Abstract
The addition of zoledronate to tumor-associated antigen (TAA)-loaded dendritic cells (DCs) promotes the activation of interferon γ-secreting Vγ9 γδ T cells, in turn eliciting TAA-specific CD8+ T-cell responses. Immunological responses induced by zoledronate-pulsed DC-based vaccines have been associated with therapeutic effects in clinical trials.
Keywords: Wilms’ tumor 1, dendritic cell, electroporation, intratumoral injection, tumor-associated antigen-derived peptide, zoledronate
Dendritic cell (DC)-based vaccines relying on several distinct methods for antigen loading have been developed for cancer immunotherapy.1 In this context, it has been shown that Vγ9 γδ T cells activated by zoledronate can link the innate and adaptive arms of the immune system via DCs so to boost the activation of tumor-associated antigen (TAA)-specific CD8+ cytotoxic T lymphocytes (CTLs).2 TAA-pulsed immature DCs (imDCs) combined with zoledronate activate Vγ9 γδ T cells while promoting the expression of CD40 ligand (CD40L) on their surface and the secretion of cytokines such as interferon γ (IFNγ), ultimately driving the expansion of TAA-specific CD8+ CTLs. We postulate that CD40L on imDC-activated Vγ9 γδ T cells in combination with zoledronate and Vγ9 γδ T cell-derived cytokines stimulate the functional maturation of imDCs, which is accompanied by the upregulation of chemokine (C-C motif) receptor 7 (CCR7) and CD62 ligand (CD62L) and promotes the proliferation of TAA-specific CD8+ CTLs.
We employed zoledronate-pulsed DCs to develop vaccines targeting various TAAs and characterized them in clinical settings (Fig. 1). In particular, we performed a Phase I/IIa clinical trial involving elderly acute myeloid leukemia (AML) patients and DCs pulsed with an HLA-A2402-restricted Wilms’ tumor 1 (WT1)-derived peptide and zoledronate.3 Three elderly HLA-A2402+ AML patients were enrolled in this study, 2 of which developed a WT1-specific immune response, as documented by skin delayed-type hypersensitivity (DTH) and/or IFNγ ELISPOT assays. Along with the elicitation of WT1-targeting immune responses, either a transient decrease in leukemic cells or disease stabilization was observed in these 2 patients. Unfortunately, the other patient dropped out of the study after the third round of vaccination owing to the rapid outgrowth of leukemic cells, correlating with the absence of WT1-specific immune responses. Recently, we employed DCs pulsed with zoledronate and an overlapping pool of WT1-derived peptides for the treatment of patients with WT1-expressing solid tumors (UMIN-CTR ID 000009447), because both CD8+ CTLs and CD4+ helper T cells targeting WT1 might (at least potentially) be induced even in the absence of HLA restriction.
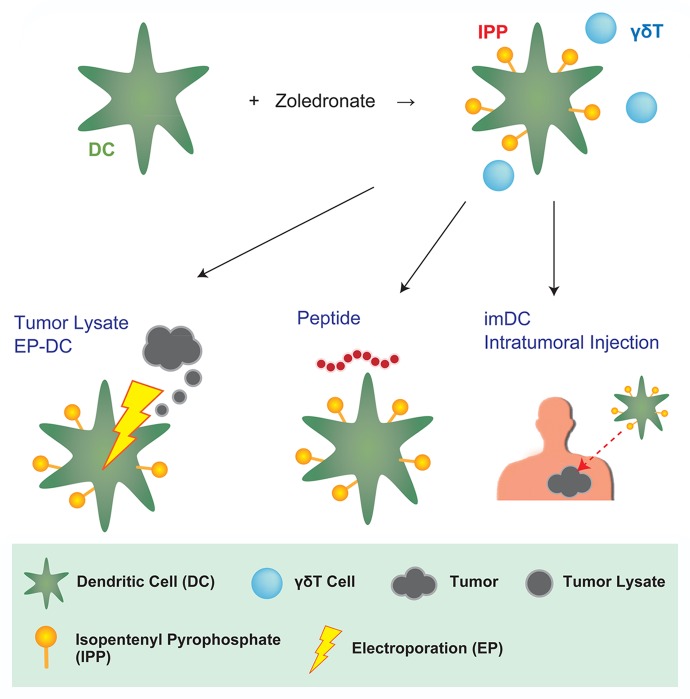
Figure 1. Zoledronate-pulsed dendritic cell-based cancer vaccines. Zoledronate is an amino-bisphosphonate that induces the accumulation of isopentenyl pyrophosphate (IPP), a ligand of the Vγ9 γδ T-cell receptor (TCR), in a variety of cells. Vγ9 γδ T cells activated by zoledronate can link the innate and adaptive branch of the immune system via dendritic cells (DCs), hence supporting the activation and proliferation of tumor-associated antigen (TAA)-specific CD8+ cytotoxic T lymphocytes (CTLs). DCs can be effectively loaded with purified TAAs, cancer cell lysates or mRNA preparations by a closed-flow electroporation (EP) system. Alternatively, loading can be performed by culturing DCs in the presence of 9–15-mer TAA-derived peptides. When it is difficult to obtain adequate amounts of TAAs for loading DC ex vivo, tumor-specific immune responses might be induced in vivo, upon the injection of immature DCs (imDCs) into neoplastic lesions.
It is well known that tumor-specific CD8 + CTLs are effectively activated once they recognize TAAs presented in the context of MHC Class I molecules along with appropriate co-stimulatory signals. Experiments in which DCs were loaded with purified TAAs or TAA-coding mRNA revealed that antigens can be presented on both MHC Class I and Class II molecules, depending on the method of antigen delivering (which influences its intracellular processing).4,5 We have previously studied electroporation (EP) as a way to deliver TAAs to DCs while preserving their viability. In fact, antigen-specific CD8+ CTLs are elicited more effectively by DCs that are electroloaded with antigens than by immature DCs that are simply cultured in the presence of antigens.6 By the closed-flow EP system, we succeeded in loading zoledronate-pulsed DCs with autologous tumor cell lysates while maintaining the viability. In a Phase I clinical trial, mild adverse events were documented when this DC vaccine was used for the treatment of advanced/recurrent cancer patients, or as an adjuvant therapy for some other types of neoplasms. However, no autoimmune responses developed upon the administration of the autologous tumor cell lysate-loaded DC-based vaccine. Among 41 patients affected by different solid tumors, vaccination was associated with an overall response rate of 4.9% and a clinical benefit rate of 31.7%. Of note, more than 90% of the patients exhibiting a clinical benefit upon vaccination manifested DTH responses. Thus, the antitumor effects of our autologous tumor cell lysate-loaded DC-based vaccine appear to be intimately associated with elicitation of tumor-specific immune responses.7
In some circumstances, it is difficult to obtain adequate amounts of TAAs for loading DCs ex vivo. In this settings, strategies that efficiently elicit tumor-specific immune responses in vivo, upon the injection of autologous DCs in neoplastic lesions that have previously been exposed to radiotherapy or chemotherapy, would be highly desirable.8 DCs can indeed efficiently engulf and process extracellular antigens shedding from apoptotic cells.9 OK432, a lyophilized preparation of a poorly virulent strain of Streptococcus pyogenes killed by penicillin, is widely used as a maturation stimulus for DCs. In a previous study, OK432-pulsed imDCs were administered intratumorally—by the endoscopic ultrasound (EUS) technique—to patients with locally advanced pancreatic cancer, aimed at potentiating the antineoplastic activity of systemic gemcitabine and conventional lymphokine-activated killer cells stimulated with anti-CD3 monoclonal antibodies.10 Three out of 5 patients enrolled in this clinical trial manifested objective clinical responses: 1) a partial remission and 2) disease stabilization lasting for more than 6 mo. In the patient undergoing partial remission, OK432-pulsed imDCs combined with gemcitabine had elicited TAA-specific CTLs, as demonstrated by IFNγ ELISPOT assays. Currently, we inject zoledronate-pulsed imDCs, instead of OK432-pulsed imDCs, into neoplastic lesions, following the protocol that was previously defined for locally advanced pancreatic cancer patients.
Of note, no serious treatment-related adverse events were documented in clinical trials involving zoledronate-pulsed DCs loaded with various TAAs. The success of cancer immunotherapy depends on the targeting of adequate TAAs and on the choice of an immunization strategy that elicits robust tumor-specific immune responses. Zoledronate-pulsed DC-based cancer vaccines represent a promising immunotherapeutic approach for the treatment of various neoplasms. This strategy is supported by recent technological progresses, such as the possibility to generate pools of overlapping TAA-derived peptides or the closed-flow EP system for the loading of DCs with tumor cell lysates.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/25636
References
- 1.Palucka K, Ueno H, Banchereau J. Recent developments in cancer vaccines. J Immunol. 2011;186:1325–31. doi: 10.4049/jimmunol.0902539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahara M, Miyai M, Tomiyama M, Mutou M, Nicol AJ, Nieda M. Copulsing tumor antigen-pulsed dendritic cells with zoledronate efficiently enhance the expansion of tumor antigen-specific CD8+ T cells via Vgamma9gammadelta T cell activation. J Leukoc Biol. 2008;83:742–54. doi: 10.1189/jlb.0307185. [DOI] [PubMed] [Google Scholar]
- 3.Kitawaki T, Kadowaki N, Fukunaga K, Kasai Y, Maekawa T, Ohmori K, et al. A phase I/IIa clinical trial of immunotherapy for elderly patients with acute myeloid leukaemia using dendritic cells co-pulsed with WT1 peptide and zoledronate. Br J Haematol. 2011;153:796–9. doi: 10.1111/j.1365-2141.2010.08490.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim KW, Kim SH, Jang JH, Lee EY, Park SW, Um JH, et al. Dendritic cells loaded with exogenous antigen by electroporation can enhance MHC class I-mediated antitumor immunity. Cancer Immunol Immunother. 2004;53:315–22. doi: 10.1007/s00262-003-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnurr M, Chen Q, Shin A, Chen W, Toy T, Jenderek C, et al. Tumor antigen processing and presentation depend critically on dendritic cell type and the mode of antigen delivery. Blood. 2005;105:2465–72. doi: 10.1182/blood-2004-08-3105. [DOI] [PubMed] [Google Scholar]
- 6.Wolfraim LA, Takahara M, Viley AM, Shivakumar R, Nieda M, Maekawa R, et al. Clinical scale electroloading of mature dendritic cells with melanoma whole tumor cell lysate is superior to conventional lysate co-incubation in triggering robust in vitro expansion of functional antigen-specific CTL. Int Immunopharmacol. 2013;15:488–97. doi: 10.1016/j.intimp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Kamigaki T, Kaneko T, Naitoh K, Takahara M, Kondo T, Ibe H, et al. Immunotherapy of autologous tumor lysate-loaded dendritic cell vaccines by a closed-flow electroporation system for solid tumors. Anticancer Res. 2013;33:2971–6. [PubMed] [Google Scholar]
- 8.Tanaka F, Yamaguchi H, Ohta M, Mashino K, Sonoda H, Sadanaga N, et al. Intratumoral injection of dendritic cells after treatment of anticancer drugs induces tumor-specific antitumor effect in vivo. Int J Cancer. 2002;101:265–9. doi: 10.1002/ijc.10597. [DOI] [PubMed] [Google Scholar]
- 9.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–74. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 10.Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, et al. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas. 2009;38:e69–74. doi: 10.1097/MPA.0b013e318197a9e3. [DOI] [PubMed] [Google Scholar]