Abstract
Objective
We sought to evaluate whether preoperative body mass index (BMI) impacts surgical outcomes, complication rates, and/or recurrence rates in women undergoing pelvic exenteration.
Methods
All women who underwent pelvic exenteration for gynecologic indications at our institution from 1993 through 2010 were included. Women were stratified into 3 groups based on BMI. Baseline characteristics, surgical outcomes, early (<60days) and late (≥60days) postoperative complications, and recurrence/survival outcomes were collected. Multivariate logistic regression analyses were performed. Kaplan-Meier survival curves were compared using log-rank test.
Results
161 patients were included (59 normal weight, 44 overweight, 58 obese). Median follow-up times were 22, 29, and 25 months. Most patients underwent total pelvic exenteration (68%); 64.6% had a vaginal reconstruction. On multivariate analysis, both overweight and obese patients had a higher risk of early superficial wound separation compared to normal weight patients – OR 10.74 (3.33–34.62, p <0.001) and OR 4.35 (1.40–13.52, p=0.011), respectively. Length of surgery was significantly longer for overweight (9.6hrs, OR 1.26, 1.02–1.55, p=0.032) and obese (10.1hrs, OR 1.24, 1.04–1.47, p=0.014) patients than for normal weight patients (8.7hrs). Late postoperative complications for patients in the normal weight, overweight, and obese groups were 47.5%, 45.5%, and 43.1% (p=0.144). There were no differences in time to recurrence (p=0.752) or overall survival (p=0.103) between groups.
Conclusion
Although operative times were longer and risk for superficial wound separation was significantly higher, pelvic exenteration appears to be feasible and safe in overweight and obese women with overall complication rates and survival outcomes comparable to normal weight women.
Introduction
First described by Brunschwig in 1948[1], pelvic exenteration involves the en bloc radical resection of the pelvic viscera and historically has been used in the management of recurrent gynecologic malignancies confined to the central pelvis. The most common indication for pelvic exenteration is central recurrence or persistence of cervical carcinoma[2]. The procedure varies based on the location and extent of disease, with the goal being to obtain negative surgical margins.
While pelvic exenteration is commonly performed with curative intent, it is associated with a significant risk of morbidity. Post-operative complications have been reported in up to 45 – 84% of cases [3–11]. Pelvic exenteration has also been associated with a significant risk of operative mortality, 2 – 10% [6, 7, 9, 12–15], although these figures have decreased in recent years [16, 17]. Despite these risks, pelvic exenteration has the potential to provide a significant survival benefit; 5-year survival rates of 20 – 60% following exenteration have been reported [4, 7, 8, 10, 13–15, 18, 19].
Obesity is a known risk factor for surgical complications including increased intra-operative blood loss, longer operative times, and increased intra-operative and post-operative complication rates. For this reason, obesity has been considered a relative contraindication to complex surgical procedures [20–23]. A recent Gynecologic Oncology Group study of patients with early stage endometrial cancer who underwent comprehensive surgical staging suggested that obesity is associated with higher non-cancer mortality and wound complications [24]. Furthermore, obesity has also been associated with lower physical well-being and quality-of-life scores following gynecologic surgery [25]. A few investigators have evaluated whether a patient’s weight affects the extent of surgery, surgical outcomes, and survival following exenteration, with conflicting results [4, 11, 14, 17]. However, these studies, did not stratify patients into weight classifications based on BMI for comparison, and one excluded morbidly obese patients (BMI > 35 kg/m2) [4]. Thus, data on the impact of obesity in the setting of pelvic exenteration are limited. The purpose of this study was to evaluate whether pre-operative BMI affects surgical outcomes, complication rates, and/or recurrence rates in women undergoing pelvic exenteration at our institution.
Methods
Following Institutional Review Board approval, a retrospective review of all women who underwent pelvic exenteration for gynecologic indications at M.D. Anderson Cancer Center between January 1993 and December 2010 was performed. Operative reports, pathology records, and clinic and hospital notes were reviewed and data were abstracted pertaining to baseline patient characteristics, surgical and pathological outcomes, early and late complications, and disease recurrence.
The cases were stratified into three weight groups (normal weight, BMI <25 kg/m2; overweight, BMI 25 kg/m2 to <30 kg/m2; and obese, BMI ≥ 30 kg/m2) as defined by the World Health Organization. Morbidly obese patients were included in the obese group as there were too few (n=13) to perform separate statistical comparisons. Post-operative complications were reported for each group as early (<60 days following exenteration) or late (≥60 days following exenteration). Complications were broadly divided into wound separation, infections (abscess, sepsis, pneumonia), urinary complications (ureteral injury, ureteral stricture, renal failure, urostomy complications), gastrointestinal complications (bowel obstruction or colostomy complications), cardiovascular complications (myocardial infarction, deep vein thrombosis, or pulmonary thromboembolism), or need for reoperation for any indication. Information on disease status (recurrence rate, time to recurrence, site of recurrence, and survival status at the time of analysis) was also collected. Time to recurrence was defined as the time interval from the exenteration to clinical or radiological diagnosis of disease recurrence. Overall survival was defined as the time interval from the exenteration to the date of death.
Univariate statistical tests of association were conducted by Fisher’s exact test for categorical variables or Kruskall-Wallis test for continuous variables. For the Fisher’s test comparing complications with BMI, two tests were performed: (1) complications < 60 days versus no complications and (2) complications ≥ 60 days versus no complications. Survival curves were designed based on the method described by Kaplan and Meier [26] and compared using a log-rank test.
Multivariate logistic regression models were used to adjust for confounding variables. Two equations were created – one to compare overweight patients to normal weight patients and another to compare obese patients to normal weight patients. Furthermore, we separately examined complications that occurred within 2 months of surgery and those that occurred more than 2 months post-surgery. In building these models, we chose all complications that were statistically significantly associated with BMI (p < 0.10) using Fisher’s exact test. Specifically, we built the model with terms for length of surgery, median estimated blood loss, wound separation, uretal stricture, renal failure and bowel obstruction. We also included ethnicitiy, hypterension and diabetes in the models as possible confounding variables that were also related to weight (p < 0.10). The full model was then modified using backward selection, keeping only those terms with p < 0.05.
Results
Between 1993 and 2010, a total of 161 pelvic exenterations were performed for oncologic indications by the gynecologic oncology service at M.D. Anderson Cancer Center. In this analysis, three patients required a second exenterative procedure and were considered twice. One patient initially had an anterior exenteration for recurrent endometrial carcinoma and 3 years later underwent a posterior exenteration for recurrent disease associated with obstruction of the rectosigmoid colon. Two patients with recurrent vulvar carcinoma initially had posterior exenterations and subsequently required anterior exenterations for disease recurrence at 10 and 21 months.
When stratified by weight group, 59 exenterations were performed in normal-weight patients, 44 in overweight patients, and 58 in obese patients. Patient demographics and pre-operative morbidities are summarized in Table 1. The majority of patients, regardless of weight, were white. There were no significant differences between groups with regard to age, tobacco use, or baseline co-morbid medical conditions except for hypertension which was more common in obese patients (36.2%, p=0.031). While exenterations in normal-weight patients were evenly distributed over the study period (29 before the year 2000 and 30 in 2000 and after), a greater proportion of overweight or obese patients underwent exenteration in the past decade. Of the 102 exenterations in overweight or obese patients in the study, 64 (69.6%) were performed in the year 2000 or after, compared to 28 (30.4%) before that date (p=0.026).
Table 1.
Baseline patient characteristics
| Normal Weight (n = 59) |
Overweight (n = 44) |
Obese (n = 58) |
p-value | |
|---|---|---|---|---|
| Mean Age at Exenteration (years) | 56.3 (24.8–85.9) | 51.8 (27.1–78.4) | 55.1 (30.4–74.0) | 0.259 |
| BMI | 21.3 (15.1–24.98) | 27.5 (25.0–29.7) | 36.4 (30.0–50.9) | |
| Race | 0.017 | |||
| White | 48 (81.4%) | 26 (59.1%) | 40 (69%) | |
| Black | 1 (1.7%) | 5 (11.4%) | 4 (6.9%) | |
| Asian | 3 (5.1%) | 0 (0%) | 0 (0%) | |
| Other | 7 (11.9%) | 13 (29.5%) | 14 (24.1%) | |
| Hispanic | 7 (11.9%) | 14 (31.8%) | 15 (25.9%) | 0.036 |
| Co-morbidities | ||||
| HTN | 9 (15.3%) | 10 (22.7%) | 21 (36.2%) | 0.031 |
| Diabetes | 3 (5.1%) | 4 (9.1%) | 10 (17.2%) | 0.096 |
| Asthma | 1 (1.7%) | 1 (2.3%) | 1 (1.17%) | >0.999 |
| Cardiac disease | 5 (8.5%) | 4 (9.1%) | 5 (8.6%) | >0.999 |
| Pulmonary disease* | 4 (6.8%) | 1 (2.3%) | 2 (3.5%) | 0.626 |
| Tobacco Use | 0.440 | |||
| Current | 10 (16.9%) | 12 (27.3%) | 8 (13.8%) | |
| Former | 11 (18.6%) | 8 (18.2%) | 15 (25.9%) | |
| Never | 38 (64.4%) | 24 (54.5%) | 35 (60.3%) | |
One missing record for obese category
Table 2 lists the clinico-pathologic features of all of the patients broken down by weight group. In the entire cohort, carcinoma of the cervix (53.4%) was the most common cancer diagnosis encountered. There were no significant differences between groups pertaining to cancer diagnosis or histologic subtype. The most common histologic subtype was squamous cell carcinoma (overall, 60.9%). Of the 161 exenterations, 124 were for recurrent disease (77.0%) and 20 for persistent (12.4%) disease. Only 17 cases (10.6%) were for primary disease. There was no significant difference in indication for surgery when the cases were stratified by weight group. The majority of cases had previously been treated with radiotherapy (70.8%) – either alone or in combination with chemotherapy. There were no significant differences between the three weight groups pertaining to treatment prior to exenteration.
Table 2.
Clinico-pathologic characteristics
| Normal Weight (n = 59) |
Overweight (n = 44) |
Obese (n = 58) |
p-value | |
|---|---|---|---|---|
| Cancer Diagnosis | 0.359 | |||
| Cervix (n=86) | 27 (45.8%) | 29 (65.9%) | 30 (51.7%) | |
| Vulva (n=21) | 8 (13.6%) | 5 (11.4%) | 8 (13.8%) | |
| Vagina (n=38) | 19 (32.2%) | 7 (15.9%) | 12 (20.7%) | |
| Uterus (n=15) | 4 (6.8%) | 3 (6.8%) | 8 (13.8%) | |
| Other (n=1) | 1 (1.7%) | 0 (0%) | 0 (0%) | |
| Histology | 0.123 | |||
| Squamous (n=98) | 31 (52.5%) | 32 (72.7%) | 35 (60.3%) | |
| Adenocarcinoma (n=36) | 15 (25.4%) | 6 (13.6%) | 15 (25.9%) | |
| Adenosquamous (n=6) | 3 (5.1%) | 3 (6.8%) | 0 (0%) | |
| Melanoma (n=8) | 5 (8.5%) | 0 (0%) | 3 (5.2%) | |
| Clear Cell (n=1) | 0 (0%) | 1 (2.3%) | 0 (0%) | |
| Other (n=10) | 4 (6.8%) | 2 (4.5%) | 4 (6.9%) | |
| Missing | 1 | 0 | 1 | |
| Indication for Surgery | 0.796 | |||
| Primary (n=17) | 8 (13.6%) | 4 (9.1%) | 5 (8.6%) | |
| Recurrent (n=124) | 44 (74.6%) | 36 (81.8%) | 44 (75.9%) | |
| Persistent (n=20) | 7 (11.9%) | 4 (9.1%) | 9 (15.5%) | |
| Prior Treatment | 0.346 | |||
| Chemoradiation | 10 (17.2%) | 10 (22.7%) | 14 (24.1%) | |
| Radiation alone | 16 (27.6%) | 12 (27.3%) | 17 (29.3%) | |
| Surgery + Radiation | 12 (20.7%) | 8 (18.2%) | 4 (6.9%) | |
| Surgery + Chemoradiation | 2 (3.4%) | 4 (9.1%) | 5 (8.6%) | |
| Surgery + Chemotherapy | 1 (1.7%) | 1 (2.3%) | 2 (3.4%) | |
| Surgery alone (non-exent) | 8 (13.8%) | 5 (11.4%) | 11 (19%) | |
| Upfront Exenteration | 9 (15.5%) | 2 (4.5%) | 5 (8.6%) | |
| Preop Radiation with Planned Exent | 0 (0%) | 2 (4.5%) | 0 (0%) | |
| Missing | 1 | 0 | 0 | |
Total pelvic exenteration (68.3%) was the most common procedure performed, followed by anterior exenteration alone (21.7%) and posterior exenteration alone (9.9%). Table 3 summarizes the exenterative procedures performed as well as the procedures used for closure of the pelvic defect or vaginal reconstruction and creation of the urinary diversion. There were no significant differences between weight groups pertaining to the type of exenteration performed (p=0.326) or for the procedure used for vaginal reconstruction. A total of 64.6% of exenterations involved vaginal reconstruction with either a gracilis or vertical rectus abdominis myocutaneous (VRAM) flap. While the gracilis flap was used more commonly than the VRAM flap in normal-weight patients (39% vs 20.3%), the VRAM was used more commonly in obese patients (29.3% gracilis flap vs. 37.9% VRAM). These differences were not statistically significant (p=0.375). Formation of an incontinent urinary conduit was the most common urinary reconstruction in all three groups. Only 29.2% of exenterations involved formation of a continent urinary diversion. Although not statistically different, an incontinent conduit was more commonly used in obese patients (81.5%, p=0.085).
Table 3.
Univariate Analysis of Surgical Characteristic
| Normal Weight (n = 59) |
Overweight (n = 44) |
Obese (n = 58) |
p-value | |
|---|---|---|---|---|
| Type of Exenteration | 0.326 | |||
| Total (n=110) | 35 (59.3%) | 34 (77.3%) | 41 (70.7%) | |
| Anterior only (n=35) | 15 (25.4%) | 7 (15.9%) | 13 (22.4%) | |
| Posterior only (n=16) | 9 (15.3%) | 3 (6.8%) | 4 (6.9%) | |
| Vaginal Reconstruction | 0.375 | |||
| None (n=30) | 15 (25.4%) | 7 (15.9%) | 8 (13.8%) | |
| Gracilis flap (n=58) | 23 (39%) | 18 (40.9%) | 17 (29.3%) | |
| VRAM (n=46) | 12 (20.3%) | 12 (27.3%) | 22 (37.9%) | |
| Omental/skin graft (n=4) | 0 (0%) | 2 (4.5%) | 2 (3.4%) | |
| Primary closure (n=21) | 8 (13.6%) | 5 (11.4%) | 1 (1.7%) | |
| Other (n=2) | 1 (1.7%) | 0 (0%) | 8 (13.8%) | |
| Urinary Procedures | 0.085 | |||
| Continent (n=42) | 17 (34.7%) | 15 (36.6%) | 10 (18.5%) | |
| Incontinent (n=102) | 32 (65.3%) | 26 (63.4%) | 44 (81.5%) | |
| Missing | 10 | 3 | 4 | |
| Surgical Measures | ||||
| EBL (mL) | 2315.5 (280–13000) | 2465.9 (500–10200) | 3033.2 (400–16500) | 0.082 |
| Length of surgery (hrs) | 8.7 (4–15.5) | 9.6 (4.3–14.3) | 10.1 (4.5–15.4) | 0.010 |
| Intraop complications (n) | 4 (6.9%) | 3 (6.8%) | 6 (10.3%) | 0.816 |
| Mean intraoperative blood transfusions (units) | 4.7 (0−21) | 4.5 (0−17) | 5.6 (0−32) | 0.717 |
| Margin Status | >0.999 | |||
| Negative (n=135) | 50 (84.7%) | 37 (84.1%) | 48 (82.8%) | |
| Positive (n=25) | 9 (15.3%) | 7 (15.9%) | 9 (15.5%) | |
| Missing | 0 | 0 | 1 | |
| Lymph Node Status | 0.482 | |||
| Negative (n=134) | 45 (76.3%) | 42 (95.5%) | 47 (81%) | |
| Positive (n=12) | 6 (10.2%) | 2 (4.5%) | 4 (6.9%) | |
| Missing | 8 | 0 | 7 | |
| Length of Stay (days) | 19.6 (7–48) | 19.2 (8–97) | 18.7 (5–63) | 0.237 |
Univariate analyses of surgical characteristics and postoperative complications are shown in Table 3 and 4. Length of surgery with increasing weight group classification was the only statistically significant difference in surgical characteristics between the groups (normal-weight, 8.7 hrs; overweight, 9.6 hrs; and obese, 10.1 hrs, p=0.010). A patient’s weight was not associated with the adequacy of the surgical resection. Superficial wound separation (p=0.0001) and total early postoperative complications (p=0.018) were significantly higher in the overweight and obese groups. On multivariate logistic regression analysis comparing overweight patients to normal-weight patients, however, early superficial wound separation (OR 10.74, 95%CI 3.33–34.62, p<0.001) and length of surgery (OR 1.26, 95%CI 1.02–1.55, p=0.032) were the only factors that were significantly increased in overweight patients. There were no significant differences in late complications between these two groups. Similarly, on multivariate logistic regression analysis comparing obese patients to normal-weight patients, early superficial wound separation (OR 4.35, 95%CI 1.40–13.52, p=0.011) and length of surgery (OR 1.24, 95%CI 1.04–1.47, p=0.014) were found to be significantly increased in obese patients. There were no significant differences in late complications between these two groups. Total late postoperative complications for patients in the normal weight, overweight, and obese groups were 47.5%, 45.5%, and 43.1% (p=0.144).
Table 4.
Univariate Analysis of Early (<60 days) and Late (≥60 days) Post-Operative Complications
| Early (<60 days) Post-Operative Complications |
Late (<60 days) Post-Operative Complications |
|||||||
|---|---|---|---|---|---|---|---|---|
| Norma l Weigh t |
Overweig ht |
Obese |
p- value |
Norma l Weigh t |
Overweig ht |
Obese |
p- value |
|
| Wound Separation | 6 (10.2%) | 20 (45.5%) | 21 (36.2%) | 0.0001 | 0 (0%) | 1 (2.3%) | 2 (3.4%) | 0.211 |
| Infection | ||||||||
| Abscess | 7 (11.9%) | 7 (15.9%) | 12 (20.7%) | 0.414 | 3 (5.1%) | 2 (4.5%) | 3 (5.2%) | >0.999 |
| Sepsis | 3 (5.1%) | 4 (9.1%) | 7 (12.1%) | >0.999 | 1 (1.7%) | 0 (0%) | 1 (1.7%) | >0.999 |
| Pneumonia | 4 (6.8%) | 5 (11.4%) | 6 (10.3%) | 0.7885 | 2 (3.4%) | 0 (0%) | 0 (0%) | 0.336 |
| Urinary | ||||||||
| Ureteral injury | 2 (3.4%) | 3 (6.8%) | 4 (6.9%) | 0.573 | 3 (5.1%) | 6 (13.6%) | 6 (10.3%) | 0.258 |
| Ureteral stricture | 0 (0%) | 4 (9.1%) | 1 (1.7%) | 0.023 | 4 (6.8%) | 1 (2.3%) | 6 (10.3%) | 0.358 |
| Renal failure | 2 (3.4%) | 1 (2.3%) | 3 (5.2%) | 0.768 | 1 (1.7%) | 1 (2.3%) | 6 (10.3%) | 0.081 |
| Urostomy complications | 14 (23.7%) | 10 (22.7%) | 18 (31%) | 0.477 | 4 (6.8%) | 9 (20.5%) | 8 (13.8%) | 0.105 |
| Gastrointestinal | ||||||||
| Bowel obstruction | 6 (10.2%) | 5 (11.4%) | 5 (8.6%) | 0.842 | 9 (15.3%) | 4 (9.1%) | 1 (1.7%) | 0.023 |
| Colostomy complication | 4 (6.8%) | 4 (9.1%) | 3 (5.2%) | 0.678 | 0 (0%) | 2 (4.5%) | 1 (1.7%) | 0.269 |
| Cardiovascular | ||||||||
| DVT/PE | 2 (3.4%) | 0 (0%) | 1 (1.7%) | 0.778 | 2 (3.4%) | 1 (2.3%) | 5 (8.6%) | 0.354 |
| MI | 1 (1.7%) | 0 (0%) | 0 (0%) | >0.999 | 0 (0%) | 0 (0%) | 1 (1.7%) | >0.999 |
| Reoperation | 7 (11.9%) | 2 (4.5%) | 6 (10.3%) | 0.460 | 0 (0%) | 1 (2.3%) | 2 (3.4%) | 0.505 |
| Total Complications | 35 (59.3%) | 36 (81.8%) | 48 (82.8%) | 0.0177 | 28 (47.5%) | 20 (45.5%) | 25 (43.1%) | 0.144 |
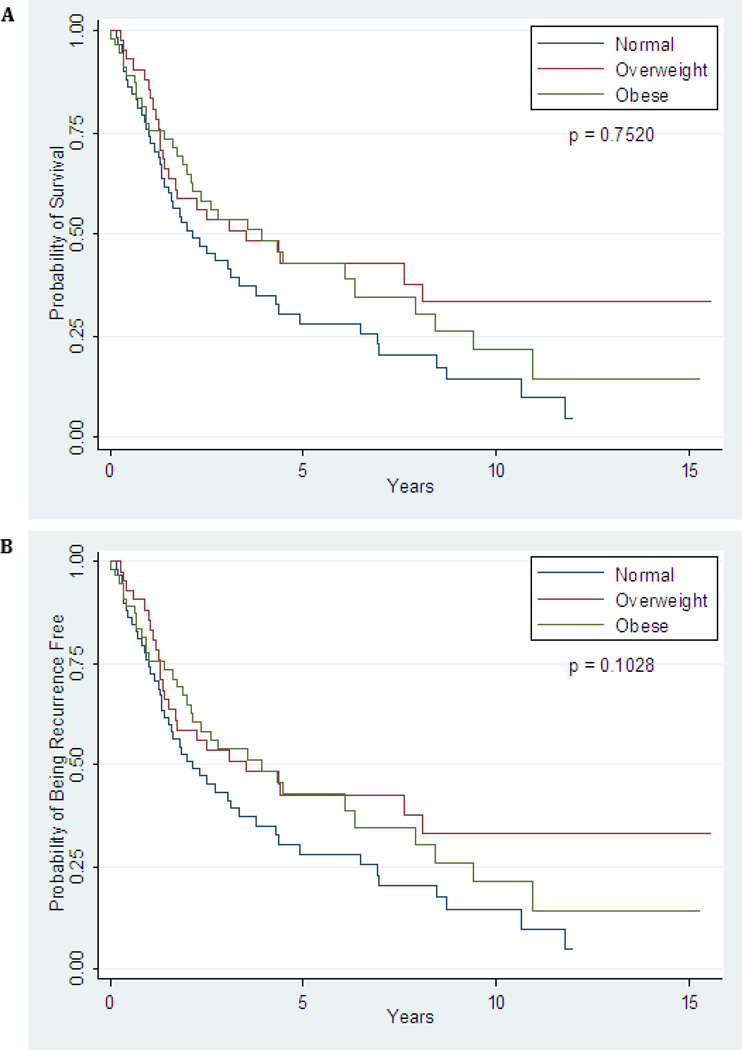
Clinical outcomes are summarized in Table 5. The median follow-up time for the entire cohort was 25 months (range, 1 – 187 months), with only two patients, both in the obese group, having been monitored for less than 60 days. A total of 84 of 161 patients developed a recurrence following exenteration. There was no difference in time-to-recurrence (p=0.065) or overall survival (0.052) when patients were stratified by primary cancer diagnosis. Prior radiation therapy also did not produce a difference in time-to-recurrence (p=0.921) or in overall survival (p=0.625). The overall recurrence rates in the normal-weight group, overweight group, and obese group were 57.4%, 48.8%, and 53.5%, respectively (p=0.679). Although patients in the overweight group appeared to have a longer median time to recurrence (5.2 years) than patients in the normal-weight (2.62 years) or obese (2.46 years) groups (Figure 1A), this difference was not statistically significant (p=0.752). The most common site of recurrence was at a distant site outside of the pelvis in all weight groups. Treatment at the time of recurrence was as follows: radiation alone (6), chemotherapy alone (20), repeat surgery alone (8), chemoradiation (12), chemotherapy following repeat surgery (6), radiation following repeat surgery (3), chemoradiation following surgery (4), hormonal therapy in combination with chemotherapy (1), and other/unknown (4) (Table 5). There was no significant difference in overall survival (p=0.103) between groups (Figure 1B). Type of exenteration did have an effect on survival outcomes, with posterior exenterations having a reduce time-to-recurrence (p=0.001) and reduced overall survival (p=0.011). However, after controlling for variables in the multivariate Cox model, BMI was still a non-significant factor in time-to-recurrence and overall survival.
Table 5.
Clinical Outcomes
| Normal Weight |
Overweight | Obese | p-value | |
|---|---|---|---|---|
| Median Follow-up (months) | 22 (2–144) | 29 (2–187) | 25 (1–183) | |
| Recurrence Rate | 57.4% | 48.8% | 53.4% | 0.679 |
| Median Time to Recurrence (yrs) | 2.62 | 5.20 | 2.46 | 0.749 |
| Site of Recurrence | 0.269 | |||
| Pelvis | 13 (41.9%) | 8 (38.1%) | 7 (21.9%) | |
| Distant | 17 (54.8%) | 13 (61.9%) | 23 (71.9%) | |
| Treatment at Recurrence | 0.990 | |||
| None | 9 (29.0%) | 4 (19.0%) | 7 (21.9%) | |
| Radiation alone | 2 (6.5%) | 1 (4.8%) | 3 (9.4%) | |
| Chemotherapy alone | 9 (29.0%) | 5 (23.8%) | 6 (18.8%) | |
| Surgery | 2 (6.5%) | 2 (9.5%) | 4 (12.5%) | |
| Chemoradiation | 4 (12.9%) | 4 (19.0%) | 4 (12.5%) | |
| Surgery + Chemotherapy | 1 (3.2%) | 2 (9.5%) | 3 (9.4%) | |
| Surgery + Radiation | 1 (3.2%) | 1 (4.8%) | 1 (3.1%) | |
| Surgery + Chemoradiation | 1 (3.2%) | 2 (9.5%) | 1 (3.1%) | |
| Chemotherapy + Hormone Therapy | 1 (3.2%) | 0 (0%) | 0 (0%) | |
| Unknown | 1 (3.2%) | 0 (0%) | 3 (9.4%) | |
| Current Status | 0.085 | |||
| NED | 13 (22%) | 19 (43.2%) | 19 (32.8%) | |
| Alive with disease | 0 (0%) | 0 (0%) | 6 (10.3%) | |
| Died of disease | 30 (50.8%0 | 17 (38.6%) | 20 (34.5%) | |
| Died of complication | 5 (8.5%) | 3 (6.8%) | 4 (6.9%) | |
| Died of other causes | 11 (18.7%) | 5 (11.4%) | 9 (15.5%) | |
Figure 1.
A: TimetoRecurrence; B: Time to Death
Discussion
To our knowledge, this study is the first to specifically evaluate the impact of BMI on surgical outcomes, postoperative complications, recurrence rates, and survival outcomes following pelvic exenteration in patients with a gynecologic malignancy. Aside from length of surgery and superficial wound separation in the early postoperative period, we found that higher BMI was not associated with increased risk of other intraoperative or postoperative complications. Importantly, increasing BMI was not associated with a higher risk of disease recurrence or shorter overall survival.
Obesity rates continue to climb throughout much of the world [27]. In the United States, approximately two-thirds of women are considered obese or overweight [28]. Inevitably, an increasing proportion of patients who present with gynecologic malignancies requiring surgical intervention will be overweight or obese. Thus, it is important that the effects of obesity on surgical outcomes and survival be investigated to better counsel patients about potential risks. We wondered whether the historical reluctance to perform complex procedures in obese patients remains valid, particularly for pelvic exenteration, one of the most technically challenging and morbid procedures performed by gynecologic oncologists. Unfortunately, data on the effects of body weight in patients undergoing exenterative surgery remain limited and conflicting [4, 11, 14, 17].
Consistent with previous reports [20–23], we found that obesity was associated with a significant increase in the length of surgery. While EBL was slightly higher in obese women this did not translate to an increased risk of intraoperative blood transfusion or intraoperative or postoperative morbidity. Length of hospital stay was also not affected by BMI. Based on our findings the clinical significance of additional time in the operating room may be minimal.
Lymph node involvement and positive surgical margins have been demonstrated to be predictors of shorter overall survival following pelvic exenteration [6, 14, 17]. There has been concern that a patient’s body habitus and BMI may affect the adequacy of surgical resection. Erkanli and colleagues [23] evaluated the effect of BMI on surgical staging of endometrial carcinoma and found that while morbidly obese patients had statistically longer operating times, the average number of lymph nodes removed did not differ from that in patients with a lower BMI. We found that there were no differences between BMI groups with regards to positive margin status or positive lymph nodes status, indicating that BMI was not associated with margin or lymph node status as markers of the adequacy of exenterative surgery for gynecologic malignancies.
The majority of patients (64.6%) underwent vaginal reconstruction with either a gracilis flap or VRAM flap. Several studies have reported that patients who undergo vaginal reconstruction with a myocutaneous flap have fewer complications following pelvic exenteration when compared to patients who do not undergo vaginal reconstruction [13, 29, 30]. The VRAM flap was introduced for vaginal reconstruction approximately a decade following routine use of the gracilis flap and provided the advantage of a longer vascular pedicle that allowed for greater mobility into the pelvis, with a presumed lower risk of flap necrosis [31]. Flap-related complications and flap loss are more common with the gracilis flap than with the VRAM flap [32]. In our study, we found that the gracilis flap was used more commonly than the VRAM flap in normal weight patients, but in obese women the VRAM was used more often. Due to the challenges of locating the skin island supplied by gracilis perforators in obese patients and the higher risk for flap necrosis, it is possible that VRAM flap reconstruction may have been preferentially selected for these patients. Another possible explanation is that a majority of the cases done in obese women (69.9%) were done after 2000 when the VRAM reconstruction was more common.
We found that overall postoperative complication rates were not significantly different between the three groups. Our overall early (59.3 – 82.8%) and late complication rates (43.1 – 47.5%) were comparable to the 45 – 84% complication rates previously reported in the literature [3–11]. We noted a greater complication rate in the early post-operative period compared to late complications, which is likely due to the difference in superficial wound separation without associated cellulitis. Overweight and obese women are more likely to be insulin resistant and have a thicker subcutaneous adipose layer at the anterior abdominal wall creating more dead-space which can contribute to poor wound healing and may explain the differences between the groups. However, associated infectious complications were not significantly different.
Large epidemiologic studies have shown that increasing weight is associated with increasing cancer mortality. A prospective study of more than 495,000 U.S. women who were cancer-free at enrollment and were observed over a 16-year period found that increased body weight was associated with a significantly increased risk of death for all cancers combined [33]. In that study, women with a BMI of 30–34.9 kg/m2 had a relative risk of death from all cancers combined of 1.23 and increasing BMI was associated with further increases in relative risk of death (RR 1.32 for BMI 35–39.9 kg/m2 and RR 1.62 for BMI ≥ 40 kg/m2, p<0.001) [33]. Furthermore, when looking at specific cancers, the most significant trends were seen in cancers of the uterine corpus and cervix, which are two common indications for exenteration. In our study, recurrence rates and overall survival did not significantly differ between weight groups. This may be due to careful patient selection and the curative intent of exenterative surgery in patients with central recurrences.
The strengths of this study are the relatively large sample size when compared to previous studies and the stratification of patients into groups based on BMI that allowed for direct comparisons. The limitations of this study include its retrospective nature and a sample size that may be too small to detect significant differences in rare outcomes. To limit the risk of confounding, we performed a multivariate logistic regression analysis. This study may also be limited by selection bias, particularly in the earlier years, as obese and morbidly obese patients may not have been offered exenteration. Those who did undergo exenteration may have been the best surgical candidates likely limiting complication rates and possibly affecting survival rates. Although this study was performed at a single institution, several different surgeons were involved over the set time period and the results may be affected by variability in surgical technique between surgeons and their level of training.
In conclusion, pelvic exenteration appears to be feasible and safe in overweight and obese women as BMI was not associated with higher complication rates or worse survival outcomes when compared to those of normal-weight women. While it is important to consider all medical co-morbidities when counseling patients about exenterative surgery, obesity alone should not be considered a contraindication to this procedure.
Highlights.
Increasing body mass index was associated with increasing length of surgery in women undergoing pelvic exenteration.
Superficial wound separation was significantly higher in overweight and obese patients compared to normal-weight women.
Preoperative BMI was not associated with long term complications, recurrence rates, or overall survival in woman after pelvic exenteration.
Acknowledgements
Sunita Patterson of MD Anderson’s Department of Scientific Publications provided editorial assistance.
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors do not have any conflicts of interest to declare.
References
- 1.Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma; a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer. 1948;1:177–183. doi: 10.1002/1097-0142(194807)1:2<177::aid-cncr2820010203>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Marnitz S, Dowdy S, Lanowska M, Schneider A, Podratz K, Kohler C. Exenterations 60 years after first description: results of a survey among US and German Gynecologic Oncology Centers. Int J Gynecol Cancer. 2009;19:974–977. doi: 10.1111/IGC.0b013e3181a8351e. [DOI] [PubMed] [Google Scholar]
- 3.Lambrou NC, Pearson JM, Averette HE. Pelvic exenteration of gynecologic malignancy: indications, and technical and reconstructive considerations. Surg Oncol Clin N Am. 2005;14:289–300. doi: 10.1016/j.soc.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Maggioni A, Roviglione G, Landoni F, Zanagnolo V, Peiretti M, Colombo N, Bocciolone L, Biffi R, Minig L, Morrow CP. Pelvic exenteration: ten-year experience at the European Institute of Oncology in Milan. Gynecol Oncol. 2009;114:64–68. doi: 10.1016/j.ygyno.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 5.McLean KA, Zhang W, Dunsmoor-Su RF, Shah CA, Gray HJ, Swensen RE, Goff BA. Pelvic exenteration in the age of modern chemoradiation. Gynecol Oncol. 2011;121:131–134. doi: 10.1016/j.ygyno.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Morley GW, Hopkins MP, Lindenauer SM, Roberts JA. Pelvic exenteration, University of Michigan: 100 patients at 5 years. Obstet Gynecol. 1989;74:934–943. [PubMed] [Google Scholar]
- 7.Hafner GH, Herrera L, Petrelli NJ. Morbidity and mortality after pelvic exenteration for colorectal adenocarcinoma. Ann Surg. 1992;215:63–67. doi: 10.1097/00000658-199201000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthopoulos AP, Manetta A, Larson JE, Podczaski ES, Bartholomew MJ, Mortel R. Pelvic exenteration: a morbidity and mortality analysis of a seven-year experience. Gynecol Oncol. 1989;35:219–223. doi: 10.1016/0090-8258(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 9.Lopez MJ, Standiford SB, Skibba JL. Total pelvic exenteration. A 50-year experience at the Ellis Fischel Cancer Center. Arch Surg. 1994;129:390–395. doi: 10.1001/archsurg.1994.01420280062008. discussion 395– 6. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg GL, Sukumvanich P, Einstein MH, Smith HO, Anderson PS, Fields AL. Total pelvic exenteration: the Albert Einstein College of Medicine/Montefiore Medical Center Experience (1987 to 2003) Gynecol Oncol. 2006;101:261–268. doi: 10.1016/j.ygyno.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, Zighelboim I. Pelvic exenteration in gynecologic oncology: a single institution study over 20 years. Gynecol Oncol. 2011;122:14–18. doi: 10.1016/j.ygyno.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marnitz S, Kohler C, Muller M, Behrens K, Hasenbein K, Schneider A. Indications for primary and secondary exenterations in patients with cervical cancer. Gynecol Oncol. 2006;103:1023–1030. doi: 10.1016/j.ygyno.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Soper JT, Berchuck A, Creasman WT, Clarke-Pearson DL. Pelvic exenteration: factors associated with major surgical morbidity. Gynecol Oncol. 1989;35:93–98. doi: 10.1016/0090-8258(89)90020-6. [DOI] [PubMed] [Google Scholar]
- 14.Shingleton HM, Soong SJ, Gelder MS, Hatch KD, Baker VV, Austin JM., Jr Clinical and histopathologic factors predicting recurrence and survival after pelvic exenteration for cancer of the cervix. Obstet Gynecol. 1989;73:1027–1034. doi: 10.1097/00006250-198906000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Lawhead RA, Jr, Clark DG, Smith DH, Pierce VK, Lewis JL., Jr Pelvic exenteration for recurrent or persistent gynecologic malignancies: a 10-year review of the Memorial Sloan-Kettering Cancer Center experience (1972–1981) Gynecol Oncol. 1989;33:279–282. doi: 10.1016/0090-8258(89)90512-x. [DOI] [PubMed] [Google Scholar]
- 16.Park JY, Choi HJ, Jeong SY, Chung J, Park JK, Park SY. The role of pelvic exenteration and reconstruction for treatment of advanced or recurrent gynecologic malignancies: Analysis of risk factors predicting recurrence and survival. J Surg Oncol. 2007;96:560–568. doi: 10.1002/jso.20847. [DOI] [PubMed] [Google Scholar]
- 17.Fleisch MC, Pantke P, Beckmann MW, Schnuerch HG, Ackermann R, Grimm MO, Bender HG, Dall P. Predictors for long-term survival after interdisciplinary salvage surgery for advanced or recurrent gynecologic cancers. J Surg Oncol. 2007;95:476–484. doi: 10.1002/jso.20686. [DOI] [PubMed] [Google Scholar]
- 18.Fotopoulou C, Neumann U, Kraetschell R, Schefold JC, Weidemann H, Lichtenegger W, Sehouli J. Long-term clinical outcome of pelvic exenteration in patients with advanced gynecological malignancies. J Surg Oncol. 2010;101:507–512. doi: 10.1002/jso.21518. [DOI] [PubMed] [Google Scholar]
- 19.Berek JS, Howe C, Lagasse LD, Hacker NF. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecol Oncol. 2005;99:153–159. doi: 10.1016/j.ygyno.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Soisson AP, Soper JT, Berchuck A, Dodge R, Clarke-Pearson D. Radical hysterectomy in obese women. Obstet Gynecol. 1992;80:940–943. [PubMed] [Google Scholar]
- 21.Anderson B, Connor JP, Andrews JI, Davis CS, Buller RE, Sorosky JI, Benda JA. Obesity and prognosis in endometrial cancer. Am J Obstet Gynecol. 1996;174:1171–1178. doi: 10.1016/s0002-9378(96)70659-2. discussion 1178– 9. [DOI] [PubMed] [Google Scholar]
- 22.Papadia A, Ragni N, Salom EM. The impact of obesity on surgery in gynecological oncology: a review. Int J Gynecol Cancer. 2006;16:944–952. doi: 10.1111/j.1525-1438.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 23.Erkanli S, Kayaselcuk F, Bagis T, Kuscu E. Impact of morbid obesity in surgical management of endometrial cancer: surgical morbidity, clinical and pathological aspects. Eur J Gynaecol Oncol. 2006;27:401–404. [PubMed] [Google Scholar]
- 24.von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma : a Gynecologic Oncology Group study. Cancer. 2006;107:2786–2791. doi: 10.1002/cncr.22351. [DOI] [PubMed] [Google Scholar]
- 25.von Gruenigen VE, Gil KM, Frasure HE, Jenison EL, Hopkins MP. The impact of obesity and age on quality of life in gynecologic surgery. Am J Obstet Gynecol. 2005;193:1369–1375. doi: 10.1016/j.ajog.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 28.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 29.Cain JM, Diamond A, Tamimi HK, Greer BE, Figge DC. The morbidity and benefits of concurrent gracilis myocutaneous graft with pelvic exenteration. Obstet Gynecol. 1989;74:185–189. [PubMed] [Google Scholar]
- 30.Jurado M, Bazan A, Alcazar JL, Garcia-Tutor E. Primary vaginal reconstruction at the time of pelvic exenteration for gynecologic cancer: morbidity revisited. Ann Surg Oncol. 2009;16:121–127. doi: 10.1245/s10434-008-0171-0. [DOI] [PubMed] [Google Scholar]
- 31.Carlson JW, Carter JR, Saltzman AK, Carson LF, Fowler JM, Twiggs LB. Gynecologic reconstruction with a rectus abdominis myocutaneous flap: an update. Gynecol Oncol. 1996;61:364–368. doi: 10.1006/gyno.1996.0157. [DOI] [PubMed] [Google Scholar]
- 32.Soper JT, Secord AA, Havrilesky LJ, Berchuck A, Clarke-Pearson DL. Comparison of gracilis and rectus abdominis myocutaneous flap neovaginal reconstruction performed during radical pelvic surgery: flap-specific morbidity. Int J Gynecol Cancer. 2007;17:298–303. doi: 10.1111/j.1525-1438.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 33.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]