allow the comparison to both the Δrei1 gene deletion mutant and the nonresistant wild-type genotype BY4742. Four microtiter plates with eight 200-µL cultures of each strain were assayed independently at each temperature. In total, the growth curves of 32 cultures of each yeast strain (eight replicates per plate) were recorded at 20-min intervals over 24-h periods (Supplemental Fig. S11). The natural logarithm (Ln) of Nt/N0 was plotted as a function of time, where N0 is the initial OD595 of each culture and Nt is the OD595 of the same culture at time t, assuming OD595 to be proportional to cell density (N). The maximum relative growth rate (µmax) was defined as the slope of the tangent at the inflection point of the semilogarithmic growth curve. The inflection point of this growth curve was found at the maximum of the first derivative function. The tangent was defined as the linear function Ln(Nt/N0) = µmaxt + b, where t is the time and b the y-axis intercept. The lag phase (λ) was defined as the x-axis intercept of this tangent and was calculated by λ = –b/µ, where Ln(Nt/N0) = 0. The unit of λ is min. The unit of µmax is min–1. Note that µmax and λ were calculated from the initial phases of the growth assays (Supplemental Fig. S11), avoiding the influence of the transition phase to stationary phase. The asymptote of the growth curve representing maximal cell density was not analyzed because of an unusual behavior of the Δrei1 mutant in stationary phase. Significance of changes compared with the BY4742 wild type or compared with the Δrei1 mutant was judged by Student’s t test. The complementation result was confirmed using the integrating destination vector pAG305GPD-ccdB (data not shown). Furthermore, we confirmed restoration of growth in the presence of kanamycine omitting the BY4742 wild type using only the Δrei1 mutant and the respective transformants with the pAG305GPD-ccdB constructs (data not shown).
Sequence data of this study were retrieved from The Arabidopsis Information Resource (http://www.arabidopsis.org/index.jsp), the Yeast Genome Database (http://www.yeastgenome.org/), or GenBank (http://www.ncbi.nlm.nih.gov/genbank) under the following accession numbers: REIL1 (At4g31420), REIL2 (At2g24500), rei1 (YBR267W), reh1 (YLR387C), ZRP9/ZNF622 (Q969S3), MELK (Q14680), B-MYB (P10244), and ASK1 (Q99683). The accession numbers, taxonomy, and protein codes of all Rei1p-like homologs investigated in the phylogenetic analysis of this study are listed in Supplemental Table S1. The accession numbers of yeast and A. thaliana genes analyzed in the coexpression study are listed in Supplemental Table S3. Note that we applied the yeast convention to report yeast proteins, genes, and mutants, e.g. Rei1p, rei1, and Δrei1, respectively.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Genomic and mRNA sequence alignment of the A. thaliana REIL genes and of orthologs from A. lyrata ssp. lyrata, T. halophila, and B. rapa ssp. pekinensis.
Supplemental Figure S2. Alignment of the REIL proteins of A. thaliana, A. lyrata ssp. lyrata, T. halophila, and B. rapa ssp. pekinensis.
Supplemental Figure S3. Phylogenic analysis of eukaryotic Rei1p-like orthologs.
Supplemental Figure S4. Reverse transcription PCR test for the absence of full-length transcripts from rosette leaves of the reil1-1, reil2-1, and reil2-2 mutants before (–flower) and after (+flower) appearance of flower buds at 10°C and at 20°C.
Supplemental Figure S5. Examples of the differential temperature control of rei1 and reh1 gene expression in yeast (Strassburg et al., 2010) compared with the temperature response of REIL1 and REIL2 transcript levels in A. thaliana (Caldana et al., 2011).
Supplemental Figure S6. Root length of reil mutants and the Col-0 wild type determined under control conditions using a vertical plate in vitro assay.
Supplemental Figure S7. Sensitivity of reil mutants and the Col-0 wild type to stress factors that are listed within the inserts of this figure.
Supplemental Figure S8. The gene sets that are positively coexpressed with REIL2 are enriched with genes that are conserved between A. thaliana and yeast.
Supplemental Figure S9. Comparative coexpression analysis of A. thaliana genes to REIL2 with the coexpression of their respective yeast orthologs with yeast rei1.
Supplemental Figure S10. Comparison of the coexpression matrix of the yeast 60S cytosolic maturation machinery to the coexpression matrix of A. thaliana paralogs.
Supplemental Figure S11. Representative growth curves, semilogarithmic plots, where the cell density is assumed to be proportional to OD595, and first derivative plot of the semilogarithmic growth curve.
Supplemental Table S1. List of eukaryotic rei1-like orthologs listed according to the abbreviations used in Supplemental Figure S3.
Supplemental Table S2. Germination rates of the reil single gene and double mutant lines compared with the Col-0 wild type at 20°C.
Supplemental Table S3. Table of A. thaliana genes that show a robust coexpression with REIL2 and a conserved coexpression of their yeast orthologs with rei1.
Supplemental Table S4. Coexpression table of the A. thaliana genes and their yeast paralogs, which are known to be functionally involved in the cytosolic maturation of the eukaryotic 60S large ribosomal subunit (compare with Fig. 6).
Supplemental Table S5. List of oligonucleotide primers used to characterize the reil1-1 (SALK_090486), reil2-1 (GK_166C10), and reil2-2 (SALK_040068) T-DNA insertion mutants.
Supplemental Document S1. List of detailed legends to Supplemental Figures S1–S11 and Supplemental Tables S1–S5.
Supplementary Material
Acknowledgments
We thank Lothar Willmitzer, Director of the department Molecular Physiology at the Max Planck Institute of Molecular Plant Physiology, for long-standing support, Dirk Walther, head of the Bioinformatics Infrastructure Group at the Max Planck Institute of Molecular Plant Physiology, for his kind support and advice in statistical and bioinformatic matters, and Weronika Ludwiczak, Max Planck Institute of Molecular Plant Physiology, for her support in the analysis of the specificity of the cold-induced growth defect of the A. thaliana reil mutants by analyzing the effect of diverse abiotic stresses.
Glossary
- COG
cluster of orthologs
- T-DNA
transfer DNA
- Col-0
ecotype Columbia
- OD595
optical density at 595 nm
- Col-0
ecotype Columbia-0
References
- Agarwal P, Arora R, Ray S, Singh AK, Singh VP, Takatsuji H, Kapoor S, Tyagi AK. (2007) Genome-wide identification of C2H2 zinc-finger gene family in rice and their phylogeny and expression analysis. Plant Mol Biol 65: 467–485 [DOI] [PubMed] [Google Scholar]
- Alberti S, Gitler AD, Lindquist S. (2007) A suite of Gateway cloning vectors for high-throughput genetic analysis in Saccharomyces cerevisiae. Yeast 24: 913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu U, Si K, Warner JR, Maitra U. (2001) The Saccharomyces cerevisiae TIF6 gene encoding translation initiation factor 6 is required for 60S ribosomal subunit biogenesis. Mol Cell Biol 21: 1453–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berná G, Robles P, Micol JL. (1999) A mutational analysis of leaf morphogenesis in Arabidopsis thaliana. Genetics 152: 729–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegert A, Mayer C, Remmert M, Söding J, Lupas AN. (2006) The MPI Bioinformatics Toolkit for protein sequence analysis. Nucleic Acids Res 34: W335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniawska Z, Espinoza C, Schlereth A, Sulpice R, Hincha DK, Hannah MA. (2008) Disruption of the Arabidopsis circadian clock is responsible for extensive variation in the cold-responsive transcriptome. Plant Physiol 147: 263–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm S, Frishman D, Mewes HW. (1997) Variations of the C2H2 zinc finger motif in the yeast genome and classification of yeast zinc finger proteins. Nucleic Acids Res 25: 2464–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J. (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldana C, Degenkolbe T, Cuadros-Inostroza A, Klie S, Sulpice R, Leisse A, Steinhauser D, Fernie AR, Willmitzer L, Hannah MA. (2011) High-density kinetic analysis of the metabolomic and transcriptomic response of Arabidopsis to eight environmental conditions. Plant J 67: 869–884 [DOI] [PubMed] [Google Scholar]
- Ciftci-Yilmaz S, Mittler R. (2008) The zinc finger network of plants. Cell Mol Life Sci 65: 1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoinet E, Jacquier A, Lutfalla G, Fromont-Racine M. (2007) The Hsp40 chaperone Jjj1 is required for the nucleo-cytoplasmic recycling of preribosomal factors in Saccharomyces cerevisiae. RNA 13: 1570–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragon F, Gallagher JEG, Compagnone-Post PA, Mitchell BM, Porwancher KA, Wehner KA, Wormsley S, Settlage RE, Shabanowitz J, Osheim Y, et al. (2002) A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417: 967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. (2006) Yeasts illustrate the molecular mechanisms of eukaryotic genome evolution. Trends Genet 22: 375–387 [DOI] [PubMed] [Google Scholar]
- Dujon B, Sherman D, Fischer G, Durrens P, Casaregola S, Lafontaine I, De Montigny J, Marck C, Neuvéglise C, Talla E, et al. (2004) Genome evolution in yeasts. Nature 430: 35–44 [DOI] [PubMed] [Google Scholar]
- Englbrecht CC, Schoof H, Böhm S. (2004) Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genomics 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (1989) PHYLIP - Phylogeny Inference Package (version 3.2). Cladistics 5: 164–166 [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9: 436–442 [DOI] [PubMed] [Google Scholar]
- Gamsjaeger R, Liew CK, Loughlin FE, Crossley M, Mackay JP. (2007) Sticky fingers: zinc-fingers as protein-recognition motifs. Trends Biochem Sci 32: 63–70 [DOI] [PubMed] [Google Scholar]
- Gartmann M, Blau M, Armache JP, Mielke T, Topf M, Beckmann R. (2010) Mechanism of eIF6-mediated inhibition of ribosomal subunit joining. J Biol Chem 285: 14848–14851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. (2000) Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell 11: 4241–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsuch PA, Pandey S, Atkin OK. (2010a) Thermal de-acclimation: how permanent are leaf phenotypes when cold-acclimated plants experience warming? Plant Cell Environ 33: 1124–1137 [DOI] [PubMed] [Google Scholar]
- Gorsuch PA, Pandey S, Atkin OK. (2010b) Temporal heterogeneity of cold acclimation phenotypes in Arabidopsis leaves. Plant Cell Environ 33: 244–258 [DOI] [PubMed] [Google Scholar]
- Guy CL, Kaplan F, Kopka J, Selbig J, Hincha DK. (2008) Metabolomics of temperature stress. Physiol Plant 132: 220–235 [DOI] [PubMed] [Google Scholar]
- Hall TM. (2005) Multiple modes of RNA recognition by zinc finger proteins. Curr Opin Struct Biol 15: 367–373 [DOI] [PubMed] [Google Scholar]
- Hannah MA, Heyer AG, Hincha DK. (2005) A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet 1: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henras AK, Soudet J, Gérus M, Lebaron S, Caizergues-Ferrer M, Mougin A, Henry Y. (2008) The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol Life Sci 65: 2334–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G, Mollá-Morales A, Pérez-Pérez JM, Kojima K, Robles P, Ponce MR, Micol JL, Tsukaya H. (2011) Differential contributions of ribosomal protein genes to Arabidopsis thaliana leaf development. Plant J 65: 724–736 [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Van Lijsebettens M, Candela H, Micol JL, Tsukaya H. (2012) Ribosomes and translation in plant developmental control. Plant Sci 191-192: 24–34 [DOI] [PubMed] [Google Scholar]
- Horsey EW, Jakovljevic J, Miles TD, Harnpicharnchai P, Woolford JL., Jr (2004) Role of the yeast Rrp1 protein in the dynamics of pre-ribosome maturation. RNA 10: 813–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. (2008) Genevestigator v3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinforma 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung NJ, Johnson AW. (2006) Nuclear recycling of the pre-60S ribosomal subunit-associated factor Arx1 depends on Rei1 in Saccharomyces cerevisiae. Mol Cell Biol 26: 3718–3727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M, Toh-e A. (2004) Ybr267w is a new cytoplasmic protein belonging to the mitotic signaling network of Saccharomyces cerevisiae. Cell Struct Funct 29: 1–15 [DOI] [PubMed] [Google Scholar]
- Jansen M, Gilmer F, Biskup B, Nagel KA, Rascher U, Fischbach A, Briem S, Dreissen G, Tittmann S, Braun S, et al. (2009) Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants. Funct Plant Biol 36: 902–914 [DOI] [PubMed] [Google Scholar]
- Johnson AW, Lund E, Dahlberg J. (2002) Nuclear export of ribosomal subunits. Trends Biochem Sci 27: 580–585 [DOI] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Sung DY, Zhao W, Popp M, Porat R, Guy CL. (2007) Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J 50: 967–981 [DOI] [PubMed] [Google Scholar]
- Kelly SM, Pabit SA, Kitchen CM, Guo P, Marfatia KA, Murphy TJ, Corbett AH, Berland KM. (2007) Recognition of polyadenosine RNA by zinc finger proteins. Proc Natl Acad Sci USA 104: 12306–12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K. (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50: 347–363 [DOI] [PubMed] [Google Scholar]
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Arpagaus S, Ban N. (2011) Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334: 941–948 [DOI] [PubMed] [Google Scholar]
- Kuzniar A, Lin K, He Y, Nijveen H, Pongor S, Leunissen JAM. (2009) ProGMap: an integrated annotation resource for protein orthology. Nucleic Acids Res 37: W428–W434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton A, Saveanu C, Decourty L, Rain JC, Jacquier A, Fromont-Racine M. (2006) A functional network involved in the recycling of nucleocytoplasmic pre-60S factors. J Cell Biol 173: 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo KY, Li ZH, Bussiere C, Bresson S, Marcotte EM, Johnson AW. (2010) Defining the pathway of cytoplasmic maturation of the 60S ribosomal subunit. Mol Cell 39: 196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AE, Hoover LA, Craig EA. (2010) The cytosolic J-protein, Jjj1, and Rei1 function in the removal of the pre-60 S subunit factor Arx1. J Biol Chem 285: 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AE, Hung NJ, Yang PZ, Johnson AW, Craig EA. (2007) The specialized cytosolic J-protein, Jjj1, functions in 60S ribosomal subunit biogenesis. Proc Natl Acad Sci USA 104: 1558–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkereit P, Gadal O, Podtelejnikov A, Trumtel S, Gas N, Petfalski E, Tollervey D, Mann M, Hurt E, Tschochner H. (2001) Maturation and intranuclear transport of pre-ribosomes requires Noc proteins. Cell 105: 499–509 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- O’Brien KP, Remm M, Sonnhammer ELL. (2005) Inparanoid: a comprehensive database of eukaryotic orthologs. Nucleic Acids Res 33: D476–D480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östlund G, Schmitt T, Forslund K, Köstler T, Messina DN, Roopra S, Frings O, Sonnhammer ELL. (2010) InParanoid 7: new algorithms and tools for eukaryotic orthology analysis. Nucleic Acids Res 38: D196–D203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panse VG, Johnson AW. (2010) Maturation of eukaryotic ribosomes: acquisition of functionality. Trends Biochem Sci 35: 260–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell KM, Bass BL. (2009) Functional redundancy of yeast proteins Reh1 and Rei1 in cytoplasmic 60S subunit maturation. Mol Cell Biol 29: 4014–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Heard J, Martin G, Reuber L, Jiang CZ, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, et al. (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290: 2105–2110 [DOI] [PubMed] [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B. (2003) An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol 53: 247–259 [DOI] [PubMed] [Google Scholar]
- Sahara T, Goda T, Ohgiya S. (2002) Comprehensive expression analysis of time-dependent genetic responses in yeast cells to low temperature. J Biol Chem 277: 50015–50021 [DOI] [PubMed] [Google Scholar]
- Sanchez DH, Szymanski J, Erban A, Udvardi MK, Kopka J. (2010) Mining for robust transcriptional and metabolic responses to long-term salt stress: a case study on the model legume Lotus japonicus. Plant Cell Environ 33: 468–480 [DOI] [PubMed] [Google Scholar]
- Schippers JHM, Mueller-Roeber B. (2010) Ribosomal composition and control of leaf development. Plant Sci 179: 307–315 [Google Scholar]
- Scholl RL, May ST, Ware DH. (2000) Seed and molecular resources for Arabidopsis. Plant Physiol 124: 1477–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong HA, Gil M, Kim KT, Kim SJ, Ha H. (2002) Phosphorylation of a novel zinc-finger-like protein, ZPR9, by murine protein serine/threonine kinase 38 (MPK38). Biochem J 361: 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong HA, Jung H, Manoharan R, Ha H. (2011) Positive regulation of apoptosis signal-regulating kinase 1 signaling by ZPR9 protein, a zinc finger protein. J Biol Chem 286: 31123–31135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong HA, Kim KT, Ha H. (2003) Enhancement of B-MYB transcriptional activity by ZPR9, a novel zinc finger protein. J Biol Chem 278: 9655–9662 [DOI] [PubMed] [Google Scholar]
- Steinhauser D, Usadel B, Luedemann A, Thimm O, Kopka J. (2004) CSB.DB: a comprehensive systems-biology database. Bioinformatics 20: 3647–3651 [DOI] [PubMed] [Google Scholar]
- Strassburg K, Walther D, Takahashi H, Kanaya S, Kopka J. (2010) Dynamic transcriptional and metabolic responses in yeast adapting to temperature stress. OMICS 14: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, Krylov DM, Mazumder R, Mekhedov SL, Nikolskaya AN, et al. (2003) The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow MF. (2010) Molecular basis of plant cold acclimation: insights gained from studying the CBF cold response pathway. Plant Physiol 154: 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukaya H, Byrne ME, Horiguchi G, Sugiyama M, Van Lijsebettens M, Lenhard M. (2013) How do ‘housekeeping’ genes control organogenesis?—Unexpected new findings on the role of housekeeping genes in cell and organ differentiation. J Plant Res 126: 3–15 [DOI] [PubMed] [Google Scholar]
- Usadel B, Nagel A, Thimm O, Redestig H, Blaesing OE, Palacios-Rojas N, Selbig J, Hannemann J, Piques MC, Steinhauser D, et al. (2005) Extension of the visualization tool MapMan to allow statistical analysis of arrays, display of corresponding genes, and comparison with known responses. Plant Physiol 138: 1195–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lijsebettens M, Vanderhaeghen R, De Block M, Bauw G, Villarroel R, Van Montagu M. (1994) An S18 ribosomal protein gene copy at the Arabidopsis PFL locus affects plant development by its specific expression in meristems. EMBO J 13: 3378–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman JM, Murphy C, Sieburth LE. (2011) BYPASS1: synthesis of the mobile root-derived signal requires active root growth and arrests early leaf development. BMC Plant Biol 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Silk WK, Schurr U. (2009) Environmental effects on spatial and temporal patterns of leaf and root growth. Annu Rev Plant Biol 60: 279–304 [DOI] [PubMed] [Google Scholar]
- Walther D, Strassburg K, Durek P, Kopka J. (2010) Metabolic pathway relationships revealed by an integrative analysis of the transcriptional and metabolic temperature stress-response dynamics in yeast. OMICS 14: 261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136: 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.