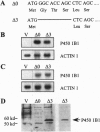
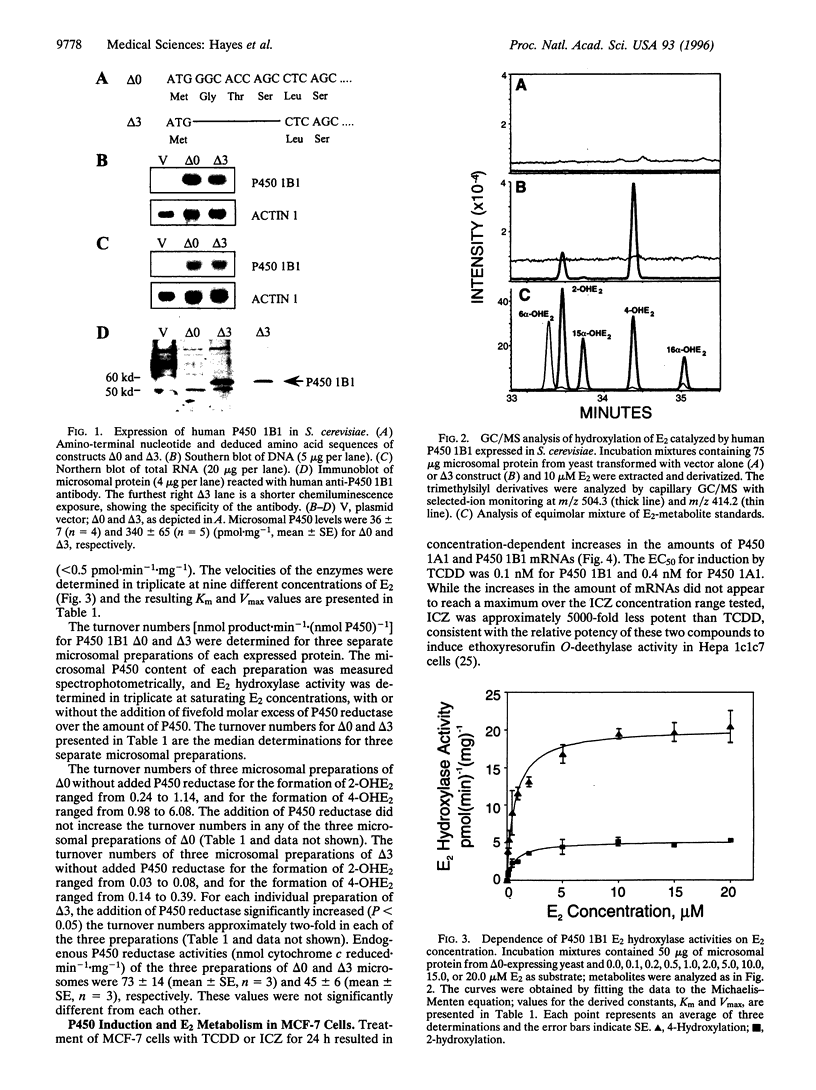
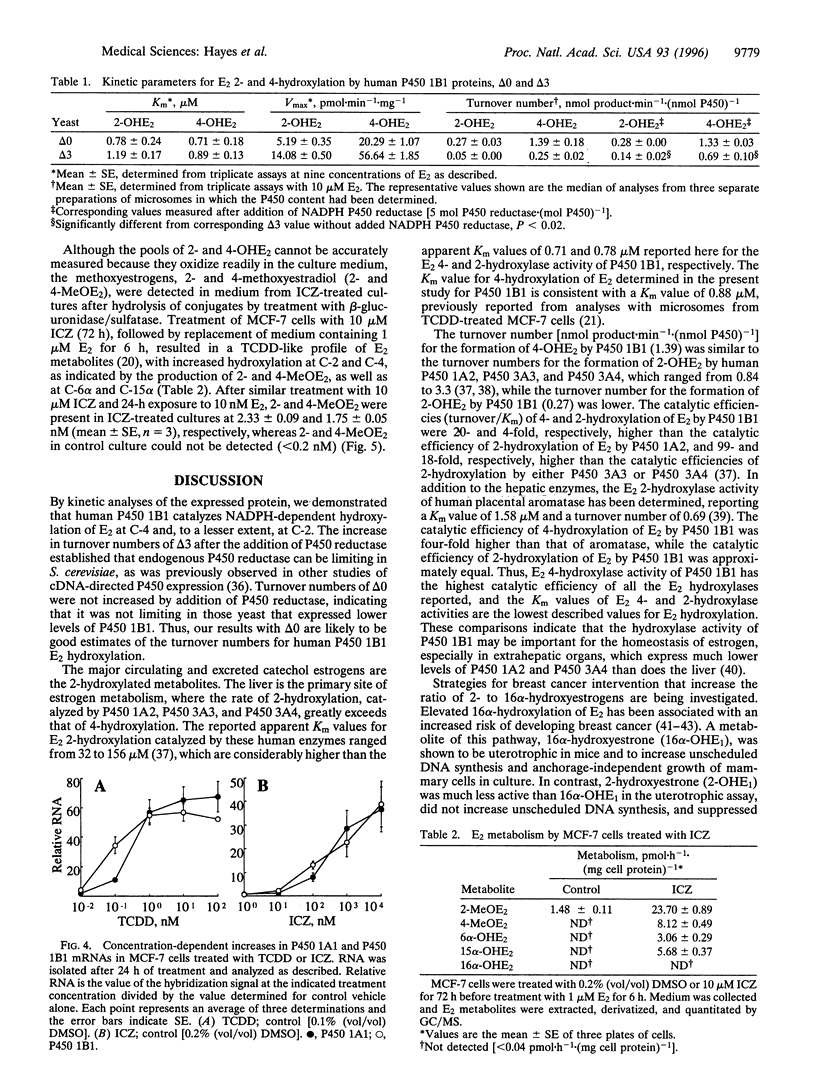
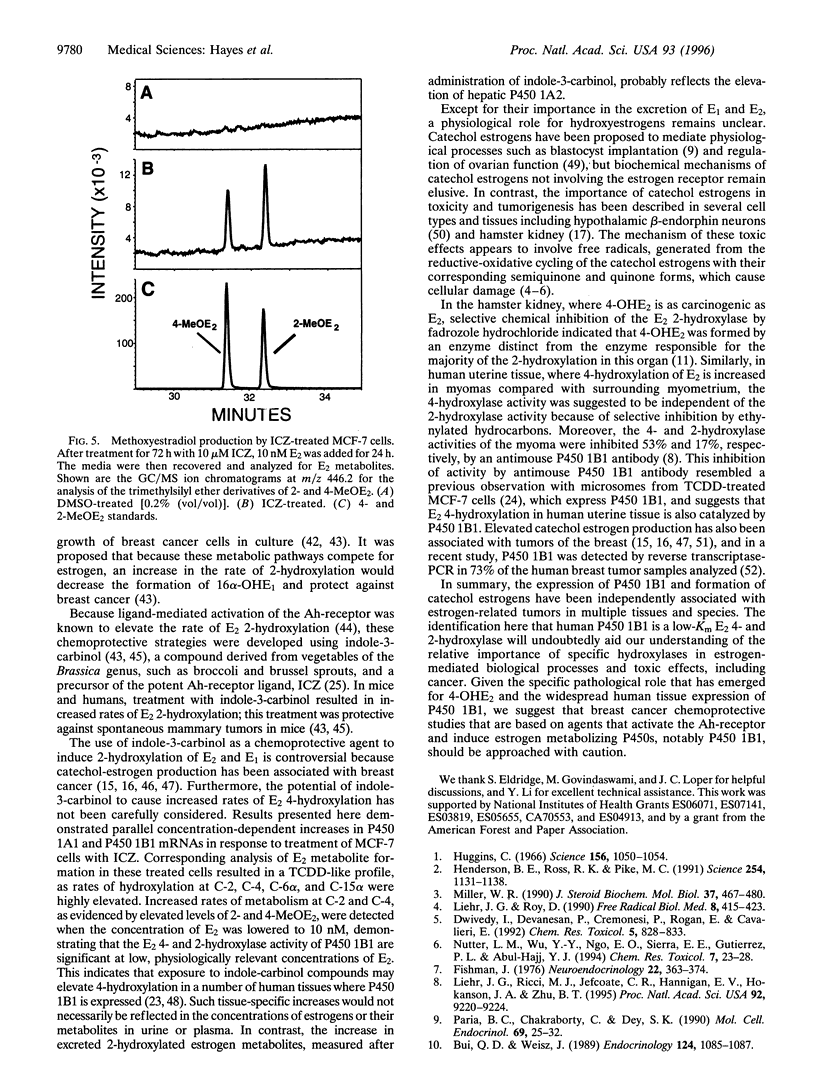
Abstract
The 4-hydroxy metabolite of 17 beta-estradiol (E2) has been implicated in the carcinogenicity of this hormone. Previous studies showed that aryl hydrocarbon-receptor agonists induced a cytochrome P450 that catalyzed the 4-hydroxylation of E2. This activity was associated with human P450 1B1. To determine the relationship of the human P450 1B1 gene product and E2 4-hydroxylation, the protein was expressed in Saccharomyces cerevisiae. Microsomes from the transformed yeast catalyzed the 4- and 2-hydroxylation of E2 with Km values of 0.71 and 0.78 microM and turnover numbers of 1.39 and 0.27 nmol product min-1.nmol P450-1, respectively. Treatment of MCF-7 human breast cancer cells with the aryl hydrocarbon-receptor ligand indolo[3,2-b]carbazole resulted in a concentration-dependent increase in P450 1B1 and P450 1A1 mRNA levels, and caused increased rates of 2-, 4-, 6 alpha-, and 15 alpha-hydroxylation of E2. At an E2 concentration of 10 nM, the increased rates of 2- and 4-hydroxylation were approximately equal, emphasizing the significance of the low Km P450 1B1-component of E2 metabolism. These studies demonstrate that human P450 1B1 is a catalytically efficient E2 4-hydroxylase that is likely to participate in endocrine regulation and the toxicity of estrogens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abul-Hajj Y. J., Thijssen J. H., Blankenstein M. A. Metabolism of estradiol by human breast cancer. Eur J Cancer Clin Oncol. 1988 Jul;24(7):1171–1178. doi: 10.1016/0277-5379(88)90124-1. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H., Gorbach S. L., Goldin B. R., Woods M. N., Dwyer J. T., Hämäläinen E. Estrogen metabolism and excretion in Oriental and Caucasian women. J Natl Cancer Inst. 1994 Jul 20;86(14):1076–1082. doi: 10.1093/jnci/86.14.1076. [DOI] [PubMed] [Google Scholar]
- Aoyama T., Korzekwa K., Nagata K., Gillette J., Gelboin H. V., Gonzalez F. J. Estradiol metabolism by complementary deoxyribonucleic acid-expressed human cytochrome P450s. Endocrinology. 1990 Jun;126(6):3101–3106. doi: 10.1210/endo-126-6-3101. [DOI] [PubMed] [Google Scholar]
- BUFFETT R. F., CLIFTON K. H., FURTH J., GADSDEN E. L. Dependent and autonomous mammotropic pituitary tumors in rats; their somatotropic features. Cancer Res. 1956 Aug;16(7):608–616. [PubMed] [Google Scholar]
- Bhattacharyya K. K., Brake P. B., Eltom S. E., Otto S. A., Jefcoate C. R. Identification of a rat adrenal cytochrome P450 active in polycyclic hydrocarbon metabolism as rat CYP1B1. Demonstration of a unique tissue-specific pattern of hormonal and aryl hydrocarbon receptor-linked regulation. J Biol Chem. 1995 May 12;270(19):11595–11602. doi: 10.1074/jbc.270.19.11595. [DOI] [PubMed] [Google Scholar]
- Bjeldanes L. F., Kim J. Y., Grose K. R., Bartholomew J. C., Bradfield C. A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradlow H. L., Michnovicz J. J., Halper M., Miller D. G., Wong G. Y., Osborne M. P. Long-term responses of women to indole-3-carbinol or a high fiber diet. Cancer Epidemiol Biomarkers Prev. 1994 Oct-Nov;3(7):591–595. [PubMed] [Google Scholar]
- Bradlow H. L., Sepkovic D. W., Telang N. T., Osborne M. P. Indole-3-carbinol. A novel approach to breast cancer prevention. Ann N Y Acad Sci. 1995 Sep 30;768:180–200. doi: 10.1111/j.1749-6632.1995.tb12121.x. [DOI] [PubMed] [Google Scholar]
- Brawer J. R., Beaudet A., Desjardins G. C., Schipper H. M. Pathologic effect of estradiol on the hypothalamus. Biol Reprod. 1993 Oct;49(4):647–652. doi: 10.1095/biolreprod49.4.647. [DOI] [PubMed] [Google Scholar]
- Bui Q. D., Weisz J. Monooxygenase mediating catecholestrogen formation by rat anterior pituitary is an estrogen-4-hydroxylase. Endocrinology. 1989 Feb;124(2):1085–1087. doi: 10.1210/endo-124-2-1085. [DOI] [PubMed] [Google Scholar]
- Dehennin L., Blacker C., Reiffsteck A., Scholler R. Estrogen 2-, 4-, 6- or 16-hydroxylation by human follicles shown by gas chromatography-mass spectrometry associated with stable isotope dilution. J Steroid Biochem. 1984 Jan;20(1):465–471. doi: 10.1016/0022-4731(84)90255-3. [DOI] [PubMed] [Google Scholar]
- Dobson M. J., Tuite M. F., Roberts N. A., Kingsman A. J., Kingsman S. M., Perkins R. E., Conroy S. C., Fothergill L. A. Conservation of high efficiency promoter sequences in Saccharomyces cerevisiae. Nucleic Acids Res. 1982 Apr 24;10(8):2625–2637. doi: 10.1093/nar/10.8.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedy I., Devanesan P., Cremonesi P., Rogan E., Cavalieri E. Synthesis and characterization of estrogen 2,3- and 3,4-quinones. Comparison of DNA adducts formed by the quinones versus horseradish peroxidase-activated catechol estrogens. Chem Res Toxicol. 1992 Nov-Dec;5(6):828–833. doi: 10.1021/tx00030a016. [DOI] [PubMed] [Google Scholar]
- Eugster H. P., Probst M., Würgler F. E., Sengstag C. Caffeine, estradiol, and progesterone interact with human CYP1A1 and CYP1A2. Evidence from cDNA-directed expression in Saccharomyces cerevisiae. Drug Metab Dispos. 1993 Jan-Feb;21(1):43–49. [PubMed] [Google Scholar]
- Fishman J., Osborne M. P., Telang N. T. The role of estrogen in mammary carcinogenesis. Ann N Y Acad Sci. 1995 Sep 30;768:91–100. doi: 10.1111/j.1749-6632.1995.tb12113.x. [DOI] [PubMed] [Google Scholar]
- Fishman J. The catechol estrogens. Neuroendocrinology. 1976;22(4):363–374. doi: 10.1159/000122645. [DOI] [PubMed] [Google Scholar]
- Gierthy J. F., Lincoln D. W., 2nd, Kampcik S. J., Dickerman H. W., Bradlow H. L., Niwa T., Swaneck G. E. Enhancement of 2- and 16 alpha-estradiol hydroxylation in MCF-7 human breast cancer cells by 2,3,7,8-tetrachlorodibenzo-P-dioxin. Biochem Biophys Res Commun. 1988 Dec 15;157(2):515–520. doi: 10.1016/s0006-291x(88)80279-1. [DOI] [PubMed] [Google Scholar]
- Han X., Liehr J. G. DNA single-strand breaks in kidneys of Syrian hamsters treated with steroidal estrogens: hormone-induced free radical damage preceding renal malignancy. Carcinogenesis. 1994 May;15(5):997–1000. doi: 10.1093/carcin/15.5.997. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Pike M. C. Toward the primary prevention of cancer. Science. 1991 Nov 22;254(5035):1131–1138. doi: 10.1126/science.1957166. [DOI] [PubMed] [Google Scholar]
- Hoffman A. R., Paul S. M., Axelrod J. Catecholestrogen synthesis and metabolism by human breast tumors in vitro. Cancer Res. 1979 Nov;39(11):4584–4587. [PubMed] [Google Scholar]
- Huggins C. Endocrine-induced regression of cancers. Science. 1967 May 26;156(3778):1050–1054. doi: 10.1126/science.156.3778.1050. [DOI] [PubMed] [Google Scholar]
- KIRKMAN H. Estrogen-induced tumors of the kidney. III. Growth characteristics in the Syrian hamster. Natl Cancer Inst Monogr. 1959 Dec;1:1–57. [PubMed] [Google Scholar]
- Lemon H. M., Heidel J. W., Rodriguez-Sierra J. F. Increased catechol estrogen metabolism as a risk factor for nonfamilial breast cancer. Cancer. 1992 Jan 15;69(2):457–465. doi: 10.1002/1097-0142(19920115)69:2<457::aid-cncr2820690231>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Li J. J., Li S. A. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed Proc. 1987 Apr;46(5):1858–1863. [PubMed] [Google Scholar]
- Liehr J. G., Fang W. F., Sirbasku D. A., Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J Steroid Biochem. 1986 Jan;24(1):353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- Liehr J. G., Ricci M. J. 4-Hydroxylation of estrogens as marker of human mammary tumors. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3294–3296. doi: 10.1073/pnas.93.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehr J. G., Ricci M. J., Jefcoate C. R., Hannigan E. V., Hokanson J. A., Zhu B. T. 4-Hydroxylation of estradiol by human uterine myometrium and myoma microsomes: implications for the mechanism of uterine tumorigenesis. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9220–9224. doi: 10.1073/pnas.92.20.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liehr J. G., Roy D. Free radical generation by redox cycling of estrogens. Free Radic Biol Med. 1990;8(4):415–423. doi: 10.1016/0891-5849(90)90108-u. [DOI] [PubMed] [Google Scholar]
- McKay J. A., Melvin W. T., Ah-See A. K., Ewen S. W., Greenlee W. F., Marcus C. B., Burke M. D., Murray G. I. Expression of cytochrome P450 CYP1B1 in breast cancer. FEBS Lett. 1995 Oct 30;374(2):270–272. doi: 10.1016/0014-5793(95)01126-y. [DOI] [PubMed] [Google Scholar]
- Miller W. R. Endocrine treatment for breast cancers: biological rationale and current progress. J Steroid Biochem Mol Biol. 1990 Nov 30;37(4):467–480. doi: 10.1016/0960-0760(90)90390-7. [DOI] [PubMed] [Google Scholar]
- Newbold R. R., Bullock B. C., McLachlan J. A. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990 Dec 1;50(23):7677–7681. [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter L. M., Wu Y. Y., Ngo E. O., Sierra E. E., Gutierrez P. L., Abul-Hajj Y. J. An o-quinone form of estrogen produces free radicals in human breast cancer cells: correlation with DNA damage. Chem Res Toxicol. 1994 Jan-Feb;7(1):23–28. doi: 10.1021/tx00037a004. [DOI] [PubMed] [Google Scholar]
- Oeda K., Sakaki T., Ohkawa H. Expression of rat liver cytochrome P-450MC cDNA in Saccharomyces cerevisiae. DNA. 1985 Jun;4(3):203–210. doi: 10.1089/dna.1985.4.203. [DOI] [PubMed] [Google Scholar]
- Osawa Y., Higashiyama T., Shimizu Y., Yarborough C. Multiple functions of aromatase and the active site structure; aromatase is the placental estrogen 2-hydroxylase. J Steroid Biochem Mol Biol. 1993 Mar;44(4-6):469–480. doi: 10.1016/0960-0760(93)90252-r. [DOI] [PubMed] [Google Scholar]
- Paria B. C., Chakraborty C., Dey S. K. Catechol estrogen formation in the mouse uterus and its role in implantation. Mol Cell Endocrinol. 1990 Feb 12;69(1):25–32. doi: 10.1016/0303-7207(90)90085-m. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kinne D., Fracchia A., Pierce V., Anderson K. E., Bradlow H. L., Fishman J. Abnormal oxidative metabolism of estradiol in women with breast cancer. Proc Natl Acad Sci U S A. 1982 May;79(9):3047–3051. doi: 10.1073/pnas.79.9.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Hayes C. L., Yamazaki H., Amin S., Hecht S. S., Guengerich F. P., Sutter T. R. Activation of chemically diverse procarcinogens by human cytochrome P-450 1B1. Cancer Res. 1996 Jul 1;56(13):2979–2984. [PubMed] [Google Scholar]
- Spicer L. J., Hammond J. M. Regulation of ovarian function by catecholestrogens: current concepts. J Steroid Biochem. 1989 Oct;33(4A):489–501. doi: 10.1016/0022-4731(89)90033-2. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Eugster H. P., Lincoln D. W., 2nd, Schuetz J. D., Schuetz E. G., Johnson J. A., Kaminsky L. S., Gierthy J. F. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1A1: a comparison of the activities induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in MCF-7 cells with those from heterologous expression of the cDNA. Arch Biochem Biophys. 1992 Mar;293(2):342–348. doi: 10.1016/0003-9861(92)90404-k. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Hayes C. L., Young N. R., Christou M., Sutter T. R., Jefcoate C. R., Gierthy J. F. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17 beta-estradiol 4-hydroxylase. J Steroid Biochem Mol Biol. 1994 Dec;51(5-6):251–258. doi: 10.1016/0960-0760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Johnson J. A., Connor S. P., Aldous K. M., Gierthy J. F. Stimulation of 17 beta-estradiol metabolism in MCF-7 cells by bromochloro- and chloromethyl-substituted dibenzo-p-dioxins and dibenzofurans: correlations with antiestrogenic activity. J Toxicol Environ Health. 1994 Apr;41(4):451–466. doi: 10.1080/15287399409531856. [DOI] [PubMed] [Google Scholar]
- Spink D. C., Lincoln D. W., 2nd, Dickerman H. W., Gierthy J. F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes an extensive alteration of 17 beta-estradiol metabolism in MCF-7 breast tumor cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6917–6921. doi: 10.1073/pnas.87.17.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter T. R., Guzman K., Dold K. M., Greenlee W. F. Targets for dioxin: genes for plasminogen activator inhibitor-2 and interleukin-1 beta. Science. 1991 Oct 18;254(5030):415–418. doi: 10.1126/science.1925598. [DOI] [PubMed] [Google Scholar]
- Sutter T. R., Tang Y. M., Hayes C. L., Wo Y. Y., Jabs E. W., Li X., Yin H., Cody C. W., Greenlee W. F. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem. 1994 May 6;269(18):13092–13099. [PubMed] [Google Scholar]
- Urban P., Cullin C., Pompon D. Maximizing the expression of mammalian cytochrome P-450 monooxygenase activities in yeast cells. Biochimie. 1990 Jun-Jul;72(6-7):463–472. doi: 10.1016/0300-9084(90)90070-w. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Lapenson D. P., Aoyama T., Gelboin H. V., Gonzalez F. J., Korzekwa K. Steroid hormone hydroxylase specificities of eleven cDNA-expressed human cytochrome P450s. Arch Biochem Biophys. 1991 Oct;290(1):160–166. doi: 10.1016/0003-9861(91)90602-f. [DOI] [PubMed] [Google Scholar]
- Weisz J., Bui Q. D., Roy D., Liehr J. G. Elevated 4-hydroxylation of estradiol by hamster kidney microsomes: a potential pathway of metabolic activation of estrogens. Endocrinology. 1992 Aug;131(2):655–661. doi: 10.1210/endo.131.2.1386303. [DOI] [PubMed] [Google Scholar]
- de Waziers I., Cugnenc P. H., Yang C. S., Leroux J. P., Beaune P. H. Cytochrome P 450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J Pharmacol Exp Ther. 1990 Apr;253(1):387–394. [PubMed] [Google Scholar]