Abstract
Purpose.
To investigate whether tissue plasminogen activator (tPA) can prevent and/or reverse steroid-induced IOP elevation in an ovine model.
Methods.
Three animal groups were subjected to bilateral steroid-induced IOP elevation using thrice daily topical ocular prednisolone administration. In the first group (N = 8), one eye each of two sheep was injected intravitreally with 100 μg, 200 μg, 500 μg, or 1 mg human recombinant tPA, while contralateral eyes received vehicle. In the second group (N = 2), one eye was injected intravitreally with tPA (100 μg), while contralateral eyes received vehicle containing L-arginine. In the third group (N = 4), each animal received intravitreal tPA in one eye concurrently with initiation of bilateral steroid administration. IOP was monitored for the duration of the experiment. Tissues from eyes of the third group were used to determine relative gene expression.
Results.
In the first and second groups, IOP decreased by 9.7 (±2.8) and 9.7 (±1.6) mm Hg, respectively, 24 hours after tPA administration. In the third group, tPA-treated eyes did not develop IOP elevation with ΔIOP of 11.8 (±1.3) mm Hg 8 days later. In all tPA-treated eyes, IOP remained low until the end of the study. mRNA levels in the trabecular meshwork were decreased for plasminogen activator tissue (PLAT), increased for matrix-metalloproteinase 1 (MMP-1), and stable for plasminogen activator inhibitor 1 (PAI-1), MMP-2, MMP-9, and MMP-13 in tPA-treated eyes compared with contralateral controls. PAI-1 mRNA levels in ciliary processes also remained similar.
Conclusions.
Recombinant human tPA is effective in both preventing and reversing steroid-induced IOP elevation in sheep. Tissue plasminogen activator may be useful as a therapeutic agent in steroid-induced glaucoma.
Keywords: intraocular pressure, tissue plasminogen activator, trabecular meshwork, extracellular matrix, matrix metalloproteinase
Recombinant human tissue plasminogen activator (tPA) is effective in both preventing and reversing steroid-induced intraocular pressure elevation in sheep. Tissue plasminogen activator may be useful as a therapeutic agent in steroid-induced glaucoma.
Introduction
Tissue plasminogen activator (tPA) is a serine protease that catalyzes the conversion of the zymogen, plasminogen, to plasmin, the major enzyme responsible for blood-clot breakdown via the proteolytic degradation of fibrin.1 This cascade can also lead to the activation of other proenzymes, including members of the matrix-metalloproteinase (MMP) family of enzymes, to their active forms.2,3 The MMPs directly degrade extracellular matrix (ECM) components, and these enzymes play a key role in the turnover and maintenance of the ECM of the trabecular meshwork (TM), a process affecting outflow facility.4,5 Because tPA is expressed, and secreted, by various organ systems including the TM6,7 and is found in the aqueous humor,8 tPA could have an important role in controlling or regulating ECM composition in the TM. Consistent with this possibility, glucocorticosteroid drugs, such as dexamethasone, which have been shown to increase the accumulation of ECM components in the TM and decrease outflow facility,9,10 also elicit reductions in tPA activity in TM organ and cell cultures,11 suggesting a linkage between these phenomena.
Glucocorticosteroids are therapeutically versatile and commonly administered as anti-inflammatory, immunosuppressive, and anti-angiogenic agents.12–15 However, glucocorticosteroids also elicit adverse ocular effects such as cataracts and increased IOP.16 Individuals susceptible to the latter side effect may require treatment for glaucoma. The phenomenon of glucocorticosteroid-induced ocular hypertension has been recognized for decades,17 and a number of predisposing risk factors have been identified among patients receiving various corticosteroid treatments.18,19 In general, it is recognized that the mechanisms by which glucocorticosteroids induce the IOP elevation involve a reduced trabecular aqueous humor outflow associated with morphologic and biochemical changes in the TM.18,19 As such, studies on the cellular processes eliciting corticosteroid-induced ocular hypertension may shed light on the cause of POAG.
In past work, we demonstrated the effectiveness of using Corriedale sheep (Ovis aries) as an animal model for glucocorticosteroid-induced ocular hypertension.20 The IOP of these animals increased approximately 2-fold within 1 to 2 weeks of topically applying 0.5% prednisolone acetate three times daily. This IOP elevation occurred with a 100% incidence in the corticosteroid-treated eyes. In the current work, we attempted to determine the effect of intravitreally administered human recombinant tPA on steroid-induced IOP elevation in sheep. This study is the first to examine the effects of exogenously administered tPA on IOP in an animal model in vivo.
Materials and Methods
Animals—Care, Husbandry, and General Experimental Procedure
All animal experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. A total of 14 healthy (female) sheep (Corriedale breed) between 12 and 24 months of age, weighing 35 to 40 kg, were selected from a local ranch in Corrientes, Argentina, for this study. The eyes and general health of the animals were considered normal by an ophthalmologist and a veterinarian, respectively. Sheep were tagged on their ear lobes for individual identification and herded from pasture whenever it was necessary to (1) topically instill prednisolone, (2) inject either tPA or vehicle intravitreally, or (3) measure IOP by applanation tonometry. For each of these procedures, the sheep were guided into a funnel corral ending in a loose-fitting yoke.20 This arrangement allowed movement and holding of the head by one person, while another either instilled the prednisolone, completed the intraocular injections, or measured IOP. Between all procedures, the animals were free to pasture.
Prednisolone Instillation Protocol
All sheep eyes received two drops of 0.5% prednisolone acetate (Ultracortenol; Novartis Ophthalmics, Hettlingen, Switzerland), three times daily (at 7 AM, 2 PM, and 7 PM) for the duration of each experiment. Such treatment induces a persistent ocular hypertension in sheep provided the instillation protocol is uninterrupted.20
Intravitreal Injection of tPA
Lyophilized tPA (Actilyse, 50 mg; Boehringer, Ingelheim, Germany) was dissolved in 0.1 mL balanced saline solution (BSS), which was then injected into the central vitreous under topical anesthesia (induced with two drops of topical Anestalcon [proparacaine, 0.5%; Alcon, Buenos Aires, Argentina]). The amounts of tPA introduced into the eye within the 0.1 mL quantity of vehicle ranged from 0.1 mg to 1 mg. For these injections, a disposable insulin syringe with a 12.7-mm, 30-gauge needle was used to penetrate the globe to the full length of the needle at a point approximately 4 mm posterior to the limbus. The angle of penetration was such that the tip of the needle was centrally located within the vitreous humor. Care was taken to avoid puncturing the lens upon injection.
In one group of animals (N = 8), the right eye of each sheep received the tPA-containing solution (0.1 mg, 0.2 mg, 0.5 mg, 1 mg, respectively; two animals per dose), whereas the left eye solely received vehicle (BSS) intravitreally, after 10 days of daily prednisolone instillations three times a day in both eyes. Prednisolone treatment continued after tPA administration for the duration of the experiment.
A second group of animals (N = 2) was treated as above with 0.1 mg tPA, but instead of BSS, the contralateral eye received 4.23 mg arginine in BSS. This amount is equivalent to the amount of arginine contained in 0.1-mg commercial tPA preparation. Prednisolone treatment initiated 7 days prior to tPA administration was bilateral as above and continued for the duration of the experiment.
A third group of animals (N = 4) received 0.1 mg tPA in one eye, while the contralateral eye received 4.23 mg arginine in BSS. Prednisolone-acetate treatment was then initiated on the same day and continued for the duration of the experiment.
Measurement of IOP of Conscious Sheep With the Handheld Perkins Applanation Tonometer
IOP was measured with a Perkins tonometer (Haag Streit USA, Mason, OH). Before the IOP measurement, two drops topical 0.5% proparacaine (Alcon) followed by two drops 0.25% fluorescein were instilled in eyes. Two sets of measurements were taken on each eye and averaged, alternating first one eye and then the other. All IOP measurements were taken between 2 PM and 4 PM every 2 or 3 days. Perkins tonometry readings were converted to mm Hg as described in detail previously.20 The IOP in both eyes of the sheep used in this study was measured prior to any treatment to establish baseline values.
Tissue Collection and Isolation of RNA
After animals were euthanized, eyes from animals in the third group were immediately enucleated. Eyes were then opened anterior to the equator using a razor blade. The lens was removed from the anterior part, and the tissue was immersed in RNA stabilizing agent (RNAlater; Ambion, Carlsbad, CA) and placed at −20°C for transportation to the United States. Upon arrival in the United States, TM and ciliary processes (CP) were dissected on ice in the presence of RNA stabilizing agent as described previously.21 Dissected tissue was homogenized, and total RNA was extracted using TRIzol reagent (Gibco, Carlsbad, CA). Briefly, the tissue was homogenized in TRIzol, and chloroform was added to separate proteins from RNA. After centrifugation, the RNA-containing supernatant was aspirated. The RNA was precipitated with isopropanol, washed with 75% ethanol, treated with DNase, and column purified using a commercial kit (RNAeasy Mini Kit; Qiagen, Valencia, CA) in accordance with the manufacturer's instructions. RNA concentrations were determined with a spectrophotometer (Nanodrop; Thermo Scientific, Wilmington, DE) and the 260:280-nm absorbance ratio was calculated to determine RNA purity.
Quantitative Real-Time PCR (qRT-PCR)
The RNA samples were reverse transcribed with random hexamers to cDNA using a reverse transcription kit (Quantitect; Qiagen) in accordance with the manufacturer's instructions. Quantitative RT-PCR was performed using a commercial kit (SYBR Green RT-PCR Reagents Kit; Applied Biosystems, Carlsbad, CA) in an ABI PRISM 7900HT sequence detector (Applied Biosystems). The sheep endogenous mRNA expression of matrix metalloproteinase-1 (MMP-1), matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), matrix metalloproteinase-13 (MMP-13), and plasminogen activator tissue (PLAT) in the TM were investigated. Plasminogen activator inhibitor 1 (PAI-1) mRNA expression was measured in both TM and CP tissues. The primer sequences used are listed in the Table. Relative quantification of gene expression was performed using the standard curve method. Mean threshold cycle (Ct) of the samples was compared among the groups by using the Ct of 18S as an internal control. The ΔCt was calculated as the difference in Ct values derived from the target gene and the 18S gene. The ΔΔCt was calculated as ΔCt of the normalized assayed genes in the treated samples minus ΔCt of normalized assayed genes in the naïve control samples. Relative expression was calculated by the 2−ΔΔCt formula.
Table.
Primer Sequences of Genes Analyzed by qRT-PCR
|
Gene |
Primer |
Sequence, 5′–3′ |
Size, bp |
| MMP-1 | Forward | AAGATGTGGAGACGGTGCAG | 144 |
| Reverse | CAGTCACTCTCAGCCCGAAG | ||
| MMP-2 | Forward | ACATACAGGATCATTGGCTACACA | 214 |
| Reverse | CGAAGGCATGAGCCAGGAG | ||
| MMP-9 | Forward | CCAGGAGAACGACGAACCAG | 248 |
| Reverse | AGTTCGCCCTCAAAGGTCTG | ||
| MMP-13 | Forward | GCCAGAACTTCCCAACCGTA | 181 |
| Reverse | GTGAAGGGCTGCACTGATCT | ||
| PLAT | Forward | CAGTGCCCAGAAGGGTTCAT | 249 |
| Reverse | GTAGCACCAGGGCTTTGAGT | ||
| PAI-1 | Forward | CTCCAAGGACCGCAACGT | 199 |
| Reverse | GCTGATCTCATCCTTGTTCCA |
Data Analysis
Differences in IOP between contralateral eyes were subjected to ANOVA with post hoc Tukey-Kramer testing. IOPs at specific individual time points were compared using t-test. Gene expression fold changes were compared with one using one-sample t-test; α = 0.05 was chosen as the level of significance.
Results
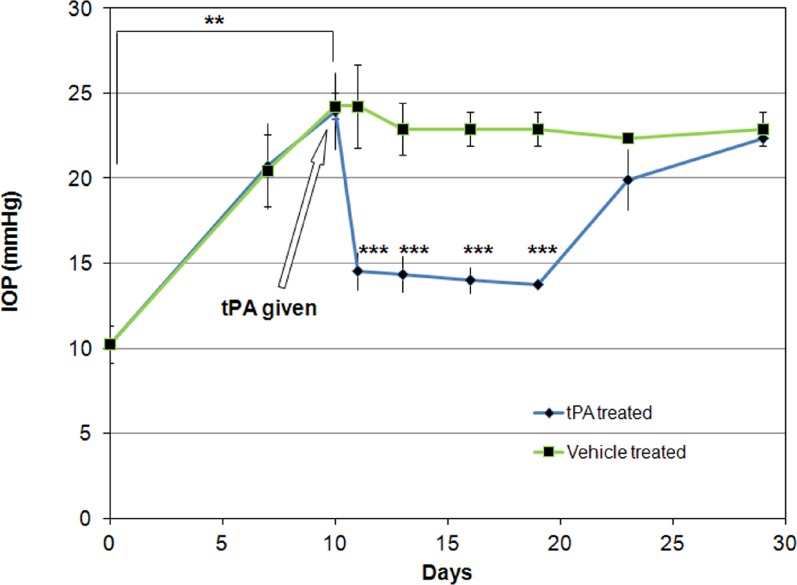
Baseline IOPs for all sheep prior to any experimental intervention was between 9 and 12 mm Hg. Treatment with prednisolone acetate for 10 days increased mean (±SD) IOP to 24.2 (±1.6) mm Hg for all animals in group 1. This pressure level was statistically significantly different from baseline mean IOP, which was 10.2 (±1.1) mm Hg (P < 0.00001, t-test) (Fig. 1).
Figure 1.
IOP (mean ± SD) in intravitreally tPA-treated and contralateral control eyes of sheep (N = 8). All eyes were also treated with prednisolone acetate starting 10 days prior to tPA administration and continuing for the duration of the experiment. Among experimental group two eyes each received tPA doses of 0.1, 0.2, 0.5, and 1 mg. IOP decreased within 24 hours in tPA-treated eyes and remained lower than that of the contralateral eyes for at least 9 days. **P < 0.0001, t-test; ***P < 0.00001 ANOVA, post hoc testing.
Treatment with tPA decreased IOP within 24 hours for all doses tested in the first group of animals to a mean (±SD) of 14.5 (±1.1) mm Hg, which was significantly lower than of the contralateral control eye, which exhibited an IOP of 24.2 (±2.4) mm Hg (P < 0.000013, paired t-test). The effect was evident for all tPA doses, independent of the dose (P > 0.27, ANOVA). IOP remained different between contralateral control eyes and tPA-treated eyes for at least 9 days, after which IOPs became similar (P < 0.00001, ANOVA, Tukey-Kramer post hoc test) (Fig. 1). Transient injection and corneal clouding was observed in five eyes but was unrelated to the dose injected (two eyes in the 1-mg dose, and one eye each in the other doses). The corneal clouding and injection was initially noted 3 days after tPA injection and resolved in all eyes within a maximum of 5 days (before IOP became similar to that of the contralateral eye).
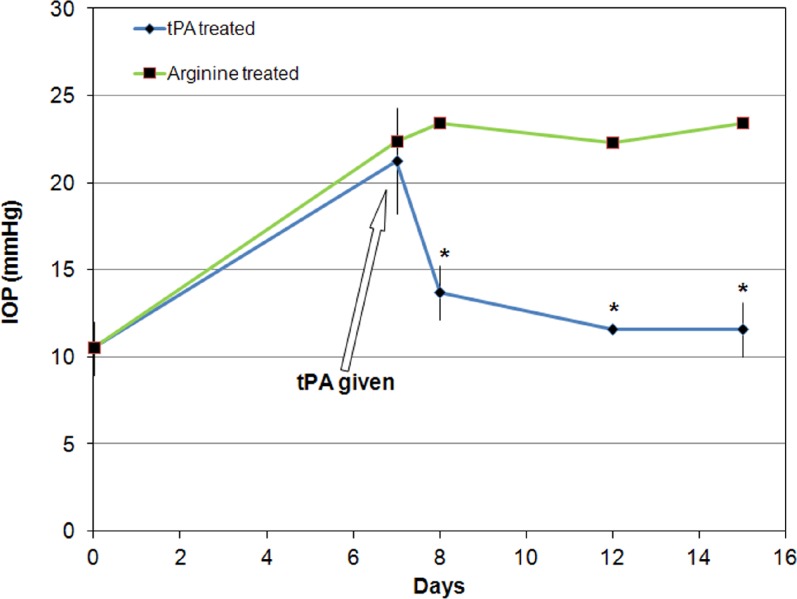
Similarly, in the animals treated with 100 μg tPA in one eye, while the contralateral eye received arginine, IOP decreased from a mean (±SD) of 21.3 (±1.5) mm Hg to a mean (±SD) of 13.7 (±0.0) mm Hg (P < 0.003, ANOVA, Tukey-Kramer post hoc test) within 24 hours in the tPA-treated eye. No significant change in IOP occurred in the arginine-treated eye with IOP remaining elevated to a mean (±SD) of 23.4 (±1.6) mm Hg (Fig. 2). No eyes (neither tPA injected nor arginine injected) showed any evidence of corneal clouding for the duration of the experiment.
Figure 2.
IOP (mean ± SD) in intravitreally tPA-treated and contralateral control eyes of sheep (N = 2). All eyes were also treated with prednisolone acetate starting 7 days prior to tPA administration and continuing for the duration of the experiment. Tissue plasminogen activator–treated eyes received 100 μg of tPA each, while contralateral eyes received the equivalent amount of arginine (4.23 mg). IOP decreased within 24 hours in tPA-treated eyes and remained lower than that of the contralateral eyes for at least 7 days. *P < 0.003, ANOVA, post hoc testing.
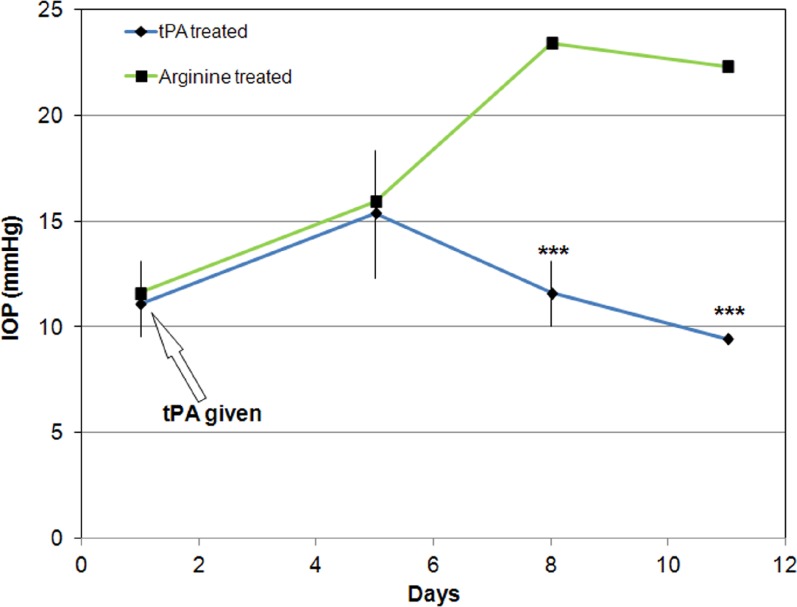
In the third group of animals that received tPA concurrently with initiation of steroid treatment, IOP remained similar in the tPA- and arginine-treated eyes until day 5, with the mean IOP of the tPA-treated and control eyes exhibiting values of 15.4 (±1.1) and 15.9 (±0.0) mm Hg (P > 0.06, paired t-test), respectively. Eight days after tPA injection, the IOP of the treated eyes declined to 11.6 (±0.0) mm Hg, while that of the control eyes increased to 23.4 (±1.3) mm Hg. The IOP difference between tPA-treated and arginine-treated eyes was significantly different over time (P < 0.00001, ANOVA) with IOP difference on days 5 and 8 being significantly different from that at earlier time points (Tukey-Kramer post hoc test). This IOP difference remained for the duration of the experiment (Fig. 3). No eyes (neither tPA injected nor arginine injected) showed any evidence of corneal clouding for the duration of the experiment.
Figure 3.
IOP (mean ± SD) in intravitreally tPA-treated and contralateral control eyes of sheep (N = 4). All eyes were also treated with prednisolone acetate starting immediately after tPA administration and continuing for the duration of the experiment. Tissue plasminogen activator–treated eyes received 100 μg of tPA each, while contralateral eyes received the equivalent amount of arginine (4.23 mg). IOP in tPA-injected eyes remained low, while it increased in arginine-treated eyes 7 days after injection. ***P < 0.00001, ANOVA, post hoc testing.
Expression of PLAT gene in the TM of tPA-treated eyes was significantly decreased (P < 0.01, t-test) compared with that of the contralateral control arginine-treated eyes in the four animals where treatment with steroids was initiated concurrently with tPA administration. PLAT mRNA expression decreased by approximately 50%. In contrast, expression of PAI-1 was not significantly different among contralateral eyes in this group of animals in either the TM or the CPs (P > 0.05, t-test) (Fig. 4).
Figure 4.
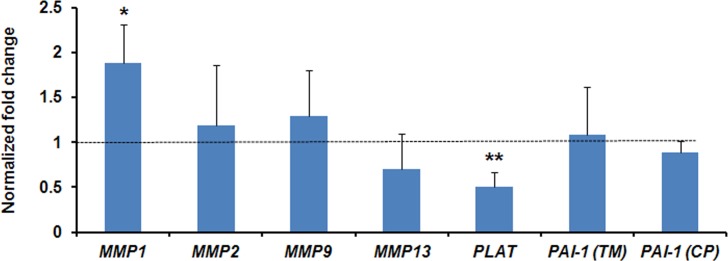
Normalized fold change (mean ± SD) expression of MMP-1, MMP-2, MMP-9, MMP-13, PLAT, and PAI-1 in trabecular meshwork (PAI-1[TM]) and PAI-1 in ciliary processes (PAI-1[CP]) in the eyes receiving tPA at the time of initiation of steroid treatment (n = 4). Fold changes were compared with 1 (dashed line). *P < 0.05, **P < 0.01, t-test.
Expression of MMP-1 in the TM of tPA-treated eyes was significantly upregulated (P < 0.05, t-test) compared with that of the contralateral control arginine-treated eyes. MMP-1 mRNA expression increased by approximately 80%. The expression of MMP-2, MMP-9, and MMP-13 was not significantly different between the groups (P > 0.05) (Fig. 4).
Discussion
The fibrinolytic system is a complex system of proteins that controls clotting of blood and subsequent dissolution of the resulting thrombus. Tissue plasminogen activator and urokinase plasminogen activator (uPA) are two separate molecules that activate plasminogen, which then becomes plasmin that degrades fibrin.1 However, tPA has also activity at the cellular level for controlling ECM remodeling and has been implicated in cell proliferation and migration.22 Tissue plasminogen activation is controlled by endogenous inhibitors (plasminogen activator inhibitors 1 and 2).2 PAI-1 has been reported to be elevated in glaucoma in the past23 and has been shown to be synthesized by both the ciliary epithelium24 and TM cells in response to TGFβ,25 a known factor that induces reduction in outflow facility.25,26 Tissue plasminogen activator has also been reported to be downregulated in organ cultures after treatment with steroids.10,11
A number of publications describe the use of human recombinant tPA either intracamerally for the acute management of excessive fibrin in the anterior segment of the eye27–30 or intravitreally for the dissolution of subretinal hemorrhages.31–33 Although IOP reductions are often mentioned, they have been attributed to the dissolution of the fibrin clot in the anterior chamber. However, to date, no attempt has been made to modulate the fibrinolytic system for therapeutic purposes in steroid-induced or open-angle glaucoma.
The present experiments were designed to determine whether intravitreal administration of tPA in a sheep-animal model of glucocorticosteroid-induced ocular hypertension could both (1) reduce the elevation in IOP after its establishment by pretreatment with the corticosteroid prednisolone and (2) prevent such IOP elevation. In the present study, prednisolone was administered by thrice-daily topical instillations, as used previously.34 With this agent, the IOP of sheep increases after approximately 7 days of treatment and remains elevated for as long as the instillation regimen is maintained (Danias J, Candia O, Gerometta R, unpublished observations, 2010). We administered human recombinant tPA by intravitreal injection. Intravitreal administration creates a depot for proteins, which are slowly eliminated in large part through the anterior segment. Although the kinetics of plasminogen in the vitreous cavity are unknown, administration of anti-VEGF antibodies results in high concentrations in the aqueous within 24 hours, which decline in a mono-exponential manner.35 Half-life for the vitreal depot has been calculated for anti-VEGF antibodies to be approximately 9 days in nonvitrectomized eyes.36 Based on the results of the current study, it is difficult to speculate what the half-life of intravitreally administered tPA may be. However, given the similar pattern of IOP change in the animals that received various doses of tPA and the effects on gene expression, it can be hypothesized that either tPA affects ECM molecules that have a very slow turnover or that the amount of tPA injected affects the rate at which it is eliminated. Testing of these hypotheses requires additional work.
The first group of animals reported in this study represented an attempt to perform a dose-response curve utilizing relatively high doses of tPA (range, 0.1–1 mg). We were surprised to find out that even the lowest dose (100 μg) caused significant and sustained (over 9 days) pressure reduction in this animal model. Because all animals (irrespective of dose) exhibited the same effect on IOP, we analyzed them as a group. Some eyes developed injection and transient corneal clouding that resolved within a maximum of 5 days. However, this was not dose dependent (observed in some eyes only at all dose levels). It is thus unclear whether effects on the cornea are the result of tPA itself, protein aggregates that may have formed during the reconstruction and injection process, or arginine that is used to ensure the commercial tPA stability. In addition, no corneal or conjunctival effects were seen in the second and third group of animals.
Tissue plasminogen activator has been used clinically in acute situations by intracameral injection usually at a dosage of 10 to 25 μg.28,37 For intravitreal use, it has been used to dissolve submacular hemorrhages at a dosage of 30 to 100 μg.32 Retinal toxicity has been reported with doses above 75 to 100 μg but has been attributed to the presence of arginine in the commercial preparations.38–40 Toxicity usually develops early and manifests as diffuse pigmentary alterations. We did not observe any pigmentary changes during the course of these experiments (even at maximal dosing), but we did not test the animals electrophysiologically and have not histologically examined the retina as our focus was the effect on IOP. However, to ensure that arginine present in the commercial tPA preparation (which can be a nitric oxide donor)41 is not responsible for the effect on IOP observed, we treated an additional two animals with the lowest dose of tPA used in the initial group (100 μg), while their contralateral eye received the equivalent amount of arginine dissolved in BSS. As for animals in the first group, tPA caused a significant and sustained (at least 8 days) drop in IOP, while contralateral eyes receiving arginine did not show any appreciable effect. This finding confirms that tPA administered intravitreally has a specific effect on reducing IOP that is elevated by steroid treatment in this animal model and is in agreement with findings in a mouse model of steroid-induced facility changes (currently in review) that shows a specific effect of tPA on outflow facility.
We also sought to determine whether tPA administration can prevent steroid-induced IOP elevation. We thus administered tPA just prior to initiation of treatment with steroids. As mentioned above, IOP elevates in the ovine model after approximately 1 week of treatment with steroids. Tissue plasminogen activator (100 μg) administered intravitreally was effective in preventing steroid-induced IOP elevation, and this effect lasted for at least 5 days.
Although tPA as a serine protease has a direct effect on a number of other enzymes, such effects are usually short lived.42,43 Tissue plasminogen activator has, however, also been shown to affect gene expression through various pathways.44,45 Since the effects of tPA on steroid-induced IOP elevation appear to be prolonged, we investigated whether tPA (either directly or indirectly) causes changes in the expression of a number of relevant genes in the TM and CP.
The fibrinolytic system is tightly regulated.1,46 Thus, we first investigated whether PAI-1 expression is affected. PAI-1 is expressed both in the CP and locally in the TM24,25 and has been proposed to affect outflow facility.26,47 Despite the fact that administration of tPA was intravitreal, we did not detect any changes in the mRNA levels of PAI-1, suggesting that the observed effect on IOP was directly the result of tPA (increased PAI-1 expression would have counteracted tPA-mediated IOP lowering, while decreased PAI-1 expression would have augmented tPA action and may have mediated the tPA effect). Our results suggest that tPA administration does not act through downregulating PAI-1 expression. Of interest, endogenous PLAT gene was downregulated in the sheep TM, suggesting the presence of a feedback loop that regulates local tPA production.
Tissue plasminogen activator is known to affect levels of MMPs in a variety of systems including the TM.10,45,48,49 Although tPA mediates plasminogen and proMMPs' activation by proteolytic cleavage, it also has effects on gene expression of MMPs.44,50–52 MMPs are a family of zinc- and calcium-dependent enzymes able to degrade ECM components. MMP-1 and MMP-13 are interstitial collagenases that degrade collagen type I, collagen type III, and collagen type IV.53,54 MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are able to degrade major components of ECM such as collagens type IV, V, VII, and X, laminins, and fibronectin. MMPs play a key role in the turnover and maintenance of the trabecular meshwork's ECM and have been shown to be involved in trabecular outflow.4,5 Because MMPs are involved in ECM turnover, and tPA can affect the levels of MMPs, we investigated whether any of the relevant MMPs were upregulated in the TM after tPA administration. Of interest, only MMP-1 mRNA was upregulated, while mRNA levels for MMP-2, MMP-9, and MMP-13 did not change. MMP-1 upregulation has been shown by our group to have a similar effect to tPA administration on IOP in this animal model.34 It is thus possible that tPA is acting upstream of MMP-1 to regulate ECM degradation, which eventually leads to IOP reduction.
It appears that some of the effects of PAI-1 on the TM are mediated through activation of MMP- 2 and MMP-9 in the TM.25 It is thus interesting that the absence of changes in PAI-1 mRNA were accompanied by a lack of changes in the mRNA levels of MMP-2 and MMP-9, both of which have been implicated in IOP elevation pathophysiology.4,55
In summary, we present evidence that tPA intravitreal administration can both decrease and prevent steroid-induced IOP elevation and that this effect appears to be related to MMP-1 upregulation. The work described in the current manuscript has been performed in the highly relevant to human disease ovine steroid-induced IOP elevation model. Although we acknowledge that, as with work on any animal model, the results may not necessarily reflect what happens in humans and need to be confirmed in ex vivo studies with human tissue, these findings may hold important therapeutic implications for steroid-induced and potentially other open-angle glaucomas.
Acknowledgments
Supported by National Eye Institute Grants EY00160, EY01867, and EY20670; an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York, to the Department of Ophthalmology, Mount Sinai School of Medicine New York; and a challenge grant from Research to Prevent Blindness, Inc., New York, New York, to the Department of Ophthalmology, SUNY Downstate Medical School.
Disclosure: R. Gerometta, P; S. Kumar, None; S. Shah, None; L. Alvarez, None; O. Candia, P; J. Danias, P
References
- 1. Lijnen HR, Collen D. Mechanisms of physiological fibrinolysis. Baillieres Clin Haematol. 1995; 8: 277–290 [DOI] [PubMed] [Google Scholar]
- 2. Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990; 6: 121–125 [DOI] [PubMed] [Google Scholar]
- 3. Murphy G, Atkinson S, Ward R, Gavrilovic J, Reynolds JJ. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann N Y Acad Sci. 1992; 667: 1–12 [DOI] [PubMed] [Google Scholar]
- 4. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998; 39: 2649–2658 [PubMed] [Google Scholar]
- 5. Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009; 88: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park JK, Tripathi RC, Tripathi BJ, Barlow GH. Tissue plasminogen activator in the trabecular endothelium. Invest Ophthalmol Vis Sci. 1987; 28: 1341–1345 [PubMed] [Google Scholar]
- 7. Shuman MA, Polansky JR, Merkel C, Alvarado JA. Tissue plasminogen activator in cultured human trabecular meshwork cells: predominance of enzyme over plasminogen activator inhibitor. Invest Ophthalmol Vis Sci. 1988; 29: 401–405 [PubMed] [Google Scholar]
- 8. Tripathi RC, Park JK, Tripathi BJ, Millard CB. Tissue plasminogen activator in human aqueous humor and its possible therapeutic significance. Am J Ophthalmol. 1988; 106: 719–722 [DOI] [PubMed] [Google Scholar]
- 9. Francois J. Corticosteroid glaucoma. Ophthalmologica. 1984; 188: 76–81 [DOI] [PubMed] [Google Scholar]
- 10. Snyder RW, Stamer WD, Kramer TR, Seftor RE. Corticosteroid treatment and trabecular meshwork proteases in cell and organ culture supernatants. Exp Eye Res. 1993; 57: 461–468 [DOI] [PubMed] [Google Scholar]
- 11. Seftor RE, Stamer WD, Seftor EA, Snyder RW. Dexamethasone decreases tissue plasminogen activator activity in trabecular meshwork organ and cell cultures. J Glaucoma. 1994; 3: 323–328 [PubMed] [Google Scholar]
- 12. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005; 353: 1711–1723 [DOI] [PubMed] [Google Scholar]
- 13. Spies CM, Strehl C, van der Goes MC, Bijlsma JW, Buttgereit F. Glucocorticoids. Best Pract Res Clin Rheumatol. 2011; 25: 891–900 [DOI] [PubMed] [Google Scholar]
- 14. LeHoang P. The gold standard of noninfectious uveitis: corticosteroids. Dev Ophthalmol. 2012; 51: 7–28 [DOI] [PubMed] [Google Scholar]
- 15. Oliver A, Ciulla TA. Corticosteroids as antiangiogenic agents. Ophthalmol Clin North Am. 2006; 19: 345–351 [DOI] [PubMed] [Google Scholar]
- 16. Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology. 2005; 112: 139–143 [DOI] [PubMed] [Google Scholar]
- 17. Gordon DM, McLean J, Koteen H, et al. The use of ACTH and cortisone in ophthalmology. Am J Ophthalmol. 1951; 34: 1675–1686 [DOI] [PubMed] [Google Scholar]
- 18. Jones R III, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006; 17: 163–167 [DOI] [PubMed] [Google Scholar]
- 19. Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye (Lond). 2006; 20: 407–416 [DOI] [PubMed] [Google Scholar]
- 20. Gerometta R, Podos SM, Danias J, Candia OA. Steroid-induced ocular hypertension in normal sheep. Invest Ophthalmol Vis Sci. 2009; 50: 669–673 [DOI] [PubMed] [Google Scholar]
- 21. Danias J, Gerometta R, Ge Y, et al. Gene expression changes in steroid-induced IOP elevation in bovine trabecular meshwork. Invest Ophthalmol Vis Sci. 2011; 52: 8636–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc Natl Acad Sci U S A. 1999; 96: 14118–14123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dan J, Belyea D, Gertner G, Leshem I, Lusky M, Miskin R. Plasminogen activator inhibitor-1 in the aqueous humor of patients with and without glaucoma. Arch Ophthalmol. 2005; 123: 220–224 [DOI] [PubMed] [Google Scholar]
- 24. Masos T, Dan JA, Miskin R. Plasminogen activator inhibitor-1 mRNA is localized in the ciliary epithelium of the rodent eye. Invest Ophthalmol Vis Sci. 2000; 41: 1006–1011 [PubMed] [Google Scholar]
- 25. Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003; 77: 757–765 [DOI] [PubMed] [Google Scholar]
- 26. Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006; 47: 226–234 [DOI] [PubMed] [Google Scholar]
- 27. Wedrich A, Menapace R, Ries E, Polzer I. Intracameral tissue plasminogen activator to treat severe fibrinous effusion after cataract surgery. J Cataract Refract Surg. 1997; 23: 873–877 [DOI] [PubMed] [Google Scholar]
- 28. Wu TT, Wang HH. Intracameral recombinant tissue plasminogen activator for the treatment of severe fibrin reaction in endophthalmitis. Eye (Lond). 2009; 23: 101–107 [DOI] [PubMed] [Google Scholar]
- 29. Erol N, Ozer A, Topbas S, Yildirim N, Yurdakul S. Treatment of intracameral fibrinous membranes with tissue plasminogen activator. Ophthalmic Surg Lasers Imaging. 2003; 34: 451–456 [PubMed] [Google Scholar]
- 30. Ozveren F, Eltutar K. Therapeutic application of tissue plasminogen activator for fibrin reaction after cataract surgery. J Cataract Refract Surg. 2004; 30: 1727–1731 [DOI] [PubMed] [Google Scholar]
- 31. Kung YH, Wu TT, Hong MC, Sheu SJ. Intravitreal tissue plasminogen activator and pneumatic displacement of submacular hemorrhage. J Ocul Pharmacol Ther. 2010; 26: 469–474 [DOI] [PubMed] [Google Scholar]
- 32. Chen CY, Hooper C, Chiu D, Chamberlain M, Karia N, Heriot WJ. Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina. 2007; 27: 321–328 [DOI] [PubMed] [Google Scholar]
- 33. Holland D, Wiechens B. Intravitreal r-TPA and gas injection in traumatic submacular hemorrhage. Ophthalmologica. 2003; 218: 64–69 [DOI] [PubMed] [Google Scholar]
- 34. Gerometta R, Spiga M-G, Borrás T, Candia OA. Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Invest Ophthalmol Vis Sci. 2010; 51: 3042–3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krohne TU, Liu Z, Holz FG, Meyer CH. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol. 2012; 154: 682–686 e2 [DOI] [PubMed] [Google Scholar]
- 36. Xu L, Lu T, Tuomi L, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013; 54: 1616–1624 [DOI] [PubMed] [Google Scholar]
- 37. Kim MH, Koo TH, Sah WJ, Chung SM. Treatment of total hyphema with relatively low-dose tissue plasminogen activator. Ophthalmic Surg Lasers. 1998; 29: 762–766 [PubMed] [Google Scholar]
- 38. Chen SN, Yang TC, Ho CL, Kuo YH, Yip Y, Chao AN. Retinal toxicity of intravitreal tissue plasminogen activator: case report and literature review. Ophthalmology. 2003; 110: 704–708 [DOI] [PubMed] [Google Scholar]
- 39. Oh H-S, Kwon OW, Chung I, et al. Retinal toxicity of commercial tissue plasminogen activator is mediated by the induction of nitric oxide in the mouse retinal primary cells. Curr Eye Res. 2005; 30: 291–297 [DOI] [PubMed] [Google Scholar]
- 40. Hrach CJ, Johnson MW, Hassan AS, Lei B, Sieving PA, Elner VM. Retinal toxicity of commercial intravitreal tissue plasminogen activator solution in cat eyes. Arch Ophthalmol. 2000; 118: 659–663 [DOI] [PubMed] [Google Scholar]
- 41. Chen J, Wollman Y, Chernichovsky T, Iaina A, Sofer M, Matzkin H. Effect of oral administration of high-dose nitric oxide donor L-arginine in men with organic erectile dysfunction: results of a double-blind, randomized, placebo-controlled study. BJU Int. 1999; 83: 269–273 [DOI] [PubMed] [Google Scholar]
- 42. Wun T-C. Plasminogen activation: biochemistry, physiology, and therapeutics. Crit Rev Biotechnol. 1988; 8: 131–148 [DOI] [PubMed] [Google Scholar]
- 43. Davydov L, Cheng JW. Tenecteplase: a review. Clin Ther. 2001; 23: 982–997 [DOI] [PubMed] [Google Scholar]
- 44. Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006; 281: 2120–2127 [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003; 9: 1313–1317 [DOI] [PubMed] [Google Scholar]
- 46. Lijnen HR. Elements of the fibrinolytic system. Ann N Y Acad Sci. 2001; 936: 226–236 [DOI] [PubMed] [Google Scholar]
- 47. Bachmann B, Birke M, Kook D, Eichhorn M, Lutjen-Drecoll E. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006; 47: 2011–2020 [DOI] [PubMed] [Google Scholar]
- 48. Ning M, Furie KL, Koroshetz WJ, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006; 66: 1550–1555 [DOI] [PubMed] [Google Scholar]
- 49. Tsuji K, Aoki T, Tejima E, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005; 36: 1954–1959 [DOI] [PubMed] [Google Scholar]
- 50. Wang X, Lee S-R, Arai K, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003; 9: 1313–1317 [DOI] [PubMed] [Google Scholar]
- 51. Ning M, Furie K, Koroshetz W, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. 2006; 66: 1550–1555 [DOI] [PubMed] [Google Scholar]
- 52. Tsuji K, Aoki T, Tejima E, et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005; 36: 1954–1959 [DOI] [PubMed] [Google Scholar]
- 53. Vincenti MP, White LA, Schroen DJ, Benbow U, Brinckerhoff CE. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): mechanisms that control enzyme activity, transcription, and mRNA stability. Crit Rev Eukaryot Gene Expr. 1996; 6: 391–411 [DOI] [PubMed] [Google Scholar]
- 54. Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999; 274: 21491–21494 [DOI] [PubMed] [Google Scholar]
- 55. Robertson JV, Siwakoti A, West-Mays JA. Altered expression of transforming growth factor beta 1 and matrix metalloproteinase-9 results in elevated intraocular pressure in mice. Mol Vis. 2013; 19: 684–695 [PMC free article] [PubMed] [Google Scholar]