Abstract
Second-generation tyrosine kinase inhibitors (TKI2) often induce molecular remission, and prolonged survival with a better tolerance in imatinib-resistant chronic myelogenous leukaemia (CML) patients. We report the case of a CML in first chronic phase who was diagnosed in August 2003 in a young 24 year-old Caucasian woman. Our patient received first imatinib and then dasatinib and nilotinib. Imatinib was well tolerated and she developed TTP/HUS on dasatinib without documented evolution of CML and finally obtained MR5.0 with nilotinib and without any side effect. This case also illustrates the absence of cross-resistance and side-effects between the different TKIs and the feasibility of kidney transplantation associated with a nilotinib treatment of CML allowing a continuing MR5.0 and no further side effects.
Keywords: TTP/HUS, Dasatinib, CML, Kidney transplantation, Nilotinib
Dear Editor,
Second-generation tyrosine kinase inhibitors (TKI2) often induce molecular remission, and prolonged survival with a better tolerance in imatinib-resistant chronic myelogenous leukaemia (CML) patients.1–3 To date, only one case of imatinib-induced thrombotic thrombocytopenic purpura/haemolytic uraemic syndrome (TTP/HUS) has been diagnosed in a case of hypereosinophilic syndrome.4 More than 50 drugs have been associated with the development of TTP5 but no TTP/HUS has been described with TKI26 yet. We report here the case of an imatinib-resistant CML presenting terminal renal failure after dasatinib-induced TTP/HUS and receiving a related renal transplantation on nilotinib.
A CML in first chronic phase was diagnosed in August 2003 in a young 24 year-old Caucasian woman with no prior medical history. Initial characteristics showed no splenomegaly, a leucocyte count of 22×109 /L, with 63% neutrophils, 15% myelemia, no circulating blast, no anaemia and a normal platelet count. Bone marrow (BM) aspirate was hypercellular with 1.5% eosinophils, 2.5% basophils, and 2.5% blasts. Cytogenetic analysis revealed 85% of Philadelphia chromosome-positive (Ph+) cells and quantitative PCR Bcr-Abl fusion transcript ratioIS (International Scale)7 was 111%. Sokal score was low, 0.57 and Hasford score was low, 0. First line imatinib mesylate (400 mg once daily) was initiated in October 2003 and well tolerated. A major molecular response (MMR or MR3.0) was obtained in February 2004 and maintained during almost 2 years (Fig. 1). In May 2006, we observed a loss of MMR without Abl mutations detected; imatinib was increased at 600 mg/day but failed to induce permanent MMR. In January 2008, imatinib was switched to a TKI2 treatment with dasatinib 100 mg daily and further MMR was achieved in a few weeks (Fig. 1). The patient did not take any other medication than dasatinib.
Fig. 1.
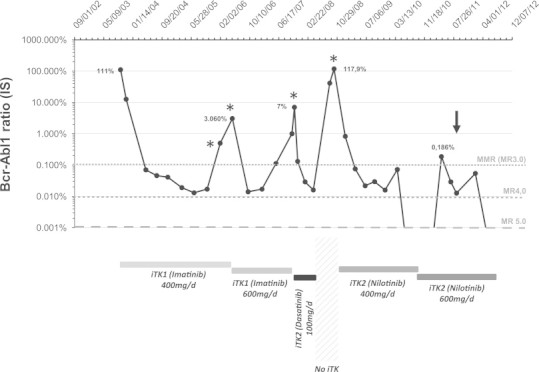
Follow-up of BCR/ABL1 transcripts assessed by RT-qPCR. Results are expressed in % according the International Scale (IS). (⁎) represent ABL mutations analysis. Arrow indicate time of renal allotransplantation.
In June 2008, TTP/HUS was diagnosed during hospitalisation in intensive care unit. Clinical and biological presentation was an association of thrombocytopenia, microangiopathic haemolytic regenerative anaemia, neurological symptoms (Glasgow coma scale score of 6) requiring intubation and respiratory assistance, acute renal failure with anuria, fever and abdominal pain. We did not notice pulmonary arterial hypertension, QTc prolongation, pericardial effusion, cardiac dysfunction or arrhythmia. Laboratory evaluations showed a 10 N serum lactate dehydrogenase (LDH) level, an elevated indirect bilirubinemia level, 50% decreased in serum haptoglobin level, presence of schizocytes on peripheral blood smears (0.5–3%), platelet count 64×109 /l, leucocytes count 18.7×109 /l, urea 27.7 mmol/l, creatinine (Cr) 703 μmol/l, creatinine clearance (Cr Cl)<10 ml/min, proteinuria 1,5 g/24 h. Bone marrow examination confirmed the absence of acceleration or blast crisis. No sign of coagulation dysfunction was noted. Lumbar puncture was sterile and proteinorachy was 0.57 g/l. No source of infection was found except for urinalysis positive to multisensible streptocoque treated with Ceftriaxone. Complement fractions were normal. Brain MRI and renal ultrasound were normal. Plasma ADAMTS13 activity level was 61%; in France, plasma ADAMTS13 activity level was checked in a reference national laboratory and the normal range is between 50% and 150%. No anti-ADAMTS 13 antibodies were detected. Lymphocytes count at the onset of TTP was 0.9×109 /l and IgH/TCR clonality was not performed. Percutaneous renal biopsy was congruent with acute tubular necrosis and TTP. The plasma level of dasatinib was not checked but dasatinib was discontinued and a series of 11 plasma exchanges was performed with corticosteroids, blood transfusions and haemodialysis.
At day +10, patient was discharged from intensive care unit to renal department (from June13rd to July 15th 2008) with less neurological troubles (persistence of few hallucinations and space-time disorientation) and no recovery of renal failure (creatinine 617 μmol/l). Persistence of anaemia (8 g/dl) with 2.7% schizocytes on peripheral smears, low level haptoglobin, 2 N increased LDH and normalized platelet count. CML was still in MMR as stated on molecular biology tests. During hospitalisation, she was on haemodialysis until 06/07/2008 and then restored spontaneous diuresis with persistent renal failure (Cr 525 μmol/l, Cr Cl 15 ml/min). The patient was discharged at day +28 from renal department with platelets in normal range (250 G/l) and no neurological symptoms.
Nilotinib 400 mg daily was first introduced in September 2008 and then increased to 600 mg daily in October 2010. We did not use any prophylactic antithrombotic treatment. Before the onset of nilotinib, clearance of creatinine was still low (15 ml/min) and low molecular weight heparins were not indicated. We did not use oral anticoagulant because of possible interactions with nilotinib and the low recovery of platelets counts. CML evaluation before nilotinib introduction showed a Bcr-Abl/Abl ratio of 41% in blood without loss of CHR and BCR-Abl domain mutation. Given the level of BCR ABL transcript in blood before the onset of nilotinib, a cytogenetic relapse is highly probable but no bone marrow aspiration was performed at this time. On nilotinib treatment, a two (0.82%) and three (0.08%) log reduction in Bcr-Abl/Abl ratios (IS) at 3 and 6 months respectively were observed with a complete cytogenetic response in BM. In March 2010, laboratory analyses showed an haemoglobin level of 12.3 g/dl and a platelet count of 198×109 /l. At latest follow-up (July 2012), the patient was in MR5.0.
Progressive aggravation of renal function occurred between July 2008 and April 2011, Cr Cl<15 ml/min and proteinuria (4 g/24 h) requiring peritoneal dialysis (PD) since May 2011. Finally, PD failed in June 2011 and in November 2011 we performed a kidney transplantation from a living related donor (her mother). Immunosuppressive regimen was composed of basiliximab (20 mg daily during 5 days), tacrolimus (0.15 mg/kg), mycofenolate mofetil (3 g daily) and corticosteroids (5 mg/kg). No recurrence of TTP/HUS occurred during kidney transplantation procedure despite the use of anticalcineurin (tacrolimus) and no other complication was reported. Currently, post-transplant renal function is stabilized at 123 μmol/l (Cr Cl 45 ml/min) with on-going immunosuppression (tacrolimus, mycophenolate mofetil); CML remains in continuous MR5.0 with nilotinib 300 mg twice daily that is well tolerated. The transient loss of MMR just before and after renal transplant is probably due to an irregular oral intake of nilotinib by the patient.
Dasatinib is a TKI2 highly effective in imatinib-resistant CML.1,3 Even if metabolism of this TKI2 is largely hepatic-dependent via CYP450 CYP3A4, few reports suggest its possible implication in acute renal failure.6 Our patient received first imatinib and then dasatinib and nilotinib. Imatinib was well tolerated and she developed TTP/HUS on dasatinib without documented evolution of CML and finally obtained MR5.0 with nilotinib and without any side effect. Direct implication of CML in the occurrence of TTP/HUS is unlikely because MMR was achieved on dasatinib before the occurrence of the first renal failure episode. Imatinib and nilotinib have equivalent profiles of kinase-inhibition (Abl kinases, c-kit, PDGF-Receptor) and few reports showed partial resolution of renal dysfunction through the dissipation of fibrosis in chronic kidney disease.8 The mechanism implicated is mediated by PDGF-R inhibition. Renal failure was already described in very few cases of imatinib treated patients and often reversible.9,10 Activity of dasatinib is quite different with an enhanced inhibition spectrum to Scr-kinases family and mechanisms implicated in dasatinib induced TTP are unknown. The medical history of the patient suggests that Abl kinase inhibition is not directly implicated in the dasatinib induced TTP/HUS. Temporal association between the initiation of dasatinib treatment and the development of TTP/HUS and the level of plasma ADAMTS13 activity level suggest a possible auto-immune mechanism more than a dose-related dasatinib toxicity. We suggest that the mechanism by which dasatinib may lead to a TTP/HUS is a direct endothelial toxicity. Dasatinib activated leucocytes adhered to endothelial cells and an adhesion mediated lysis of these cells occurred with an alteration of activity and metabolism of vWF and platelet aggregation, resulting in TTP/HUS.11
To our knowledge it is the first report of a dasatinib TTP/HUS with terminal renal failure. This case also illustrates the absence of cross-resistance and side-effects between the different TKIs and the feasibility of kidney transplantation associated with a nilotinib treatment of CML allowing a continuing MR5.0 and no further side effects.
Contributor Information
Eric Deconinck, Email: edeconinck@chu-besancon.fr.
Fabrice Larosa, Email: flarosa@chu-besancon.fr.
References
- 1.Hochhaus A, Baccarani M, Deininger M., Apperley J.F., Lipton J.H., Goldberg S.L. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, Giles FJ, Bhalla KN. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2011;117:1141–1145. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Megan Cornelison A, Kantarjan H, Cortes J, Jabbour E. Outcome of treatment of chronic myeloid leukemia with second-generation tyrosine kinase inhibitors after imatinib failure. Clinical Lymphoma Myeloma and Leukemia. 2011;11:S101–S110. doi: 10.1016/j.clml.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziyad al aly, Philoctete Ashley Jennifer M., Gellens Mary E., Gonzalez Esther A. Thrombotic thrombocytopenic purpura in a patient treated with imatinib mesylate: true association or mere coincidence? American Journal of Kidney Diseases. 2005;45:762–768. doi: 10.1053/j.ajkd.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Dlott JS, Danielson CF, Blue-Hnidy DE, mc Carthy LJ. Drug-induced thrombotic thrombocytopenic purpura/hemolytic uraemic syndrome: a consise review. Therapeutic Apheresis and Dialysis. 2004;8:102–111. doi: 10.1111/j.1526-0968.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 6.Holstein SA, Stokes JB, Hohl RJ. Renal failure and recovery associated with second-generation Bcr-Abl kinase inhibitors in imatinib-resistant chronic myelogenous leukemia. Leukemia Research. 2009;33:344–347. doi: 10.1016/j.leukres.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, Schiffer C, Jones D., Cortes J. Monitoring the response and course of chronic myeloid leukemia in the modern era of BCR-ABL tyrosine kinase inhibitors: practical advice on the use and interpretation of monitoring methods. Blood. 2008;111:1774–1780. doi: 10.1182/blood-2007-09-110189. [DOI] [PubMed] [Google Scholar]
- 8.Lyoda M, Shibata T, Hirai Y, Kuno Y, Akizawa T. Nilotinib attenuates renal injury and prolongs survival in chronic kidney disease. Journal of the American Society of Nephrology. 2011;22:1486–1496. doi: 10.1681/ASN.2010111158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitiyakara C, Atichartakarn V. Renal failure associated with a specific inhibitor of BCR-ABL tyrosine kinase, STI 571. Nephrology Dialysis Transplantation. 2002;17:685–687. doi: 10.1093/ndt/17.4.685. [DOI] [PubMed] [Google Scholar]
- 10.Gafter-Gvili A, Ram R, Gafter U, Shpilberg O, Raanani P. Renal failure associated with tyrosine kinase inhibitors-case report and review of the literature. Leukemia Research. 2010;34:123–127. doi: 10.1016/j.leukres.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Mannucci PM, Lombardi R, Lattuada A, Ruggenenti P, Viganò GL, Barbui T. Enhanced proteolysis of plasma von Willebrand factor in thrombotic thrombocytopenic purpura and the hemolytic uraemic syndrome. Blood. 1989;74(3):978–983. August 15. [PubMed] [Google Scholar]


