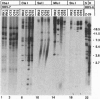
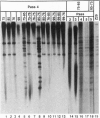
Abstract
Human herpesviruses 6 and 7 (HHV-6 and HHV-7) are prevalent lymphotropic viruses that infect more than 80% of children at infancy or during early childhood. Infection ranges from asymptomatic to severe disease. HHV-6B causes exanthem subitum. The virus can be recovered from peripheral blood mononuclear cells during the acute phase of exanthem subitum, but the host remains latently infected throughout life. In immunocompromised patients undergoing kidney, liver, or bone marrow transplantation latent HHV-6B is reactivated, at times causing severe or fatal disease. Here, we describe the establishment of an in vitro system for reactivation of HHV-6B and HHV-7 from latency. HHV-7 is reactivated from latently infected peripheral blood mononuclear cells by T-cell activation. HHV-6B could not be reactivated under similar conditions; however, the latent HHV-6B could be recovered after the cells were infected with HHV-7. Once reactivated, the HHV-6B genomes became prominent and the HHV-7 disappeared. We conclude that HHV-7 can provide a transacting function(s) mediating HHV-6 reactivating from latency. Understanding the activation process is critical for the development of treatments to control the activation of latent viruses so as to avoid these sometimes life threatening infections in transplant recipients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Balachandran N., Josephs S. F., Hung C. L., Krueger G. R., Kramarsky B., Salahuddin S. Z., Gallo R. C. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991 Oct;184(2):545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- Asano Y., Suga S., Yoshikawa T., Yazaki T., Uchikawa T. Clinical features and viral excretion in an infant with primary human herpesvirus 7 infection. Pediatrics. 1995 Feb;95(2):187–190. [PubMed] [Google Scholar]
- Asano Y., Yoshikawa T., Suga S., Yazaki T., Kondo K., Yamanishi K. Fatal fulminant hepatitis in an infant with human herpesvirus-6 infection. Lancet. 1990 Apr 7;335(8693):862–863. doi: 10.1016/0140-6736(90)90983-c. [DOI] [PubMed] [Google Scholar]
- Aubin J. T., Collandre H., Candotti D., Ingrand D., Rouzioux C., Burgard M., Richard S., Huraux J. M., Agut H. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J Clin Microbiol. 1991 Feb;29(2):367–372. doi: 10.1128/jcm.29.2.367-372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Amelse R. E., Zhou W. W., Chang C. K. Identification of proteins specific for human herpesvirus 6-infected human T cells. J Virol. 1989 Jun;63(6):2835–2840. doi: 10.1128/jvi.63.6.2835-2840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneman Z. N., Ablashi D. V., Li G., Eger-Fletcher M., Reitz M. S., Jr, Hung C. L., Brus I., Komaroff A. L., Gallo R. C. Human herpesvirus 7 is a T-lymphotropic virus and is related to, but significantly different from, human herpesvirus 6 and human cytomegalovirus. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10552–10556. doi: 10.1073/pnas.89.21.10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. B., Inoue N., Kite-Powell K., Zaki S., Pellett P. E. Frequent isolation of human herpesvirus 7 from saliva. Virus Res. 1993 Jul;29(1):91–98. doi: 10.1016/0168-1702(93)90128-a. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Drobyski W. R., Russler S. K., Tapper M. A., Knox K. K., Ash R. C. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991 Jul 20;338(8760):147–149. doi: 10.1016/0140-6736(91)90137-e. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Knox K. K. Human herpesvirus 6 (HHV-6) isolation from bone marrow: HHV-6-associated bone marrow suppression in bone marrow transplant patients. Blood. 1994 Nov 15;84(10):3307–3310. [PubMed] [Google Scholar]
- Cone R. W., Huang M. L., Hackman R. C. Human herpesvirus 6 and pneumonia. Leuk Lymphoma. 1994 Oct;15(3-4):235–241. doi: 10.3109/10428199409049719. [DOI] [PubMed] [Google Scholar]
- Di Luca D., Katsafanas G., Schirmer E. C., Balachandran N., Frenkel N. The replication of viral and cellular DNA in human herpesvirus 6-infected cells. Virology. 1990 Mar;175(1):199–210. doi: 10.1016/0042-6822(90)90200-b. [DOI] [PubMed] [Google Scholar]
- Downing R. G., Sewankambo N., Serwadda D., Honess R., Crawford D., Jarrett R., Griffin B. E. Isolation of human lymphotropic herpesviruses from Uganda. Lancet. 1987 Aug 15;2(8555):390–390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- Drobyski W. R., Knox K. K., Majewski D., Carrigan D. R. Brief report: fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med. 1994 May 12;330(19):1356–1360. doi: 10.1056/NEJM199405123301905. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Katsafanas G. C., Wyatt L. S., Yoshikawa T., Asano Y. Bone marrow transplant recipients harbor the B variant of human herpesvirus 6. Bone Marrow Transplant. 1994 Nov;14(5):839–843. [PubMed] [Google Scholar]
- Frenkel N., Schirmer E. C., Katsafanas G., June C. H. T-cell activation is required for efficient replication of human herpesvirus 6. J Virol. 1990 Sep;64(9):4598–4602. doi: 10.1128/jvi.64.9.4598-4602.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Schirmer E. C., Wyatt L. S., Katsafanas G., Roffman E., Danovich R. M., June C. H. Isolation of a new herpesvirus from human CD4+ T cells. Proc Natl Acad Sci U S A. 1990 Jan;87(2):748–752. doi: 10.1073/pnas.87.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U. A., Carrigan D. R., Carss A. L., Arno J. Two groups of human herpesvirus 6 identified by sequence analyses of laboratory strains and variants from Hodgkin's lymphoma and bone marrow transplant patients. J Gen Virol. 1993 Apr;74(Pt 4):613–622. doi: 10.1099/0022-1317-74-4-613. [DOI] [PubMed] [Google Scholar]
- Hidaka Y., Okada K., Kusuhara K., Miyazaki C., Tokugawa K., Ueda K. Exanthem subitum and human herpesvirus 7 infection. Pediatr Infect Dis J. 1994 Nov;13(11):1010–1011. [PubMed] [Google Scholar]
- Human herpesvirus-6 strain groups: a nomenclature. Arch Virol. 1993;129(1-4):363–366. doi: 10.1007/BF01316913. [DOI] [PubMed] [Google Scholar]
- Irving W. L., Ratnamohan V. M., Hueston L. C., Chapman J. R., Cunningham A. L. Dual antibody rises to cytomegalovirus and human herpesvirus type 6: frequency of occurrence in CMV infections and evidence for genuine reactivity to both viruses. J Infect Dis. 1990 May;161(5):910–916. doi: 10.1093/infdis/161.5.910. [DOI] [PubMed] [Google Scholar]
- Lopez C., Pellett P., Stewart J., Goldsmith C., Sanderlin K., Black J., Warfield D., Feorino P. Characteristics of human herpesvirus-6. J Infect Dis. 1988 Jun;157(6):1271–1273. doi: 10.1093/infdis/157.6.1271. [DOI] [PubMed] [Google Scholar]
- Lusso P., Secchiero P., Crowley R. W., Garzino-Demo A., Berneman Z. N., Gallo R. C. CD4 is a critical component of the receptor for human herpesvirus 7: interference with human immunodeficiency virus. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3872–3876. doi: 10.1073/pnas.91.9.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. J., Littler E., Arrand J. R., Jordan D., Mallick N. P., Johnson R. W. Human herpesvirus 6 infection in renal-transplant recipients. N Engl J Med. 1989 Jun 8;320(23):1560–1561. [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Schirmer E. C., Wyatt L. S., Yamanishi K., Rodriguez W. J., Frenkel N. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5922–5926. doi: 10.1073/pnas.88.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchiero P., Carrigan D. R., Asano Y., Benedetti L., Crowley R. W., Komaroff A. L., Gallo R. C., Lusso P. Detection of human herpesvirus 6 in plasma of children with primary infection and immunosuppressed patients by polymerase chain reaction. J Infect Dis. 1995 Feb;171(2):273–280. doi: 10.1093/infdis/171.2.273. [DOI] [PubMed] [Google Scholar]
- Sutherland S., Christofinis G., O'Grady J., Williams R. A serological investigation of human herpesvirus 6 infections in liver transplant recipients and the detection of cross-reacting antibodies to cytomegalovirus. J Med Virol. 1991 Mar;33(3):172–176. doi: 10.1002/jmv.1890330306. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Kondo T., Torigoe S., Okada S., Mukai T., Yamanishi K. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J Pediatr. 1994 Jul;125(1):1–5. doi: 10.1016/s0022-3476(94)70113-x. [DOI] [PubMed] [Google Scholar]
- Wyatt L. S., Balachandran N., Frenkel N. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J Infect Dis. 1990 Oct;162(4):852–857. doi: 10.1093/infdis/162.4.852. [DOI] [PubMed] [Google Scholar]
- Wyatt L. S., Frenkel N. Human herpesvirus 7 is a constitutive inhabitant of adult human saliva. J Virol. 1992 May;66(5):3206–3209. doi: 10.1128/jvi.66.5.3206-3209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt L. S., Rodriguez W. J., Balachandran N., Frenkel N. Human herpesvirus 7: antigenic properties and prevalence in children and adults. J Virol. 1991 Nov;65(11):6260–6265. doi: 10.1128/jvi.65.11.6260-6265.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K. Human herpesvirus 6. Microbiol Immunol. 1992;36(6):551–561. doi: 10.1111/j.1348-0421.1992.tb02055.x. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Nakashima T., Suga S., Asano Y., Yazaki T., Kimura H., Morishima T., Kondo K., Yamanishi K. Human herpesvirus-6 DNA in cerebrospinal fluid of a child with exanthem subitum and meningoencephalitis. Pediatrics. 1992 May;89(5 Pt 1):888–890. [PubMed] [Google Scholar]