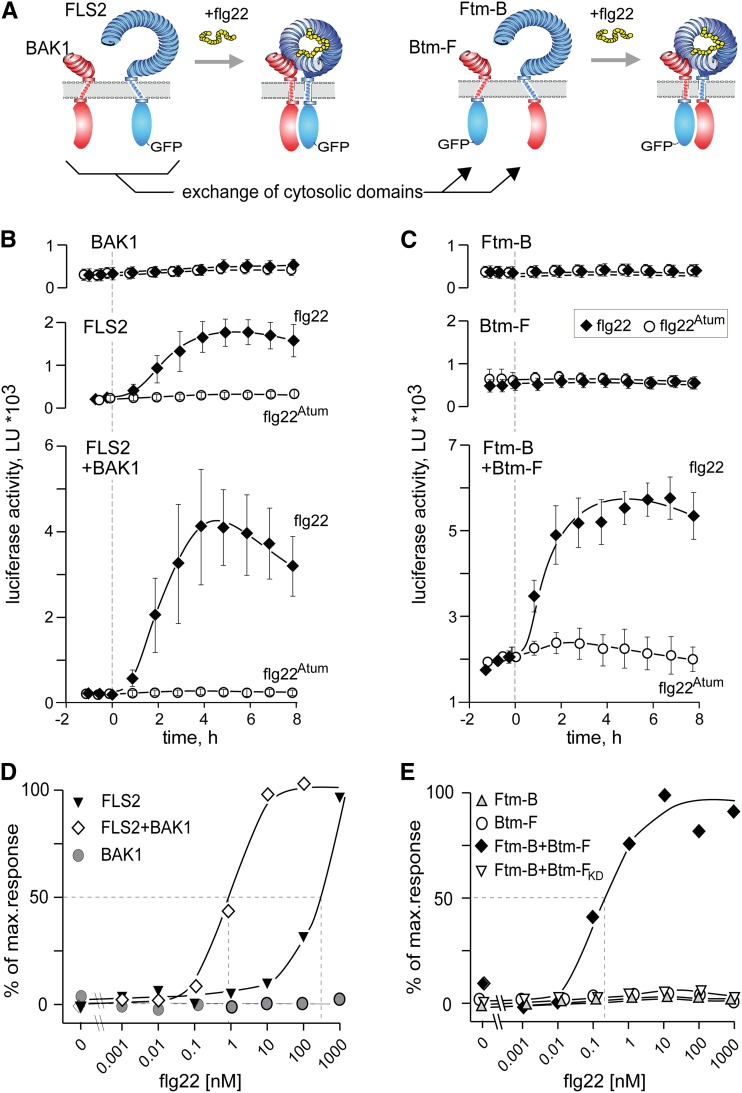
Receptor/coreceptor pairs with swapped cytosolic domains are fully functional, demonstrating importance of heteromer formation as molecular switch-on for intracellular signaling.
Abstract
Receptor kinases sense extracellular signals and trigger intracellular signaling and physiological responses. However, how does signal binding to the extracellular domain activate the cytoplasmic kinase domain? Activation of the plant immunoreceptor Flagellin sensing2 (FLS2) by its bacterial ligand flagellin or the peptide-epitope flg22 coincides with rapid complex formation with a second receptor kinase termed brassinosteroid receptor1 associated kinase1 (BAK1). Here, we show that the receptor pair of FLS2 and BAK1 is also functional when the roles of the complex partners are reversed by swapping their cytosolic domains. This reciprocal constellation prevents interference by redundant partners that can partially substitute for BAK1 and demonstrates that formation of the heteromeric complex is the molecular switch for transmembrane signaling. A similar approach with swaps between the Elongation factor-Tu receptor and BAK1 also resulted in a functional receptor/coreceptor pair, suggesting that a “two-hybrid-receptor assay” is of more general use for studying heteromeric receptor complexes.
Cell surface receptors are chemical sensors, often with an exquisite specificity and sensitivity, which detect extracellular signals and initiate corresponding intracellular response programs. Many of these receptors are transmembrane proteins with an extracellular ligand-binding domain and an intracellular protein kinase domain. Higher plants, such as Arabidopsis (Arabidopsis thaliana), have several hundred genes encoding receptor like kinases (Shiu and Bleecker, 2001; Shiu and Li, 2004). How are these receptor like kinases activated by their ligands, and how do they initiate a subsequent intracellular signaling cascade? In our work, we used the leucine-rich repeat receptor kinase (LRR-RK) Flagellin sensing2 (FLS2), which specifically detects bacterial flagellin or its peptide epitope flg22 at subnanomolar concentrations (Gomez-Gomez and Boller, 2000; Gómez-Gómez et al., 2001; Chinchilla et al., 2006). FLS2 undergoes heteromeric complex formation with brassinosteroid receptor1 associated kinase1 (BAK1) within seconds after application of the flagellin-derived peptide ligand flg22 (Chinchilla et al., 2007; Heese et al., 2007; Schulze et al., 2010). Thus, BAK1 might act as a coreceptor of FLS2. However, as previously observed (Chinchilla et al., 2007; Roux et al., 2011), FLS2 is still functional in the absence of BAK1, although with a reduced efficiency. This raises the question whether the ligand-induced heteromeric complex has merely an enhancing effect or whether association with BAK1 or a functional substitute acts as the essential switch-on for transmembrane signaling of FLS2. BAK1 is one of the five members that form the somatic embryogenesis receptor kinase (SERK) family (Albrecht et al., 2008), and other members of this family might partially substitute for BAK1 (Roux et al., 2011). However, a rigorous genetic approach to delineate the role of these potential substitutes is not feasible because triple mutants (serk1 serk3 serk4) and quadruple mutants (serk1 serk2 serk3 serk4) exhibit severe general phenotypes of dwarfing or even lethality at the early embryo stage (He et al., 2007; Gou et al., 2012) that might be due to the important role of SERKs in plant developmental processes (Li et al., 2002; Nam and Li, 2002).
To address the role of the heteromeric complex with BAK1 in the absence of other interfering SERKs, we took a two-hybrid-receptor approach based on the premise that the apoplastic and cytoplasmic domains of FLS2 and BAK1 function in a modular manner. A heteromeric complex might thus also form and function when the roles of FLS2 and BAK1 are reversed by reciprocal swapping of their cytoplasmic protein kinase domains (Fig. 1A, schematic view).
Figure 1.
Heteromeric complex formation of FLS with BAK1 switches on flagellin-dependent transmembrane signaling. A, Model for the flg22-dependent heteromeric receptor complex. Schematic representation of FLS2 (blue), BAK1 (red), and the chimeric receptor constructs Ftm-B and Btm-F. Ftm-B comprises the extracellular and the transmembrane domains of FLS2 (blue) and the cytoplasmic domain of BAK1 (red). The receptor chimera Btm-F represents the reciprocal construct with the cytoplasmic part of BAK1 replaced by that of FLS2. The cytoplasmic domain of FLS2 was C-terminally tagged with a GFP. B and C, Functional comparison of native FLS2 and BAK1 (B) with the two hybrid receptors Ftm-B and Btm-F (C). The experiments show luciferase activity in Arabidopsis fls2 bak1-4 double mutant protoplasts cotransformed with pFRK1::Luciferase as a reporter and the receptor constructs indicated. At 0 h (dashed line) protoplasts were treated with 100 nm of flg22 (black diamonds) or 100 nm of the inactive analog flg22Atum (white circles). Light emission of the protoplasts was measured with a luminometer. Values represent averages and sds of three replicates. Data shown are representative for at least three independent repetitions of the experiments with all constructs. D and E, Dose-response relationship for flg22-dependent induction of pFRK1::Luciferase in protoplasts expressing the receptor constructs indicated. Values represent increase in luciferase activity after 5 h of treatment as percentage of the increase observed with FLS2 plus BAK1 treated with saturating doses of greater than or equal to 10 nm flg22. Comparison of (half-maximal stimulation values in D and E shows that cells coexpressing Ftm-B plus Btm-F responded at least as sensitive to flg22 as cells coexpressing FLS2 plus BAK1. A combination of Ftm-B with the kinase dead version Btm-FKD was not functional, even when treated with 1,000 nm flg22. LU, Light units.
RESULTS
FLS2 and BAK1 Also Function after Reciprocal Swaps of Their Kinase Domains
Swapping the cytoplasmic domains between the LRR-RKs FLS2 and BAK1 resulted in the receptor chimera Ftm-B, consisting of the FLS2 ectodomain including FLS2 transmembrane domain and the cytoplasmic part of BAK1, and the reciprocal receptor chimera Btm-F, consisting of the ectodomain and the transmembrane domain of BAK1 and the cytoplasmic domain of FLS2, respectively (Fig. 1A; Supplemental Fig. S1). In contrast to tagging FLS2 (Zipfel et al., 2004), a C-terminal GFP tag was reported to compromise the functionality of BAK1 (Ntoukakis et al., 2011), and the receptor constructs with the kinase of BAK1 were used without a tag. To test the functionality of the chimeric receptors, we used mesophyll protoplasts from Arabidopsis leaves transformed with a reporter gene encoding Luciferase under the control of the flg22-responsive promoter of flg22-induced receptor-like kinase1 (pFRK1) as a well-established and highly sensitive monitoring system (Yoo et al., 2007; Albert et al., 2010; Mueller et al., 2012). Protoplasts derived from Arabidopsis fls2 bak1 double mutant plants, transformed with a gene encoding BAK1, exhibited low background of luciferase activity, and this activity did not increase in response to flg22 (Fig. 1B). By contrast, protoplasts transformed with authentic FLS2 exhibited clear induction of luciferase after treatment with 100 nm flg22 but not after treatment with the inactive peptide flg22Atum (Felix et al., 1999; Albert, 2013; Fig. 1B, middle). Similarly, transformation with FLS2 still resulted in a functional response to flg22 in protoplasts of Arabidopsis efr fls2 bak1-5 serk4 that lack functionality of two of the SERKs (Supplemental Fig. S2). This confirms results of previous publications (Chinchilla et al., 2007; Roux et al., 2011) that indicated functionality of FLS2 not to be strictly dependent on BAK1. Nevertheless, in protoplasts lacking BAK1, a distinctly higher response to flg22 was observed after cotransformation with FLS2 plus BAK1 than after transformation with FLS2 alone (Fig. 1B, bottom). Expression of either one of the chimeric receptors Btm-F or Ftm-B, respectively, did not confer responsiveness to flg22 (Fig. 1C). Importantly, however, protoplasts expressing a combination of Ftm-B plus Btm-F rapidly and strongly responded to flg22 (Fig. 1C), demonstrating that the combination of the chimeric receptors with the reciprocal swaps can fully reconstitute flg22 perception.
Equal Responsiveness of Authentic and Chimeric Receptor Pairs
A quantitative analysis with testing of different concentrations of flg22 confirmed that double mutants of fls2 bak1 transformed with BAK1 alone did not respond to flg22, even at the highest concentration of 1,000 nm tested (Fig. 1D). Cells transfected with FLS2 alone were nonresponsive to concentrations of up to 10 nm flg22, but clearly responded to higher concentrations (Fig. 1D). Cells transfected with FLS2 plus BAK1 were approximately 300 times more sensitive and showed half-maximal response at a concentration of approximately 1 nm flg22 (Fig. 1D). Cells with Ftm-B alone or Btm-F alone did not show any response to flg22, even at the highest concentrations of 1,000 nm tested. By contrast, cells with the chimeric forms Ftm-B plus Btm-F proved at least as sensitive to flg22 as cells with the authentic forms FLS2 plus BAK1 and clearly responded with an increase in luciferase activity down to concentrations of approximately 0.1 nm flg22 (Fig. 1E). As reported for FLS2 (Asai et al., 2002), functionality of the chimeric form Btm-F was dependent on a functional kinase, and the kinase dead version Btm-FKD was unable to substitute for Btm-F when tested in combination with Ftm-B (Fig. 1E; Supplemental Fig. S3).
Ligand-Dependent Complex Formation of the Chimeric Receptors
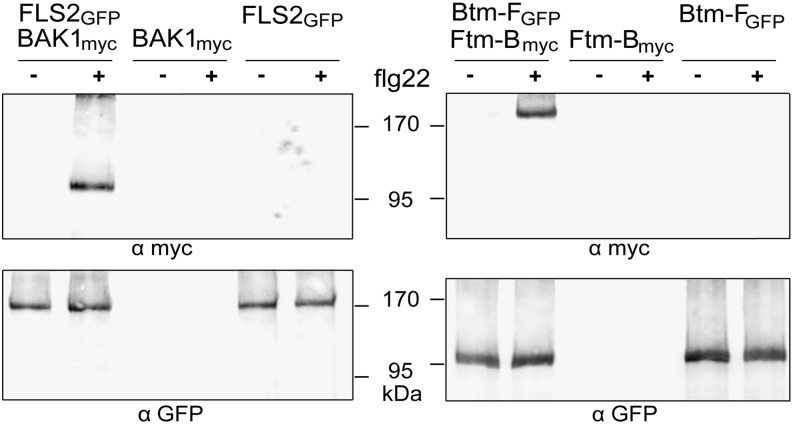
Receptor activation by flg22 coincides with rapid formation of a stable complex between FLS2 and BAK1 (Chinchilla et al., 2007). This can be conveniently tested by transient expression of receptors in Nicotiana benthamiana followed by immunoprecipitation (Roux et al., 2011). For testing complex formation of the chimeric receptors, myc tags were added to the C termini of BAK1 and Ftm-B (Supplemental Fig. S1). Leaves either treated with flg22 for 2 min or not were analyzed for the myc-tagged proteins in the immunoprecipitates obtained with antibodies against the GFP tags present on FLS2 and Btm-F (Fig. 2). The results clearly demonstrate that Ftm-B and Btm-F undergo the same rapid and ligand-dependent complex formation as authentic FLS2 and BAK1.
Figure 2.
Ligand-induced receptor complexes FLS2/BAK1 and Ftm-B/Btm-F. Coimmunoprecipitation assays with receptor constructs expressed in N. benthamiana leaves. Detergent-solubilized extracts from leaves, treated for 2 min with 100 nm flg22 as indicated, were immunoprecipitated via the GFP tag present at the C terminus of FLS2 and Btm-F. Western blots were developed with antibodies against the myc tag on BAK1 and Ftm-B (top) or the GFP tag present on FLS2 and Btm-F (bottom).
Complex Formation of EFR and BAK1 with Swapped Kinases Switch on Signaling as Well
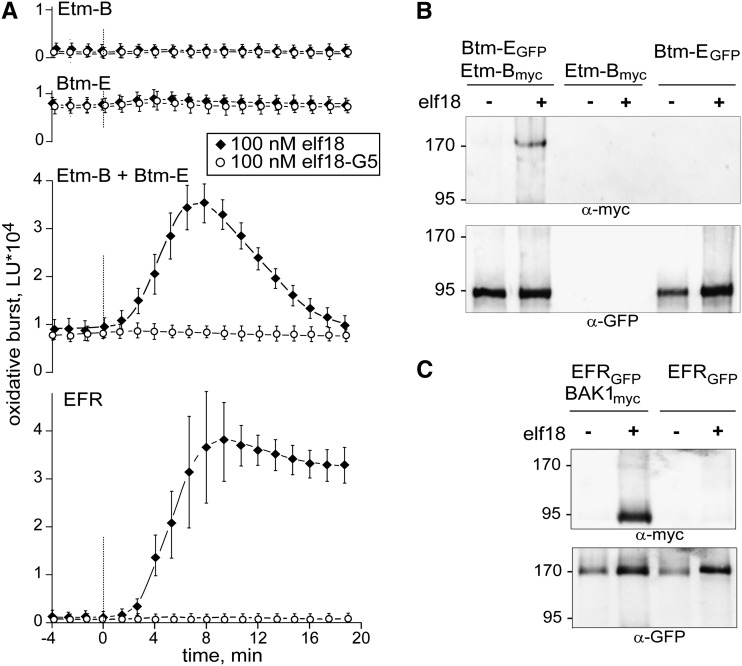
N. benthamiana has no ortholog of the Arabidopsis receptor kinase EFR (for elongation factor-Tu receptor) but can be rendered responsive to the EF-Tu-derived elf18 peptide by transformation with constructs encoding the LRR-RK EFR from Arabidopsis (Zipfel et al., 2006). We thus constructed Etm-B and Btm-E (Supplemental Fig. S1), chimeric receptors with swapped cytoplasmic domains, to test the importance of heteromeric complex formation for activation of EFR. As reported previously (Zipfel et al., 2006), leaves of N. benthamiana expressing authentic EFR gain responsiveness to elf18 and respond with rapid activation of an oxidative burst (Fig. 3A, bottom). Leaves expressing either Etm-B or Btm-E alone exhibited no response to elf18. Expression of Btm-E, either alone or together with Etm-B, caused a somewhat elevated background of reactive oxygen species (ROS) also in the absence of elf18 (Fig. 3A). Nevertheless, the leaves with a combination of Etm-B plus Btm-E clearly responded with induction of an oxidative burst to application of elf18 (Fig. 3A). Thereby, induction of an elf18-dependent oxidative burst was paralleled by phosphorylation of mitogen-activated protein (MAP) kinases in leaves expressing Etm-B plus Btm-E but not in leaves expressing each of the constructs alone (Supplemental Fig. S4). Also, coimmunoprecipitation experiments show that Btm-E and Etm-B, much like the authentic pair of EFR and BAK1 (Fig. 3, B and C), undergo rapid ligand-dependent complex formation.
Figure 3.
Functionality of the chimeric EFR-BAK1 receptors Etm-B and Btm-E. A, Oxidative burst measurements in N. benthamiana leaves expressing Etm-B, Btm-E, a combination of Etm-B plus Btm-E, or EFR. Leaf pieces were treated with 100 nm elf18 or 100 nm of the inactive analog elf18G5, and ROS production was measured as light units (LU) by a luminometer for 20 min. Data show average and sd for three replicates. Experiments shown are representative for at least five independent repetitions of the experiments with all constructs. B and C, Elf18-induced complexes Etm-B/Btm-E and EFR/BAK1. Coimmunoprecipitation assays with receptor constructs expressed in N. benthamiana leaves. Detergent-solubilized extracts from leaves, treated for 2 min with 100 nm elf18 as indicated, were immunoprecipitated via the GFP tag present at the C terminus of EFR and Btm-E. Western blots were developed with antibodies against the myc tag on BAK1 and Etm-B (top) or the GFP tag present on EFR and Btm-E (bottom).
DISCUSSION AND CONCLUSION
Heteromer formation with BAK1 has first been described for the receptor kinase BRI1 (Li et al., 2002; Nam and Li, 2002; Santiago et al., 2013). The ligand-induced complex BRI1/BAK1 undergoes sequential mutual transphosphorylation of both partners (Wang et al., 2008; Oh et al., 2009, 2010). Similarly, ligand-induced complex formation of BAK1 with EFR, FLS2, and Peptide1 receptor1 has also been found to coincide with de novo phosphorylations of the complex partners in vivo (Schulze et al., 2010; Schwessinger et al., 2011). While much of the current research on these receptor kinases is focused on phosphorylation sites and their relevance for activation of downstream signaling (Oh et al., 2009, 2010; Schwessinger et al., 2011; Yan et al., 2012), our approach with two hybrid receptors concentrated on the initial step in signaling that brings the receptor and coreceptor in close contact.
Previous investigations with chimeric receptors focused either on swapping of the extracellular domain to get insights into ligand perception (Wulff et al., 2001, 2004; Mueller et al., 2012) or on the cytosolic kinase domain to reprogram the cellular response output (He et al., 2000; Albert et al., 2010). Here, we used the receptor pairs FLS2/BAK1 and EFR/BAK1 with reciprocal swaps of their cytoplasmic domains to study the function of heteromer formation for transmembrane signal activation. Both chimeric receptor pairs were found to form ligand-dependent heteromeric complexes and to exhibit full functionality and sensitivity with respect to activation of immune signaling in response to their ligands. With both chimeric receptor pairs, however, we observed a somewhat increased basal level of response in the absence of ligand that correlated with the presence of Btm-F or Btm-E, respectively (Figs. 1C and 3A). Mechanisms that usually control and inhibit the signaling output of BAK1, such as interaction with BAK1 interacting receptor kinase (Gao et al., 2009), might not work properly with these chimeric receptors. Alternatively, these chimeric forms of BAK1 might interfere with other receptor-mediated processes that depend on BAK1. Interference with regulatory processes might also explain the failure of our attempts to obtain stably transformed Arabidopsis plants expressing the chimeric receptors Btm-F or Btm-E. Transformation with constructs encoding these receptors resulted in very few seedlings with a functional selection marker, and none of them accumulated detectable levels of Btm-F or Btm-E protein. Importantly though, the elevated background did not affect functionality of the receptor/coreceptor pairs in the transient expression systems.
The use of two hybrid complex partners established in this work serves as a tool to exclude interference by redundant components such as the other SERKs. While useful for future studies, such as testing which of the SERKs can functionally substitute for BAK1 and identifying the subdomains of the receptors relevant for complex formation, this approach should also be more generally applicable on other receptors that undergo heteromeric interactions.
Our findings show that the extracellular and the intracellular domains of FLS2, EFR, and BAK1 act as modules that can match also when reciprocally interchanged between the partners of the heteromeric complexes. These experiments also provide direct evidence that heteromer formation, rather than having an enhancing or facilitating effect, acts as a closing switch for receptor activation and transmembrane signaling. Thus, we postulate that BAK1 and other SERKs have a general and indispensable role as transmembrane activators for signal-binding receptors like FLS2, EFR, and, likely, many other receptor kinases. The two-hybrid-receptor technique applied in this report might be of more general use, inspiring studies of ligand-binding receptors and transmembrane signaling in research areas beyond plant immunity.
MATERIALS AND METHODS
Receptor Construction
Chimeric receptors were generated by fusion of two separate PCR products via overlapping primers as described (Albert et al., 2010). Sequence parts of fusion regions are indicated in Supplemental Figure S1. BAK1, FLS2, and the chimeric receptors were recombined into the pK7FWG2.0 expression vector (http://www.psb.ugent.be) using Gateway technology (Invitrogen). FLS2 and Btm-F were fused to a C-terminal GFP tag. The myc-tagged versions of Ftm-B, Etm-B, and BAK1 were expressed in the pGWB20 vector. The kinase dead version Btm-FKD was constructed via introducing point mutations indicated in Supplemental Figure S2.
Functionality Assays
Transient expression in leaf mesophyll protoplasts of fls2 bak1-4 double and efr fls2 bak1-5 serk4 quadruple mutant plants were performed as described (Yoo et al., 2007). Aliquots of 100,000 protoplasts were cotransformed with 25 μg plasmid DNA encoding FRK1::luciferase (Yoo et al., 2007) and 1.25 to 5 μg plasmid DNA encoding FLS2, BAK1, or the chimeric receptors. Protoplasts were resuspended in W5 solution with 0.2 mm luciferin (d-Luciferin, firefly [Photinus pyralis], P.J.K.), and 100-μL aliquots containing 10,000 protoplasts were distributed in a 96-well plate. After 16 h, the cells were treated with flg22 or flg22Atum, and luciferase activity was monitored as light emitted from the protoplasts in a luminometer (Mithras LB 940). For analysis of the oxidative burst, leaf samples of transiently transformed Nicotiana benthamiana were analyzed for ROS production in a luminol-dependent assay as described (Albert et al., 2010).
Coimmunoprecipitation
Agrobacterium tumefaciens carrying the plasmids encoding the appropriate constructs were infiltrated in different combinations (optical density at 600 nm = 0.1) into N. benthamiana leaves. After approximately 36 to 40 h of incubation leaves were infiltrated with 200 nm flg22, and material was harvested 2 min later. Membrane proteins were solubilized as described (Chinchilla et al., 2007) and immune adsorbed via the GFP tag to GFP-Trap beads (ChromoTek, IZB Martinsried). Western blots were developed with anti-GFP antibodies or antibodies against the myc tag followed by staining with secondary antibodies coupled to alkaline phosphatase and CDP-Star (Roche) as substrate. Chemiluminescence was detected with a CCD camera (Viber Louromat, PeqLAB).
MAP Kinase Phosphorylation
N. benthamiana leaves transiently expressing the receptor constructs were infiltrated with 100 nm elf18 or 100 nm elf18G5, respectively, and leaf samples were analyzed for phosphorylated MAP kinases on Western blots using Phospho-P44/42 MAPK (Erk1/2; Thr-202/Tyr-204) Antibody (Cell Signaling Technologies).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At5g46330 (FLS2; AtFLS2), At5g20480 (EFR), and At4g33430 (BAK1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Schematic view of hybrid-receptor constructs.
Supplemental Figure S2. Functionality of FLS2 in bak1 serk4 protoplasts.
Supplemental Figure S3. Ftm-B is not functional in combination with kinase dead version of Btm-F.
Supplemental Figure S4. Ligand-dependent phosphorylation of MAP kinases.
Supplementary Material
Acknowledgments
We thank Isabell Albert for critically reading the manuscript and discussion and Ana Dominguez for providing the efr fls2 bak1-5 serk4 Arabidopsis mutant plants.
Glossary
- LRR-RK
Leu-rich repeat receptor kinase
- ROS
reactive oxygen species
- MAP
mitogen-activated protein
References
- Albert M. (2013) Peptides as triggers of plant defence. J Exp Bot (in press) [DOI] [PubMed] [Google Scholar]
- Albert M, Jehle AK, Mueller K, Eisele C, Lipschis M, Felix G. (2010) Arabidopsis thaliana pattern recognition receptors for bacterial elongation factor Tu and flagellin can be combined to form functional chimeric receptors. J Biol Chem 285: 19035–19042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht C, Russinova E, Kemmerling B, Kwaaitaal M, de Vries SC. (2008) Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE proteins serve brassinosteroid-dependent and -independent signaling pathways. Plant Physiol 148: 611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18: 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, et al. (2009) Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Bauer Z, Boller T. (2001) Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13: 1155–1163 [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gomez L, Boller T. (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gou X, Yin H, He K, Du J, Yi J, Xu S, Lin H, Clouse SD, Li J. (2012) Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet 8: e1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J. (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17: 1109–1115 [DOI] [PubMed] [Google Scholar]
- He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J. (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288: 2360–2363 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. (2002) BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110: 213–222 [DOI] [PubMed] [Google Scholar]
- Mueller K, Bittel P, Chinchilla D, Jehle AK, Albert M, Boller T, Felix G. (2012) Chimeric FLS2 receptors reveal the basis for differential flagellin perception in Arabidopsis and tomato. Plant Cell 24: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J. (2002) BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110: 203–212 [DOI] [PubMed] [Google Scholar]
- Ntoukakis V, Schwessinger B, Segonzac C, Zipfel C. (2011) Cautionary notes on the use of C-terminal BAK1 fusion proteins for functional studies. Plant Cell 23: 3871–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Kota U, Goshe MB, Clouse SD, Huber SC. (2009) Tyrosine phosphorylation of the BRI1 receptor kinase emerges as a component of brassinosteroid signaling in Arabidopsis. Proc Natl Acad Sci USA 106: 658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Wu X, Zhao Y, Clouse SD, Huber SC. (2010) Autophosphorylation of Tyr-610 in the receptor kinase BAK1 plays a role in brassinosteroid signaling and basal defense gene expression. Proc Natl Acad Sci USA 107: 17827–17832 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C. (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Henzler C, Hothorn M. (2013) Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science 341: 889–892 [DOI] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. (2010) Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem 285: 9444–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. (2011) Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet 7: e1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. (2001) Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 2001: re22. [DOI] [PubMed] [Google Scholar]
- Shiu SH, Li WH. (2004) Origins, lineage-specific expansions, and multiple losses of tyrosine kinases in eukaryotes. Mol Biol Evol 21: 828–840 [DOI] [PubMed] [Google Scholar]
- Wang X, Kota U, He K, Blackburn K, Li J, Goshe MB, Huber SC, Clouse SD. (2008) Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell 15: 220–235 [DOI] [PubMed] [Google Scholar]
- Wulff BB, Kruijt M, Collins PL, Thomas CM, Ludwig AA, De Wit PJ, Jones JD. (2004) Gene shuffling-generated and natural variants of the tomato resistance gene Cf-9 exhibit different auto-necrosis-inducing activities in Nicotiana species. Plant J 40: 942–956 [DOI] [PubMed] [Google Scholar]
- Wulff BB, Thomas CM, Smoker M, Grant M, Jones JD. (2001) Domain swapping and gene shuffling identify sequences required for induction of an Avr-dependent hypersensitive response by the tomato Cf-4 and Cf-9 proteins. Plant Cell 13: 255–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Ma Y, Liu D, Wei X, Sun Y, Chen X, Zhao H, Zhou J, Wang Z, Shui W, et al. (2012) Structural basis for the impact of phosphorylation on the activation of plant receptor-like kinase BAK1. Cell Res 22: 1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.