Abstract
Early nutritional support is an essential component of burn care to prevent ileus, stress ulceration, and the effects of hypermetabolism. The American Burn Association practice guidelines state that enteral feedings should be initiated as soon as practical. The authors sought to evaluate compliance with early enteral nutrition (EN) guidelines, associated complications, and hospitalization outcomes in a prospective multicenter observational study. They conducted a retrospective review of mechanically ventilated burn patients enrolled in the prospective observational multicenter study “Inflammation and the Host Response to Injury.” Timing of initiation of tube feedings was recorded, with early EN defined as being started within 24 hours of admission. Univariate and multivariate analyses were performed to distinguish barriers to initiation of EN and the impact of early feeding on development of multiple organ dysfunction syndrome, infectious complications, days on mechanical ventilation, intensive care unit (ICU) length of stay, and survival. A total of 153 patients met study inclusion criteria. The cohort comprised 73% men, with a mean age of 41 ± 15 years and a mean %TBSA burn of 46 ± 18%. One hundred twenty-three patients (80%) began EN in the first 24 hours and 145 (95%) by 48 hours. Age, sex, inhalation injury, and full-thickness burn size were similar between those fed by 24 hours vs after 24 hours, except for higher mean Acute Physiology and Chronic Health Evaluation II scores (26 vs 23, P = .03) and smaller total burn size (44 vs 54% TBSA burn, P = .01) in those fed early. There was no significant difference in rates of hyperglycemia, abdominal compartment syndrome, or gastrointestinal bleeding between groups. Patients fed early had shorter ICU length of stay (adjusted hazard ratio 0.57, P = 0.03, 95% confidence interval 0.35–0.94) and reduced wound infection risk (adjusted odds ratio 0.28, P = 0.01, 95% confidence interval 0.10–0.76). The investigators have found early EN to be safe, with no increase in complications and a lower rate of wound infections and shorter ICU length of stay. Across institutions, there has been high compliance with early EN as part of the standard operating procedure in this prospective multicenter observational trial. The investigators advocate that initiation of EN by 24 hours be used as a formal recommendation in nutrition guidelines for severe burns, and that nutrition guidelines be actively disseminated to individual burn centers to permit a change in practice.
Severe burn injury results in a prolonged hypermetabolic and catabolic state that persists as long as 1 year after injury.1 Early nutritional support has become a critical component of early management of injured patients to prevent ileus, stress ulceration, and the effects of hypermetabolism. International nutrition support guidelines advocate that enteral feedings should occur early in critically ill patients who have a functioning gastrointestinal tract, but what is considered early varies significantly.2–5 The Canadian Clinical Practice Guidelines recommend starting enteral nutrition (EN) within 24 to 48 hours after admission to the intensive care unit (ICU) in critically ill patients.3 The Eastern Association for the Surgery of Trauma recommends that intragastric feedings be started as soon as possible in burn patients after admission, because delayed enteral feeding (>18 hours) results in a high rate of gastroparesis and the need for intravenous nutrition.4 Similarly, the American Burn Association (ABA) advocates early EN as soon as practical.5
The practice of early enteral feeding was one of the standard practice guidelines adopted by the Inflammation and Host Response to Injury (Glue Grant). The Glue Grant standard operating procedures state that EN should be started as soon as possible after admission.6 However, both ABA and Glue Grant guidelines lack a specific time frame for initiation, allowing for variations in practice to be a potential barrier to uniformity within a prospective multicenter trial.
Increasing emphasis is now placed on practice guidelines, protocols, and bundles in all areas of critical care, including burns.7–9 The impact of guidelines in burn critical care, however, is unknown, as few studies have evaluated compliance. Recognized barriers to compliance include patient and injury factors, logistical issues, and individual institutional practices or provider preferences. We sought to evaluate compliance with early EN, possible barriers to implementation, associated complications, and hospitalization outcomes in a prospective multicenter observational study.
METHODS
Study Design
The Inflammation and Host Response to Injury is a collaborative program supported by the National Institute of General Medical Sciences designed to better define the proteomic and genomic response to injury. In the context of this large cohort study, we set out 1) to evaluate current practices in EN and examine the logistical, patient, injury, resuscitation, or provider characteristics that may contribute to a delay in EN and 2) to examine the relationship between early EN and hospital outcomes. The principal exposure of interest was early EN, defined as initiation of tube feeding within the first 24 hours postburn injury. Outcomes of interest were complications associated with early feeding, multiple organ dysfunction syndrome, infectious complications, ventilator days, ICU length of stay (LOS), and in-hospital mortality.
Patients and Data Collection
Eligible subjects included all mechanically ventilated adults with complete outcome data enrolled in the burn arm of the “Inflammation and Host Response to Injury” program from March 2004 to September 2009. We chose mechanically ventilated adults to evaluate the most severely ill cohort and to eliminate variations in supplementing oral nutrition with tube feeding.10 Criteria for adult patient enrollment into each of the five participating sites study were age 18 years or older, burn size ≥20% TBSA, no other concomitant trauma, and admission within 96 hours of injury. Patients who were not resuscitated and placed on comfort care were not eligible for enrollment. Patients and their families were interviewed about their medical history and when available, medical records before injury were also reviewed to learn about medical illnesses.
With respect to clinical management, investigators from all participating sites agreed to adhere to standard management protocols and defined all clinical variables to be prospectively recorded, as previously described.6 Clinical data were collected by trained nurse abstractors and entered into a web-based data collection platform specifically adapted for this program. Data integrity was evaluated centrally and by external review as previously described.10 For this study, we abstracted data on all enrolled mechanically ventilated adult patients with complete hospitalization records as of September 1, 2009. Approval for the study was granted by the Glue Grant administrative core and by the University of Washington Institutional Review Board.
Data Analysis
We compared baseline patient and injury characteristics between patients who received and who did not receive early EN, including age, sex, ethnicity, %TBSA burn, injury etiology, presence of inhalation injury, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Denver multiorgan failure score10 at 24 hours and maximal Denver score, resuscitation volume, urine output, and development of abdominal compartment syndrome. Total fluid volume (including colloid and crystalloid) administered in the first 24 hours after injury was examined as a ratio over the volume predicted by the Parkland formula (4 ml/kg/%TBSA). Development of complications, days requiring mechanical ventilation, ICU LOS, and mortality were recorded for each patient.
Categorical variables, reported as proportions, were compared with χ2 tests. Continuous variables are reported as mean ± SD. Means were compared using Student’s t-test if a normal distribution was detected. Nonparametric variables were compared with Wilcoxon rank-sum test where appropriate. Logistic regression analyses were performed to assess the impact of early EN on complications and hospitalization outcomes.
To adjust for confounding variables and assess possible effect modification, multiple logistic regression analyses were performed, including all variables that demonstrated significant association with the outcome of interest in the bivariate analyses (at the P<.20 level). Time-to-event analyses were performed by Cox proportional hazard ratio model. The model included known factors that contribute to burn outcomes (age, %TBSA, and presence of inhalation injury), as well as participating center, to control for potential variability among participating sites. All statistical analyses were performed with the use of STATA 10 (College Station, TX), a statistical software package. Actual P values are reported.
RESULTS
At the time of our analysis, there were a total of 229 enrolled adult subjects with complete and validated data. We restricted the study population to 153 mechanically ventilated subjects on admission. This cohort comprised 73% men, with a mean age of 41 ± 15 years and a mean %TBSA burn of 46 ± 18%. Of these 153 patients, 123 patients (80%) received EN by 24 hours, and 145 patients (95%) received EN by 48 hours. Four participating centers initiated EN via gastric tubes, whereas one site used postpyloric feeding tubes.
Potential Barriers to Early Feeding
We attempted to systemically evaluate logistical, patient, and injury factors that may have impacted the timing of EN initiation (Table 1). Differences in age, sex, inhalation injury, and full-thickness burn size were not statistically significant between groups, with the exception that patients fed in the first 24 hours had a higher mean APACHE II score (26 vs 23, P = .03), despite smaller overall burn size (44 vs 54% TBSA burn, P = .01). From a logistical standpoint, there was no difference in mean time from injury to admission between groups (3.4 vs 4.0 hours, P = .28), and the time of day that patients were injured and admitted were similar between groups (P = 0.80 and 0.96, respectively). No patients had associated abdominal trauma that would influence the decision to feed.
Table 1.
Patient demographics for early enteral nutrition in intubated adults
| Variable Mean (SD) or % |
All (n = 153) |
Early EN (n = 123) |
EN After 24 hr (n = 30) |
P |
|---|---|---|---|---|
| Age (yr) | 41 (15) | 41 (15) | 41 (16) | .92 |
| Percent male | 73 | 73 | 73 | .99 |
| Percent TBSA burn | 46 (18) | 44 (18) | 54 (20) | .01 |
| Percent full-thickness burn | 34 (19) | 34 (18) | 35 (22) | .70 |
| Inhalation injury | 56 | 59 | 43 | .13 |
| APACHE II | 25 (7) | 26 (7) | 23 (7) | .03 |
EN, enteral nutrition; APACHE, Acute Physiology and Chronic Health Evaluation.
To assess the resuscitation factors that might have influenced the timing of EN, we examined fluid requirements, development of abdominal compartment syndrome, hyperglycemia, insulin requirements, vasopressors, and early complications and found no statistical differences between the two groups (Table 2). Specifically, use of vasopressors was not associated with initiation of EN, as 10% of patients in each group received vasopressors in the first 24 hours.
Table 2.
Resuscitation factors
| Variable Mean (SD) or % | All (n = 153) | Early EN (n = 123) | EN After 24 hr (n = 30) | P |
|---|---|---|---|---|
| Fluid resuscitation (observed/expected) | 1.4 (0.6) | 1.4 (0.6) | 1.3 (0.6) | .22 |
| Abdominal compartment syndrome | 4.6 | 4.9 | 3.3 | .72 |
| 24-hr high glucose (mg/dl) | 191 (59) | 193 (58) | 185 (65) | .54 |
| 24-hr insulin requirement (U) | 247 (260) | 230 (254) | 316 (278) | .11 |
| Received pressors in first 24 hr | 10.5 | 10.6 | 10.0 | .93 |
| ALI at 24 hr | 65 | 62 | 77 | .14 |
| AKI at 24 hr | 26 | 28 | 20 | .40 |
| MODS at 24 hr | 11 | 10 | 13 | .57 |
ALI, acute lung injury; AKI, acute kidney injury; MODS, multiple organ dysfunction syndrome.
Outcomes
Next, we compared injury outcomes between those who received EN and those who did not. There was no increased incidence of gastrointestinal bleeding, abdominal compartment syndrome, need for laparotomy, or ventilator-associated pneumonia (Table 3). We also evaluated the influence of early EN on time to ventilator-associated pneumonia development and found that early EN was not a significant predictor (adjusted hazard ratio [HR] = 1.23, P = .41, 95% confidence interval [CI] 0.74–2.04). In addition, length of mechanical ventilation, incidence of bloodstream and total infectious complications, multiple organ dysfunction syndrome, and survival were similar between the two groups. Patients fed early did have a shorter ICU LOS (40.7 vs 52.5 days, P = .03) and decreased wound infection rates (54.5 vs 80%, P = .01).
Table 3.
Hospital Outcomes with Early Enteral Nutrition
| Variable | All (n = 153) |
EN 24 (n = 123) |
EN After 24 (n = 30) |
P |
|---|---|---|---|---|
| VAP (%) | 69.9 | 71.5 | 63.3 | .38 |
| ARDS (%) | 54.2 | 57.8 | 40.0 | .13 |
| MODS (%) | 32.0 | 30.1 | 40.0 | .30 |
| Vent days >21 (%) | 49.0 | 45.5 | 63.3 | .08 |
| Blood infection (%) | 62.7 | 56.1 | 90.0 | .15 |
| Wound infection (%) | 59.5 | 54.5 | 80.0 | .01 |
| Infectious complication (%) | 86.9 | 86.2 | 90.0 | .58 |
| GI bleed (%) | 4.6 | 4.1 | 6.7 | .54 |
| ICU LOS (d) | 43.0 | 40.7 | 52.5 | .03 |
| Survival (%) | 76.5 | 78.9 | 66.7 | .16 |
VAP, ventilator-associated pneumonia; ARDS, acute respiratory distress syndrome; MODS, multiple organ dysfunction syndrome; ICU LOS, intensive care unit length of stay.
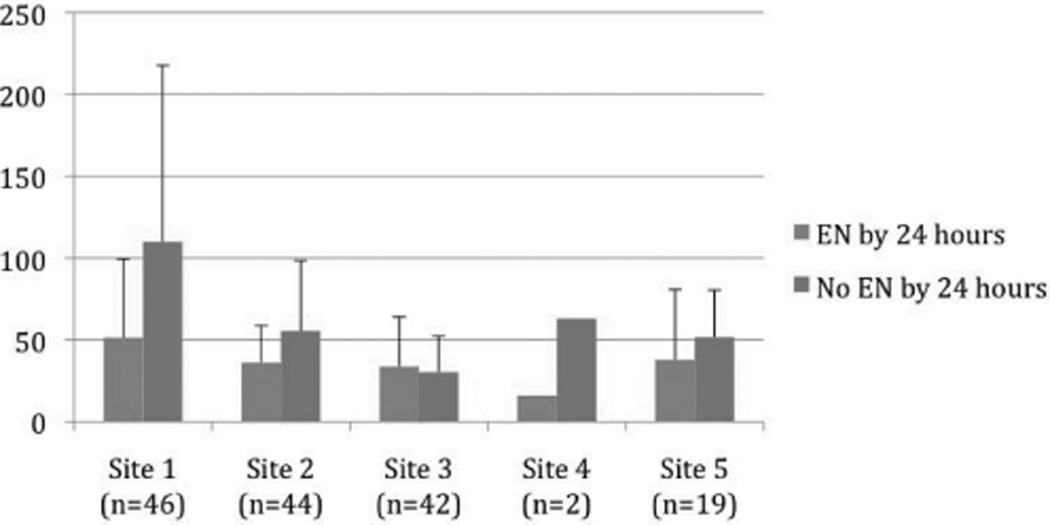
After adjustment for age, %TBSA burn, inhalation injury, and participating burn center, patients fed in the first 24 hours still had a shorter ICU LOS (adjusted HR = 0.57, P = .03, 95% CI 0.35–0.94) and lower wound infection risk (OR = 0.28, P = .01, 95% CI 0.10–0.76). Participating sites did not significantly influence the outcomes of wound infections, development of pneumonia, or prolonged mechanical ventilation. Participating sites, however, had a modifying effect on ICU LOS (adjusted HR = 0.85, P = .04, 95% CI 0.74–0.99; Table 4). As shown in Figure 1, mean ICU LOS was shorter for patients fed within 24 hours at four sites and nearly equivalent at the fifth burn center.
Table 4.
Analysis of factors associated with higher ICU LOS
| Variable | Adjusted Hazard Ratio |
P | 95% CI |
|---|---|---|---|
| Early EN initiation | 0.57 | .03 | 0.35–0.94 |
| Age (per yr) | 0.99 | .41 | 0.98–1.00 |
| TBSA (per %) | 1.02 | <.01 | 1.01–1.03 |
| Inhalation injury | 1.62 | <.01 | 0.35–0.94 |
| Participating site | 0.85 | .04 | 0.74–0.99 |
EN, enteral nutrition; ICU LOS, intensive care unit length of stay; CI, confidence interval.
Figure 1.
Mean intensive care unit length of stay (days) by participating center.
DISCUSSION
Nutrition is a key aspect of outcome after burn injury. Accordingly, the ABA guidelines state that “enteral feedings should be initiated as soon as practical.”5 In this multicenter prospective study, the standard operating procedures similarly emphasize the importance of early nutrition, recommending that “enteral nutrition should be started as soon as possible.”6 When examining adherence to these guidelines, we found a high compliance among participating centers with 80% (123) to 95% (145) of patients being fed in the first 24 to 48 hours.
We examined the potential barriers to initiating feeds within the first day of admission, looking for logistical, patient, injury, or resuscitation factors that may have impacted initiation of feeding. However, we found no significant differences between those fed within 24 hours and those after 24 hours. Thus, they likely were not the principal barriers for early EN initiation. Therefore, we postulate that provider factors likely accounted for the variation in practice.
Early enteral nutritional support in severe burns has demonstrated numerous advantages, such as increased caloric intake, insulin secretion and protein retention,11 improved bowel mucosal integrity,12 and decreased incidence of stress gastritis.13 A recent review of patients treated over 30 years indicates that modern resuscitation combined with early EN has nearly eliminated the incidence of significant stress ulceration.13 However, it has been difficult to show impact on nutritional, metabolic, or biochemical markers and clinical outcomes, such as LOS, infection rates, and mortality.14
There have been many concerns regarding early enteral feeding in critically injured patients such as the potential for intestinal necrosis in patients undergoing resuscitation11,14 or fear for aspiration and associated pneumonia, although a recent study showed no increased risk of aspiration or pneumonia in patients who were fed early.15 We believe that many aspirations, especially microaspirations, occur frequently and may not become apparent until infection is present. Thus, we defined ventilator-associated pneumonia as the clinically important endpoint that encompasses complications related to aspiration events. Also, we found no harmful effects of early feeding of severely burned patients, as there was no increased incidence of gastrointestinal bleeding, laparotomy for abdominal compartment syndrome, or ischemic bowel, even while 10% of patients received vasopressors during resuscitation.
We also examined the potential impact of earlier enteral feeding on injury outcome. Two previous small trials of early EN in burn patients have shown no difference between early and late feeding for LOS, ICU days, the number of infections, or mortality.14,16 Conversely, Hart et al17 have shown a significant decrease in skeletal muscle catabolism with early EN and burn wound excision, independent of the metabolic rate. Although our study may suggest some benefit to early EN, prospective evaluations with well-defined EN protocols are needed to answer whether early EN has positive effects on wound infection rates, duration of mechanical ventilation, and ICU LOS. Creation of specifically worded practice guidelines with protocolized initiation of EN may help unify practice patterns and facilitate future attempts to answer these questions.
It is certainly valid to ask the question, “Is there really a difference between feeding at 20 hours and 25 hours?” Some practitioners are advocating waiting until after resuscitation and feeding at 48 to 72 hours. Perhaps to find a difference between groups, very early feeding would need to be performed. Animal studies have shown safety in feeding 2 hours after injury, with ability to decrease the hypermetabolic and catabolic response to injury as well as decreased bacterial translocation,18–22 but human studies have failed to replicate this finding.
This study has several limitations. Our study is retrospective in nature and across multiple institutions. Although we have systematically assessed patient, injury, and logistical factors that may affect the timing of EN initiation, potential systematic variability between centers not associated with the start of tube feedings might have contributed to the observed outcome differences. Standard operating protocols for the Glue Grant project were intended to decrease variability. However, center variability still significantly contributed to outcomes, as exemplified by the observed center effect on ICU LOS. Also, we did not have data on the amount of calories provided to each subject per day, whether individual patients achieved caloric goals (and how soon), nor did we capture data on metabolic and nutritional parameters at regular intervals. As such, we were unable to assess whether those subjects who were started on early EN achieved nutritional goals earlier or whether early EN influenced postburn hypermetabolism. In addition, in the immediate resuscitation period, patients are often transferred from nonburn facilities and require initial stabilization, including bedside procedures and wound care. Thus, it can be logistically difficult to initiate feeding as quickly as can be performed in an animal study. The dataset did not specify the exact hour of initiation of EN and thus did not permit subgroup analysis within those fed by 24 hours. As the majority of patients were fed within 48 hours (still considered early compared with many studies that have looked at late feeding), there may be little difference between those fed late on the day of admission and early on the second day.
Experience with nutrition guidelines in critical care indicates that ICUs with a feeding protocol achieve more adequate EN and that an active dissemination of clinical practice guidelines is much more effective than passive.23,24 These elements highlight that lack of a unit-specific protocol or awareness of a practice guideline constitutes barriers to its implementation. Therefore, we advocate that initiation of EN by 24 hours be used as a formal recommendation in nutrition guidelines for severe burns and that nutrition guidelines be actively disseminated to individual burn centers to permit a change in practice.
CONCLUSION
We have found high compliance with early EN in this prospective, multicenter observational trial, such that 80% (123) to 95% (145) of patients were fed in the first 24 to 48 hours, respectively, although some variation exists among participating institutions. There was no demonstrable harm from early EN and perhaps measurable benefits, with noted decreases in ICU LOS and incidence of wound infections. We advocate that initiation of EN by 24 hours be used as a formal recommendation in nutrition guidelines for severe burns and that nutrition guidelines be actively disseminated to individual burn centers to permit a change in practice.
ACKNOWLEDGMENTS
The investigators acknowledge the contribution of the Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award 2-U54-GM062119 from the National Institute of General Medical Sciences, the National Center for Research Resources (NCCR) Grant 1KL2RR025015-01, and the David and Nancy Auth-Washington Research Foundation Endowment.
Supported by the National Institute of General Medical Sciences. This article was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the NIGMS.
REFERENCES
- 1.Perreira CT, Murphey KD, Herndon DN. Altering metabolism. J Burn Car Rehabil. 2005;26:194–199. [PubMed] [Google Scholar]
- 2.Kreymann K, Berger M, Deutz N, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210–223. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Perenter Enteral Nutr. 2003;27:355–373. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs DG, Jacobs DO, Kudsk KA, et al. EAST Practice Management Guidelines Workgroup. Practice management guidelines for nutritional support of the trauma patient. J Trauma. 2004;57:660–678. doi: 10.1097/01.ta.0000135348.48525.a0. discussion 679. [DOI] [PubMed] [Google Scholar]
- 5.Evidence-Based Guidelines Group ABA. Practice Guidelines for Burn Care. J Burn Care Rehabil. 2001;22:59S–66S. [Google Scholar]
- 6.Silver G, Klein M, Herndon D, et al. Inflammation and the Host Response to Trauma, Collaborative Research Program. Standard operating procedures for the clinical management of patients enrolled in a prospective study of Inflammation and the Host Response to Thermal Injury. J Burn Care Res. 2007;28:222–230. doi: 10.1097/BCR.0B013E318031AA44. [DOI] [PubMed] [Google Scholar]
- 7.Mosier M, Pham T. American Burn Association practice guidelines for prevention, diagnosis, and treatment of ventilator-associated pneumonia (VAP) in burn patients. J Burn Care Res. 2009;30:910–928. doi: 10.1097/BCR.0b013e3181bfb68f. [DOI] [PubMed] [Google Scholar]
- 8.Pham TN, Cancio LC, Gibran NS. American Burn Association practice guidelines burn shock resuscitation. J Burn Care Res. 2008;29:257–266. doi: 10.1097/BCR.0b013e31815f3876. [DOI] [PubMed] [Google Scholar]
- 9.Gibran NS Committee on Organization and Delivery of Burn Care, American Burn Association. Practice Guidelines for burn care, 2006. J Burn Care Res. 2006;27:437–438. doi: 10.1097/01.BCR.0000226084.26680.56. [DOI] [PubMed] [Google Scholar]
- 10.Klein MB, Hayden D, Elson C, et al. The association between fluid administration and outcome following major burn: a multicenter study. Ann Surg. 2007;245:622–628. doi: 10.1097/01.sla.0000252572.50684.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschlich M, Jenkins M, Mayes T, Khoury J, Kagan RJ, Warden GD. The 2002 Clinical Research Award. An evaluation of the safety of early vs delayed enteral support and effects on clinical, nutritional, and endocrine outcomes after severe burns. J Burn Care Rehabil. 2002;23:401–415. doi: 10.1097/01.BCR.0000036588.09166.F1. [DOI] [PubMed] [Google Scholar]
- 12.Peng Y, Yuan Z, Xiao G. Effects of early enteral feeding on the prevention of enterogenic infection in severely burned patients. Burns. 2001;27:145–149. doi: 10.1016/s0305-4179(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 13.Xiao S, Zhu S, Xia Z, et al. Prevention and treatment of gastrointestinal dysfunction following severe burns: a summary of recent 30-year clinical experience. World J Gastroenterol. 2008;14:3231–3235. doi: 10.3748/wjg.14.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peck M, Kessler M, Cairns B, Chang YH, Ivanova A, Schooler W. Early enteral nutrition does not decrease hypermetabolism associated with burn injury. J Trauma. 2004;57:1143–1149. doi: 10.1097/01.ta.0000145826.84657.38. [DOI] [PubMed] [Google Scholar]
- 15.Venter M, Rode H, Sive A, Visser M. Enteral resuscitation and early enteral feeding in children with major burns—effect on McFarlane response to stress. Burns. 2007;33:464–471. doi: 10.1016/j.burns.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Chiarelli A, Enzi G, Casadei A, Baggio B, Valerio A, Mazzoleni F. Very early nutrition supplementation in burned patients. Am J Clin Nutr. 1990;51:1035–1039. doi: 10.1093/ajcn/51.6.1035. [DOI] [PubMed] [Google Scholar]
- 17.Hart DW, Wolf SE, Chinkes DL, et al. Effects of early excision and aggressive enteral feeding on hypermetabolism, catabolism, and sepsis after severe burn. J Trauma. 2003;54:755–761. doi: 10.1097/01.TA.0000060260.61478.A7. [DOI] [PubMed] [Google Scholar]
- 18.Mochizuki H, Trocki O, Dominioni L, Brackett KA, Joffe SN, Alexander JW. Mechanism of prevention of postburn hypermetabolism and catabolism by early enteral feeding. Ann Surg. 1984;200:297–310. doi: 10.1097/00000658-198409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominioni L, Trocki O, Mochizuki H, Brackett KA, Joffe SN, Alexander JW. Prevention of severe postburn hypermetabolism and catabolism by immediate intragastric feeding. J Burn Care Rehabil. 1984;5:106–112. [Google Scholar]
- 20.Gianotti O, Nelson JL, Alexander JW, Chalk CL, Pyles T. Post injury hypermetabolic response and magnitude of translocation: prevention by early enteral nutrition. Nutrition. 1994;3:225–231. [PubMed] [Google Scholar]
- 21.Inoue S, Epstein MD, Alexander JW, Trocki O, Jacobs P, Gura P. Prevention of yeast translocation across the gut by a single enteral feeding after burn injury. JPEN J Parenter Enteral Nutr. 1989;13:565–571. doi: 10.1177/0148607189013006565. [DOI] [PubMed] [Google Scholar]
- 22.Braga M, Gianotti L, Constantini E, et al. Impact of enteral nutrition of intestinal bacterial translocation and mortality in burned mice. Clin Nutr. 1994;13:256–261. doi: 10.1016/0261-5614(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 23.Heyland DK, Dhaliwal R, Day A, Jain M, Drover J. Validation of the Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients: results of a prospective observational study. Crit Care Med. 2004;32:2260–2266. doi: 10.1097/01.ccm.0000145581.54571.32. [DOI] [PubMed] [Google Scholar]
- 24.Jain M, Heyland D, Dhaliwal R, et al. Dissemination of the Canadian clinical practice guidelines for nutrition support: results of a cluster randomized controlled trial. Crit Care Med. 2006;34:2362–2369. doi: 10.1097/01.CCM.0000234044.91893.9C. [DOI] [PubMed] [Google Scholar]