Abstract
Inflammatory mediators can play a dual role in oncogenesis and tumor progression. CX3CL1, a chemokine previously implicated in natural killer cell- and CD8+ T cell-mediated antitumor immune responses, has now been identified as a promoter of ERBB2-expressing breast carcinomas as it cross-activates members of the epidermal growth factor receptor family.
Keywords: chemokine, cross-activation, ERK, fractalkine, inflammation, MAPK, tumor promotion
One hallmark of cancer is the robust proliferative potential of malignant cells, stemming from mutations in genes that control cell cycle progression and apoptosis.1 However, the development of clinically relevant neoplasms is not only the result of genetic alterations in transformed cells, but also of their interactions with the local microenvironment. In this context, a prominent role is played by chemokines, a family of inflammatory proteins secreted by most, if not all, malignant and stromal cells.
Chemokines can exert pro- and antitumor effects, via both cell-intrinsic and cell-extrinsic mechanisms. Thus, chemokines not only regulate the survival, proliferative potential, and metastatic attitude of malignant cells, but also modulate angiogenesis and provide cues for the recruitment of mesenchymal and immune cells to the tumor bed. Such an heterogeneous panel of biological functions is often reflected by paradoxical, context-dependent effects.2 Models of spontaneous carcinogenesis are therefore needed to study the effects of distinct chemokines on the biology of specific tumors.
Although most chemokines are normally secreted in the extracellular space, chemokine (C-X3-C motif) ligand 1 (CX3CL1, also known as fractalkine or neurotactin) is produced as a membrane-anchored precursor that can be cleaved by ADAM family metalloproteases in an inducible or constitutive manner. Membrane-tethered CX3CL1 and its receptor, CX3CR1 (a G protein-coupled receptor), function as adhesion molecules in vitro, in physiological flow conditions, whereas the shed form of CX3CL1 acts as a soluble chemoattractant for CX3CR1+ cells.3 Transgenic mice expressing the wild-type CX3CL1 precursor or an obligatory soluble CX3CL1 mutant revealed that membrane-tethered and soluble CX3CL1 have specific functions in vivo.4
On one hand, the CX3CL1/CX3CR1 signaling axis transduces pro-survival, proliferative, and metastatic signals, thus favoring tumor progression. On the other hand, CX3CL1/CX3CR1 mediate antitumor effects by enhancing natural killer (NK) cell- and CD8+ T lymphocyte-mediated tumor-specific immune responses.5,6 Moreover, we have recently demonstrated that CX3CL1 can promote the progression of breast carcinoma by operating in concert with members of the epidermal growth factor receptor (EGFR) family.7
CX3CL1 is a transcriptional target of p53.8 Given its capacity to prime antitumor immune responses, one could hypothesize that CX3CL1 operates as a cell-extrinsic tumor suppressor downstream of p53. In support of this view, 3 independent in silico studies (GEOprofile Database GSE3744, GDS3324; EMBL-EBI Database GEOD10780) showed that CX3CL1 is downregulated in primary human breast cancers as compared with healthy breast tissue. CX3CL1 is also downregulated in spontaneous breast tumors that arise in MMTV-neu mice (which overexpress the rat ERBB2 ortholog, neu, in the mammary gland). Surprisingly, the transgenic re-expression of CX3CL1 in MMTV-neu breast tumors neither favors disease regression nor affects tumor growth, but rather promotes the onset of new oncogenic lesions in non-transduced mammary glands.7
How does the re-expression of CX3CL1 increase tumor multiplicity in MMTV-neu mice? One possibility is that increased levels of CX3CL1 in the tumor microenvironment boost the metastatic potential of neoplastic cells, which then colonize other mammary glands. CX3CL1 stimulates the migratory capacity of CX3CR1+ breast cancer cells in vitro, upregulates SNAI2 (previously known as SLUG), and causes the intracellular delocalization of E-cadherin through the canonical pertussis toxin (PTX)-sensitive, CX3CR1-dependent pathway. Although these results suggest that CX3CL1 stimulate the epithelial-mesenchymal transition and invasiveness in vitro, CX3CL1 does not seem to promote metastatic dissemination in vivo, as we found no tumor cells in sentinel lymph nodes nor we were able to document an infiltration of the basal lamina in the novel tumors forming in CX3CL1-transduced MMTV-neu mice.
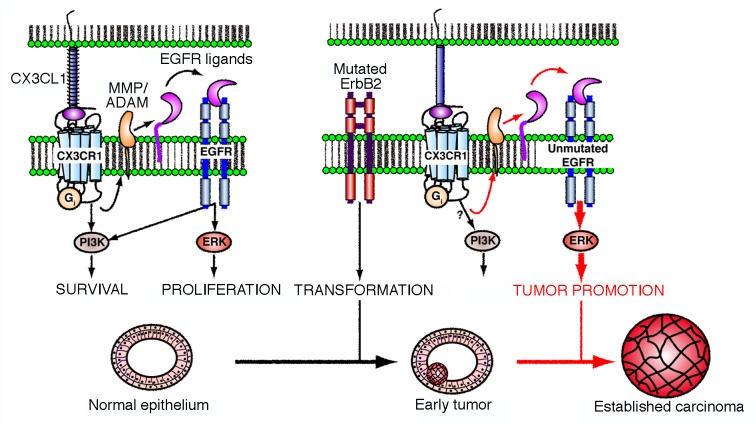
MMTV-neu mice normally develop focal adenocarcinomas, while multifocal lesions arise after long latency periods. Thus, CX3CL1 might accelerate the expansion of pre-neoplastic lesions, hence increasing tumor multiplicity. The intratumoral injection of a CX3CL1-encoding virus induces a systemic increase in CX3CL1 levels. However, even though inflammation has been described as a cell-extrinsic mechanism to promote tumorigenesis,1 we observed no differences in the inflammatory infiltrate associated with CX3CL1 expression in pre-neoplastic or established lesions. In contrast, we found that CX3CL1 promotes the activation of the EGFR by stimulating the matrix metalloproteinase (MMP)-dependent shedding of membrane-tethered EGF precursors. Thus, CX3CL1 induces the activation of both EGFR and extracellular signal-regulated kinases (ERKs) in (pre-) neoplastic cells spontaneously arising in MMTV-neu mice, in a PTX-insensitive manner.
The bidirectional crosstalk between structurally distinct receptor classes (such as G protein-coupled receptor and tyrosine kinase receptors) had previously been described in the context of cancer,9,10 yet the relevance of this functional interaction remains poorly defined. To address this issues, we developed neu-dependent and -independent models of spontaneous breast cancer on CX3CL1-deficient or -proficient genetic backgrounds. We observed that approximately 30% of MMTV-neu/Cx3cl1−/− mice do not develop tumors by 15 mo of age, and those that do so manifest a significant delay in tumor onset and a significant reduction in tumor burden as compared with control animals. In contrast, the lack of CX3CL1 did not affect tumor onset and tumor burden in a neu-independent breast cancer model (MMTV-PyMT mice).
The results implicate CX3CL1 in the development of spontaneous murine EGFR-dependent breast carcinomas (Fig. 1). CX3CL1 is not involved in tumor initiation, which is presumably due to neu mutations, but promotes the progression of small (pre-)neoplastic lesions by cross-activating the EGFR-ERK signaling axis. The direct activation of this mitogenic pathway by the polyomavirus middle T antigen (PyMT) might explain why breast carcinomas arising in MMTV-PyMT mice grow similarly on CX3CL1-deficient and CX3CL1-proficient genetic backgrounds.
Figure 1. CX3CL1 stimulates the progression of breast carcinoma. In mammary epithelial cells, chemokine (C-X3-C motif) ligand 1 (CX3CL1) favors the activation of epidermal growth factor receptor (EGFR) family members by stimulating the cleavage of membrane-tethered EGF precursors by ADAM matrix metalloproteinases (MMPs). This appears to activate the extracellular signal-regulated kinase (ERK) signaling cascade downstream of the CX3CL1 receptor (CX3CR1) in untransformed and neoplastic ERBB2+ cells. Although CX3CL1 is not needed for the development and physiological functions of the mammary gland, the CX3CL1-induced transactivation of EGFR plays a major role in the progression of early ERBB2+ breast carcinomas. This cell-intrinsic tumor-promoting activity of CX3CL1 might be associated with qualitative and/or quantitative changes in the strength, frequency or breadth of ERK-conveyed signals in transformed cells. However, established carcinomas become independent of this cross-activation circuit for progression. PI3K, phosphoinositide-3-kinase.
Our study raises several questions. First, it will be important to understand why the upregulation of CX3CL1 in the tumor microenvironment can have such diverse functional consequences (i.e., tumor rejection by the immune6 and accelerated tumor progression upon the activation of cancer cell-intrinsic signaling cascades7). CX3CL1-mediated antitumor immune responses have mainly been documented in xenograft tumor models. In this setting, the inoculation of cancer cells may per se trigger some extent of inflammation. Conversely, autochthonous tumors are poorly inflammatory/immunogenic due to central and peripheral tolerance mechanisms. Second, it will be important to determine whether CX3CL1 enhances the proliferation and/or the self-renewal capacity of tumor-initiating cells. Finally, the translational relevance of these findings must be established, as ERBB2 is amplified and (wild-type or mutant) EGFR is overexpressed in ~30% and ~50% of human breast carcinomas, respectively. Detailed studies on the mutual interaction between the ERBB2 and EGFR signaling axes could provide deep insights into the progression of breast carcinomas and perhaps lead to the identification of novel targets for therapeutic interventions.
Disclosure of Potential Conflicts of Interest
This work was supported by the Ministry of Science (SAF2011-24453) and the Comunidad de Madrid (INMUNOTHERCAN, S2010/BMD-2326).
Glossary
Abbreviations:
- CX3CL1
chemokine (C-X3-C motif) ligand 1
- EGF
epidermal growth factor
- EGFR
EGF receptor
- ERK
extracellular signal-regulated protein kinase
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- NK
natural killer
- PTX
pertussis toxin
- PyMT
polyomavirus middle T antigen
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25669
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.González-Martin A, Mira E, Mañes S. CCR5 as a potential target in cancer therapy: inhibition or stimulation? Anticancer Agents Med Chem. 2012;12:1045–57. doi: 10.2174/187152012803529637. [DOI] [PubMed] [Google Scholar]
- 3.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30. doi: 10.1016/S0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 4.Kim KW, Vallon-Eberhard A, Zigmond E, Farache J, Shezen E, Shakhar G, et al. In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood. 2011;118:e156–67. doi: 10.1182/blood-2011-04-348946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudin F, Nasreddine S, Donnadieu AC, Emilie D, Combadière C, Prévot S, et al. Identification of the chemokine CX3CL1 as a new regulator of malignant cell proliferation in epithelial ovarian cancer. PLoS One. 2011;6:e21546. doi: 10.1371/journal.pone.0021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang L, Hu HD, Hu P, Lan YH, Peng ML, Chen M, et al. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007;14:1226–34. doi: 10.1038/sj.gt.3302959. [DOI] [PubMed] [Google Scholar]
- 7.Tardáguila M, Mira E, García-Cabezas MA, Feijoo AM, Quintela-Fandino M, Azcoitia I, et al. CX3CL1 promotes breast cancer via transactivation of the EGF pathway. Cancer Res. 2013;73:4461–73. doi: 10.1158/0008-5472.CAN-12-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiraishi K, Fukuda S, Mori T, Matsuda K, Yamaguchi T, Tanikawa C, et al. Identification of fractalkine, a CX3C-type chemokine, as a direct target of p53. Cancer Res. 2000;60:3722–6. [PubMed] [Google Scholar]
- 9.Mira E, Lacalle RA, González MA, Gómez-Moutón C, Abad JL, Bernad A, et al. A role for chemokine receptor transactivation in growth factor signaling. EMBO Rep. 2001;2:151–6. doi: 10.1093/embo-reports/kve027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]