Root border-like cells of flax and Arabidopsis activate innate immunity responses to elicitors involving both callose deposition and cell wall extensin reorganization.
Abstract
Plant pathogens including fungi and bacteria cause many of the most serious crop diseases. The plant innate immune response is triggered upon recognition of microbe-associated molecular patterns (MAMPs) such as flagellin22 and peptidoglycan. To date, very little is known of MAMP-mediated responses in roots. Root border cells are cells that originate from root caps and are released individually into the rhizosphere. Root tips of Arabidopsis (Arabidopsis thaliana) and flax (Linum usitatissimum) release cells known as “border-like cells.” Whereas root border cells of pea (Pisum sativum) are clearly involved in defense against fungal pathogens, the function of border-like cells remains to be established. In this study, we have investigated the responses of root border-like cells of Arabidopsis and flax to flagellin22 and peptidoglycan. We found that both MAMPs triggered a rapid oxidative burst in root border-like cells of both species. The production of reactive oxygen species was accompanied by modifications in the cell wall distribution of extensin epitopes. Extensins are hydroxyproline-rich glycoproteins that can be cross linked by hydrogen peroxide to enhance the mechanical strength of the cell wall. In addition, both MAMPs also caused deposition of callose, a well-known marker of MAMP-elicited defense. Furthermore, flagellin22 induced the overexpression of genes involved in the plant immune response in root border-like cells of Arabidopsis. Our findings demonstrate that root border-like cells of flax and Arabidopsis are able to perceive an elicitation and activate defense responses. We also show that cell wall extensin is involved in the innate immunity response of root border-like cells.
Plant root tips constantly release metabolically active border cells into the rhizosphere (Hawes et al., 2000). Border cells have been defined as cells that separate from the root tip of higher plants and disperse individually into suspension immediately after their contact with water (Hawes et al., 2000, 2003). These cells play a fundamental role in controlling the interaction of plant roots with neighboring organisms within the soil (Hawes et al., 2000; Gunawardena and Hawes, 2002; Cannesan et al., 2011). Upon their separation from the root cap, border cells become uniquely differentiated, producing proteins and metabolites that are distinct from those made by the root cap cells (Brigham et al., 1995; Wen et al., 2007).
Root border cells impact plant health and survival by protecting the root meristem from pathogenic infection, as has been clearly demonstrated for pea (Pisum sativum) against the fungus Nectria haematococca (Gunawardena and Hawes, 2002; Gunawardena et al., 2005). Border cells have also been shown to repel pathogenic bacteria by means of their secreted mucilage (Hawes et al., 2000). During their detachment from the root cap, border cells of legumes export a large number of proteins, the secretome, containing antimicrobial enzymes, including chitinase, peptidase, and glucanase (Wen et al., 2007, 2009). Furthermore, pea border cells secrete other compounds such as extracellular DNA, the phytoalexin pisatin, and arabinogalactan proteins that contribute to root protection against soil-borne pathogens (Wen et al., 2009; Cannesan et al., 2011, 2012; Hawes et al., 2011).
Arabidopsis (Arabidopsis thaliana) root tips release the so-called border-like cells that are different from border cells in both organization (i.e. they remain attached to each other instead of being isolated as individual cells) and number (a few hundreds instead of thousands; Vicré et al., 2005; Durand et al., 2009; Driouich et al., 2012). Root border-like cells are produced by other Brassicaceae species including Brassica napus and radish (Raphanus sativus; Driouich et al., 2007, 2010, 2012), although they are not specific to the Brassicaceae family. Indeed, it has been recently shown that border-like cells are also produced by a few other species, including flax (Linum usitatissimum), an agronomically important crop that belongs to the Linaceae family (Driouich et al., 2012).
Root border-like cell walls of Arabidopsis are specifically enriched in pectic and arabinogalactan protein epitopes (Vicré et al., 2005). While pectic polysaccharides present at the cell surface of border-like cells have been found to control their attachment to each other, arabinogalactan proteins were shown to control the interactions between root border-like cells and the soil bacteria Rhizobium spp. (Vicré et al., 2005). In addition, recent studies have provided fresh evidence of the involvement of arabinogalactan proteins as well as extensins in the interaction of root cells with other microbial pathogens, including oomycetes and fungi (Xie et al., 2011; Cannesan et al., 2012).
While the protective function of “classical” border cells has been mostly studied in pea (Hawes et al., 2000; Gunawardena and Hawes, 2002; Pan et al., 2004), there is no information available so far regarding the function of root border-like cells in either Arabidopsis or flax plants (Vicré et al., 2005; Driouich et al., 2007, 2010). Considering the importance of classical root border cells in root protection and plant defense, we hypothesized that border-like cells could also play a significant role in plant-microbe interactions. Thus, as border cells do, border-like cells could possibly be involved in local root defense, providing a protection to the vulnerable root meristem against soil-borne pathogens. However, it is not known whether border-like cells are able to specifically perceive and respond to pathogens.
Plants recognize pathogens through sensing of conserved microbial epitopes called microbe-associated molecular patterns (MAMPs), such as bacterial flagellin (Felix et al., 1999) and components derived from microbe cell walls (e.g. chitin, peptidoglycan, lipopolysaccharides, etc.). The recognition of MAMPs through specific plant receptors triggers the activation of a collaborative defense response to restrict pathogen growth. This primary innate immune response includes the induction of mitogen-activated protein kinase signaling, transcriptional reprogramming, production of reactive oxygen species (ROS; Boller and Felix, 2009; Millet et al., 2010), and modifications of cell wall structure via deposition of callose (Hao et al., 2008) or accumulation of Hyp-rich glycoproteins such as extensin (Wojtaszek et al., 1995; Ribeiro et al., 2006). Most of this knowledge has come from a number of studies performed on leaves. To date, there is relatively little information available on cell responses to MAMPs in roots (Attard et al., 2010; Millet et al., 2010). These studies have highlighted that (1) root responses often differ from what is observed in leaves and (2) the MAMP response in roots is tissue specific and therefore highly complex (Millet et al., 2010; Cannesan et al., 2012).
One of the main objectives of this study was to determine whether border-like cells could sense and respond to MAMPs, as they are among the first structures of plant roots to interact with soil microorganisms. To this end, we have examined the response of flax and Arabidopsis border-like cells to flagellin22 (flg22) and peptidoglycan (PGN) from Bacillus subtilis, two well-characterized MAMPs that are widely used in various plant systems, including Arabidopsis (Felix et al., 1999; Kunze et al., 2004; Mueller et al., 2012). Using microscopical and immunocytochemical techniques in conjunction with specific probes as well as Arabidopsis mutants, we show that border-like cells of both plant species are able to respond specifically to MAMPs by producing ROS, including superoxide (O2·–) and singlet oxygen (1O2), and deposition of callose, a well-known marker of defense. In addition, significant accumulation and alteration of extensin epitopes within the cell wall are also observed upon elicitation.
RESULTS
Characterization of Flax Root Border-Like Cells and Their Cell Walls
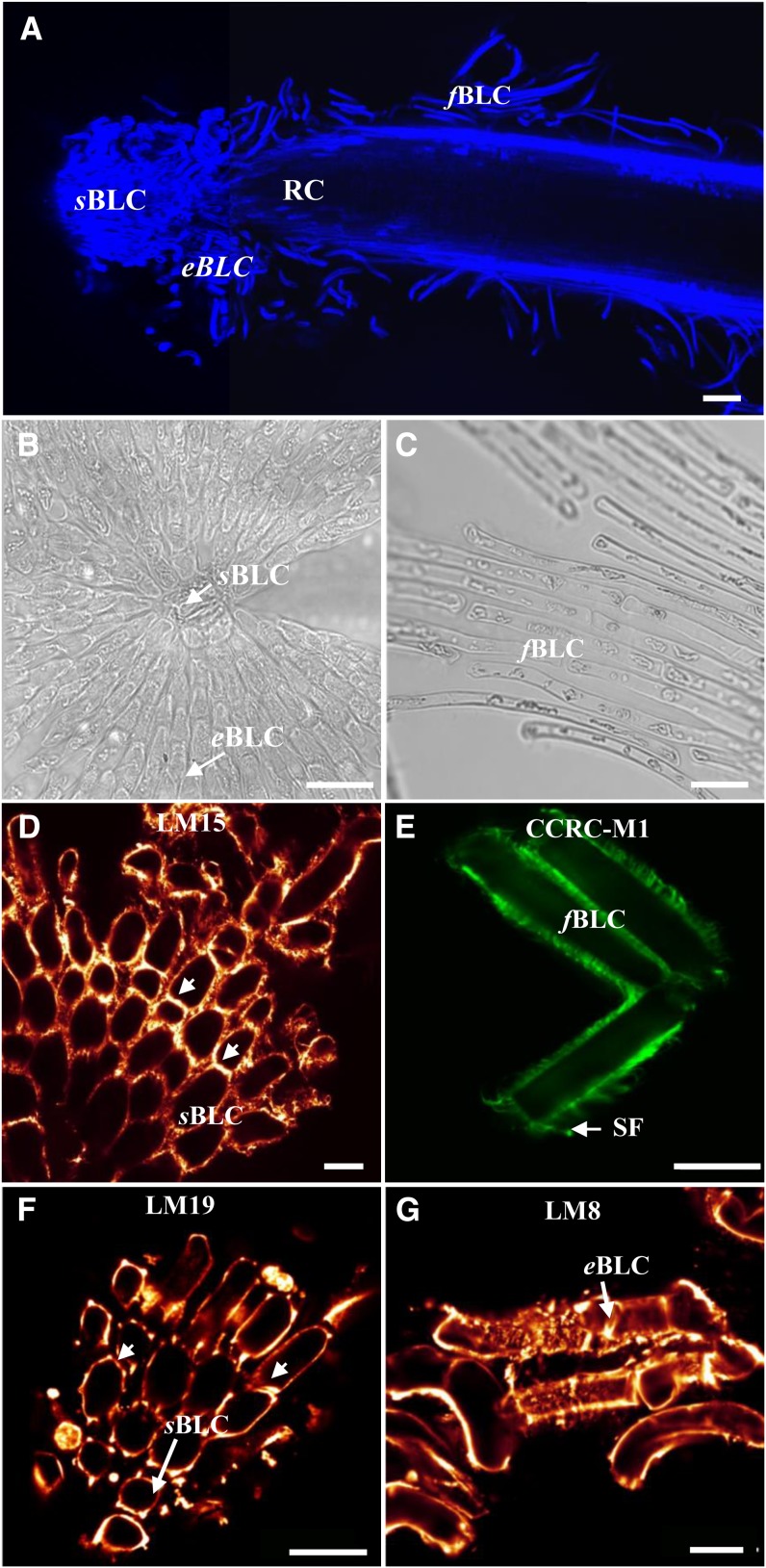
It is now well established that Arabidopsis root tips release viable border-like cells (Vicré et al., 2005). Such cells have also been observed in other Brassicaceae species and in flax, an economically important crop in the Linaceae family (Driouich et al., 2007, 2012). Here, we further characterize flax border-like cells. Under our experimental conditions, flax root border-like cells appear at the root tip at 2 d of growth, reaching a number of 416 ± 14 (Supplemental Table S1), which increased to reach a maximum of 8,250 ± 2,308 at 6 d. In terms of number, flax root produces many more border-like cells than the Brassicaceae species Arabidopsis (116 ± 10), B. napus (375 ± 137), and radish (907 ± 75; Driouich et al., 2012). Similar to Arabidopsis, flax root border-like cells consist of several layers of cells that remain attached to the root tip or are released in the vicinity of the root (Fig. 1, A–C). In terms of morphology, only two cell types have been observed in Arabidopsis, spherical and elongated cells (Vicré et al., 2005). In flax, it was possible to distinguish three populations of root border cells based on their morphological features: the spherical border-like cells present at the very tip of the root, the elongated border-like cells, and the filamentous border-like cells (Fig. 1, B and C; Supplemental Table S2).
Figure 1.
Microscopical characterization of root border-like cells from flax. Calcofluor staining of the root tip shows border-like cell organization. A, In flax, three distinct populations of root border-like cells occur: spherical border-like cells (sBLC), elongated border-like cells (eBLC), and filamentous border-like cells (fBLC). RC, Root cap. B and C, Micrographs showing the morphology of the spherical border-like cells and the elongated border-like cells released from the root tip (B) and the filamentous border-like cells along the root surface (C). D to G, Fluorescence micrographs of border-like cells from flax immunostained with mAbs recognizing cell wall polysaccharide epitopes. D and E, Immunodetection of xyloglucan epitopes recognized by the mAbs LM15 (D) and CCRC-M1 (E). Arrowheads indicate cell walls with higher intensity of fluorescence. Note the presence of filamentous structures (SF) stained with the mAb CCRC-M1. F and G, Flax border-like cells immunolabeled with LM19 (F) and LM8 (G), recognizing homogalacturonan and xylogalacturonan epitopes, respectively. Arrowheads indicate areas with higher intensity of fluorescence. Root border-like cell walls are strongly labeled with all the antibodies. Bars = 100 μm (A), 20 µm (B, D, F, and G), 40 µm (C), and 8 μm (E).
Using immunocytochemistry and various anti-cell wall antibodies, we have found that all of these cell types secrete substantial amounts of cell wall matrix polysaccharides (Fig. 1, D–G). These include xyloglucan epitopes, as revealed by the monoclonal antibodies (mAbs) LM15 (Marcus et al., 2008) and CCRCM1 (Puhlmann et al., 1994; Fig. 1, D and E), and pectins including homogalacturonan and xylogalacturonan, as revealed by the mAbs LM19 (Verhertbruggen et al., 2009) and LM8 (Willats et al., 2004), respectively (Fig. 1, F and G). It is interesting that the intensity of LM15 fluorescence increases along regions of contact between two adjacent border-like cells (Fig. 1D, arrowheads). These microdomains within the cell wall are particularly important in border-like cell organization, as they are resistant to the general cell wall degradation occurring during root cap cell separation. Also, areas of high intensity were found to be associated with corner junctions when using LM19. Finally, the abundance of xylogalacturonan labeling is interesting, as xylogalacturonan [formed by α-(1-4)-linked galacturonic acid and the high degree of substitution by Xyl] is thought to confer enhanced resistance to enzymatic degradation by pathogens (Willats et al., 2004; Jensen et al., 2008).
Viability of Border-Like Cells
The major goal of this study was to determine whether root border-like cells are able to perceive elicitors and initiate defense responses in both Arabidopsis and flax. Thus, prior to any experimentation toward this goal, it is necessary to determine whether the cells are viable.
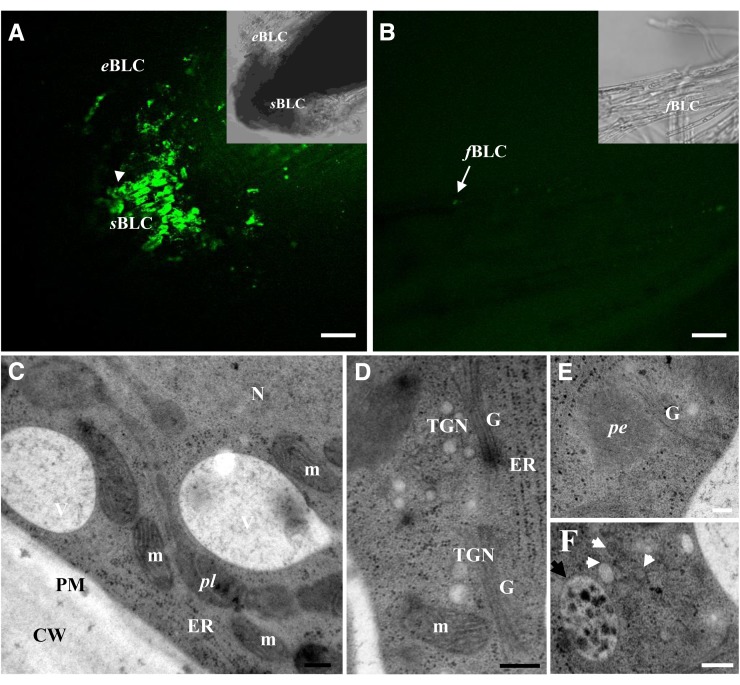
We have previously shown that Arabidopsis root tips release border-like cells that remain viable and metabolically active up to 24 h after detachment (Vicré et al., 2005). Here, we have assessed the viability of root border-like cells of Arabidopsis using calcein acetoxy-methyl ester (calcein-AM) staining up to 96 h after separation from the root cap (Supplemental Fig. S1). Detached root border-like cells were placed in one-half-strength Murashige and Skoog liquid medium (1/2 MS) for 24 and 48 h before staining with calcein-AM. As shown in Supplemental Figure S1, the cells present a strong fluorescence, indicating that they are viable. However, we also observed that the intensity of fluorescence decreases in detached root border-like cells at 72 after release. Similarly, freshly released root border-like cells of flax are also shown to be viable, although they present a distinct pattern of fluorescence compared with Arabidopsis cells (Fig. 2). As shown in Figure 2, fluorescence staining is associated with spherical and elongated cells, but filamentous cells are not or are faintly stained, indicating that only the spherical cells are viable. These findings prompted us to investigate in more detail the ultrastructure of spherical and elongated root border-like cells from flax. Electron microscopy examination was performed on ultrathin sections from root border-like cells prepared by high-pressure freezing and subsequent freeze substitution. As illustrated in Figure 2, spherical cells present at the very root tip showed a dense cytoplasm, large nuclei, small vacuoles, and a well-defined plasma membrane adherent to the cell wall. Elongated cells differed from their spherical counterparts by the presence of a large central vacuole occupying the whole cell volume, whereas the cytoplasm formed a thin layer appressed between the tonoplast and the plasma membrane (data not shown). There was no obvious sign of programmed cell death, such as deconstruction of organelles, cytoplasmic vacuolation, separation of the plasma membrane from the cell wall, and degenerated plasmodesmata. Both spherical and elongated border-like cells were characterized by the presence of numerous organelles, including mitochondria, peroxisomes, multivesicular bodies, endoplasmic reticulum, and Golgi membranes. The high amount of small vesicles in the cytoplasm reflects an important Golgi activity. Together, these findings strongly suggest that spherical and elongated border-like cells of flax are metabolically active cells, as reported previously for border-like cells of Arabidopsis (Vicré et al., 2005).
Figure 2.
Root border-like cells of flax are released as living cells. A and B, Root border-like cells stained with calcein-AM. The presence of fluorescence is indicative of the cell viability. Note the presence of fluorescence in spherical border-like cells and elongated border-like cells in A. No fluorescence is observed in filamentous border-like cells. C to F, Ultrastructural organization of spherical border-like cells. Cytoplasm showing different organelles, including Golgi stacks (G), endoplasmic reticulum (ER), mitochondria (m), and nucleus (N). White arrowheads indicate secretory vesicles, and the black arrowhead shows a multivesicular body. CW, Cell wall; eBLC, elongated border-like cells; fBLC, filamentous border-like cells; pe, peroxisome; pl, plastid; PM, plasma membrane; sBLC, spherical border-like cells; TGN, trans-Golgi network. Bars = 40 μm (A and B) and 200 nm (D–F). [See online article for color version of this figure.]
Production of ROS in Response to the MAMPs flg22 and PGN
It is known that recognition of MAMPs such as flg22 (Millet et al., 2010) by plant cells activates the innate immune system and that one of the earliest defense responses is the production of ROS. To date, it is unknown whether root border-like cells (and, to a larger extent, root border cells) are able to trigger a response to MAMPs. Therefore, we assessed the production of ROS (including hydrogen peroxide [H2O2] and singlet oxygen) following treatment with flg22 and PGN in both flax and Arabidopsis border-like cells over time using microscopy and two different fluorescent probes (see below).
Response to flg22
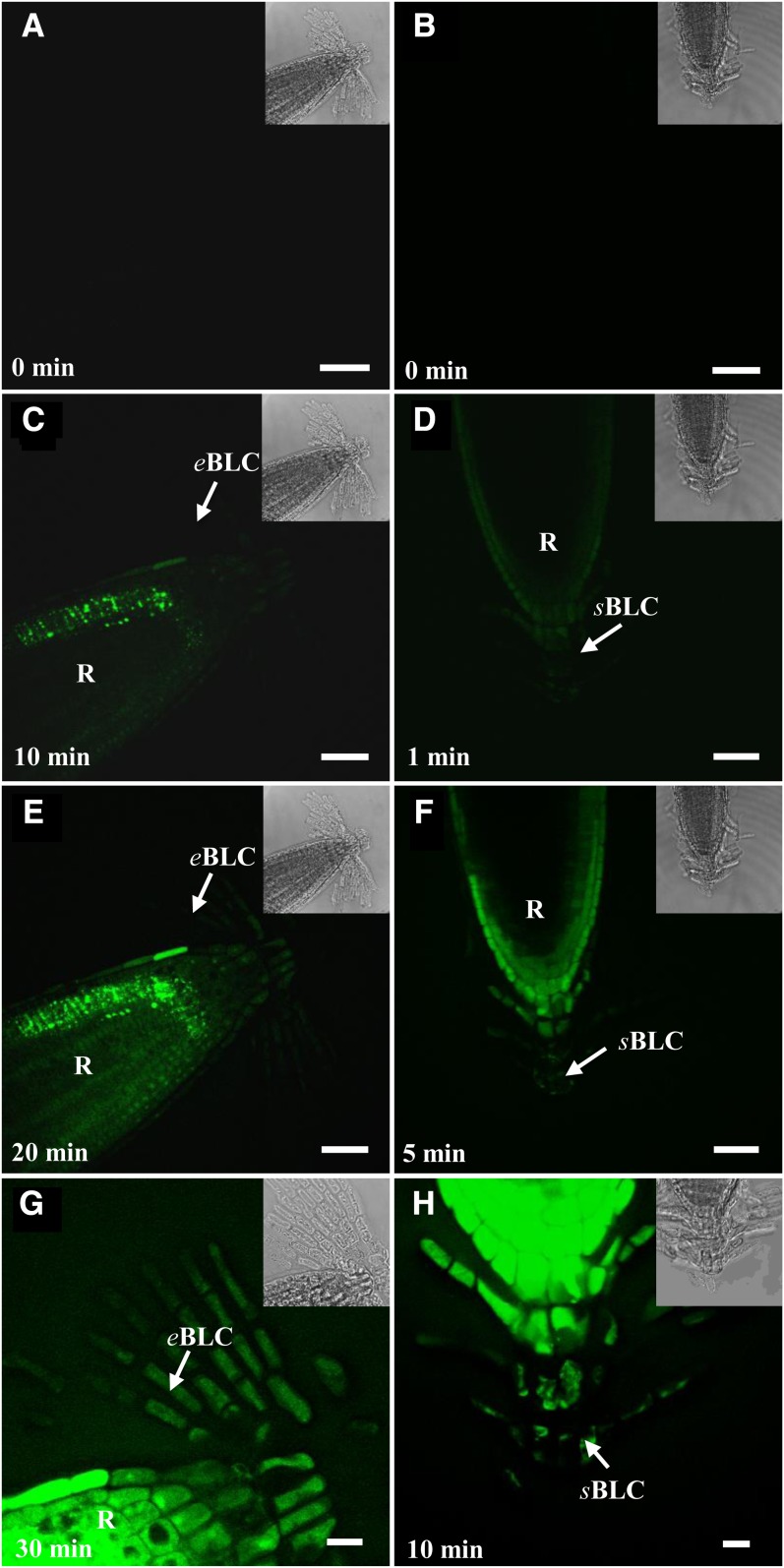
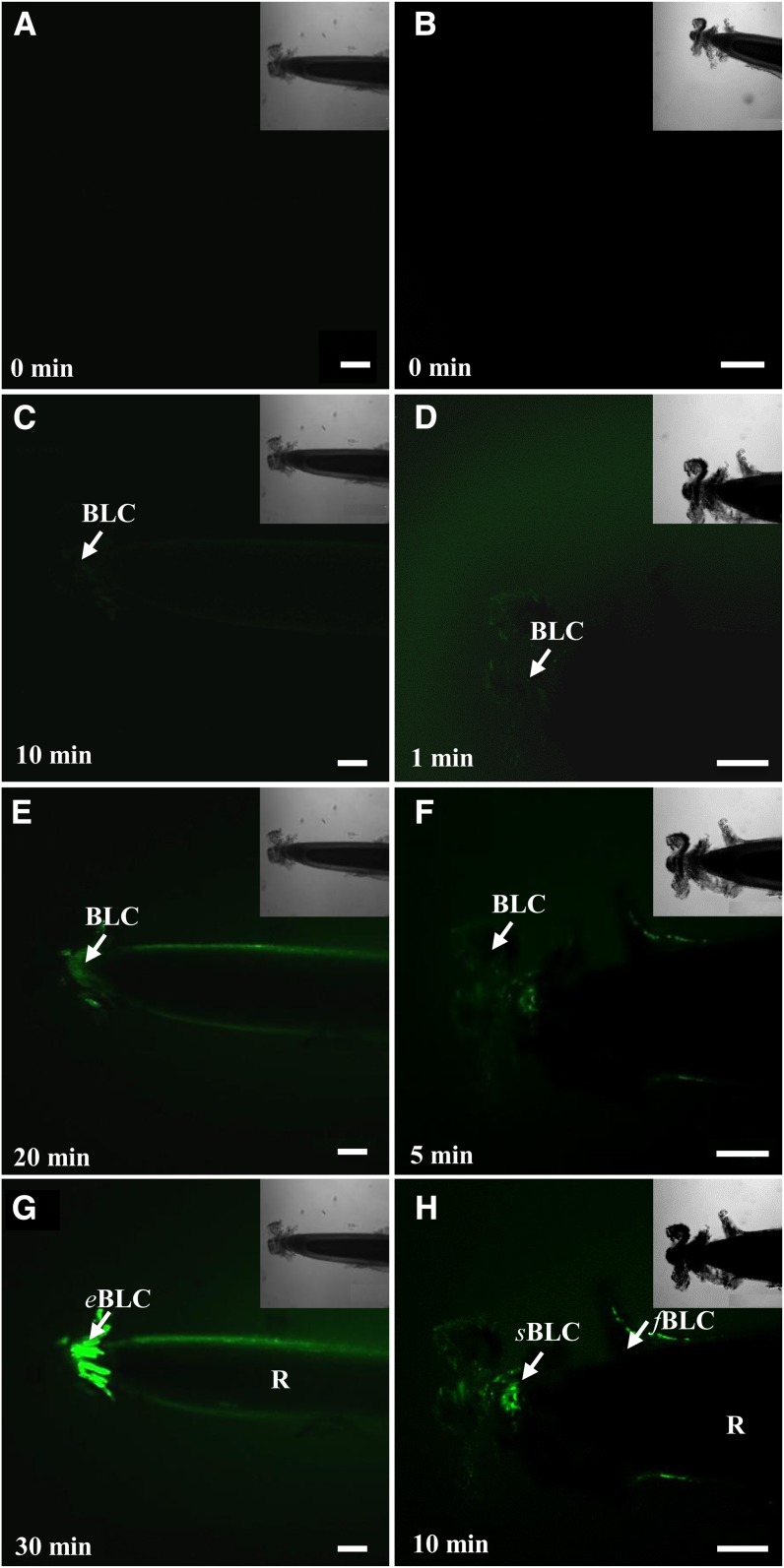
As shown in Figure 3A, no autofluorescence was detected in root border-like cells in the absence of the MAMPs. In contrast, upon exposure of the roots to flg22, fluorescence was detectable within 10 min in Arabidopsis cells with the broad probe 5,6-chloromethyl-2′,7′-dichlorodihydrofluorescein-diacetate acetyl ester (CMH2DCFDA) and increased over time to become extensive by 30 min (Fig. 3G). It is worth noting that total ROS production also occurs in response to elicitors in blocks of border-like cells that are completely detached from the root tip (Fig. 4). Furthermore, MAMP-induced production of ROS still occurred in isolated root border-like cells that were placed up to 48 h in 1/2 MS only (Supplemental Fig. S2).
Figure 3.
Time-course production of ROS in root border-like cells from Arabidopsis after treatment with 1 μm flg22 and staining with fluorescent probes. Observations were made by laser confocal scanning microscopy. Roots were stained with the probe CMH2DCFDA (A, C, E, and G) to visualize the overall ROS and with the probe SOSG (B, D, F, and H) to specifically visualize the 1O2. Fluorescence is indicative of ROS occurrence. Arrows point to border-like cells. eBLC, Elongated border-like cells; R, root; sBLC, spherical border-like cells. Bars = 40 μm. [See online article for color version of this figure.]
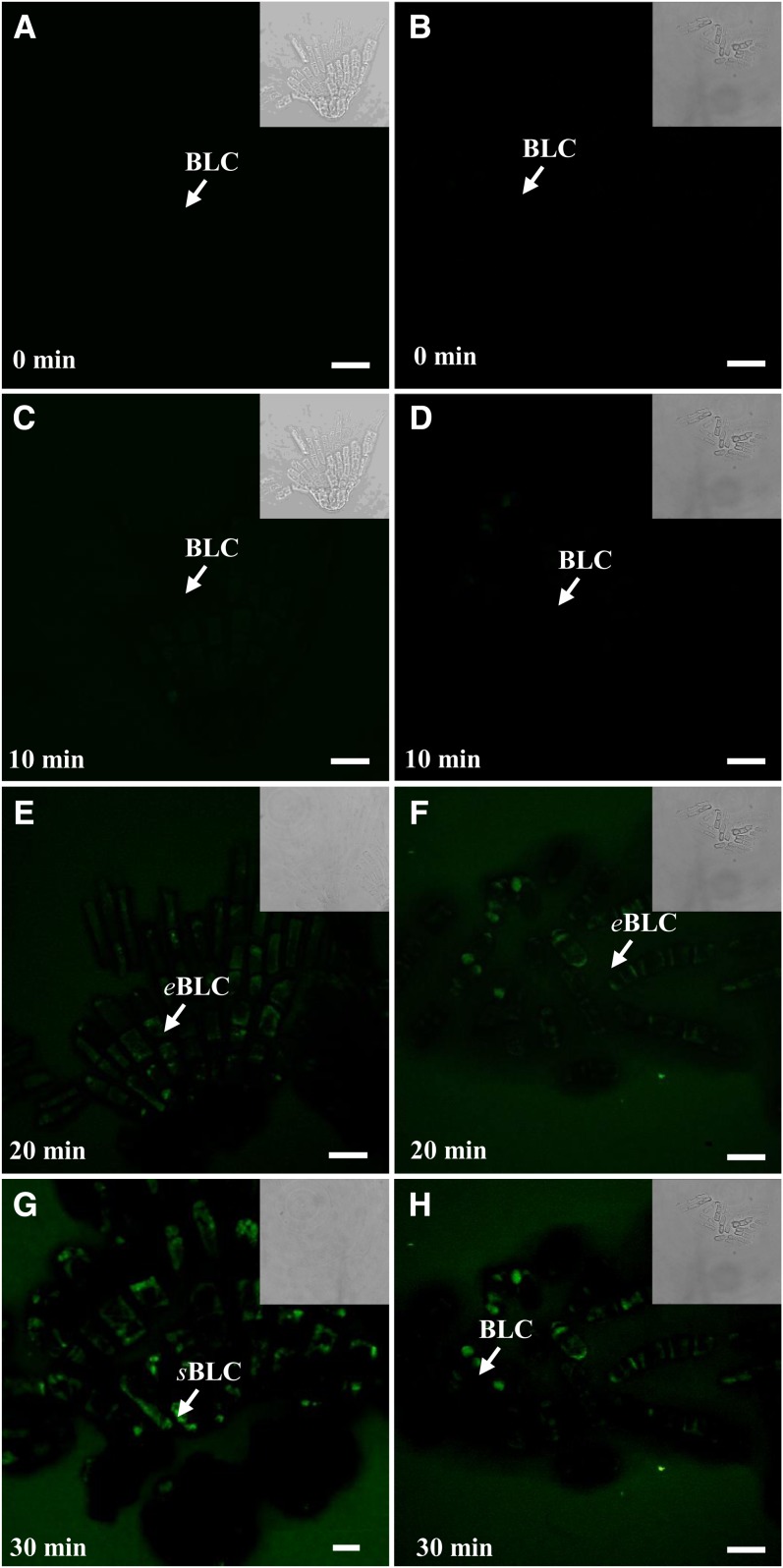
Figure 4.
Time-course production of ROS in isolated blocks of border-like cells after separation from the root tip of Arabidopsis following treatment with 1 μm flg22 (A, C, E, and G) and 100 µg mL−1 PGN (B, D, F, and H). Border-like cells were loaded with the probe CMH2DCFDA and observed by laser confocal scanning microscopy. White arrows point to border-like cells (BLC). eBLC, Elongated border-like cells; sBLC, spherical border-like cells. Bars = 20 μm (A–C and E–H) and 10 µm (D). [See online article for color version of this figure.]
In addition, detection of 1O2 in Arabidopsis cells was performed with the highly specific probe Singlet Oxygen Sensor Green (SOSG; Flors et al., 2006). Fluorescence appeared rapidly and, remarkably, within only 1 min of treatment with flg22 and increased over time quite substantially in all border-like cells as well as in the root proper (Fig. 3). The newly produced layers of cells were highly reactive to the elicitor than the older ones (Fig. 3H). Salin and Bridges (1981) have previously suggested that wound-induced chemiluminescence of soybean (Glycine max) roots could originate from the production of 1O2. However, to our knowledge, this is the first report of the detection of 1O2 in Arabidopsis root cells in response to a MAMP.
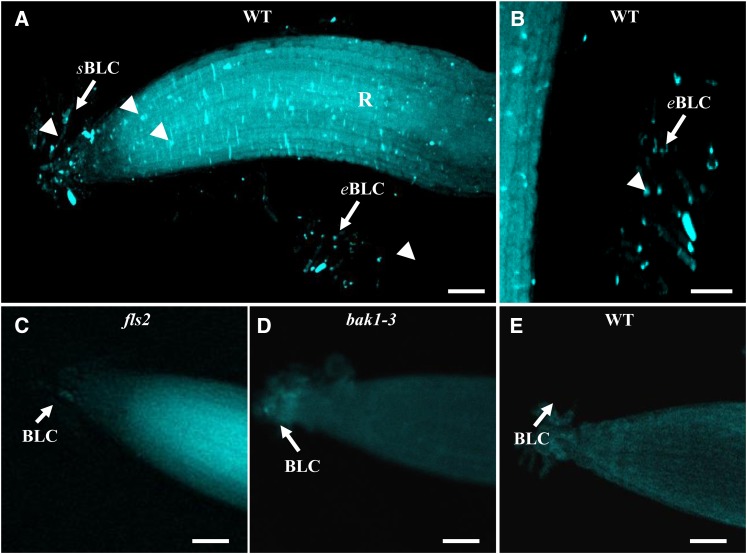
To check for the specificity of the response, we have analyzed ROS production in Arabidopsis flagellin-sensitive2 (fls2) and brassinosteroid insenstive1-associated kinase (bak1)-3 mutants, lacking a functional flg22 receptor, FLS2, and an associated receptor kinase, BAK1, respectively (Gómez-Gómez et al., 2001; Chinchilla et al., 2007). The response of root border-like cells to flg22 was completely abolished in the fls2 mutant (Supplemental Fig. S3) and strongly diminished in the bak1 mutant (Supplemental Fig. S4). In addition, controls were also performed using the respiratory burst oxidase homolog (rboh)DF double mutant of Arabidopsis affected in the NADPH oxidase catalytic subunits required for ROS production (Torres et al., 2002). As shown in Supplemental Figure S3, reduced fluorescence was detected in the atrbohDF mutant in response to flg22 treatment.
Similar to Arabidopsis, treatment with flg22 also triggers an oxidative burst in root border-like cells of flax as revealed by both probes, CMH2DCFDA and SOSG (Supplemental Fig. S5). In addition, and as for Arabidopsis, the ROS detected with the probe SOGS appeared earlier than those detected with the CMH2DCFDA probe.
Response to PGN
We also investigated the accumulation of ROS of root border-like cells of both species in response to PGN. ROS-dependent fluorescence of CMH2DCFDA was detected within 20 min of elicitation for both Arabidopsis and flax (Fig. 5; Supplemental Fig. S5). In contrast, detection of 1O2 with the SOGS probe occurred earlier (i.e. between 5 and 10 min) after elicitation with PGN.
Figure 5.
Time course of ROS production in root border-like cells from flax after treatment with 100 μg mL−1 PGN and staining with fluorescent probes. Observations were made by laser confocal scanning microscopy. Roots were stained with the probe CMH2DCFDA (A, C, E, and G) to visualize the overall ROS produced and with the probe SOSG (B, D, F, and H) to specifically visualize the 1O2. Fluorescence is indicative of ROS occurrence. The white arrows point to border-like cells (BLC). Fluorescence indicates the presence of ROS. eBLC, Elongated border-like cells; fBLC, filamentous border-like cells; R, root; sBLC, spherical border-like cells. Bars = 100 μm. [See online article for color version of this figure.]
Together, these data demonstrate that border-like cells released from both Arabidopsis and flax root tips are able to produce ROS quite rapidly in response to the MAMPs, flg22, and PGN.
Response to Fungal MAMPs
The production of ROS in border-like cells of both species was also assessed in response to treatments with fungal elicitors, including chitin, fusaric acid, and a mycelium extract from Fusarium oxysporum, a soil-borne pathogen that affects flax roots among other plants. As summarized in Supplemental Tables S3 and S4, these elicitors induced an oxidative burst in border-like cells of both species.
It is important to note that in all experiments, ROS were not detected in border-like cells of both species in control experiments carried out with omission of the MAMPs (Supplemental Figs. S6 and S7). Furthermore, control experiments using hypochlorite (1% [w/v] NaOCl, commercial bleach; adapted from Choi and Hu, 2008), known to cause intracellular production of H2O2, induced a positive staining by both probes, CMH2DCFDA and SOSG (Supplemental Figs. S8 and S9).
Effects of MAMPs on Two Cell Wall Markers of Plant Defense Activity
Callose Deposition
Callose deposition is a well-established cellular marker of plant defense (Millet et al., 2010; McCann et al., 2012). To ascertain whether flg22 and PGN induce immune activity in root border-like cells of Arabidopsis and flax, we investigated callose production by staining the cells with aniline blue. As illustrated in Figure 6 and Supplemental Figure S10A, callose deposition was detected in root border-like cells of both plant species after 48 h of elicitation with flg22 (i.e. presence of typical punctate aggregates stained in blue). Interestingly, detached root border-like cells of Arabidopsis placed in 1/2 MS for 48 h are still able to produce callose in response to elicitation with flg22 (Supplemental Fig. S11). This response was abolished in fls2 and bak1-3 Arabidopsis mutants (Fig. 6). In contrast, PGN did not trigger any callose deposition in root border-like cells of both species (data not shown).
Figure 6.
Elicited-deposition of callose in root border-like cells of wild-type (WT) Arabidopsis (A, B, and E) or mutants (C and D). Callose staining is shown for root tips treated with 1 μm flg22 (A–D) or not treated (E). Small fluorescent spots are detectable in elicited root border-like cells. Note the absence of staining in Arabidopsis fls2 and bak1-3 mutants treated with 1 μm flg22 (C and D). Callose is stained with aniline blue. Arrowheads indicate callose deposition. The white arrows point to border-like cells (BLC). eBLC, Elongated border-like cells; R, root; sBLC, spherical border-like cells. Bars = 50 μm (A and C–E) and 25 µm (B). [See online article for color version of this figure.]
Extensin Epitope Modification
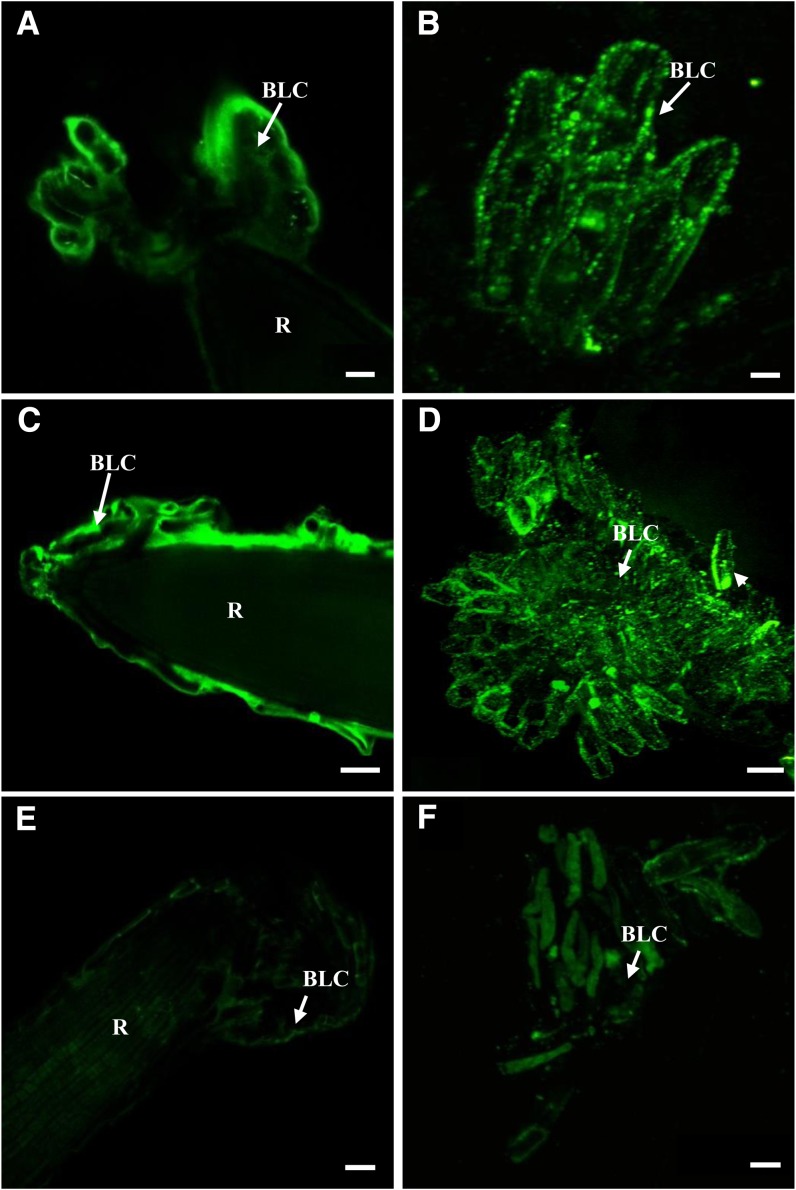
The accumulation of extensin in cell walls is known to occur as a response to biotic stress (Brisson et al., 1994; Guzzardi et al., 2004; Deepak et al., 2007; Xie et al., 2011). To investigate the impact of the MAMPs on extensin, we examined the distribution of extensin epitopes in border-like cells of Arabidopsis and flax using the mAb LM1 (Smallwood et al., 1995) and immunofluorescence microscopy.
In nonelicited Arabidopsis and as shown in Figure 7, the cell surface of border-like cells is uniformly labeled with LM1 with apparent equal intensity. Treatment with the PGN did not affect the distribution of LM1-recognized epitopes, as labeling was uniform, similar to nonelicited cells. Unlike in Arabidopsis, in nontreated flax root, LM1 labeling appeared heterogenous at the cell surface of border-like cells (Fig. 7). The punctate fluorescence observed suggests the occurrence of cell wall microdomains that are highly enriched in extensin. In contrast and interestingly, treatment of flax cells with PGN induced the formation of larger aggregates in the cell wall (recognized by the LM1 antibody) as compared with nontreated cells (Fig. 7D).
Figure 7.
Immunostaining of extensin epitopes at the surface of border-like cells of Arabidopsis (A, C, and E) and flax (B, D, and F) with the mAb LM1. Roots of Arabidopsis and flax were treated with buffer only (A and B), with 100 µg mL−1 PGN (C and D), or with 1 µm flg22 (E and F). Note the presence of large aggregates of fluorescence in D and that there is less fluorescence observed in E and F. Arrows point to border-like cells (BLC). R, Root. Bars = 20 μm (A, C, E, and F), 8 μm (B), and 100 μm (D). [See online article for color version of this figure.]
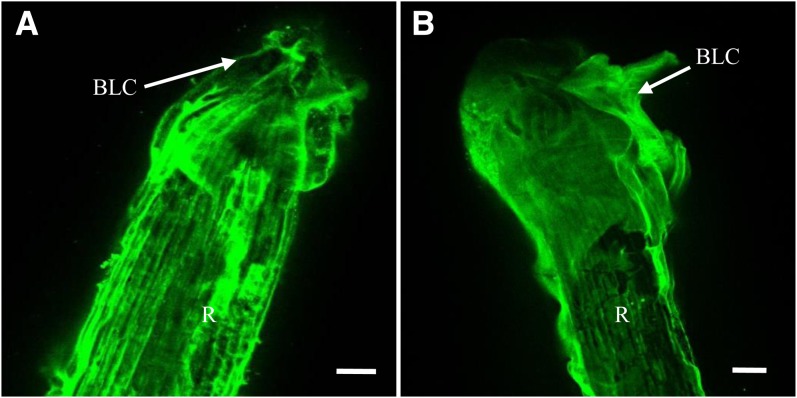
Surprisingly, the effect of elicitation with flg22 on the pattern of labeling with LM1 was different from that found in response to PGN. Elicitation with flg22 almost completely abolished LM1 labeling at the cell surface of both Arabidopsis and flax border-like cells (Fig. 7, E and F). Such a loss of LM1 labeling did not occur in atrbohDF or in fls2 mutants of Arabidopsis in response to flg22 (Fig. 8). The antiextensin LM1 labeling was uniformly distributed, similar to that observed in nonelicited atrbohDF root border-like cells.
Figure 8.
Immunostaining of extensin epitopes with the mAb LM1 at the surface of border-like cells of atrbohDF mutant treated with buffer only (A) or with 1 µm flg22 (B). Arrows point to border-like cells (BLC). R, Root. Bars = 40 μm. [See online article for color version of this figure.]
Effects of MAMPs on Defense Gene Activation in Root Border-Like Cells of Arabidopsis
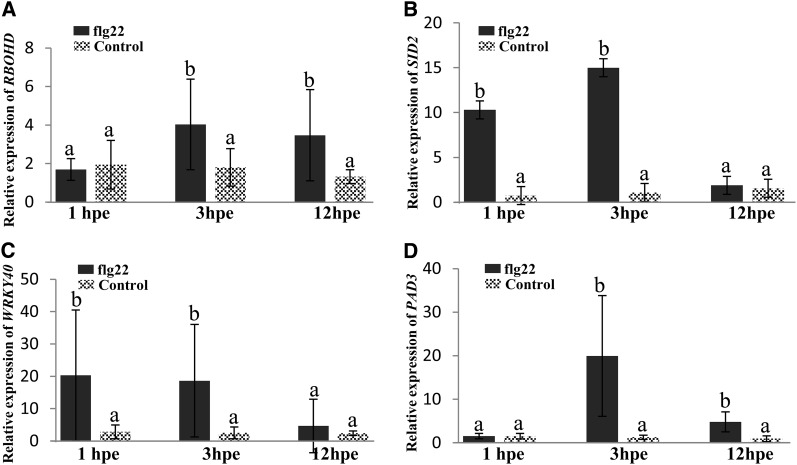
In order to investigate whether MAMPs activate defense gene markers in root border-like cells, we examined the expression changes of some selected genes in response to flg22 over a time course by real-time quantitative reverse transcription (qRT)-PCR. The Arabidopsis RBOHD gene encoding a NADPH oxidase is crucial for ROS production in response to pathogens (Pogány et al., 2009). After flg22 treatment, the expression of RBOHD is up-regulated from 3 h after elicitation (Fig. 9A). MAMP recognition generally triggers the activation of the salicylic acid pathway (Roux et al., 2011). An induction of SALICYLIC ACID INDUCTION DEFICIENT2 (SID2), one of the essential genes involved in salicylic acid biosynthesis, is observed already 1 h after flg22 application and is stronger at 3 h of treatment (Fig. 9B). We also assessed the level of expression of WRKY40, a transcription factor reported to regulate the expression of jasmonic acid signaling genes (Pandey et al., 2010). A 17-fold elevated WRKY40 transcript level is detected in flg22-elicited border-like cells as compared with the control (Fig. 9C). PHYTOALEXIN DEFICIENT3 (PAD3) is a cytochrome P450 enzyme involved in the last biosynthetic step of camalexin, the major phytoalexin produced by Arabidopsis (Pandey et al., 2010; Saga et al., 2012). Increase in PAD3 transcript accumulation transiently occurs at 3 h after elicitation (Fig. 9D).
Figure 9.
Relative expression levels of RBOHD (A), SID2 (B), WRKY40 (C) and PAD3 (D) in Columbia border-like cells in response to flg22 elicitation. hpe, Hours post elicitation. Gene expression values are presented as relative expression. The reference gene used in this study was ACTIN2. Data represent two biological replicates including three technical replicates per sample for each condition. Different letters indicate significant differences between mean values, whereas means with the same letters are not significantly different. P < 0.005 (nonparametric test, Mann-Whitney).
Together, these data demonstrate that in root border-like cells, flg22 activates several genes involved in the immune response.
DISCUSSION
Understanding the cellular and molecular events underlying plant responses to pathogens at the root level is of high importance to developing new strategies for plant protection against diseases. Whereas mechanisms of plant defense involving the aerial parts are well documented, there is little knowledge of root responses to soil-borne pathogens (Attard et al., 2010; Millet et al., 2010; Lakshmanan et al., 2012; Wang et al., 2012). Extrapolation of defense responses from the leaves to the root system should be used carefully, since growing evidence suggests that molecular mechanisms are quite different between roots and aerial organs (Attard et al., 2010; Millet et al., 2010). Recent studies have established the existence of tissue-specific responses in roots to both MAMPs and pathogen inoculation (Millet et al., 2010; Cannesan et al., 2011). For instance, the innate immunity response triggered in the root elongation zone, which is highly susceptible to pathogens, differs from other root zones.
Root border cells are also atypical in that they act as “sentries” specialized in the protection of vital root tissues such as the root cap and meristem against pathogen attacks (Hawes et al., 2003; Wen et al., 2009; Cannesan et al., 2012). We hypothesized that border-like cells such as the ones released by Arabidopsis root tips and flax would play a significant role in the protection of the root much like border cells do. Here, we have examined the response of root border-like cells to MAMPs in the plant models Arabidopsis and flax. Flax is a particularly promising model to study the function of border-like cells in root defense, as flax root caps release larger quantities of root border-like cells compared with other Brassicaceae species, and flax production is considerably threatened by soil-borne pathogens causing serious yield losses (Lorenc-Kukuła et al., 2009).
It is now well established that Arabidopsis and flax root tips release border-like cells instead of border cells, as in pea (Vicré et al., 2005). Whereas root border cells of the legume pea are clearly involved in defense against fungal pathogens (Hawes et al., 2000), the function of root border-like cells in root protection has not been studied until now. As part of an initial investigation into root defense mechanisms in flax, our study here revealed the presence of living root border-like cells that detach from the root. Different populations of root border-like cells (spherical, elongated, and filamentous cells) could be distinguished based on morphological analysis. Staining of flax root tips with calcein-AM, a vital dye, demonstrated that freshly released spherical and elongated root border-like cells were still viable, whereas filamentous border-like cells probably underwent a programmed cell death. In addition, ultrastructural characterization of both spherical and elongated root border cells at the subcellular level supported that these cells are metabolically active. Different populations of cells were also reported for root border-like cells of Arabidopsis and classical root border cells of pea (Vicré et al., 2005; Cannesan et al., 2011). The production of spherical pea root border cells together with the synthesis of the phytoalexin pisatin were shown to increase in roots colonized by the pathogenic oomycete Aphanomyces euteiches. It was hypothesized that only spherical border cells were involved in root tip protection against the oomycete. Therefore, it is tempting to speculate that the different populations of root border-like cells observed in flax and Arabidopsis are not equally involved in root tip protection. How and to what extent these populations of cells contribute to root defense will await further investigations.
The MAMP-induced ROS production by border-like cells was directly monitored by fluorescence microscopy using fluorescent probes. Our data showed both early and late production of ROS by Arabidopsis and flax cells in response to the selected MAMPs. Interestingly, the production of 1O2 using the highly specific probe SOSG was detected in root border-like cells of both species. So far, imaging the 1O2 production with the probe SOSG has been reported in diatoms and leaves of higher plants (Flors et al., 2006) but never in roots. In leaves, 1O2 is mainly a product of photosynthesis that is synthesized under light via chlorophylls acting as photosensitizers (Flors et al., 2006; Triantaphylidès and Havaux 2009). It has been speculated that, upon pathogen attack, 1O2 is generated either by phytotoxins such as phototoxic phytoalexins or from the activation of a plasma membrane NADPH oxidase (Apel and Hirt, 2004; Flors and Nonell, 2006). An elicitor derived from fungal cell walls induces the generation of 1O2 in stem cell cultures of ginseng (Panax ginseng Xu et al., 2005). 1O2 production was suggested in wounded soybean roots due to peroxidases (Salin and Bridges, 1981). The addition of H2O2 induces 1O2 production in freshly cut radish root slices and cell suspension isolated from radish roots (Rastogi and Pospísil, 2010). To our knowledge, the detection of 1O2 in root border-like cells of flax and Arabidopsis is the first example of its occurrence in an intact (i.e. unwounded) plant root tissue in response to a MAMP. We have no evidence of the source of 1O2 production in root cells observed here, but it could originate from an activation of plasma membrane NADPH oxidases. The role of 1O2 is still not well known as compared with other ROS, but recent findings suggest a function in signaling and its involvement in the response to pathogens (Vellosillo et al., 2010). A cross talk between 1O2- and H2O2-dependent signaling has been reported to occur during certain environmental stress conditions (Laloi et al., 2007). It is thus possible that 1O2 is a major signaling factor of root border-like cells under biotic stress conditions.
The oxidative burst is an early response to pathogen attacks and/or MAMP perception (Hückelhoven 2007; Boller and Felix, 2009). However, ROS production is not only involved in response to pathogenic attacks but is a rather common feature of biotic interactions (including beneficial interactions) and abiotic stress (Møller and Sweetlove, 2010; Torres, 2010; Huang et al., 2012). Callose deposition is widely used as a cellular marker of the activation of the innate immunity response in plants (Millet et al., 2010; McCann et al., 2012). For instance, novel elicitors from bacterial pathogens were recently identified using callose detection as a positive control of the plant defense response (McCann et al., 2012). Callose is well known to play a major role in defense by providing localized reinforcement of the cell wall, particularly at the sites of pathogen attack, to reduce or prevent pathogen invasion (Hardham et al., 2007; Hückelhoven 2007; Underwood, 2012). In this study, in addition to ROS, we found that callose is synthesized by root border-like cells of both species in response to elicitors. It has been shown that callose accumulates in Arabidopsis roots upon infiltration with specific treatments producing the formation of 1O2, O2·−, and H2O2 (Vellosillo et al., 2010). Thus, 1O2 production by border-like cells is to be correlated to callose deposition observed upon elicitation in order to strengthen the cell walls. Furthermore, we show that flg22 treatment up-regulates genes involved in plant defense signaling and camalexin biosynthesis in root border-like cells of Arabidopsis. These findings support the hypothesis that root border-like cells might function in root defense against soil-borne pathogens as classical border cells do.
Another cell wall polymer that we have investigated is extensin. We found that modifications in the distribution of extensin epitopes occurred in root border-like cells of both flax and Arabidopsis in response to the MAMPs used. Extensins are cell wall Hyp-rich glycoproteins that were shown to accumulate in roots interacting with pathogenic microbes (Velasquez et al., 2011, 2012; Xie et al., 2011; Hirao et al., 2012). More especially, extensins contribute to cell wall physical properties by creating an insoluble extensin network mediated by H2O2 and particular peroxidases termed extensin peroxidases (Kieliszewski and Lamport, 1994; Pereira et al., 2011). The degree of success in preventing invasion does not depend only on the delivery of polymers into the cell wall but also on the cross linking of polymers that form physical and chemical barriers against pathogen penetration (Smallwood et al., 1995; Hardham et al., 2007). An increase in oxidative cross linking of an extensin-like hyroxyproline-rich glycoprotein (HRGP) has been reported to occur in response to the elicitation of soybean. Insolubilization of extensin was correlated with an increase in cell wall resistance to digestion by pathogens (Bradley et al., 1992; Brisson et al., 1994; Ribeiro et al., 2006). It is possible that ROS production in root border-like cells in response to elicitation causes an increase in H2O2 substrate levels and promotes oxidative extensin cross linking within the cell wall. This hypothesis is supported by the finding that the pattern of LM1 labeling is not affected by flg22 elicitation in root border-like cells of the atrbohDF mutant. This mutant is affected in the NADPH oxidase catalytic subunits and produces very little or no ROS in response to elicitors. The effect of elicitation on LM1 epitope distribution in root border-like cells was different depending on the plant species and the nature of the treatment. Such modifications in the distribution of LM1 epitopes reflect either an in muro deposition of extensin or their reorganization into an extensin network resulting in the formation of large aggregates of fluorescence (Fig. 7D). Another consequence of the insolubilization of extensin polymers could be the nonaccessibility of the epitopes to the antibody LM1, which could explain the observed loss of fluorescence in response to flg22.
To summarize, this study demonstrates that root border-like cells released by Arabidopsis and flax are able to perceive MAMPs and activate defense responses even after their complete detachment from the root cap. ROS production occurs evenly in both Arabidopsis and flax border-like cells. Cell wall alteration and reorganization also occur in border-like cells in response to elicitation. Together, our findings suggest a reinforcement of the cell wall architecture involving both callose deposition and extensin reorganization. Additionally, we show that the responses of root border-like cells vary (1) between the species, flax versus Arabidopsis, and (2) according to the nature of the MAMP used. What are the future directions? Evidence has accumulated suggesting that the group of HRGPs including extensins and arabinogalactan proteins are key factors in regulating plant-microbe interactions (Balestrini et al., 1996; Vicré et al., 2005; Seifert and Roberts, 2007; Xie et al., 2011; Cannesan et al., 2012, Nguema Ona et al., 2012, 2013). For instance, arabinogalactan protein extracts from root caps were recently proposed as an antiparasitic compound against pathogenic oomycetes (Cannesan et al., 2012). As a consequence, root border-like cells provide a promising root cell model to unravel molecular interactions between HRGPs and soil-borne microorganisms and to isolate new antimicrobial compounds or damage-associated molecular patterns. However, the specific roles of individual extensins in the root response to pathogens remain to be established (Gaspar et al., 2001; van Hengel and Roberts, 2002; Xie et al., 2011). Transcriptomic analyses, for instance, together with quantitative real-time PCR of transcripts expressed in isolated root border-like cells under a biotic stress, would help in identifying specific HRGPs involved in root protection.
MATERIALS AND METHODS
Plant Material
Plant materials used were wild-type Arabidopsis (Arabidopsis thaliana ecotype Columbia) and the mutants fls2 (Gómez-Gómez et al., 2001; SALK_062054C), bak1-3 (Chinchilla et al., 2007; SALK_034523C), and atrbohDF (Torres et al., 2002) and flax (Linum usitatissimum ‘Barbara’). Seeds were surface sterilized and sown onto Murashige and Skoog medium containing 1% (w/v) Bacto Agar (Durand et al., 2009). Plates with seeds were placed in 16-h-day/8-h-night cycles (120 μE m−2 s−1, 21°C) and vertically to avoid the loss of border-like cells (Vicré et al., 2005; Cannesan et al., 2012). Root border-like cells, from 10-d-old Arabidopsis seedlings and 5- to 6-d-old flax seedlings, were used in this study. Freshly detached border-like cells were collected by adding 1/2 MS on poly-l-Lys-coated slides.
For experiments on isolated border-like cells, detached cells were collected from the root tip and placed in the same medium for 24, 48, 72, and 96 h prior to examination.
MAMPs
The MAMPs used in this study include the synthetic peptide flg22 (Felix et al., 1999) synthesized by Dr. J. Leprince (PRIMACEN platform, University of Rouen). MAMP preparations were made from mycelium extracts of Fusarium oxysporum (Hano et al., 2006). MAMPs were used at the following concentrations: 1 µm flg22 (Millet et al., 2010), 100 µg mL−1 Bacillus subtilis peptidoglycan (Sigma-Aldrich), and 100 µg mL−1 chitin (Sigma-Aldrich).
Histochemical Staining and Light Microscopy
Whole seedlings were mounted on a microscopic slide, and a droplet of water was added on the root apex to visualize the presence of root border-like cells using bright-field microscopy (Leica Microsystems DC 3000 microscope). Roots were mounted on microscope slides in a drop of water for examination using bright-field microscopy. Vital staining of root border-like cells was performed with 5 µm calcein AM (Sigma-Aldrich) as described previously by Vicré et al. (2005). Border-like cells were stained for 60 min, carefully washed in deionized water, and observed using a microscope equipped with UV fluorescence (excitation filter, 490 nm; emission filter, 520 nm). Images were acquired with a Leica DCF 300 FX camera.
Detection of ROS
In situ detection of general ROS was performed by staining root border-like cells with the probe CMH2DCFDA, which is nonfluorescent in its reduced form. In living cells, cell esterases convert CMH2DCFDA into 2′,7′-dichlorodihydrofluorescein diacetate, which is subsequently oxidized by ROS into the fluorescent form 2′,7′-dichlorofluorescein in the presence of ROS (Hempel et al., 1999; Kristiansen et al., 2009). Roots or detached border-like cells were mounted on a microscope slide with 20 mm phosphate buffer, pH 6.1, and then incubated with 30 µL of 5 µm CMH2DCFDA. The absence of fluorescence was checked by laser scanning confocal microscopy (Leica TCS SP2 AOBS; excitation filter, 488 nm; barrier filter, 510 nm; 550 mV). Roots were treated with 100 µL of a diluted elicitor solution (1 µm flg22 or 100 µg mL−1 peptidoglycan), and the time-course formation of ROS was monitored. To further investigate the ROS production in root border-like cells, the cell 1O2 known to be produced in response to wounding was detected using the SOSG reagent (Flors et al., 2006). Roots were incubated with 30 µL of 50 µm SOSG according to the protocol of Flors et al. (2006), and elicitation was performed as described above. The time-course production of 1O2 was monitored by confocal laser microscopy (Leica TCS SP2 AOBS; excitation filter, 488 nm; barrier filter, 550 nm; 550 mV). As a control, the accumulation of ROS was monitored in fls2 and bak1-3 mutants, which are insensitive to flg22. Root border cells from flax were jettisoned by dipping flax root into a droplet of water.
Detection of Callose Formation
Staining of callose was performed using aniline blue (Sigma-Aldrich) at a concentration of 1 mg L−1 according to Millet et al. (2010). For experiments involving elicitation, roots were treated with 100 µL of a diluted elicitor solution (1 µm flg22 or 100 µg mL−1 peptidoglycan) for 48 h. Acetic acid:ethanol (1:3) was added to the seedlings for 4 h, and the fixative solution was changed several times followed by ethanol (75%, v/v) for 2 h, ethanol (50%, v/v) for 2 h, ethanol (25%, v/v) for 2 h, and finally deionized water overnight (Daudi et al., 2012). Roots were incubated in 10% (w/v) NaOH and placed at 37°C for 4 h. Roots were stained with aniline blue (0.1%, w/v) in 108 mm sodium phosphate (pH 11) overnight. Roots were mounted in antifade solution (Citifluor AF2; Agar Scientific) and examined using a confocal laser microscope (Leica TCS SP2 AOBS; excitation filter, 359 nm; barrier filter, 461 nm).
Immunofluorescence Localization of Cell Wall Polysaccharide Epitopes
The monoclonal antibodies specific for cell wall polysaccharides used in this study were LM15 (Marcus et al., 2008), CCRC-M1 (Puhlmann et al., 1994), LM19 (Verhertbruggen et al., 2009), LM8 (Willats et al., 2004), and LM1 (Smallwood et al., 1995). Roots were fixed for 30 min in 4% (w/v) paraformaldehyde in 50 mm PIPES, 1 mm CaCl2, pH 7, and immunolabeled according to Willats et al. (2001). Roots were washed in 50 mm PIPES, 1 mm CaCl2, pH 7, and incubated for 30 min in a blocking solution of 3% (w/v) bovine serum albumin with 0.01 m phosphate-buffered saline. After being carefully rinsed in phosphate-buffered saline containing 0.05% (v/v) Tween 20 (PBST), roots were incubated overnight at 4°C in LM15 (1:5), CCRC-M1 (1:5), LM19 (1:5), LM8 (1:5), or LM1 (1:5) diluted in PBST (0.05%, v/v) containing normal goat serum (1:30) as described previously by Vicré et al. (2005). After five washes with 0.05% PBST, roots were incubated with anti-rat IgG (1:50 dilution in 0.1% [v/v] PBST) coupled to fluorescein isothiocyanate (Sigma) for 2 h. After washing in 0.05% PBST, roots were mounted in antifading agent (Citifluor; Agar Scientific) and examined using confocal laser microscopy (Leica TCS SP2 AOBS; excitation filter, 488 nm; barrier filter, 510 nm; 550 mV). For elicitation, roots were first treated with 100 µL of a diluted elicitor solution (1 µm flg22 or 100 µg mL−1 peptidoglycan) for 48 h prior to immunolabeling. Control experiments in which the primary antiserum was omitted were performed following the same procedure.
Ultrastructural Analysis by Electron Microscopy
Root tips from 5-d-old flax seedlings were prepared as described previously (Chevalier et al., 2010; Follet-Gueye et al., 2012). Briefly, samples were immersed in 0.2 m Suc and high-pressure frozen with the HPF-EM PACT I freezer (Leica). Then, root tips were transferred to a freeze substitution automate (EM-AFS Leica) for cryosubstitution in 1% (w/v) osmium diluted in anhydrous acetone. Finally, samples were infiltrated and embedded in Spurr resin. Using an ultracut EM-UC6 (Leica), thin sections (70 nm) were mounted on formvar-coated nickel grids. Sections were classically stained 10 min with 0.5% (w/v) uranyl acetate diluted in methanol and 10 min in lead citrate. Sections were observed with a Philips FEI Tecnai 12 Biotwin transmission electron microscope operating at 80 kV with an ES500W Erlangshen CCD camera (Gatan).
RNA Isolation and Real-Time qRT-PCR
Real-time qRT-PCR analyses were performed for measuring ROBHD, SID2, WRKY40, and PAD3 transcript accumulation in root border-like cells of Arabidopsis upon 1, 3, and 12 h of flg22 treatment. Mock inoculations were carried out using phosphate buffer (20 mm, pH 6.1). Root border cells from 1,000 seedlings per condition were collected for RNA extraction. Two biological replicates including three technical replicates per sample were done for each condition. The RNA extraction was performed using the RNeasy Micro Kit (Qiagen) following the manufacturer’s instruction manual. The gene-specific primers for the genes ROBHD (At5g47910; forward, 5′-CTGGACACGTAAGCTCAGGA-3′, reverse, 5′-GCCGAGACCTACGAGGAGTA-3′), SID2 (At1g74710; forward, 5′-GAGACTTACGAAGGAAGATGATGAG-3′, reverse, 5′-TGATCCCGACTGCAAATTCACTCTC-3′), WRKY40 (At1g80840; forward, 5′-GATCCACCGACAAGTGCTTT-3′, reverse, 5′-AGGGCTGATTTGATCCCTCT-3′), and PAD3 (At3g26830; forward, 5′-TGCTCCCAAGACAGACAATG-3′, reverse, 5′-GTTTTGGATCACGACCCATC-3′) were synthesized (Eurogentec). Primers were validated according to the protocol of Bookout and Mangelsdorf (2003). Complementary DNAs were synthesized from 1 ng of total RNA using the QuantiTect Reverse Transcription kit (Qiagen). qRT-PCR was performed using a qRT-PCR ABI PRISM 7500 machine and fast SYBR Green Master Mix (Applied Biosystems). The program used for qRT-PCR was as follows: 20 s at 95°C, 40 cycles of 3 s at 95°C and 15 s at 95°C, followed by a melt curve of 1 min at 60°C, 15 s at 95°C, and 15 s at 60°C. Expression values were normalized to that of ACTIN2. Statistical significances were calculated by using the Mann-Whitney test (Siegel and Castellan, 1988).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers At3g18780 (ACTIN2), At4g33430 (BAK1), At5g46330 (FLS2), At5g47910 (ROBHD), At1g74710 (SID2), At1g80840 (WRKY40), At3g26830 (PAD3).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Root border cells are stained with calcein-AM after being detached from the root cap for 24, 48, 72, and 96 h.
Supplemental Figure S2. Production of ROS by detached root border-like cells of Arabidopsis after 24, 48, 72, and 96 h of release from the root cap.
Supplemental Figure S3. Time-course production of ROS in root border-like cells from Arabidopsis mutants fls2 and atrboh after treatment with 1 μm flg22.
Supplemental Figure S4. Time-course production of ROS in root border-like cells from Arabidopsis mutants bak1-3 and fls2 after treatment with 1 μm flg22.
Supplemental Figure S5. Time course of ROS production in root border-like cells from flax after treatment with 1 µm flg22.
Supplemental Figure S6. Negative control production of ROS in root border-like cells from Arabidopsis after treatment with 20 mm phosphate buffer.
Supplemental Figure S7. Negative control production of ROS in root border-like cells from flax after treatment with 20 mm phosphate buffer.
Supplemental Figure S8. Positive control production of ROS in root border-like cells from Arabidopsis after treatment with 1% (v/v) bleach.
Supplemental Figure S9. Positive control production of ROS in root border-like cells from flax after treatment with 1% (v/v) bleach.
Supplemental Figure S10. Elicited deposition of callose detected by aniline blue staining in root border-like cells of flax.
Supplemental Figure S11. Elicited-deposition of callose detected by aniline blue staining in detached root border-like cells of Arabidopsis.
Supplemental Table S1. Production of root border-like cells in flax.
Supplemental Table S2. Morphological features of root border-like cells from flax.
Supplemental Table S3. Production of ROS in root border-like cells from flax in response to fungal elicitors.
Supplemental Table S4. Production of ROS in root border-like cells from Arabidopsis in response to fungal elicitors.
Supplementary Material
Acknowledgments
We thank Jean Paul Knox (University of Leeds) for providing some of the mAbs used in this study. We are grateful to Sophie Bernard and Carole Plasson (Laboratoire Glycobiologie et Matrice Extracellulaire Végétale, University of Rouen) for excellent technical assistance with plant cultures and electron microscopy.
Glossary
- MAMP
microbe-associated molecular pattern
- ROS
reactive oxygen species
- PGN
peptidoglycan
- mAb
monoclonal antibody
- 1/2 MS
one-half-strength Murashige and Skoog liquid medium
- H2O2
hydrogen peroxide
- CMH2DCFDA
5,6-chloromethyl-2′,7′-dichlorodihydrofluorescein-diacetate acetyl ester
- 1O2
singlet oxygen
- SOSG
Singlet Oxygen Sensor Green
- qRT
quantitative reverse transcription
- PBST
phosphate-buffered saline containing 0.05% (v/v) Tween 20
- HGRP
hyroxyproline-rich glycoprotein
References
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Attard A, Gourgues M, Callemeyn-Torre N, Keller H. (2010) The immediate activation of defense responses in Arabidopsis roots is not sufficient to prevent Phytophthora parasitica infection. New Phytol 187: 449–460 [DOI] [PubMed] [Google Scholar]
- Balestrini R, Hahn MG, Faccio A, Mendgen K, Bonfante P. (1996) Differential localization of carbohydrate epitopes in plant cell walls in the presence and absence of arbuscular mycorrhizal fungi. Plant Physiol 111: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Mangelsdorf DJ. (2003) Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl Recept Signal 1: e012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ. (1992) Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70: 21–30 [DOI] [PubMed] [Google Scholar]
- Brigham LA, Woo HH, Nicoll SM, Hawes MC. (1995) Differential expression of proteins and mRNAs from border cells and root tips of pea. Plant Physiol 109: 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C. (1994) Function of oxidative cross‐linking of cell wall structural proteins in plant disease resistance. Plant Cell 6: 1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannesan MA, Durand C, Burel C, Gangneux C, Lerouge P, Ishii T, Laval K, Follet-Gueye ML, Driouich A, Vicré-Gibouin M. (2012) Effect of arabinogalactan proteins from the root caps of pea and Brassica napus on Aphanomyces euteiches zoospore chemotaxis and germination. Plant Physiol 159: 1658–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannesan MA, Gangneux C, Lanoue A, Giron D, Laval K, Hawes M, Driouich A, Vicré-Gibouin M. (2011) Association between border cell responses and localized root infection by pathogenic Aphanomyces euteiches. Ann Bot (Lond) 108: 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier L, Bernard S, Ramdani Y, Lamour R, Bardor M, Lerouge P, Follet-Gueye ML, Driouich A. (2010) Subcompartment localization of the side chain xyloglucan-synthesizing enzymes within Golgi stacks of tobacco suspension-cultured cells. Plant J 64: 977–989 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Choi O, Hu Z. (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42: 4583–4588 [DOI] [PubMed] [Google Scholar]
- Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP. (2012) The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24: 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepak S, Shailasree S, Kini RK, Hause B, Shetty SH, Mithöfer A. (2007) Role of hydroxyproline-rich glycoproteins in resistance of pearl millet against downy mildew pathogen Sclerospora graminicola. Planta 226: 323–333 [DOI] [PubMed] [Google Scholar]
- Driouich A, Cannesan MA, Dardelle F, Durand C, Plancot B, Bernard S, Follet-Gueye ML, Vicré-Gibouin M (2012) Unity is strength: the power of border cells and border-like cells in relation with plant defense. In JM Vivanco, F Baluska, eds, Secretions and Exudates in Biological Systems, Signaling and Communication in Plants. Springer-Verlag, Berlin, Heidelberg, pp 91–107 [Google Scholar]
- Driouich A, Durand C, Cannesan MA, Percoco G, Vicré-Gibouin M. (2010) Border cells versus border-like cells: are they alike? J Exp Bot 61: 3827–3831 [DOI] [PubMed] [Google Scholar]
- Driouich A, Durand C, Vicré-Gibouin M. (2007) Formation and separation of root border cells. Trends Plant Sci 12: 14–19 [DOI] [PubMed] [Google Scholar]
- Durand C, Vicré-Gibouin M, Follet-Gueye ML, Duponchel L, Moreau M, Lerouge P, Driouich A. (2009) The organization pattern of root border-like cells of Arabidopsis is dependent on cell wall homogalacturonan. Plant Physiol 150: 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Flors C, Fryer MJ, Waring J, Reeder B, Bechtold U, Mullineaux PM, Nonell S, Wilson MT, Baker NR. (2006) Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green. J Exp Bot 57: 1725–1734 [DOI] [PubMed] [Google Scholar]
- Flors C, Nonell S. (2006) Light and singlet oxygen in plant defense against pathogens: phototoxic phenalenone phytoalexins. Acc Chem Res 39: 293–300 [DOI] [PubMed] [Google Scholar]
- Follet-Gueye ML, Mollet JC, Vicré-Gibouin M, Bernard S, Chevalier L, Plancot B, Dardelle F, Ramdani Y, Coimbra S, Driouich A (2012) Immuno-glyco-imaging in plant cells: localization of cell wall carbohydrate epitopes and their biosynthesizing enzymes. In H Dehghani, ed, Applications of Immunocytochemistry. In Tech, Rijeka, pp 297–320 [Google Scholar]
- Gaspar Y, Johnson KL, McKenna JA, Bacic A, Schultz CJ. (2001) The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Mol Biol 47: 161–176 [PubMed] [Google Scholar]
- Gómez-Gómez L, Bauer Z, Boller T. (2001) Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13: 1155–1163 [PMC free article] [PubMed] [Google Scholar]
- Gunawardena U, Hawes MC. (2002) Tissue specific localization of root infection by fungal pathogens: role of root border cells. Mol Plant Microbe Interact 15: 1128–1136 [DOI] [PubMed] [Google Scholar]
- Gunawardena U, Rodriguez M, Straney D, Romeo JT, VanEtten HD, Hawes MC. (2005) Tissue-specific localization of pea root infection by Nectria haematococca: mechanisms and consequences. Plant Physiol 137: 1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzardi P, Genot G, Jamet E. (2004) The Nicotiana sylvestris extensin gene, Ext 1.2A, is expressed in the root transition zone and upon wounding. Biochim Biophys Acta 1680: 83–92 [DOI] [PubMed] [Google Scholar]
- Hano C, Addi M, Bensaddek L, Crônier D, Baltora-Rosset S, Doussot J, Maury S, Mesnard F, Chabbert B, Hawkins S, et al. (2006) Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 223: 975–989 [DOI] [PubMed] [Google Scholar]
- Hao P, Liu C, Wang Y, Chen R, Tang M, Du B, Zhu L, He G. (2008) Herbivore-induced callose deposition on the sieve plates of rice: an important mechanism for host resistance. Plant Physiol 146: 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardham AR, Jones DA, Takemoto D. (2007) Cytoskeleton and cell wall function in penetration resistance. Curr Opin Plant Biol 10: 342–348 [DOI] [PubMed] [Google Scholar]
- Hawes MC, Bengough G, Cassab G, Ponce G. (2003) Root caps and rhizosphere. J Plant Growth Regul 21: 352–367 [Google Scholar]
- Hawes MC, Curlango-Rivera G, Wen F, White GJ, Vanetten HD, Xiong Z. (2011) Extracellular DNA: the tip of root defenses? Plant Sci 180: 741–745 [DOI] [PubMed] [Google Scholar]
- Hawes MC, Gunawardena U, Miyasaka S, Zhao X. (2000) The role of root border cells in plant defense. Trends Plant Sci 5: 128–133 [DOI] [PubMed] [Google Scholar]
- Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM. (1999) Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med 27: 146–159 [DOI] [PubMed] [Google Scholar]
- Hirao T, Fukatsu E, Watanabe A. (2012) Characterization of resistance to pine wood nematode infection in Pinus thunbergii using suppression subtractive hybridization. BMC Plant Biol 12: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Ma SL, Bai LP, Zhang L, Ma H, Jia P, Liu J, Zhong M, Guo ZF. (2012) Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep 39: 969–987 [DOI] [PubMed] [Google Scholar]
- Hückelhoven R. (2007) Cell wall-associated mechanisms of disease resistance and susceptibility. Annu Rev Phytopathol 45: 101–127 [DOI] [PubMed] [Google Scholar]
- Jensen JK, Sørensen SO, Harholt J, Geshi N, Sakuragi Y, Møller I, Zandleven J, Bernal AJ, Jensen NB, Sørensen C, et al. (2008) Identification of a xylogalacturonan xylosyltransferase involved in pectin biosynthesis in Arabidopsis. Plant Cell 20: 1289–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieliszewski MJ, Lamport DT. (1994) Extensin: repetitive motifs, functional sites, post-translational codes, and phylogeny. Plant J 5: 157–172 [DOI] [PubMed] [Google Scholar]
- Kristiansen KA, Jensen PE, Møller IM, Schulz A. (2009) Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol Plant 136: 369–383 [DOI] [PubMed] [Google Scholar]
- Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, Felix G. (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan V, Kitto SL, Caplan JL, Hsueh YH, Kearns DB, Wu YS, Bais HP. (2012) Microbe-associated molecular patterns-triggered root responses mediate beneficial rhizobacterial recruitment in Arabidopsis. Plant Physiol 160: 1642–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. (2007) Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenc-Kukuła K, Zuk M, Kulma A, Czemplik M, Kostyn K, Skała J, Starzycki M, Szopa J. (2009) Engineering flax with the GT family 1 Solanum sogarandinum glycosyltransferase SsGT1 confers increased resistance to Fusarium infection. J Agric Food Chem 57: 6698–6705 [DOI] [PubMed] [Google Scholar]
- Marcus SE, Verhertbruggen Y, Hervé C, Ordaz-Ortiz JJ, Farkas V, Pedersen HL, Willats WG, Knox JP. (2008) Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann HC, Nahal H, Thakur S, Guttman DS. (2012) Identification of innate immunity elicitors using molecular signatures of natural selection. Proc Natl Acad Sci USA 109: 4215–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, Ausubel FM. (2010) Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22: 973–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller IM, Sweetlove LJ. (2010) ROS signalling: specificity is required. Trends Plant Sci 15: 370–374 [DOI] [PubMed] [Google Scholar]
- Mueller K, Bittel P, Chinchilla D, Jehle AK, Albert M, Boller T, Felix G. (2012) Chimeric FLS2 receptors reveal the basis for differential flagellin perception in Arabidopsis and tomato. Plant Cell 24: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema-Ona E, Coimbra S, Vicré-Gibouin M, Mollet JC, Driouich A. (2012) Arabinogalactan proteins in root and pollen-tube cells: distribution and functional aspects. Ann Bot (Lond) 110: 383–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguema-Ona E, Vicré-Gibouin M, Cannesan MA, Driouich A. (2013) Arabinogalactan proteins in root-microbe interactions. Trends Plant Sci 18: 440–449 [DOI] [PubMed] [Google Scholar]
- Pan JW, Ye D, Wang LL, Hua J, Zhao GF, Pan WH, Han N, Zhu MY. (2004) Root border cell development is a temperature-insensitive and Al-sensitive process in barley. Plant Cell Physiol 45: 751–760 [DOI] [PubMed] [Google Scholar]
- Pandey SP, Roccaro M, Schön M, Logemann E, Somssich IE. (2010) Transcriptional reprogramming regulated by WRKY18 and WRKY40 facilitates powdery mildew infection of Arabidopsis. Plant J 64: 912–923 [DOI] [PubMed] [Google Scholar]
- Pereira CS, Ribeiro JM, Vatulescu AD, Findlay K, MacDougall AJ, Jackson PA. (2011) Extensin network formation in Vitis vinifera callus cells is an essential and causal event in rapid and H2O2-induced reduction in primary cell wall hydration. BMC Plant Biol 11: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogány M, von Rad U, Grün S, Dongó A, Pintye A, Simoneau P, Bahnweg G, Kiss L, Barna B, Durner J. (2009) Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis-Alternaria pathosystem. Plant Physiol 151: 1459–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhlmann J, Bucheli E, Swain MJ, Dunning N, Albersheim P, Darvill AC, Hahn MC. (1994) Generation of monoclonal antibodies against plant cell-wall polysaccharides. I. Characterization of a monoclonal antibody to a terminal alpha-(1→2)-linked fucosyl-containing epitope. Plant Physiol 104: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi A, Pospísil P. (2010) Effect of exogenous hydrogen peroxide on biophoton emission from radish root cells. Plant Physiol Biochem 48: 117–123 [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Pereira CS, Soares NC, Vieira AM, Feijó JA, Jackson PA. (2006) The contribution of extensin network formation to rapid, hydrogen peroxide-mediated increases in grapevine callus wall resistance to fungal lytic enzymes. J Exp Bot 57: 2025–2035 [DOI] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C. (2011) The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga H, Ogawa T, Kai K, Suzuki H, Ogata Y, Sakurai N, Shibata D, Ohta D. (2012) Identification and characterization of ANAC042, a transcription factor family gene involved in the regulation of camalexin biosynthesis in Arabidopsis. Mol Plant Microbe Interact 25: 684–696 [DOI] [PubMed] [Google Scholar]
- Salin ML, Bridges SM. (1981) Chemiluminescence in wounded root tissue: evidence for peroxidase involvement. Plant Physiol 67: 43–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert GJ, Roberts K. (2007) The biology of arabinogalactan proteins. Annu Rev Plant Biol 58: 137–161 [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ (1988) Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill, New York [Google Scholar]
- Smallwood M, Martin H, Knox JP. (1995) An epitope of rice threonine- and hydroxyproline-rich glycoprotein is common to cell wall and hydrophobic plasma-membrane glycoproteins. Planta 196: 510–522 [DOI] [PubMed] [Google Scholar]
- Torres MA. (2010) ROS in biotic interactions. Physiol Plant 138: 414–429 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantaphylidès C, Havaux M. (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14: 219–228 [DOI] [PubMed] [Google Scholar]
- Underwood W. (2012) The plant cell wall: a dynamic barrier against pathogen invasion. Front Plant Sci 3: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hengel AJ, Roberts K. (2002) Fucosylated arabinogalactan-proteins are required for full root cell elongation in Arabidopsis. Plant J 32: 105–113 [DOI] [PubMed] [Google Scholar]
- Velasquez M, Salter JS, Dorosz JG, Petersen BL, Estevez JM. (2012) Recent advances on the posttranslational modifications of EXTs and their roles in plant cell walls. Front Plant Sci 3: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez SM, Ricardi MM, Dorosz JG, Fernandez PV, Nadra AD, Pol-Fachin L, Egelund J, Gille S, Harholt J, Ciancia M, et al. (2011) O-Glycosylated cell wall proteins are essential in root hair growth. Science 332: 1401–1403 [DOI] [PubMed] [Google Scholar]
- Vellosillo T, Vicente J, Kulasekaran S, Hamberg M, Castresana C. (2010) Emerging complexity in reactive oxygen species production and signaling during the response of plants to pathogens. Plant Physiol 154: 444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhertbruggen Y, Marcus SE, Haeger A, Ordaz-Ortiz JJ, Knox JP. (2009) An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr Res 344: 1858–1862 [DOI] [PubMed] [Google Scholar]
- Vicré M, Santaella C, Blanchet S, Gateau A, Driouich A. (2005) Root border-like cells of Arabidopsis: microscopical characterization and role in the interaction with rhizobacteria. Plant Physiol 138: 998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Schornack S, Marsh JF, Gobbato E, Schwessinger B, Eastmond P, Schultze M, Kamoun S, Oldroyd GE. (2012) A common signaling process that promotes mycorrhizal and oomycete colonization of plants. Curr Biol 22: 2242–2246 [DOI] [PubMed] [Google Scholar]
- Wen F, VanEtten HD, Tsaprailis G, Hawes MC. (2007) Extracellular proteins in pea root tip and border cell exudates. Plant Physiol 143: 773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen F, White GJ, VanEtten HD, Xiong Z, Hawes MC. (2009) Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiol 151: 820–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Knox JP. (2001) In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 213: 37–44 [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Steele-King CG, Marcus SE, Mort A, Huisman M, van Alebeek GJ, Schols HA, Voragen AG, Le Goff A, et al. (2004) A xylogalacturonan epitope is specifically associated with plant cell detachment. Planta 218: 673–681 [DOI] [PubMed] [Google Scholar]
- Wojtaszek P, Trethowan J, Bolwell GP. (1995) Specificity in the immobilisation of cell wall proteins in response to different elicitor molecules in suspension-cultured cells of French bean (Phaseolus vulgaris L.). Plant Mol Biol 28: 1075–1087 [DOI] [PubMed] [Google Scholar]
- Xie D, Ma L, Samaj J, Xu C. (2011) Immunohistochemical analysis of cell wall hydroxyproline-rich glycoproteins in the roots of resistant and susceptible wax gourd cultivars in response to Fusarium oxysporum f. sp. benincasae infection and fusaric acid treatment. Plant Cell Rep 30: 1555–1569 [DOI] [PubMed] [Google Scholar]
- Xu X, Hu X, Neill SJ, Fang J, Cai W. (2005) Fungal elicitor induces singlet oxygen generation, ethylene release and saponin synthesis in cultured cells of Panax ginseng C. A. Meyer. Plant Cell Physiol 46: 947–954 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.