An efflux-type boron transporter facilitates efficient borate cross-linking of rhamnogalacturonan II in cell walls and root cell elongation under boron deficiency.
Abstract
Boron (B) is required for cross linking of the pectic polysaccharide rhamnogalacturonan II (RG-II) and is consequently essential for the maintenance of cell wall structure. Arabidopsis (Arabidopsis thaliana) BOR1 is an efflux B transporter for xylem loading of B. Here, we describe the roles of BOR2, the most similar paralog of BOR1. BOR2 encodes an efflux B transporter localized in plasma membrane and is strongly expressed in lateral root caps and epidermis of elongation zones of roots. Transfer DNA insertion of BOR2 reduced root elongation by 68%, whereas the mutation in BOR1 reduced it by 32% under low B availability (0.1 µm), but the reduction in shoot growth was not as obvious as that in the BOR1 mutant. A double mutant of BOR1 and BOR2 exhibited much more severe growth defects in both roots and shoots under B-limited conditions than the corresponding single mutants. All single and double mutants grew normally under B-sufficient conditions. These results suggest that both BOR1 and BOR2 are required under B limitation and that their roles are, at least in part, different. The total B concentrations in roots of BOR2 mutants were not significantly different from those in wild-type plants, but the proportion of cross-linked RG-II was reduced under low B availability. Such a reduction in RG-II cross linking was not evident in roots of the BOR1 mutant. Thus, we propose that under B-limited conditions, transport of boric acid/borate by BOR2 from symplast to apoplast is required for effective cross linking of RG-II in cell wall and root cell elongation.
Boron (B) is an essential trace element for plants (Warington, 1923). B deficiency and toxicity have negative effects on plant growth and development. Insufficient B availability affects the quality and quantity of agricultural production (Shorrocks, 1997) through the inhibition of root elongation, leaf expansion, and fertility (for review, see Loomis and Durst, 1992; Marschner, 1995; Dell and Huang, 1997; Miwa and Fujiwara, 2010). A number of physiological studies suggest that B deficiency affects cell elongation rather than cell division in the growing portions of plants (Dell and Huang, 1997). There is also evidence that cross linking of the pectic polysaccharide rhamnogalacturonan II (RG-II) in cell walls is the primary physiological role of B in plants (O’Neill et al., 2004).
When the B supply is limited, most B in plants is localized in the cell wall. Matoh et al. (1993) isolated a B-polysaccharide complex from the cell walls of radish (Raphanus sativus) roots, which was later identified as an RG-II-B complex (Ishii and Matsunaga, 1996; Kobayashi et al., 1996; O’Neill et al., 1996). The importance of RG-II in reproduction has been demonstrated through the analysis of an Arabidopsis (Arabidopsis thaliana) mutant incapable of synthesizing CMP-3-deoxy-d-manno-2-octulosonic acid, an activated form of an RG-II-specific monosaccharide (Kobayashi et al., 2011). Borate forms a cis-diol ester with apiosyl residues in one of the four side chains of the two RG-II monomers to generate the RG-II-B dimer (Ishii et al., 1999). The Arabidopsis mutant murus1 contains reduced levels of Fuc, a glycosyl residue of RG-II (O’ Neill et al., 2001), and has altered RG-II structure (Pabst et al., 2013). Compared with the wild type, murus1 exhibits reduced cross linking of RG-II by borate and reduced leaf expansion under sufficient levels of B (O’ Neill et al., 2001). The application of high concentrations of B restores both the cross linking of RG-II and leaf expansion in this mutant, suggesting that the cross linking of RG-II by borate is essential for normal leaf expansion (O’Neill et al., 2001).
For B to be utilized by plants, it must be transported from the soil and through the roots to the shoots. In addition to passive diffusion across membranes (Marschner, 1995; Dordas and Brown, 2000), B transport is facilitated by boric acid channels, which are major intrinsic proteins. Takano et al. (2006) identified nodulin26-like intrinsic protein5;1 (NIP5;1) in Arabidopsis as a boric acid channel required for efficient uptake into root cells under B-limited conditions. Arabidopsis NIP6;1, the gene most similar to NIP5;1, contributes to the preferential distribution of B in young shoot tissues (Tanaka et al., 2008).
BOR1 was the first B transporter identified through an analysis of the Arabidopsis bor1-1 mutant that requires high levels of B, 30 and 100 µm B supply for normal leaf expansion and fertility, respectively (Noguchi et al., 1997; Takano et al., 2002). Shoot growth in bor1-1 plants is severely inhibited under low-B conditions, but root growth is not (Takano et al., 2001). Borate RG-II cross linking in shoots is reduced in bor1-1 under the low-B condition, correlating with the reduced B concentrations in total shoots and shoot cell wall fraction (Noguchi et al., 2003). The bor1-1 mutant has no apparent defect in B uptake by roots but is impaired in efficient root-to-shoot translocation of the mineral under low-B conditions (Noguchi et al., 2000; Takano et al., 2002). BOR1 encodes an efflux-type B transporter that is localized in the plasma membrane and is expressed in various root cells, including those in the endodermis (Takano et al., 2002, 2010). Thus, BOR1 is required for effective xylem loading under B-limited conditions, and BOR1 is also likely to be involved in the preferential distribution of B to young leaves (Takano et al., 2001). Interestingly, BOR1 accumulation is regulated by B conditions at the posttranscriptional level, and the protein is recycled between the plasma membrane and endosomes under low-B conditions. In addition, it is transported to the vacuole for degradation when B levels are high (Takano et al., 2005, 2010). Ubiquitination of the Lys-590 residue in BOR1 is required for this degradative process (Kasai et al., 2011).
There are six BOR1 paralogs in the Arabidopsis genome. Overexpression of BOR4 confers high B tolerance in plants, suggesting that it is involved in B tolerance through the export of the mineral out of symplast (Miwa et al., 2007; Miwa and Fujiwara, 2011). BOR4 is stably accumulated in the plasma membrane at toxic levels of B (Miwa et al., 2007).
Here, we report an investigation of the role of BOR2, the paralog most similar to BOR1, in B transport in Arabidopsis. Based on analyses of the transport activity, expression, and the phenotypes of transfer DNA (T-DNA) insertion mutants, we propose a new function for an efflux B transporter in root cell elongation under B-limited conditions.
RESULTS
B Transport Activity of BOR2 in Yeast
In the Arabidopsis genome, six genes are predicted to encode proteins with greater than 60% amino acid sequence similarity to BOR1 (Arabidopsis Genome Initiative, 2000; Nakagawa et al., 2007b). We obtained full-length complementary DNAs (cDNAs) for all six paralogs. Based on the extent of amino acid sequence similarity to BOR1, we previously named the six paralogs At3g62270, At3g06450, At1g15460, At1g74810, At5g25430, and At4g32510 as BOR2, BOR3, BOR4, BOR5, BOR6, and BOR7, respectively (Nakagawa et al., 2007b).
The paralog BOR2 is most similar to BOR1. The predicted open reading frame (ORF) in the BOR2 cDNA consists of 703 amino acids with 90% sequence identity to BOR1. BOR2 contains 10 to 11 putative transmembrane regions according to Aramemnon (http://aramemnon.botanik.uni-koeln.de/), as is the case for BOR1. BOR1 and BOR2 reside on chromosomes 2 and 3, respectively, and correspond to regions of genome duplication (Arabidopsis Genome Initiative, 2000). Both BOR1 and BOR2 contain 12 exons and 11 introns, and their exon-intron break points are identical.
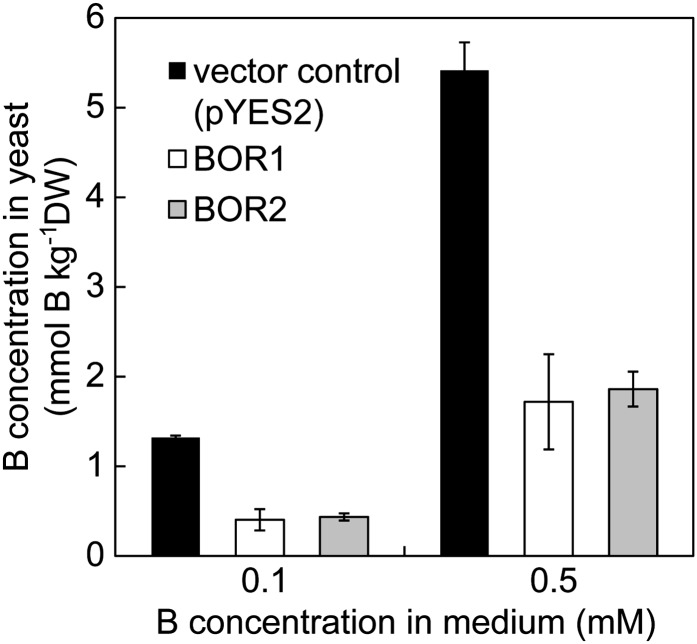
BOR2 was expressed in a yeast (Saccharomyces cerevisiae) strain lacking the BOR1 homolog ScBOR1 (Takano et al., 2007) under the control of the GAL1 promoter to determine the B transport activity of BOR2. Cells at midlog phase were harvested and incubated for 60 min in medium containing 0.1 or 0.5 mm boric acid. Following incubation, the cells were washed twice in cold water, boiled, and then centrifuged. The concentration of B in the supernatant was determined to estimate the amount of soluble B in the cells. The B concentrations in yeast cells expressing BOR2 were about 30% to 35% of those in cells carrying the empty vector (Fig. 1), i.e. the B concentrations in cells expressing BOR2 were reduced to one-third of those of the vector control. This outcome is similar to that obtained when performing the same experiments with BOR1 (Takano et al., 2002), suggesting that BOR2 also encodes a functional efflux-type B transporter.
Figure 1.
The B concentrations in yeast cells expressing BOR1 or BOR2. The concentrations of soluble B in yeast cells (mmol B kg–1 dry weight) are shown, given as the means ± sd from three independent transformants. DW, Dry weight.
Cell-Type Specificity of BOR2 Expression
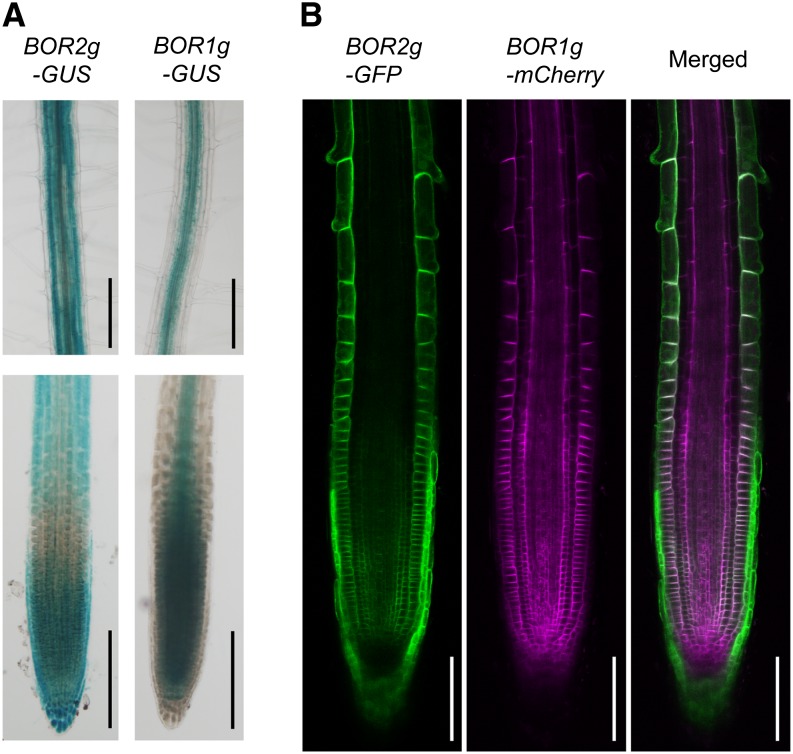
To examine the cell-type specificity of BOR2 expression, we generated transgenic Arabidopsis lines BOR2genome-GUS and BOR1genome-GUS that carry GUS fused to genomic fragments corresponding to the promoter and ORF at the C termini (Fig. 2A). When the plants were grown under low-B conditions, GUS activity derived from BOR2-GUS was detected throughout the root, with staining in the meristematic and elongation zones, and in cortical cells in the mature portion of the roots (Fig. 2A). GUS activity derived from BOR1-GUS was detected in root meristematic and transition zones and around vascular tissues during root cell maturation (Fig. 2A). To further clarify the cell-specific expression of BOR1 and BOR2, we established transgenic Arabidopsis plants carrying both BOR2genome-GFP and BOR1genome-mCherry to coexpress BOR2-GFP and BOR1-mCherry fusion protein under the control of their own promoter (Fig. 2B). BOR2-GFP was predominantly observed in lateral root caps and epidermis of elongation zones. BOR2-GFP was also detected in all cells of the root meristematic zone, but little was observed in transition zones. BOR1-mCherry was expressed in meristem and transition zones and the epidermis and endodermis of elongation zones but not in lateral root caps. This result for BOR1-mCherry is mostly consistent with previous reports of studies that employed BOR1-GFP (Takano et al., 2010). These observations suggest that cell specificity of BOR2 expression overlaps, in part, that of BOR1 but also shows some differences.
Figure 2.
Cell specificity of BOR2 expression. A, GUS histochemical staining of roots of transgenic Arabidopsis roots carrying BOR2genome-GUS (BOR2g-GUS) and BOR1genome-GUS (BOR1g-GUS). Bars = 200 µm. B, GFP and mCherry imaging of a transgenic Arabidopsis root carrying both BOR2genome-GFP (BOR2g-GFP) and BOR1genome-mCherry (BOR1g-mCherry). Bars = 100 µm. Plants were grown in solid media containing 0.3 µm boric acid for 4 d.
Subcellular Localization of BOR2
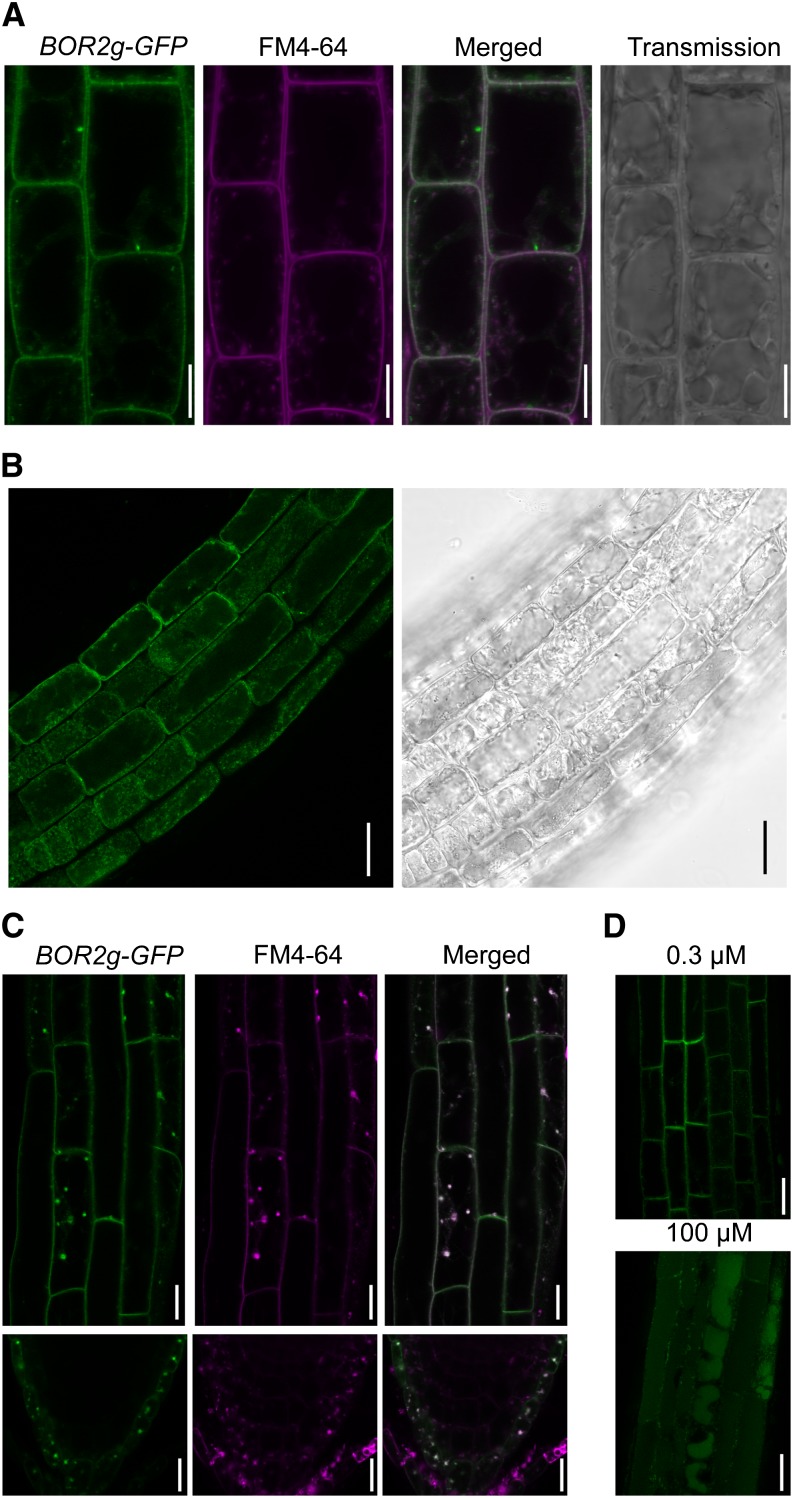
In the transgenic lines carrying BOR2genome-GFP, GFP fluorescence was detected at the cell periphery (Fig. 2B). Visualization of the plasma membrane with FM4-64 revealed that BOR2-GFP colocalized with FM4-64 staining and was not localized to the tonoplast (Fig. 3A). These observations demonstrate that BOR2 localizes to the plasma membrane. Upon exposure to 400 mm mannitol for 3 min to induce plasmolysis, BOR2-GFP was observed in the shrunken protoplasm (Fig. 3B). This shows that BOR2 is nonstably localized to the plasma membrane and is easily internalized to the cytoplasm.
Figure 3.
Subcellular localization of BOR2 in the transgenic lines carrying BOR2genome-GFP (BOR2g-GFP). A, GFP imaging and FM4-64 staining of root epidermis of the transgenic Arabidopsis. Plants were grown in solid media containing 0.3 µm boric acid for 9 d and treated with 4 µm FM4-64 for several minutes. Bars = 10 µm. B, GFP imaging of root epidermis exposed to 400 mm mannitol for 3 min for plasmolysis. GFP (left) and bright field (right) are shown. Plants were grown in solid media containing 1 µm boric acid for 5 d. Bars = 25 µm. C, GFP imaging of the transgenic root epidermis and root tip treated with BFA. Five-day-old plants grown under 0.3 µm boric acid were exposed to 50 µm CHX for 30 min and 25 µm FM4-64 for 2 min, washed, and supplied with 50 µm CHX and 50 µm BFA for 30 min. GFP (left), FM4-64 (middle), and a merged image (right) are shown. Bars = 25 µm. D, GFP imaging of the transgenic root epidermis transferred to high B conditions. Plants were grown in solid media containing 0.3 µm boric acid for 9 d and then transferred to the media containing 0.3 or 100 µm boric acid. They were incubated for 150 min under the dark condition. Bars = 25 µm.
BOR1 exhibits polar localization in the inner (inward) domain of the plasma membrane in all types of root cells (Takano et al., 2010; Fig. 2B). As was the case with BOR1, BOR2-GFP shows polarity toward the inner plasma membrane domain in the cells of the meristematic zone (Fig. 2B), although polar localization of BOR2-GFP was weak in epidermis of the elongation zone (Fig. 2B).
To investigate the recycling of BOR2, we exposed the transgenic BOR2genome-GFP plants to brefeldin A (BFA) in the presence of the protein synthesis inhibitor cycloheximide (CHX) under low-B conditions. BFA is an inhibitor of guanine-nucleotide exchange factors for ADP-ribosylation factor, which inhibits exocytosis but not endocytosis, and causes endosomal aggregation (Robinson et al., 2008). BOR2-GFP was found to be colocalized in the BFA body with FM4-64, an endocytic tracer, in the epidermis of the elongation zone and lateral root caps (Fig. 3C), suggesting that BOR2-GFP cycles between the plasma membrane and endosomes in a manner similar to BOR1 (Takano et al., 2005) under low-B conditions.
In the presence of high B concentrations, BOR1 is degraded in the vacuole via the endocytic pathway (Takano et al., 2005). The transgenic plants carrying BOR2genome-GFP were transferred from the media containing 0.3 µm boric acid to media containing either 0.3 or 100 µm boric acid and incubated in darkness for 150 min. GFP fluorescence was detected in the plasma membrane in the presence of 0.3 µm B, but GFP was observed in vacuoles in medium containing 100 µm B (Fig. 3D). These results suggest that the accumulation of BOR2 is also regulated at the level of protein degradation in response to B conditions, similar to BOR1.
Growth of Loss-of-Function Mutants under Low-B Conditions
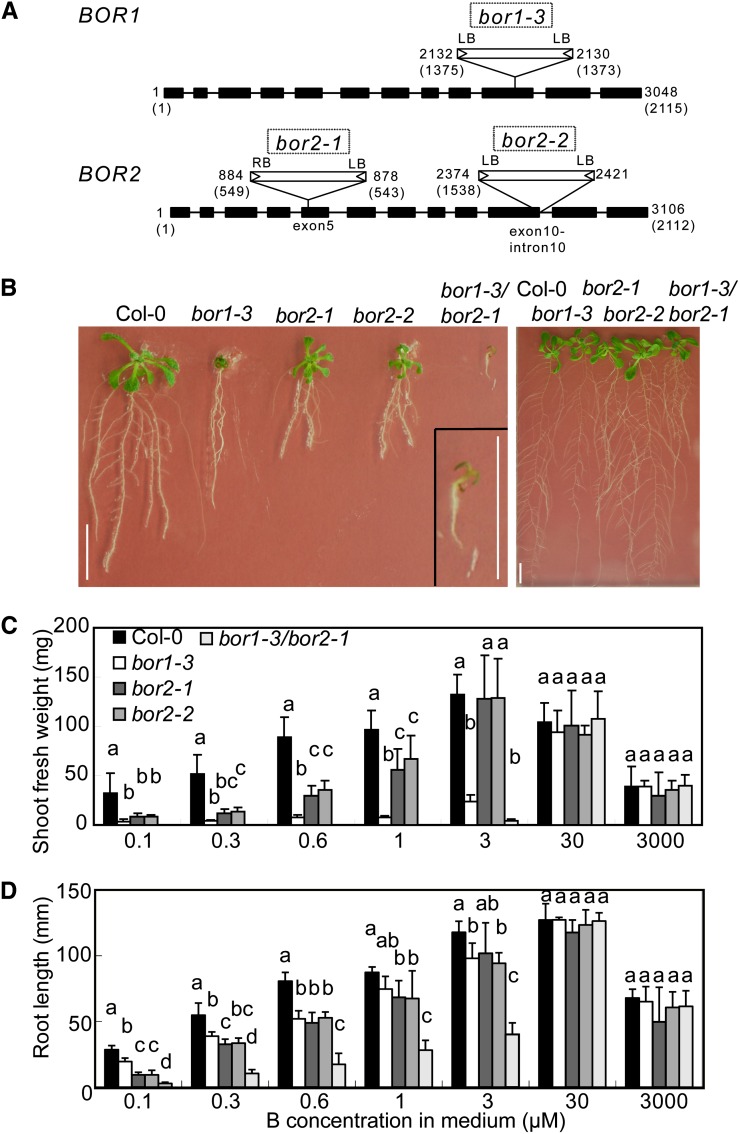
To examine the effects of BOR2 function loss on plant growth and B transport, we established transgenic Arabidopsis lines homozygous for T-DNA insertions in BOR1 or BOR2 (Alonso et al., 2003; Rosso et al., 2003) and determined the sites of the insertions. The lines bor1-3 (SALK_37312), bor2-1 (SALK_56473), and bor2-2 (GABI 527H04) were all found to carry T-DNA insertions in the exons of the corresponding genes (Fig. 4A; Kasai et al., 2011). The growth of the T-DNA insertion mutant bor1-3 was not significantly different from that of bor1-1, which carries a point mutation in the BOR1 ORF.
Figure 4.
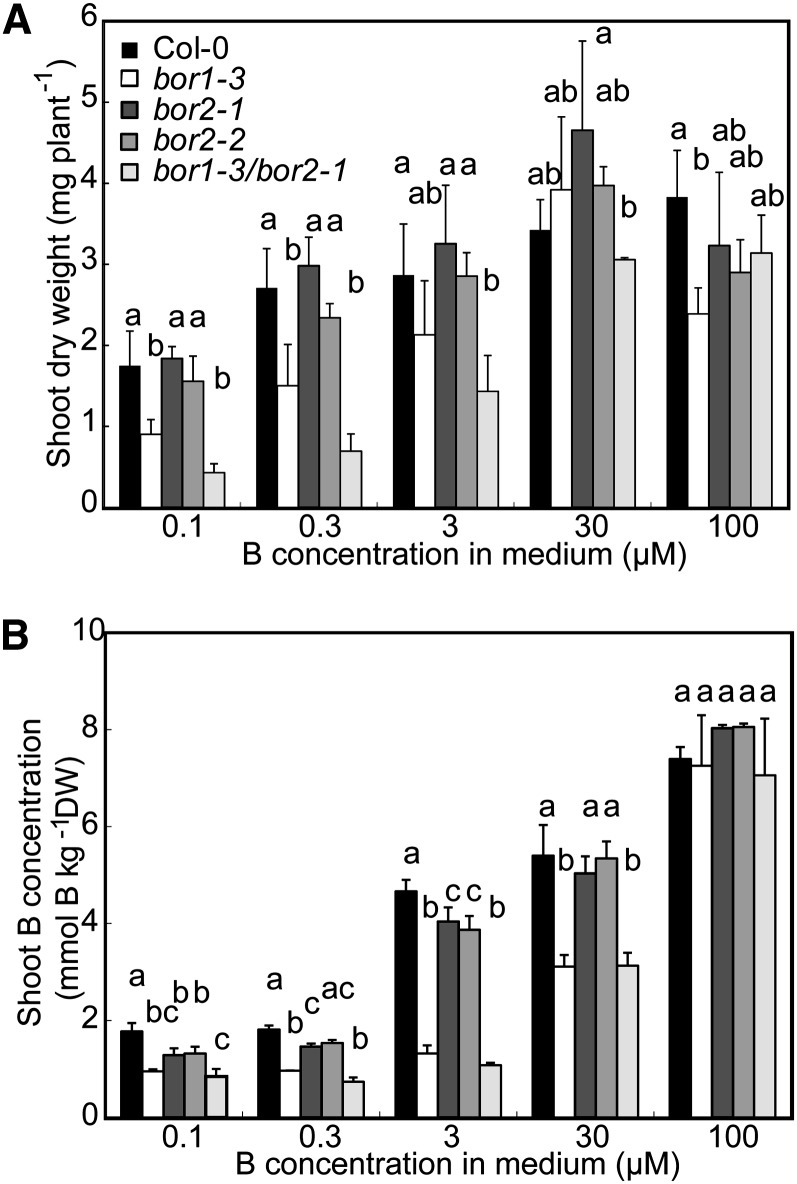
Characterization of T-DNA insertion mutants and their growth in media containing various concentrations of B. A, Schematic representation of T-DNA insertions in the BOR1 and BOR2 genes and the exon-intron structures of the genes. Black boxes and bars indicate exons and introns, respectively. The numbers shown at the right borders (RB) and the left borders (LB) correspond to the relative nucleotide positions from the first codon (+1) in the genomic sequences. The numbers in brackets are the nucleotide positions in the cDNA sequences. B, Fourteen-day-old plants of the T-DNA insertion lines grown in solid media supplemented with 0.1 µm (left) and 30 µm boric acid (right). A magnified view of bor1-3/bor2-1 is shown at the lower right of the left section. Bars = 10 mm. C, Fresh weights of aerial portions of the T-DNA insertion lines collected from plants grown for 21 d on solid media containing various concentrations of boric acid. Means ± sd are shown (n = 3–12). D, Primary root lengths of the T-DNA insertion lines grown for 14 d on solid media containing various concentrations of boric acid. Means ± sd are shown (n = 3–19). In C and D, different letters above each bar indicate a significant difference among the lines grown in the same B concentration (P < 0.05, Tukey’s test).
Wild-type ecotype Columbia (Col-0), bor1-3, bor2-1, bor2-2, and bor1-3/bor2-1 plants were grown on solid media containing between 0.1 and 3,000 µm boric acid. The growth of the bor2-1 and bor2-2 mutant plants was less than that of wild-type plants on medium containing less than or equal to 1 µm boric acid (Fig. 4, B–D). The growth patterns of bor2-1 and bor2-2 were similar, strongly suggesting that these defects are caused by the disruption of BOR2.
The shoot fresh weights of bor2-1 and bor2-2 were 26% and 25% (0.1 µm), 24% and 26% (0.3 µm), 34% and 34% (0.6 µm), and 58% and 69% (1 µm), respectively, of those of wild-type plants when the B supply was limited (Fig. 4C). In bor1-3 plants, shoot growth was severely reduced to approximately 10% (0.1, 0.3, 0.6, and 1 µm) and 17% (3 µm) of those of the wild type under B-limited conditions, as reported previously for bor1-1 (Noguchi et al., 1997; Takano et al., 2001). The extent of shoot growth inhibition in bor2-1 and bor2-2 was not as severe as that in bor1-3 (Fig. 4, B and C).
Root growth, by contrast, was more severely affected in the bor2-1 and bor2-2 mutants than in the bor1-3 mutants (Fig. 4B). The lengths of the primary roots in bor2-1 and bor2-2 were reduced to 32% and 33% (0.1 µm), 59% and 61% (0.3 µm), 61% and 65% (0.6 µm), and 78% and 77% (1 µm), respectively, of that in wild-type plants (Fig. 4D). These growth defects did not occur when plants were grown under sufficient B concentrations (30 µm), suggesting that BOR2 functions mainly under low-B conditions.
bor1-3/bor2-1 double mutant plants had much more severe growth defects than any of the single insertion lines (Fig. 4, B–D). The fresh shoot weights of double mutants grown under boric acid concentrations less than or equal to 1 µm were not measured because of poor growth. The double mutant plants grew normally in the presence of 30 µm boric acid. These results suggest that BOR1 and BOR2 have major and at least partially overlapping roles in shoot and root growth under B-limited conditions.
B Accumulation in the Aerial Portions of BOR2 Mutant Plants
Because BOR1 is involved in xylem loading of B, we determined the amounts of B in the aerial portions of wild-type and mutant plants grown hydroponically in the presence of 0.1, 0.3, 3, 30, or 100 µm boric acid to explore the functions of BOR2 in B translocation. There were no significant differences in the shoot dry weights between BOR2 mutants and the wild type under all conditions tested (Fig. 5A). The shoots of bor2-1 and bor2-2 plants grown in medium containing less than or equal to 3 µm boric acid contained lower amounts of B than the shoots of wild-type plants, but there were no significant differences in the B contents in plants grown in medium containing greater than or equal to 30 µm boric acid (Fig. 5B). However, in bor1-3 and bor1-3/bor2-1 double mutant plants, there were significant reductions in the B contents in the aerial portions in media containing 0.1, 0.3, 3, or 30 µm boric acid. Hence, BOR2 likely contributes to the root-to-shoot translocation of B under limited B supply conditions, but its contribution is much smaller than that of BOR1.
Figure 5.
B concentrations in aerial portions of BOR2 mutant plants. Col-0, bor1-3, bor2-1, bor2-2, and bor1-3/bor2-1 plants were grown hydroponically for 30 d in media containing 0.1, 0.3, 3, 30, or 100 µm boric acid. The dry weights (A) and the B concentrations (B) of the aerial portions of the plants were determined. Means ± sd are shown (n = 3–5). Different letters above each bar indicate a significant difference among lines receiving the same B treatment (P < 0.05, Tukey’s test). DW, Dry weight.
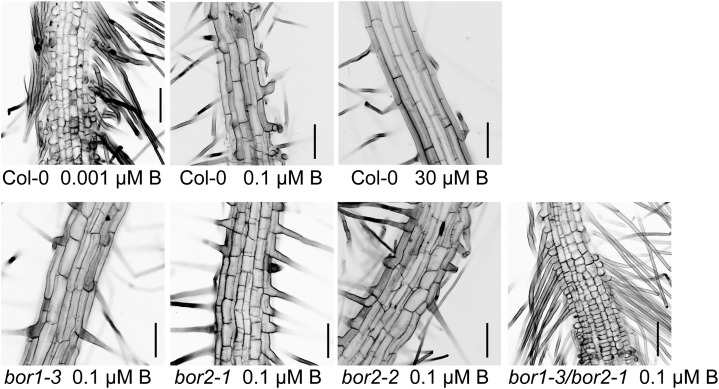
Reduction in Root Epidermal Cell Length in BOR2 Mutant Lines Grown under B-Limited Conditions
Because root growth was more affected in BOR2 mutants than in the BOR1 mutant in solid media under B-limiting conditions (Fig. 4, B and D), we observed root cells after low B exposure to determine the nature of the root growth inhibition in BOR2 mutant plants. Plants were first grown with 30 µm boric acid for 4 d and then transferred to media containing 0.001 (wild-type plants only), 0.1, or 30 µm boric acid. After 2 d, we examined the epidermal cells in the root hair zones, which developed after the transfer (Fig. 6). In plants transferred to medium containing 0.1 µm boric acid, the axial lengths of the root epidermal cells in bor1-3, bor2-1, bor2-2, and bor1-3/bor2-1 were reduced compared with those in the wild type. The double mutant had the greatest reduction in cell length, followed in order by bor2-1 and bor2-2, with the least reduction in bor1-3. In bor2-1, bor2-2, and bor1-3/bor2-1 plants transferred to medium containing 0.1 µm boric acid, the root epidermal cells were not only short but were also swollen and irregular in shape.
Figure 6.
Root cell elongation in mutant lines in response to changes in the B concentration of the medium. Plants were grown for 4 d in solid media containing 30 µm boric acid, transferred to medium supplemented with 0.001, 0.1, or 30 µm boric acid, and incubated for 2 d. Epidermal cells of root hair zone that developed after the medium transfer were observed. Bars = 100 µm.
Wild-type plants exposed to more severe B depletion conditions (0.001 µm boric acid) also had reduced root cell lengths and swollen root cells. The impaired cell growth seen in wild-type plants grown in 0.001 µm boric acid is similar to that seen in the double mutants grown in 0.1 µm boric acid. Thus, root cell elongation was at least inhibited by the BOR2 mutation when the plants were transferred from B-sufficient to B-deficient medium, similar to the severe effects of B deficiency on wild-type plants.
Reduced RG-II-B Dimer Formation in BOR2 Mutant Plants Grown in B-Limited Medium
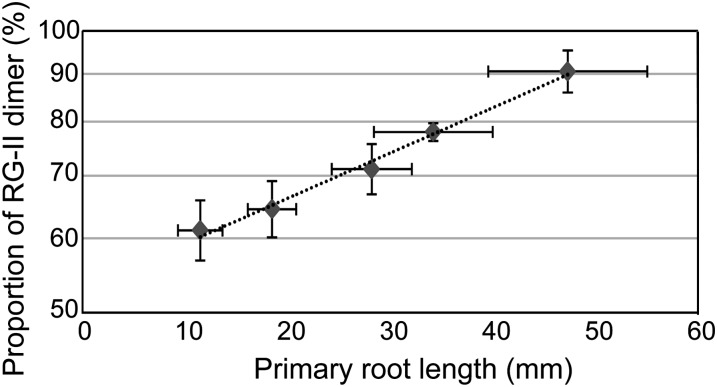
One primary function of B is the cross linking of the pectic polysaccharide RG-II in cell walls (O’Neill et al., 2001). We first determined the relationship between borate cross linking of RG-II and the root growth of wild-type Arabidopsis plants under low-B conditions (Fig. 7). When wild-type plants were grown in solid media containing 0.001, 0.1, 0.3, 1, or 30 µm boric acid for 8 d, the primary root lengths were reduced along with the decreased B concentrations in the media and were positively correlated with the proportion of RG-II dimer. It is noted that approximately 60% of RG-II in roots was cross linked when root elongation was severely inhibited in medium containing 0.001 µm boric acid. The graph suggests that at least 50% cross linking of RG-II is required to maintain root growth.
Figure 7.
Relation between primary root lengths and proportion of RG-II dimer. Col-0 plants were grown in solid media containing 0.001, 0.1, 0.3, 1, and 30 µm boric acid for 8 d. Cell wall fractions were isolated, and the relative proportions of RG-II-B dimer to total RG-II were determined. Relative proportion of RG-II-B (%) is shown in a longitudinal axis as a log scale, and an approximation straight line is presented. Mean ± sd are shown (n = 52–239 for primary root length, n = 3–4 for RG-II measurement). Primary root lengths were reduced along with the decreased B concentrations in the media, and each dot corresponds to the data obtained from plants grown under 0.001, 0.1, 0.3, 1, and 30 µm boric acid supply.
Then, we determined the B concentrations and formation of RG-II cross links in roots to investigate the causes of the growth defects observed in BOR2 T-DNA mutant plants (Table I). There were no significant differences in the concentrations of total B in the roots of bor2-1, bor2-2, and wild-type plants; however, the relative proportions of the RG-II-B dimer to the total RG-II in bor2-1 and bor2-2 were reduced to 45%, whereas that of wild-type plants was reduced to 54% when plants were grown on medium supplemented with 0.1 µm boric acid. At 0.1 µm boric acid, the B concentrations of the roots of bor1-3 and bor1-3/bor2-1 were 15% and 20% lower than in wild-type plants, respectively. The rate of formation of the RG-II-B dimer in bor1-3 roots was not significantly different from that in wild-type plants. In bor1-3/bor2-1, the relative proportions of the RG-II-B dimer were reduced to levels similar to those in bor2-1 and bor2-2. All lines had greater than 90% RG-II-B dimer formation when grown on medium supplemented with 30 µm boric acid. In summary, the BOR2 mutation did not affect the concentrations of total B in roots but influenced the formation of RG-II-B dimers in roots exposed to limited B supply. Assuming that a reduction of even 10% in RG-II cross linking significantly affects root growth (Fig. 7), the reduction in RG-II-B dimer formation from 54% to 45% (Table I) is likely to be a major cause of the reduced cell elongation seen in T-DNA mutant plants of BOR2 grown under low-B conditions.
Table I. RG-II-B dimer formation and B concentration of Arabidopsis roots grown under low-B and normal-B conditions.
Plants were grown on solid media containing 0.1 and 30 µm boric acid for 10 to 14 d. Cell wall fraction was prepared from plant roots and then treated with EPG. The solution of EPG-soluble material was fractionated by size-exclusion chromatography equipped with a refractive index detector. Relative proportions of RG-II-B dimer to total RG-II and B concentration of whole roots are shown (means ± sd, n = 3–4). Different superscript letters show significant difference (P < 0.05, Tukey’s test).
| Plant line |
B Concentration in Roots |
Relative Proportion of RG-II-B Dimer |
||
|---|---|---|---|---|
| 0.1 µm
B Concentration in Medium |
30 µm B Concentration in Medium | 0.1 µm B Concentration in Medium | 30 µm B Concentration in Medium | |
| mmol kg–1 dry wt | % | |||
| Col-0 | 0.39 ± 0.03a | 1.44 ± 0.17 | 54.0 ± 1.1a | 90.1 ± 4.1 |
| bor1-3 | 0.33 ± 0.01ab | 1.54 ± 0.04 | 52.8 ± 0.4a | 90.0 ± 1.1 |
| bor2-1 | 0.36 ± 0.04ab | 1.28 ± 0.15 | 42.5 ± 0.5b | 91.6 ± 2.1 |
| bor2-2 | 0.37 ± 0.02ab | 1.53 ± 0.05 | 45.7 ± 1.0b | 92.0 ± 2.3 |
| bor1-3/bor2-1 | 0.31 ± 0.03b | 1.43 ± 0.12 | 45.1 ± 0.9b | 90.4 ± 1.5 |
DISCUSSION
BOR2 Is Essential for Root Growth under Low-B Conditions
BOR2 was characterized as an efflux-type B transporter localized to the plasma membrane, which is also true for BOR1. However, the major physiological roles of BOR1 and BOR2 in plants are different: BOR1 functions in xylem loading of B, whereas BOR2 functions in root cell elongation under B-limited conditions.
The shoot B concentrations were lower in BOR2 mutant plants than in wild-type plants, although the reduction was greater in bor1-3 under low-B conditions (Fig. 5B). Hence, BOR2 also functions in the transport of B to shoots, but the extent of this contribution, if any, is minor compared with that of BOR1. The predominant expression of BOR1 in the endodermis and its polar localization in the inner plasma membrane domain (Takano et al., 2010; Fig. 2B) are likely to be major factors in B transport into the stele, which is a prerequisite for subsequent transport of B from the root to the shoot via the xylem.
It is interesting to note that BOR1 and BOR2 are both efflux-type B transporters exhibiting polar localization in plasma membrane and internalization and that tissue specificity of their expressions are in part overlapping to each other (Figs. 2 and 3), while their major physiological roles are different: BOR1 for xylem loading and BOR2 for cross linking of pectins (this study). Although we cannot rule out the possibility that there may be a difference in transport properties and/or differential regulation of proteins, given the evidence, it is reasonable to surmise that the major reason for the differences in physiological roles between BOR1 and BOR2 are the differential tissue-specific patterns.
In addition to its minor contribution to the root-to-shoot translocation of B, BOR2 was found to be required for normal root growth (Fig. 4, B and D), particularly for root cell elongation (Fig. 6), under conditions of limited B supply. The expression of BOR2 in the meristematic zone and the epidermis of the elongation zone (Fig. 2) is likely to support root cell elongation. The high-B-induced degradation of BOR2 (Fig. 3D) is consistent with the importance of BOR2 under low-B conditions.
Borate Cross Linking of RG-II Is Important for Root Growth
One of the physiological roles of B in shoots is the cross linking of RG-II chains in the cell wall (O’Neill et al., 2001). Although this role has yet to be demonstrated definitively in roots, the preferential distribution of B in root cell walls and the presence of RG-II-B suggest that B also functions in the cross linking of RG-II molecules in roots. B is distributed primarily to cell walls prior to its accumulation in the soluble fraction of Arabidopsis roots under low-B conditions (Noguchi et al., 2000). The RG-II-B complex is known to occur in radish roots (Kobayashi et al., 1996) and pumpkin (Cucurbita maxima) roots (Matsunaga and Ishii, 2006).
In a study of pumpkin roots exposed to low-B conditions, RG-II-B dimer formation decreased along with the root dry weight (Matsunaga and Ishii, 2006). In pumpkin roots subjected to B-depleted conditions (no addition of B), the fraction of RG-II forming the borate complex was about 55% (Matsunaga and Ishii, 2006). Under these conditions, the pumpkin root dry weight was about 50% lower than under the B-sufficient conditions. A positive correlation was also found between root growth and the proportion of RG-II-B formation under a wide range of B-deficient conditions in our study of wild-type Arabidopsis plants (Fig. 7). The proportion of RG-II cross linking reported for pumpkin roots is comparable to our present data for wild-type Arabidopsis roots (Fig. 7; Table I). These observations also support the idea that the occurrence of RG-II-B is a determinant of root growth, especially root cell elongation, as B depletion inhibits root cell elongation (Fig. 6). Furthermore, our study suggests that 50% RG-II cross linking is likely to be a lower limit to support plant root elongation.
The total root B concentration was not significantly different in bor2-1 and bor2-2 under limited B supply, but the fraction of RG-II forming the borate complex in bor2-1 and bor2-2 was reduced to 43% to 46%, whereas that of the wild type under the same B condition was 54% (Table I). It is reasonable to assume that the further reduction of borate-cross-linked RG-II caused by the mutation in BOR2 under B-deficient conditions was detrimental to cell elongation and resulted in reduced root elongation (Figs. 4 and 6). Given all of these observations, we propose that decreased RG-II-B dimer formation is the major inhibitor of root cell elongation in BOR2 mutants grown in limited B.
The Role of BOR2 in B Transport for Efficient RG-II-B Complex Formation: A Model
Immunocytochemical analysis in radish roots using antibodies against RG-II suggested that RG-II is present in all root tip cells and that it is located extremely close to the plasma membrane beneath the cell wall (Matoh et al., 1998; Matoh, 2001). RG-II-B dimers form spontaneously in vitro, and the reaction is stimulated by the addition of divalent cations (O’Neill et al., 1996; Ishii et al., 1999). Under physiological conditions, B is present mainly as boric acid in solution; boric acid is a Lewis acid that interacts with water molecules to form borate anion (B[OH]3 + H2O = B[OH]4– + H+; pKa = 9.24; Power and Woods, 1997). Borate anion forms a cis-diol ester with the apiosyl residues in RG-II (Ishii and Ono, 1999) and is thus likely preferred over boric acid as the substrate for RG-II dimer formation. However, molecular mechanisms for borate RG-II dimerization are still largely unknown, i.e. the site and the substrates of the reaction in vivo.
Root cell elongation is also severely reduced in loss-of-function mutants of NIP5;1, a boric acid channel for B uptake that is required for growth under B-limited conditions (Takano et al., 2006). GFP-NIP5;1 expressed under the control of its own promoter was detected in the root cap and epidermal cells in the elongation zone of the roots (Takano et al., 2010). These findings suggest that B transport via the apoplast and simple diffusion across the plasma membrane are not able to satisfy the demand for B during root cell elongation under B-limited conditions. Because BOR2 is expected to export B from the cytosol, it is reasonable to propose that under conditions of limited B supply, the B required for root cell elongation comes mainly from the cytosol through BOR2.
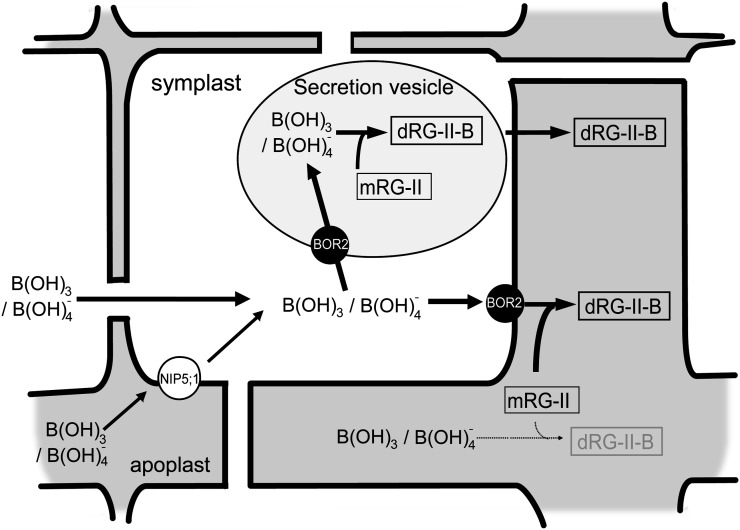
A model for BOR2 function is presented in Figure 8. In medium containing low B concentrations, the B concentrations seem insufficient for RG-II-B dimer formation in apoplast. One model is that a portion of the B entering cells through NIP5;1 or by passive diffusion and the boric acid/borate is supplied by the BOR2 exporter to the RG-II monomer in apoplast, resulting in efficient dimer formation. RG-II is likely present near the plasma membrane; thus, a B exporter located in this membrane would be able to directly control RG-II cross linking. It is also reasonable to assume that there is a specific interaction, either directly or indirectly, between BOR2 and a protein, if any, that facilitates borate cross linking to establish the efficient transport of boric acid/borate into RG-II.
Figure 8.
Schematic model of the roles of BOR2 in root cells. A possible role for BOR2 in root meristems under conditions of low-B supply is shown. One hypothesis is that boric acid/borate is exported out of the cell by BOR2 to the apoplast, where it is used in the efficient cross linking of RG-II molecules. Another possibility is that BOR2 concentrates boric acid/borate into secretion vesicles to facilitate dimerization of RG-II in the vesicles, and then the dimeric RG-II is secreted to the apoplast. The thickness of the arrows signifies the efficiency of the reaction. mRG-II, RG-II monomer; dRG-II-B, RG-II-B dimer.
It is also possible that RG-II-B forms in the secretion vesicles. We demonstrated that BOR2-GFP was localized to endosomal aggregations following BFA treatment under B-limited conditions (Fig. 3C), suggesting that a significant quantity of BOR2 is located in the secretory/recycling pathway between the plasma membrane and trans-Golgi network. It is possible that BOR2 localized to the secretion vesicles transports boric acid/borate into them, resulting in increased B concentrations in these compartments and facilitating RG-II-B dimer formation.
In conclusion, we demonstrated that BOR2 is indispensable for root growth and RG-II-B cross linking in cell walls under B-limited conditions. Our findings are an example of the involvement of mineral transporters in cell wall formation and maintenance during normal plant growth.
MATERIALS AND METHODS
Plant Culture and Transformation
Arabidopsis (Arabidopsis thaliana) wild-type (Col-0) was from our laboratory stock. Plants were grown on solid media (Fujiwara et al., 1992) in which the B concentrations were adjusted with boric acid. To reduce B contamination, ultrapure water by Milli-Q Synthesis A-10 (Merck Millipore) and autoclavable polypropylene bottles were used. For growth tests, the solid media contained 2% (w/v) Suc and 1.5% (w/v) gellan gum (Wako Pure Chemicals), and for RG-II dimer formation in the wild type, the media contained 1% (w/v) Suc and 1% (w/v) gellan gum. Surface-sterilized seeds were sown on the solid media and incubated for two days at 4°C. The plates were then placed vertically and incubated at 22°C under a 16-h-light /8-h-dark cycle. For the determination of B amounts in shoots, Arabidopsis plants were grown hydroponically as described (Takano et al., 2001). To generate transgenic Arabidopsis, plasmids were introduced into Agrobacterium tumefaciens strain GV3101 (C58C1Rifr) pMP90 (Gmr) and transformation was performed by the floral dip method. For selection of transformants, solid media that contain one-half strength of Murashige Skoog salt, 1% (w/v) Suc and 0.2% (w/v) gellan gum, and hygromycin (20 mg L–1) was used. For selection of plants expressing GFP or mCherry-tagged proteins, T1 plants resistant to hygromycin were transferred to solid media (Takano et al., 2005) containing 0.3 µm boric acid, and fluorescence-positive plants were selected under a macrozoom fluorescent microscope (MVX10; Olympus).
Cloning of Full-Length cDNA
Full-length cDNAs for all of six paralogs were obtained, and DNA sequences of the clones were determined. The cDNAs for At1g15460, At3g06450, and At3g62270 were obtained from the RIKEN Genomic Sciences Center, and those for At1g74810, At4g32510, and At5g25430 were isolated by reverse transcription-mediated PCR. cDNA of At1g74810 was obtained from Arabidopsis roots, and those of At4g32510 and At5g25340 were obtained from flowers.
Generation of Yeast Expressing BOR2 and Determination of B Concentrations in Yeast Cells
BOR2 cDNA ORF was amplified using KOD Plus DNA polymerase (TOYOBO) with the primer set (5′-TGAGAGGTACCAGAGCCATGGAAGAGACTTTTGTTCCGTTTGA-3′ and 5′-GTTTTAGCATGCTCATTTCGACGATGATGATGGGT-3′). The PCR products were digested with KpnI and SphI and inserted into the corresponding sites in pYES2, resulting in pTF524 (BOR2 coding sequence in pYES2). The plasmids pTF477 (BOR1), pTF524 (BOR2), and pYES2 were introduced into yeast (Saccharomyces cerevisiae) strain ΔScBOR1 (Winzeler et al., 1999). Transformants were selected on synthetic minimal medium (Sherman, 1991) supplemented with 2% (w/v) Glc, 20 mg L–1 His, 30 mg L–1 Leu, and 20 mg L–1 Met. The yeast medium used in this experiment was synthetic minimum medium containing 2% (w/v) Gal. Fifty milliliters of culture at the midlog phase were transferred to a 50-mL plastic tube and centrifuged at 3,000g for 5 min to collect the yeast cells. The cells were mixed with 20 mL medium (adjusted to pH 5.5 with Tris) containing 0.1 or 0.5 mm boric acid and incubated for 60 min. Sampling of the soluble fraction in the yeast cells and determination of the B concentrations were conducted as described by Takano et al. (2002). Experiments were performed with three independent transformants.
T-DNA Insertion Mutants and Determination of T-DNA Insertion Sites
T2 seeds of bor1-3 (SALK_37312), bor2-1 (SALK_56473), and bor2-2 (GABI 527H04) were provided by the Salk Institute (Alonso et al., 2003) and the Max Planck Institute (Rosso et al., 2003). From the T2 plants provided, homozygous lines were selected by PCR and the flanking sequences were determined by sequencing. All of the plant lines were in the Col-0 background. The primers used for bor1-3 and bor2-1 were described in Kasai et al. (2011). The primers for bor2-2, 5′-ATTGCCAATTGAAGTCAAAG-3′ (forward) and 5′-TTTTACCGACATTTGACATGGTA-3′ (reverse) and the GABI left border, 5′-ATATTGACCATCATACTCATTGC-3′, were used.
Generation of Transgenic Plants Expressing GUS and Fluorescence Protein Fusions under the Control of Own Promoter
For generation of BOR2 construct, the genomic nucleotides 23053503 to 23059413 of chromosome 3 sequences corresponding to putative promoter region (2.8 kb) and ORF (3.1 kb) was amplified by PCR using primers 5′-CACCTCTAGATGAATAATTAATAT-3′ and 5′-TTTCGACGATGATGATGGGTTTAA-3′. The fragment was cloned into pENTR/D-TOPO (Invitrogen) and then into pMDC163 (Curtis and Grossniklaus, 2003) and pGWB504 (Nakagawa et al., 2007a) for GUS and GFP fusion by LR reaction in Gateway technology (Invitrogen), respectively. The resulting plasmids were named as pKM20 for BOR2genome-GUS and pST1 for BOR2genome-GFP. mCherry fragment was amplified by PCR with the primers 5′-CGCCTCGACTCTAGAATGGTGAGCAAGGGCGAGGA-3′ and 5′-GGGAAATTCGAGCTCCTACTTGTACAGCTCGTCCA-3′ using wave1R (Geldner et al., 2009) as a template. The fragment was cloned into the XbaI and SacI sites of pMDC163 by a In-Fusion HD cloning kit (Takara). The resulting Gateway destination vector containing mCherry was named as pKI1. For the BOR1 construct, the genomic sequence corresponding to promoter and ORF was amplified with the primers 5′-AAAAAGCAGGCTCGGGAGATGACTAACAACGACAC-3′ and 5′-AGAAAGCTGGGTGTTCGATGACGACTGGTTCAAGG-3′. The DNA fragment was cloned into pDONR/zeo by BP reaction and then into pKI1 and pMDC164 (Curtis and Grossniklaus, 2003) for mCherry and GUS fusion by LR reaction with Gateway technology (Invitrogen), resulting in pKI2 for BOR1genome-mCherry and BOR1-pMDC164 for BOR1genome-GUS, respectively. pKM20 and pST1 were introduced into bor2-1, and BOR1-pMDC164 was introduced into bor1-1. bor1-3/bor2-1 were cotransformed with pST1 and pKI2. GFP fluorescence in the transgenic lines carrying BOR2genome-GFP (pST1) was similar to the cotransformed lines carrying both BOR2genome-GFP (pST1) and BOR1genome-mCherry (pKI2; Supplemental Fig. S1A; Fig. 2B). Introduction of the construct BOR2genome-GFP (pST1) into bor2-1 restored the bor2-1 growth under 0.3 µm boric acid in T2 generation, suggesting that BOR2-GFP was functional (Supplemental Fig. S1B).
GUS Staining
GUS staining was carried out with transgenic plants in T2 generation for BOR2genome-GUS and in T3 for BOR1genome-GUS. Plants were grown for 4 d in medium containing 0.3 µm boric acid. GUS staining was conducted as described in Shibagaki et al. (2002). Optical images were taken with a light microscope (BX50WI; Olympus).
GFP Imaging
Laser scanning confocal microscopy was performed using TCS-SP8 equipped with a water-immersed ×40 lens (Leica Microsystems). For imaging of GFP and FM4-64 (Molecular Probes), excitation wavelength was 488 nm and the detection wavelengths were 500 to 540 nm for GFP and greater than 640 nm for FM4-64. Dual-color images of GFP and mCherry were acquired by sequential line switching, allowing the separation of channels by both excitation and emission with the following excitation and detection wavelengths: 488 nm and 505 to 530 for GFP and 552 nm and 600 to 700 for mCherry. The plants were grown on vertically placed solid medium (Takano et al., 2005) containing 0.3 or 1 μm boric acid, 2% (w/v) Suc, and 1.5% (w/v) gellan gum (Wako Pure Chemicals) for 4 to 9 d. As an endocytic tracer, a styryl dye FM4-64 [N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino) phenyl) hexatrienyl) pyridinium dibromide] was used. FM4-64 was prepared as 10 mm stock solution in water and used at 4 µm, except for the experiments using BFA (Sigma). For plasmolysis, plants were exposed to 400 mm mannitol for 3 min. Stock solution of CHX (Sigma) and BFA were prepared at 50 mm in water and 50 mm in dimethyl sulfoxide, respectively. For BFA treatment, plants were grown in solid media containing 1 µm boric acid for 5 d. Then, knife-cut root tips (10 mm) were incubated with 50 µm CHX for 30 min and then 25 µm FM4-64 for 2 min in a plastic tube with a lid open. After washing the roots, they were treated with 50 µm CHX and 50 µm BFA for 30 min. To expose high-B concentrations, plants were first grown in solid media containing 0.3 µm boric acid for 9 d. Then, the plants were transferred to the media containing 0.3 or 100 µm boric acid and incubated in darkness for 150 min. Solutions for each treatment were prepared in the liquid medium (Takano et al., 2005).
Observation of Root Cells under Low-B Conditions
Plants were grown for 4 d in solid media containing 30 µm boric acid and transferred to medium supplemented with 0.001 (only for Col-0), 0.1, or 30 µm boric acid. Positions of primary root tip at 24 h after the medium transfer were marked. Two days after the medium transfer, epidermal cells located at the marked portion were observed. Plant roots were cut and treated with 10 µg mL–1 propidium iodide (Molecular Probes). They were observed using a confocal laser microscopy Zeiss LSM 510 with a 543-nm excitation wavelength and an emission window of greater than 560 nm for propidium iodide.
Analysis of B Concentrations in Shoots and Roots
For B measurements of shoots, plants were grown hydroponically in media supplemented with 0.1, 0.3, 3, 30, or 100 µm boric acid (Takano et al., 2001). Aerial portions of 30-d-old hydroponically cultured plants were harvested. For roots, plants grown on solid media containing 0.1 or 30 µm boric acid for 10 to 14 d were harvested. The plant tissues were dried at 60°C for more than 48 h, and then the dry weight was measured. The tissues were put in 8-mL Teflon tubes and digested at 130°C for 3 h with 1 mL of concentrated HNO3. The acid-digested samples were dissolved with 3 mL of the solution containing 5 μg L−1 beryllium in 0.08 n HNO3 and diluted with the same solution as appropriate. The B concentrations were then determined by inductively coupled plasma mass spectrometry (model SPQ9000; SII).
Determination of RG-II-B Dimer Ratio
To determine RG-II dimer formation in wild-type plants, plants were grown in solid media containing 0.001, 0.1, 0.3, 1, and 30 µm boric acid for 8 d. For one sample for cell wall extraction, 80 plants (0.001 and 0.1 µm) and 40 plants (0.3, 1, and 30 µm) were used. For comparison of RG-II dimerization among mutant lines, plants were grown on solid media containing 0.1 or 30 µm boric acid for 10 to 14 d. To prepare a cell wall fraction, roots were harvested and homogenized in 80% (v/v) ethanol. Insoluble residues after centrifugation were washed with 80% (v/v) ethanol twice, 99.5% (v/v) ethanol, chloroform/methanol twice, acetone, and water twice. Alcohol-insoluble residues considered as cell wall materials were treated with 0.1 m NaOH for more than 4 h at 4°C to saponify methyl and acetyl esters and then adjusted to pH 5.0 with 10% (v/v) acetic acid. They were digested with endopolygalacturonase (EPG) for 24 h at 4°C or 16 h at 35°C. We have already confirmed that EPG treatment at 4°C did not make differences from that at 35°C in the case of Arabidopsis wild-type roots. After centrifugation, the supernatant was filtered through a 0.2- or 0.45-µm membrane. They were subjected to size-exclusion HPLC/refractive index detector (Hitachi High Technologies Corporation) and analyzed as described in Matsunaga and Ishii (2006). The relative proportions of RG-II-B dimer to total RG-II were estimated from the peak area corresponding to RG-II-B dimer to the integration of the respective peak area of RG-II monomer and RG-II-B dimer.
Sequence data from this article can be found in the GenBank/EMBL databases under accession numbers GU971377 (BOR6, At5g25430) and GU971378 (BOR7, At4g32510).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Functional complementation of Arabidopsis carrying BOR2genome-GFP (BOR2g-GFP).
Supplementary Material
Acknowledgments
We thank Tsuyoshi Nakagawa and Niko Geldner for providing vectors, the technical assistance of Yuko Kawara and Kayoko Aizawa, Japanese Society for the Promotion of Science Research Fellowships for Young Scientists, and Hokkaido University Leader Development System in the Basic Interdisciplinary Research Areas.
Glossary
- RG-II
rhamnogalacturonan II
- B
boron
- T-DNA
transfer DNA
- cDNA
complementary DNA
- ORF
open reading frame
- BFA
brefeldin A
- CHX
cycloheximide
- Col-0
ecotype Columbia
- EPG
endopolygalacturonase
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell B, Huang LB. (1997) Physiological response of plants to low boron. Plant Soil 193: 103–120 [Google Scholar]
- Dordas C, Brown PH. (2000) Permeability of boric acid across lipid bilayers and factors affecting it. J Membr Biol 175: 95–105 [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S. (1992) Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol 99: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner N, Dénervaud-Tendon V, Hyman DL, Mayer U, Stierhof YD, Chory J. (2009) Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J 59: 169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Matsunaga T. (1996) Isolation and characterization of a boron-rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydr Res 284: 1–9 [Google Scholar]
- Ishii T, Matsunaga T, Pellerin P, O’Neill MA, Darvill A, Albersheim P. (1999) The plant cell wall polysaccharide rhamnogalacturonan II self-assembles into a covalently cross-linked dimer. J Biol Chem 274: 13098–13104 [DOI] [PubMed] [Google Scholar]
- Ishii T, Ono H. (1999) NMR spectroscopic analysis of the borate diol esters of methyl apiofuranosides. Carbohydr Res 321: 257–260 [Google Scholar]
- Kasai K, Takano J, Miwa K, Toyoda A, Fujiwara T. (2011) High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J Biol Chem 286: 6175–6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Kouzu N, Inami A, Toyooka K, Konishi Y, Matsuoka K, Matoh T. (2011) Characterization of Arabidopsis CTP:3-deoxy-d-manno-2-octulosonate cytidylyltransferase (CMP-KDO synthetase), the enzyme that activates KDO during rhamnogalacturonan II biosynthesis. Plant Cell Physiol 52: 1832–1843 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matoh T, Azuma JI. (1996) Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol 110: 1017–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WD, Durst RW. (1992) Chemistry and biology of boron. Biofactors 3: 229–239 [PubMed] [Google Scholar]
- Marschner H. (1995) Mineral Nutrition of Higher Plants, Ed 2. Academic Press, San Diego [Google Scholar]
- Matoh T (2001) Boron in plant nutrition and cell wall development. In N Ae, J Arihara, K Okada, A Srinivasan, eds, Plant Nutrient Acquisition, New Perspective. Springer-Verlag, Tokyo, pp 227–250 [Google Scholar]
- Matoh T, Ishigaki KI, Ohno K, Azuma JI. (1993) Isolation and characterization of a boron-polysaccharide complex from radish roots. Plant Cell Physiol 34: 639–642 [Google Scholar]
- Matoh T, Takasaki M, Takabe K, Kobayashi M. (1998) Immunocytochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant Cell Physiol 39: 483–491 [Google Scholar]
- Matsunaga T, Ishii T. (2006) Borate cross-linked/total rhamnogalacturonan II ratio in cell walls for the biochemical diagnosis of boron deficiency in hydroponically grown pumpkin. Anal Sci 22: 1125–1127 [DOI] [PubMed] [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. (2007) Plants tolerant of high boron levels. Science 318: 1417. [DOI] [PubMed] [Google Scholar]
- Miwa K, Fujiwara T. (2010) Boron transport in plants: co-ordinated regulation of transporters. Ann Bot (Lond) 105: 1103–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Fujiwara T. (2011) Role of overexpressed BOR4, a boron exporter, in tolerance to high level of boron in shoots. Soil Sci Plant Nutr 57: 558–565 [Google Scholar]
- Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, et al. (2007a) Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 71: 2095–2100 [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T. (2007b) Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 19: 2624–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Dannel F, Heidrun P, Römheld V, Hayashi H, Fujiwara T. (2000) Defect in root-shoot translocation of boron in Arabidopsis thaliana mutant bor1-1. J Plant Physiol 156: 756–761 [Google Scholar]
- Noguchi K, Ishii T, Matsunaga T, Kakegawa K, Hayashi H, Fujiwara T. (2003) Biochemical properties of the cell wall in the Arabidopsis mutant bor1-1 in relation to boron nutrition. J Plant Nutr Soil Sci 166: 175–178 [Google Scholar]
- Noguchi K, Yasumori M, Imai T, Naito S, Matsunaga T, Oda H, Hayashi H, Chino M, Fujiwara T. (1997) bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol 115: 901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MA, Eberhard S, Albersheim P, Darvill AG. (2001) Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science 294: 846–849 [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Ishii T, Albersheim P, Darvill AG. (2004) Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu Rev Plant Biol 55: 109–139 [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P. (1996) Rhamnogalacturonan II, a pectic polysaccharide in the walls of growing plant cells, forms a dimer that is covalently cross-linked by a borate ester. J Biol Chem 271: 22923–22930 [DOI] [PubMed] [Google Scholar]
- Pabst M, Fischl RM, Brecker L, Morelle W, Fauland A, Köfeler H, Altmann F, Léonard R. (2013) Rhamnogalacturonan II structure shows variation in the side chains monosaccharide composition and methylation status within and across different plant species. Plant J 76: 61–72 [DOI] [PubMed] [Google Scholar]
- Power PP, Woods WG. (1997) The chemistry of boron and its speciation in plants. Plant Soil 193: 1–13 [Google Scholar]
- Robinson DG, Jiang LW, Schumacher K. (2008) The endosomal system of plants: charting new and familiar territories. Plant Physiol 147: 1482–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B. (2003) An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol 53: 247–259 [DOI] [PubMed] [Google Scholar]
- Sherman F. (1991) Getting started with yeast. Methods Enzymol 194: 3–21 [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP. (2002) Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29: 475–486 [DOI] [PubMed] [Google Scholar]
- Shorrocks VM. (1997) The occurrence and correction of boron deficiency. Plant Soil 193: 121–148 [Google Scholar]
- Takano J, Kobayashi M, Noda Y, Fujiwara T. (2007) Saccharomyces cerevisiae Bor1p is a boron exporter and a key determinant of boron tolerance. FEMS Microbiol Lett 267: 230–235 [DOI] [PubMed] [Google Scholar]
- Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T. (2005) Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci USA 102: 12276–12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T. (2002) Arabidopsis boron transporter for xylem loading. Nature 420: 337–340 [DOI] [PubMed] [Google Scholar]
- Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T. (2010) Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc Natl Acad Sci USA 107: 5220–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T. (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18: 1498–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Yamagami M, Noguchi K, Hayashi H, Fujiwara T. (2001) Preferential translocation of boron to young leaves in Arabidopsis thaliana regulated by the BOR1 gene. Soil Sci Plant Nutr 47: 345–357 [Google Scholar]
- Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T. (2008) NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20: 2860–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warington K. (1923) The effect of boric acid and borax on the broad bean and certain other plants. Ann Bot (Lond) 37: 629–672 [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.