Abstract
We experienced a patient with angioimmunoblastic T-cell lymphoma (AITL) without Epstein–Barr virus-positive B (EBV-B) cells at initial presentation who progressed to AITL with expansion of EBV-B cells at relapse. Based on the results of repeated biopsy, the patient was successfully treated with rituximab in combination with chemotherapy at relapse. A repeat biopsy may be necessary to determine the optimum therapeutic strategy at relapse, particularly for patients with suspected expansion of B cell and/or EBV-B cells. Although a recent report found no significant prognostic advantage of rituximab, it is one of the active drugs for selected patients with AITL.
Keywords: Angioimmunoblastic T-cell lymphoma (AITL), Epstein–Barr virus, EBV-DNA, Rituximab, R-CHOP
Case report
In May 2010, a 63-year-old Japanese male was referred to our hospital with skin rash, fever, general fatigue, bilateral pharyngitis, multiple superficial lymphadenopathies, and weight loss of 10 kg in 2 months. His white blood cell count was 9.8×109/L, of which 1.5% were atypical lymphoid cells. His hemoglobin level was 9.7 g/dL and his platelet count was 79×109/L. His serum albumin, lactate dehydrogenase, and C-reactive protein levels were 24 g/L, 363 IU/L, and 11.54 mg/dL, respectively. The results of other liver and renal function tests were <1.5 times the upper limits of normal. Although his serum immunoglobulin (Ig) G, IgA, and IgM levels were 3359, 246, and 252 mg/dL, respectively, there was no evidence of monoclonal gammopathy. The Coombs test was positive, and tests for human immunodeficiency virus and human T-lymphotropic virus type I were negative. Epstein–Barr virus (EBV) antibody to viral capsid antigen (EBVVCA) IgM was negative but EBVVCA IgG was positive. EBV antibody to nuclear antigen (EBNA) IgG was also positive. As positron emission tomography (PET)/computed tomography (CT) images revealed multiple superficial, intra-abdominal, and mesenteric lymphadenopathies that were accompanied by hepatosplenomegaly, a pharyngeal biopsy was performed. Microscopically, the biopsied tissue contained atypical, neoplastic CD3-positive T cells admixed with CD20-negative and EBV-encoded small RNA (EBER)-negative B cells (Fig. 1a). Based on these findings, the patient was diagnosed with peripheral T-cell lymphoma, not otherwise specified. Therefore, he was treated with six cycles of chemotherapy consisting of cyclophosphamide, adriamycin, vincristine, and prednisolone (CHOP). After chemotherapy, his blood test results had improved and there was no evidence of hepatosplenomegaly or lymphadenopathy on CT images, nor was there any abnormal accumulation of [18F]-fluorodeoxyglucose (18F FDG) on PET scans. The patient achieved complete remission (CR) in November 2010. However, 1 month later, disease recurrence was confirmed. Thereafter, he received low-dose etoposide, prednisolone, and cyclophosphamide, followed by low-dose methotrexate (MTX) and cyclosporine (CsA). Despite this regimen, we found enlargement of his lymph nodes, particularly of his left axillary lymph nodes, as their diameter was >10 cm at this time (Fig. 2a). His serum monoclonal IgM level had also increased to 5445 mg/dL. The patient's Eastern Cooperative Oncology Group performance status (PS) was 4. A repeat biopsy of the left axillary lymph node was performed in August 2011. A large number of non-neoplastic, CD20- and EBER-positive B cells and neoplastic CD3-positive T cells were found in the biopsy specimen (Fig. 1b). Therefore, the patient was finally diagnosed with angioimmunoblastic T-cell lymphoma (AITL). Based on the diagnosis of AITL with proliferation of CD20- and EBER-positive B cells, the patient was treated with rituximab monotherapy. Although the lymph node did not shrink rapidly, his serum IgM level decreased to 4200 mg/dL following two cycles of once-weekly rituximab. He was then treated with four cycles of CHOP combined with rituximab (R-CHOP). The enlarged lymph nodes almost disappeared after this treatment, and his serum IgM level decreased to 800 mg/dL. Abnormal 18F FDG accumulation was not detected on PET scans, although a small (2 cm) lymph node was detected in the left axillary lymph node on a CT scan taken after four cycles of R-CHOP in January 2012 (Fig. 2b). The patient's condition improved dramatically, reaching a PS of 0 at this time. He ultimately completed six cycles of R-CHOP, and CR was maintained for 6 months until relapse in June 2012.
Fig. 1.
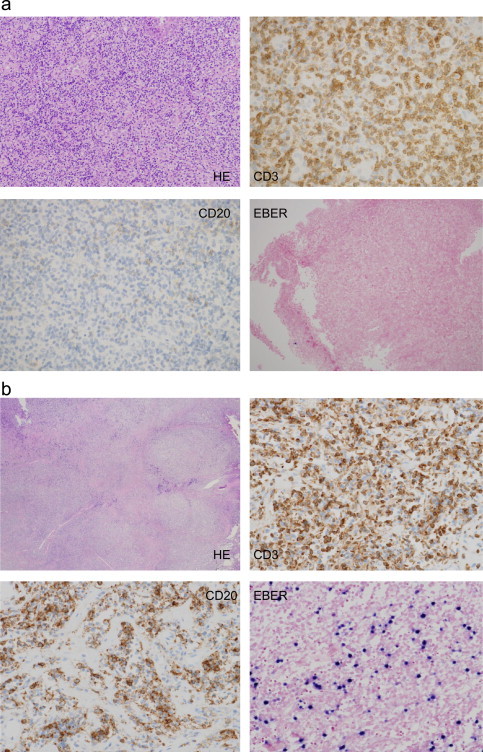
(a) Images of the pharyngeal biopsy samples taken at the initial presentation. Abnormal lymphoid cells were found in hematoxylin and eosin-stained biopsy samples. These abnormal lymphoid cells were positive for CD3 and negative for CD20 and EBER and (b) images of the left axillary lymph node biopsy samples. The lymph node contained a large number of neoplastic CD3 positive T-cells admixed with non-neoplastic, CD20 and EBER positive B-cells.
Fig. 2.
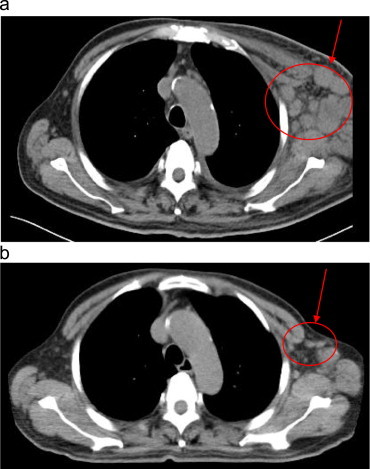
(a) CT scan of the patient taken at relapse of AITL and (b) CT scan of the patient taken after completion of the R-CHOP regimen.
Advani et al. reported that CsA was associated with good overall and durable responses.1 Although the mechanism remains unclear, CsA may inhibit the function of T cells, including their production of cytokines involved in the progression of AITL. Consequently, immunosuppressive therapies might exacerbate EBV infection and the occurrence of secondary cancer. Targeted therapies, such as rituximab, might have a potential role in the treatment of AITL. Rituximab may eradicate EBV-B cells and activated B cells, and may prevent the progression to AITL. However, the authors of a multicenter phase II trial conducted by the Groupe d' Etude des Lymphomes de l' Adulte concluded that R-CHOP did not improve prognosis as compared with standard CHOP.2 EBV infection and proliferation of EBV-B cells are usually detected in patients with AITL, especially in advanced stages of the disease.3 These clinical features are thought to occur secondary to immunodeficiency.4 Zhou et al. reported that a higher tissue EBV load was correlated with disease progression and B cell clonality.3 Delfau-Laure et al. reported that a higher EBV load tended to be associated with a shorter progression-free survival, although the presence of EBV copies in peripheral blood mononclear cells (PBMC) was not associated with the response to R-CHOP.2
We should discuss whether the presence of EBV-DNA in PBMC is more useful than repeated lymph node biopsy for determining the optimum salvage strategy at the time of relapse. EBV is generally detected at the initial diagnosis of AITL in most patients. Therefore, the detection of EBV-DNA in PBMC, alone, may be insufficient to determine the treatment starategy at relapse. If EBV-DNA in PBMC is negative or if its level is low at initial diagnosis of AITL, and if a higher EBV load is detected in these patients at relapse, these observations might indicate EBV infection/reactivation and proliferation of EBV-B cells, consistent with disease progression. However, EBV-positive lymphoproliferative disorder or diffuse large B-cell lymphoma were reported in some patients with AITL.5,6 Therefore, although repeated lymph node biopsy is invasive, it should be considered for patients with AITL who have high EBV loads or if expansion of B cells and/or EBV-B cells is suspected at relapse. The results of such tests may also help clinicians to differentiate between AITL and other EBV-positive disorders.
Our patient with AITL, without expansion of EBV-B cells at initial presentation, was treated with CHOP, followed by low-dose MTX and CsA upon early relapse after the CHOP regimen. The proliferation of EBV-B cells was found in a repeat lymph node biopsy taken at relapse following MTX and CsA therapy. Therefore, we treated the patient with R-CHOP, which was very effective. Although a repeat lymph node biopsy is rarely performed after definitive diagnosis of AITL, we strongly recommend that it is performed in selected patients with AITL.
Contribution
All authors contributed to the writing and preparation of the manuscript.
References
- 1.Advani R, Horwitz S, Zelenetz A, Horning SJ. Angioimmunoblastic T cell lymphoma: treatment experience with cyclosporine. Leukemia and Lymphoma. 2007;48:521–525. doi: 10.1080/10428190601137658. [DOI] [PubMed] [Google Scholar]
- 2.Delfau-Larue MH, de Leval L., Joly B., Plonquet A, Challine D, Parrens M. Targeting intratumoral B cells with rituximab in addition to CHOP in angioimmunoblastic T-cell lymphoma. A clinicobiological study of the GELA. Haematologica. 2012;97:1594–1602. doi: 10.3324/haematol.2011.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Attygalle AD, Chuang SS, Diss T, Ye H, Liu H. Angioimmunoblastic T-cell lymphoma: histological progression associated with EBV and HHV6B viral load. British Journal of Haematology. 2007;138:44–53. doi: 10.1111/j.1365-2141.2007.06620.x. [DOI] [PubMed] [Google Scholar]
- 4.Attygalle AD, Chuang SS, Diss TC, Du MQ, Isaacson PG, Dogan A. Distinguishing angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified, using morphology, immunophenotype and molecular genetics. Histopathology. 2007;50:498–508. doi: 10.1111/j.1365-2559.2007.02632.x. [DOI] [PubMed] [Google Scholar]
- 5.Zettl A, Lee SS, Rüdiger T, Starostik P, Marino M, Kirchner T. Epstein–Barr virus-associated B-cell lymphoproliferative disorders in angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. American Journal of Clinical Pathology. 2002;117:368–379. doi: 10.1309/6UTX-GVC0-12ND-JJEU. [DOI] [PubMed] [Google Scholar]
- 6.Skugor ND, Perić Z, Vrhovac R, Radić-Kristo D, Kardum-Skelin I, Jaksić B. Diffuse large B-cell lymphoma in patient after treatment of angioimmunoblastic T-cell lymphoma. Collegium Antropologicum. 2010;34:241–245. [PubMed] [Google Scholar]


