Abstract
Traditionally, bone has been viewed as a relatively static tissue only fulfilling mechanical and scaffolding function. In the past decade however, this classical view of the bone has considerably evolved towards a more complex picture. It is now clear that the skeleton is not only a recipient for hormonal input but it is also an endocrine organ itself. Through the secretion of an osteoblast-derived molecule, osteocalcin, the skeleton regulates glucose homeostasis and male reproductive functions. When undercarboxylated, osteocalcin acts following its binding to a G-coupled receptor, GPRC6A, on pancreatic β cells to increase insulin secretion, on muscle and white adipose tissue to promote glucose homeostasis and on Leydig cells of the testis to favor testosterone biosynthesis. More recently, it was also shown that osteocalcin acts via a pancreas-bone-testis axis that regulates, independently of and in parallel to the hypothalamus-pituitary-testis axis, male reproductive functions by promoting testosterone biosynthesis. Lastly, in trying to expand the biological relevance of osteocalcin from mouse to human, it was shown that GPRC6A is a potential new susceptibility locus for primary testicular failure in humans. Altogether, these results shed new light on the importance of the endocrine role of the skeleton and also provide credence to the search for additional endocrine functions of this organ.
The classical view of the bone physiology
The skeleton is essential for locomotion and is defined primarily by its mechanical and scaffolding properties. This is critical for vertebrates to maintain a constant bone mass with high bone quality and excellent biomechanical properties. This is achieved by the ability of the bone to constantly renew itself through a mechanism called bone remodeling 1,2. Bone remodeling is a biphasic process including the destruction of the preexisting bone (bone resorption mediated by the osteoclasts), followed by a second phase of de novo formation of the bone, (bone formation mediated by the osteoblasts) 2-4. Importantly, these two phases not only occur sequentially but also in a balanced manner to keep a constant bone mass throughout life. A mis-regulation of this balance leads to diseases, the most frequent being osteoporosis, which is caused by an increase of bone resorption in comparison to bone formation 1,2,4-7. The regulation of bone (re)modeling is complex and involves mechanical stimuli, locally produced factors and many hormones. For instance, sex steroid hormones play a crucial role during the bone growth spurts of puberty, and for maintenance of bone mass 3,7-11
The novel dimension to the bone physiology
Bone remodeling occurs throughout life in dozens of location in the skeleton, which is also one of the organs covering the largest surface in our body. Both the cellular events it entails and the surface covered by the skeleton suggest that this physiological process is costly energy-wise. Clinical observations support, fully, this view of bone (re)modeling. Specifically, the absence of food intake, as in anorectic children causes a near-total arrest of growth and low bone mass in adulthood 12-14. Moreover, and unrelated to food intake, it has been known for a long time that the growth and integrity of both the female and the male skeleton are influenced by sex steroid hormones. The biological importance of this regulation is best exemplified by the fact that gonadal failure triggers bone loss in both genders and leads to osteoporosis in post-menopausal women 15,16. Taken together, this view of bone (re)modeling and these clinical observations suggest that there may be a coordinated regulation of bone mass or growth, energy metabolism and reproduction 1,17.
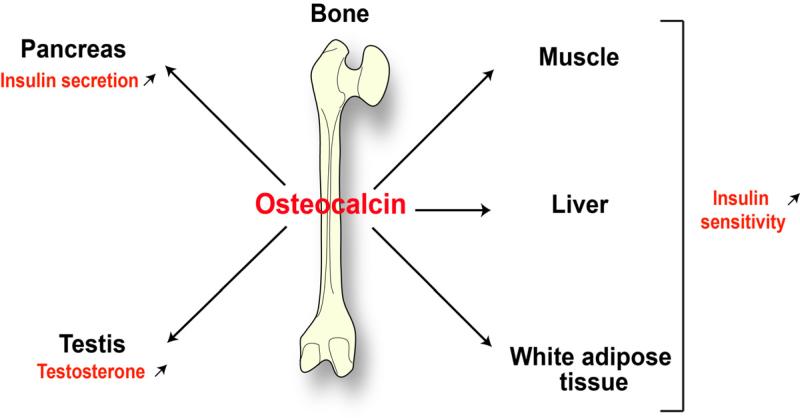
Many genetic-based studies have shown that this hypothesis is true in both rodents and humans. The skeleton secretes at least two hormones. First, fibroblast growth factor 23 (FGF 23) regulating mineral metabolism through its control of the phosphate homeostasis that is intimately linked to bone health 18,19. Second, an osteoblast-specific secreted protein, osteocalcin, when undercarboxylated, acts as a multifunctional hormone. Osteocalcin acts on pancreatic β cells to increase their proliferation and insulin secretion 17,20,21. It also promotes glucose homeostasis by acting in various tissues, such as muscle, liver and fat 17,20,21 (Figure 1).
Figure 1. The skeleton is an endocrine organ.
The endocrine regulation of energy metabolism and male reproduction by the bone is mediated by osteocalcin, an osteoblast-specific secreted molecule. Osteocalcin regulates energy metabolism by increasing insulin secretion, favoring pancreatic-β-cell proliferation, and increasing insulin sensitivity in various tissues. In addition, it promotes male reproductive function by stimulating testosterone synthesis in Leydig cells.
Subsequently, two groups working independently showed that mice lacking the insulin receptor in osteoblasts (InsRosb–/– mice) were glucose intolerant and insulin insensitive when fed on normal chow; that is, they were a phenocopy of the osteocalcin-gene-deficient mice 22-24. Because mice lacking the insulin receptor in skeletal muscle or white adipose tissue do not display glucose intolerance when fed a normal diet 25,26, insulin must act in additional tissues to achieve glucose homeostasis. The fact that bone is such a tissue legitimizes the notion that this tissue is necessary for glucose homeostasis. In addition, InsRosb–/– mice had significantly less biologically active (undercarboxylated) osteocalcin in their sera, revealing that insulin signaling in osteoblasts is a determinant of osteocalcin bioactivity 25,26. In a manner that is both elegant and economical, insulin uses the interplay between osteoblasts and osteoclasts for that purpose. Specifically, insulin inhibits the expression in osteoblasts of the gene encoding osteoprotegerin (Opg) 23, which hampers osteoclast differentiation. In other words, insulin signaling in osteoblasts favors bone resorption, a process that occurs at pH 4.5 27. Acidic pH is the only mechanism known to achieve decarboxylation of proteins 28, therefore, bone resorption decarboxylates and activates osteocalcin 23 (Figure 2). Thus, in a feed forward loop, insulin signaling in osteoblasts promotes its own secretion by activating osteocalcin. Furthermore, mice and humans in which bone resorption is genetically impaired show a decrease in the undercarboxylated form of osteocalcin, resulting in glucose intolerance 23 (Figure 2).
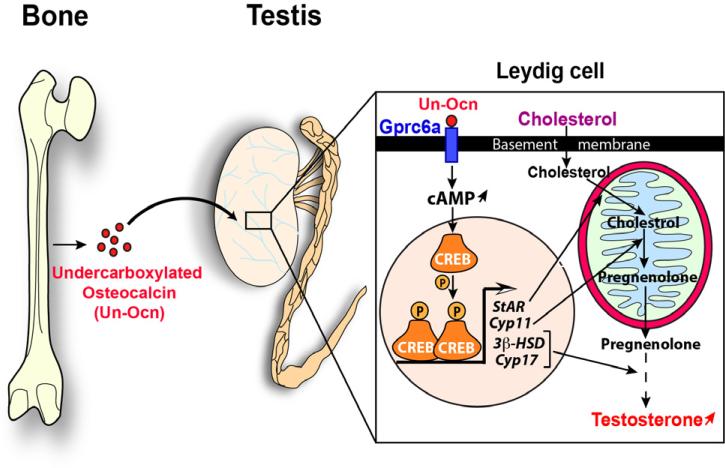
Figure 2. Molecular mode of action of osteocalcin in regulating testosterone production.
Osteocalcin has an unusual mode of activation that relies on the interplay between two specific bone cells: Osteoblasts and Osteoclasts. The osteoblasts produce and secrete an inactive form of this molecule (carboxylated) that is stored in the extracellular bone matrix (ECM). The activity of the osteoclasts create resorption lacunae in the ECM inducing a low PH (4.5) which is necessary and sufficient to bio-activate osteocalcin by promoting its undercarboxylation. The mechanism by which osteocalcin is activated is regulated in osteoblasts by insulin signaling, which inhibits the expression in osteoblasts of the gene encoding osteoprotegerin (Opg), which hampers osteoclast differentiation. Following its binding to Gprc6a expressed in Leydig cells, osteocalcin favors cAMP production that leads to the activation of the transcription factor CREB (cAMP response element binding). CREB activates the expression of several genes encoding the enzymes that are necessary for testosterone biosynthesis, such as StAR, Cyp11a, 3 -HSD and Cyp17. Steroidogenic acute regulatory protein (StAR) is crucial for transport of cholesterol to mitochondria where biosynthesis of steroids is initiated. Cyp11a encodes the cholesterol side-chain cleavage enzyme (P450scc) that catalyzes the first and rate-limiting step, which converts cholesterol to pregnenolone. 3 -HSD and Cyp17 encode two enzymes required during the conversion of pregnenolone to testosterone. Testosterone is a sex steroid hormone require for many aspects of testicular functions, such as germ cell survival and spermatogenesis.
The discovery of the hormonal functions of osteocalcin raised multiple questions with great biological and medical importance. Chief among them was to elucidate the signaling events triggered by this hormone in target cells. A prerequisite to address this question was the identification of a specific receptor for osteocalcin. Because most hormones have several functions, the next question was whether this was the case for osteocalcin.
Osteocalcin favors testosterone production by the Leydig cells of the testis
The well-known regulation of bone remodeling by gonads 5,6,9,10,29 suggested also that bone may in turn, through its endocrine capacity, affect reproductive functions in one or both genders. Verifying this hypothesis would further enhance the emerging concept that bone, energy metabolism and reproduction are coordinately regulated.
To test the validity of this hypothesis, a cell biology approach showed that osteoblasts secrete factor(s) that could markedly increase testosterone production by testis explants and primary Leydig cells 30. The specificity of this function was verified in three ways: First, the supernatant of osteoblast cultures could not enhance sex steroid production in ovary explants. Second, they could not induce estradiol production by Leydig cells. Third, no other mesenchymal cell type shared this ability with osteoblasts 30. This novel role of osteoblasts was verified recently in vivo 31.
The fact that osteocalcin is a bone-derived hormone and that Osteocalcin−/− mice aforementioned bred poorly suggested that ability of osteoblast culture supernatant could be due to osteocalcin. Testing this hypothesis relied on the use of a gain of function model for osteocalcin (Esp−/− mice) and a loss-of-function one (Osteocalcin−/− mice) 21. Osteocalcin-deficient mice showed a decrease in testes, epidydimedes and seminal vesicles weights whereas the weight of these organs was increased in Esp-deficient mice. The spermogram of male osteocalcin-deficient mice showed a 50% decrease in sperm count, while the one of male Esp-deficient mice showed a 30% increase in this parameter. Leydig cell maturation appears to be halted in absence of Osteocalcin 30. These features suggested that osteocalcin might favor testosterone synthesis. Again, this was verified by the simple but powerful co-culture assay, and then in vivo 30. Indeed, circulating testosterone levels are low in Osteocalcin−/− and high in Esp−/− mice. Consistent with the fact that the supernatant of osteoblast cultures do not affect estradiol production, osteocalcin also does not affect the expression of genes encoding the aromatase enzymes, and estrogen levels are within the normal range in Esp−/− and Osteocalcin−/− mice 30. To formally establish that osteocalcin regulates testosterone production as a bone-derived hormone and not as a testis-secreted growth factor, mice lacking Osteocalcin only in osteoblasts were generated. Male Osteocalcinosb −/− mice had the same testosterone production defect as the classical Osteocalcin −/− mice, while deletion of Osteocalcin in Leydig cells did not affect male fertility 30. Taken together these experiments established that osteocalcin is a bone-derived hormone favoring fertility in male mice by promoting Leydig cell maturation and testosterone production (Figure 1 and 3). In other words it verified that for at least one gender there is an endocrine regulator of reproduction by the skeleton. It also illustrates the existence of major differences in the regulation of fertility between male and female mice.
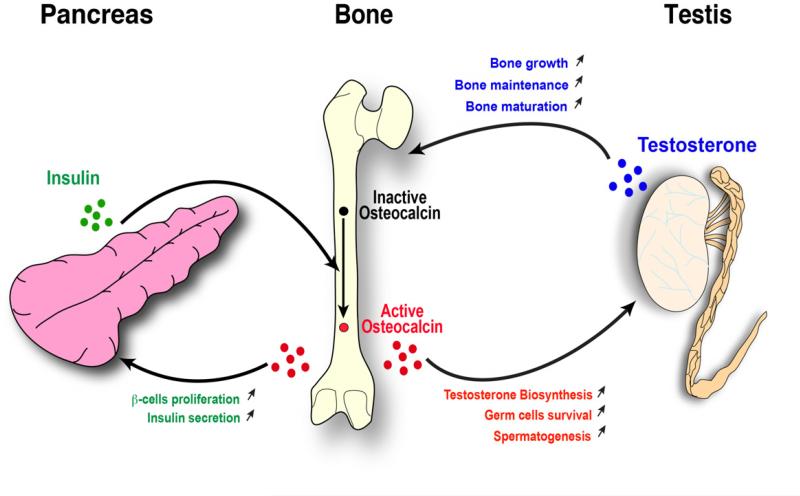
Figure 3. Osteocalcin-stimulated testosterone biosynthesis is positively regulated by insulin signaling in osteoblasts.
Insulin signaling in osteoblasts stimulates the bio-activation of osteocalcin. In a feedback loop control, undercarboxylated active osteocalcin then stimulates insulin secretion by the -cells of the pancreatic islets, promotes insulin sensitivity in peripheral organs and favors testosterone biosynthesis in Leydig cells of the testis. Testosterone in turn favors bone growth, maintenance and maturation.
With hindsight, these observations were both surprising and expected. They were surprising because bone is not classically seen as an endocrine organ much less one regulating reproduction. They were surprising also because of the absence of regulation of fertility in females. On the other hand, they were expected because the feedback rule that applies to most endocrine regulations suggested that given the fact that sex steroid hormones regulate bone mass in both genders, such a feedback regulation might exist. In broader terms, the existence of this function increases the importance of osteocalcin as a hormone.
Osteocalcin reproductive function is mediated by a G-coupled receptor, Gprc6a
In the molecular era, the identification of a novel hormone begs immediately the question of its mechanism of action. A prerequisite to answering this question is to characterize a receptor to which this hormone would bind specifically on its target cells. In the case of osteocalcin, this was achieved through a two-step strategy taking advantage of the fact that osteocalcin regulates fertility in males but not in females 30.
In the first step it was asked what the signal transduction pathway affected by osteocalcin is in two target cells, the β-cell of the pancreas and the Leydig cell of the testis. This approach identified the production of cAMP as the only intracellular signaling event triggered reproducibly by osteocalcin in these two cell types (Figure 2). We interpreted this result as suggesting that the, or an, osteocalcin receptor is probably a G protein coupled receptor (GPCR) linked to adenylate cyclase. Therefore, in the second step of this experimental strategy, taking advantage of the dichotomy of the functions of osteocalcin between males and females, we asked whether there were orphan GPCRs expressed at a higher level (5-fold higher) in testes than in ovaries. Out of more than a hundred orphan GPCRs submitted to this test, twenty two of them were more expressed in testes than in ovaries and only four were expressed predominantly or only in Leydig cells 30. One of these four orphan GPCRs, Gprc6a, was a particularly good candidate to be an osteocalcin receptor, since its inactivation in mice results in metabolic and reproductive phenotypes similar to those seen in Osteocalcin−/− mice 7,30,32-34. Furthermore, and although it was never tested through any binding assays, it has been proposed that Gprc6a was a calcium sensing receptor working better in the presence of osteocalcin 32-34.
Although the aforementioned result could not be reproduced, several criteria formally identified Gprc6a as an osteocalcin receptor present in Leydig cells 7,30. First, there is direct binding of osteocalcin to WT but not to Gprc6a-deficient Leydig cells; second, osteocalcin increases cAMP production in WT but not in Gprc6a-deficient Leydig cells; third, and more poignantly, a Leydig cell-specific deletion of Gprc6a revealed a reproduction phenotype caused by low testosterone production that was similar if not identical to the one seen in the case of osteocalcin inactivation; fourth, in an even more convincing experiment compound heterozygous mice lacking one copy of Osteocalcin and one copy of Gprc6a had a reproduction phenotype identical in all aspects to the one seen in Osteocalcin−/− or Gprc6a−/− mice. The identification of Gprc6a led subsequently to the realization that CREB is a transcriptional effector of osteocalcin regulation of testosterone biosynthesis by favoring the expression of key enzymes of this biosynthetic pathway in Leydig cells 30 (Figure 2). The identification of Gprc6a now allows addressing many more questions; chief among them is that we may be in a position to identify functions of osteocalcin. It allows also to perform a more sophisticated dissection of osteocalcin's molecular mode of action in known and yet to be identified target cells.
Osteocalcin regulates male fertility independently of the hypothalamo-pituitary-axis
The main endocrine pathway regulating male fertility is the hypothalamo-pituitary axis, in which luteinizing hormone (LH), a heterodimer between an α-subunit common to several peptide hormones and a β-subunit specific to LH favors testosterone biosynthesis 35-38. Although less severe, the reproductive phenotype of Osteocalcin−/− and Gprc6a−/− male mice bears resemblance to the one seen in Lhb−/− (LH-deficient) male mice as they are both characterized by a defect in testosterone synthesis and testosterone-dependent events 30,37. Yet, a remarkable feature of the reproduction phenotype observed in Osteocalcin−/− or Gprc6a−/− mice is that it develops in the face of an increase in circulating levels of LH 35,37,39. This situation raised the following question: Does osteocalcin act downstream of LH or does the realization that osteocalcin regulates male fertility reveal the existence of two different pathways, both necessary for male fertility, one pituitary-dependent and one bone-dependent?
Addressing this question, it was shown first that circulating levels of the active form (undercarboxylated) of osteocalcin were not lower in Lhb−/− than in WT male mice and daily injections of osteocalcin for one month in 6 week-old Lhb−/− male mice did not normalize circulating testosterone levels 49. Second, histological analysis of testes of 10 week-old Lhb−/− male mice injected with osteocalcin failed to show any improvement in spermatogenesis, testis size and weight or a reversal of their Leydig cells hypoplasia 49. Taken together these experiments indicate that the regulation of testosterone synthesis by osteocalcin does not depend on a measurable influence of Gprc6a on Lh expression.
Conceivably however, LH could be required for osteocalcin stimulation of testosterone biosynthesis by Leydig cells. Yet, two experimental evidences suggested that this is not the case. First, the positive effect of osteocalcin on testosterone synthesis in Leydig cells was recorded when cells were maintained in serum free medium, i.e., in total absence of LH 49. Second, in cell culture LH does not regulate expression of Osteocalcin or the gene modifying it in osteoblasts. In summary these results support the notion that osteocalcin regulates male fertility independently of the hypothalamo-pituitary-axis, they also failed to provide any evidence that LH regulates Osteocalcin expression 49.
Bone resorption as a determinant of osteocalcin reproductive function in the mouse
Dissociating pituitary-dependent from bone-dependent regulation of male fertility suggests the existence of a second axis regulating this function and raises the question of the identity of upstream regulators of osteocalcin reproductive function. That the ability of osteocalcin to favor glucose homeostasis is determined by osteoclastic bone resorption raised the question of whether male fertility was another physiological function to be added to the credit of bone resorption 23. Testing this hypothesis using loss-of-function and gain-of-function mouse models of bone resorption, it was shown that bone resorption is a physiological determinant of osteocalcin's regulation of testosterone production and male reproductive function through its ability to activate osteocalcin 49. In addition, it was recently shown in humans that osteocalcin and the bone turnover is associated with testosterone circulating levels in the general population and in patients with bone disorders 40,41. The data presented so far support a model in which osteoclast-mediated bone resorption regulates male fertility in mice through the decarboxylation and the activation of osteocalcin. This model combined with previously published observations also implies that bone resorption is required not only for male fertility, but also for the control of energy metabolism 22-24.
The cardinal role of bone resorption in the regulation of testosterone production provided the opportunity to look for these additional upstream regulators of osteocalcin reproductive function. As described previously, insulin signaling in osteoblasts enhances osteocalcin activity, which in turn favors insulin secretion. Consequently, insulin signaling in osteoblasts might influence testosterone biosynthesis in an osteocalcin-dependent manner 49.
This possibility was addressed by analyzing male mice lacking the gene encoding the insulin receptor selectively in osteoblasts (InsRosb−/−) 23. These animals, that have less active osteocalcin, demonstrated a decrease in testes size and weight, both in epidydimides and seminal vesicle weights and in sperm count and circulating testosterone levels. Lastly, this observation was firmly confirmed by generating and testing compound mutant mice lacking one allele of InsR in osteoblasts and one allele of either Osteocalcin or Gprc6a. Here again, testis, epididymides, and seminal vesicle weights, and sperm count and circulating levels of testosterone, in these compound mutant mice demonstrated abnormalities that were similar to those seen in InsRosb−/−, Osteocalcin−/− or even greater relevance, in Gprc6a−/− mice 30,32,49. Taken together, these observations strongly suggested the existence of a pancreas-bone-testis axis in the control of male reproductive functions that acts in parallel to the hypothalamus-pituitary-testis axis 49 (Figure 3).
Conservation of the reproductive function of osteocalcin in human
A second legitimate question that has plagued osteocalcin research since its recognition as a hormone in rodents has been to determine whether it also has an endocrine function in humans. The function of osteocalcin as a regulator of testosterone production has been recently extended to humans. It was shown that osteocalcin and the bone turnover is associated with testosterone circulating levels in general population and in patients with bone disorders 40. Moreover, Dr. Khosla's group has also shown that there is a significant association between serum osteocalcin and testosterone levels during mid-puberty in males 41. They postulate that this axis may be most relevant during rapid skeletal growth in adolescent human males to help maximize bone size. However, while there is a growing body of evidence that osteocalcin serum levels are a reliable indicator of the degree of insulin secretion, insulin sensitivity and circulating serum testosterone levels in humans 42-44, until recently, there was no genetic evidence establishing that osteocalcin fulfills its endocrine functions in humans.
The identification of an osteocalcin receptor and the realization that osteocalcin influences male fertility, provided an opportunity to tackle this issue. In fact, the fertility phenotype of the Osteocalcin−/− mice, mainly characterized by a subfertility, mediocre spermogram, low circulating testosterone levels and high circulating LH levels 30, is the exact phenocopy of a rare but well defined syndrome in human called peripheral testicular insufficiency 45-48. Thus, a systematic genomic analysis of Osteocalcin and GPRC6A loci of a cohort of 59 patients with this syndrome was initiated with the goal to identify loss-of-function mutation in Osteocalcin or its receptor that would explain their clinical presentation 49.
As a result of this genomic analysis, two patients in this cohort harbored a point mutation T>A transversion in exon 4 of GPRC6A 49. This missense mutation results in an amino acid substitution (F464Y) in a highly conserved region of one of the transmembrane domain of GPRC6A and prevents its localization to the cell membrane, therefore resulting in a loss of function of this receptor 49. Furthermore, three different cell-based assays indicated that this mutation also acts in a dominant negative manner in cells 49. Lastly, it was noted that both patients harboring this substitution-mutation in GPRC6A originated form the same region of the globe, shared a history of glucose intolerance and displayed similar defects in reproductive hormones. Indeed, these patients presented a metabolic syndrome characterized by an increase in body mass index, as well as a glucose intolerance determined by hyperinsulinemia after fasting, a glucose tolerance test, and an insulin tolerance test 49. Many of these features are seen in mice lacking Osteocalcin or Gprc6a in all cells 7,30,32-34,47. It is thus interesting that the F464Y variant was not observed in 1,000 controls. Careful phenotypic analysis of individuals carrying the F464Y allele may clarify the spectrum of associated metabolic, cardiovascular and reproductive defects. Taken together these results indicate the importance of osteocalcin signaling in human and suggest that GPRC6A may be a new susceptibility locus for primary testicular failure in humans, a disease whose cause is often un-indentified. Results of this initial foray in the genetic analysis of osteocalcin functions in humans should be viewed as a stepping-stone to perform a more systematic analysis in a larger patient population with primary testicular failure as well as in patients with glucose intolerance or metabolic syndromes.
The known functions of the skeleton indicate that skeleton physiology affects many more organs and functions than just skeleton itself, as it affects glucose homeostasis, energy expenditure and fertility. These novel functions of bone underscore the notion that the skeleton is an important member of the endocrine network affecting multiple functions in the body. Moreover, they also raise the strong possibility that other endocrine functions of the skeleton have yet to be described.
Highlights: “Regulation of male fertility by the bone-derived hormone osteocalcin”.
- A novel dimension to the bone physiology: the skeleton secretes two hormones
- Osteocalcin, a bone-derived hormone, favors testosterone production by the Leydig cells of the testis
- Osteocalcin regulates male fertility independently of the hypothalamo-pituitary-axis
- Bone resorption as a determinant of osteocalcin reproductive function in the mouse
- Conservation of the reproductive function of osteocalcin in human
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mas. Cell metabolism. 2006;4:341–348. doi: 10.1016/j.cmet.2006.10.008. doi:10.1016/j.cmet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 3.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. doi:10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinology and metabolism clinics of North America. 2005;3:1015–1030. xi. doi: 10.1016/j.ecl.2005.07.009. doi:10.1016/j.ecl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. doi:10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 7.Oury F. A crosstalk between bone and gonads. Annals of the New York Academy of Sciences. 2012;1260:1–7. doi: 10.1111/j.1749-6632.2011.06360.x. doi:10.1111/j.l749-6632.2011.06360.x. [DOI] [PubMed] [Google Scholar]
- 8.Khosla S, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. The Journal of clinical endocrinology and metabolism. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 9.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocrine reviews. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 10.Vanderschueren D, et al. Androgens and bone. Endocrine reviews. 2004;25:389–425. doi: 10.1210/er.2003-0003. doi:10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 11.Venken K, et al. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21:576–585. doi: 10.1359/jbmr.060103. doi:10.1359/jbmr.060103. [DOI] [PubMed] [Google Scholar]
- 12.Legroux-Gerot I, Vignau J, Collier F, Cortet B. Bone loss associated with anorexia nervosa. Joint, bone, spine: revue du rhumatisme. 2005;72:489–495. doi: 10.1016/j.jbspin.2004.07.011. doi:10.1016/j.jbspin.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Misra M, Klibanski A. Bone health in anorexia nervosa. Current opinion in endocrinology, diabetes, and obesity. 2011;18:376–382. doi: 10.1097/MED.0b013e32834b4bdc. doi:10.1097/MED.0b013e32834b4bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra M, Klibanski A. Bone metabolism in adolescents with anorexia nervosa. Journal of endocrinological investigation. 2011;34:324–332. doi: 10.3275/7505. doi:10.3275/7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riggs BL, Khosla S, Melton LJ., 3rd A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- 16.Riggs BL, Melton LJ., 3rd Involutional osteoporosis. N Engl J Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- 17.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. doi:10.1038/naturel0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori M, Shimizu Y, Fukumoto S. Minireview: fibroblast growth factor 23 in phosphate homeostasis and bone metabolism. Endocrinology. 2011;152:4–10. doi: 10.1210/en.2010-0800. doi:10.1210/en.2010-0800. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu Y, Fukumoto S, Fujita T. Evaluation of a new automated chemiluminescence immunoassay for FGF23. Journal of bone and mineral metabolism. 2012;30:217–221. doi: 10.1007/s00774-011-0306-4. doi:10.1007/s00774-011-0306-4. [DOI] [PubMed] [Google Scholar]
- 20.Karsenty G, Oury F. Biology without walls: the novel endocrinology of bone. Annual review of physiology. 2012;74:87–105. doi: 10.1146/annurev-physiol-020911-153233. doi:10.1146/annurev-physiol-020911-153233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. doi:10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–575. doi: 10.1016/j.bone.2011.04.017. doi:10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferron M, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. doi:10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulzele K, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. doi:10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bluher M, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Developmental cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 26.Bruning JC, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Molecular cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 27.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Experimental cell research. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- 28.Poser JW, Price PA. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. The Journal of biological chemistry. 1979;254:431–436. [PubMed] [Google Scholar]
- 29.Kousteni S, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. doi:10.1126/science.l074935. [DOI] [PubMed] [Google Scholar]
- 30.Oury F, et al. Endocrine Regulation of Male Fertility by the Skeleton. Cell. 2011;144:796–809. doi: 10.1016/j.cell.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshikawa Y, et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:2012–2025. doi: 10.1002/jbmr.417. doi:10.1002/jbmr.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pi M, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PloS one. 2008;3:e3858. doi: 10.1371/journal.pone.0003858. doi:10.1371/journal.pone.0003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pi M, Quarles LD. Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology. 2012;153:2062–2069. doi: 10.1210/en.2011-2117. doi:10.1210/en.2011-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. Journal of bone and mineral research ia the official journal of the American Society for Bone and Mineral Research. 2011;26:1680–1683. doi: 10.1002/jbmr.390. doi:10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar TR. Functional analysis of LHbeta knockout mice. Molecular and cellular endocrinology. 2007;269:81–84. doi: 10.1016/j.mce.2006.10.020. doi:10.1016/j.mce.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Bousfield GR, Ward DN. Evidence for two folding domains in glycoprotein hormone alpha-subunits. Endocrinology. 1994;135:624–635. doi: 10.1210/endo.135.2.7518386. [DOI] [PubMed] [Google Scholar]
- 37.Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17294–17299. doi: 10.1073/pnas.0404743101. doi:10.1073/pnas.0404743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annual review of biochemistry. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. doi:10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 39.Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocrine reviews. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- 40.Hannemann A, et al. Osteocalcin is associated with testosterone in the general population and selected patients with bone disorders. Andrology. 2013;1:469–474. doi: 10.1111/j.2047-2927.2012.00044.x. doi:10.1111/j.2047-2927.2012.00044.x. [DOI] [PubMed] [Google Scholar]
- 41.Kirmani S, et al. Relationship of testosterone and osteocalcin levels during growth. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:2212–2216. doi: 10.1002/jbmr.421. doi:10.1002/jbmr.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez-Real JM, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. The Journal of clinical endocrinology and metabolism. 2009;94:237–245. doi: 10.1210/jc.2008-0270. doi:10.1210/jc.2008-0270. [DOI] [PubMed] [Google Scholar]
- 43.Im JA, Yu BP, Jeon JY, Kim SH. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clinica chimica acta; international journal of clinical chemistry. 2008;396:66–69. doi: 10.1016/j.cca.2008.07.001. doi:10.1016/j.cca.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Saleem U, Mosley TH, Jr., Kullo IJ. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1474–1478. doi: 10.1161/ATVBAHA.110.204859. doi:10.1161/ATVBAHA.110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burns KH, Matzuk MM. Minireview: genetic models for the study of gonadotropin actions. Endocrinology. 2002;143:2823–2835. doi: 10.1210/endo.143.8.8928. [DOI] [PubMed] [Google Scholar]
- 46.Glass AR, Vigersky RA. Testicular reserve of testosterone precursors in primary testicular failure. Fertility and sterility. 1982;38:92–96. doi: 10.1016/s0015-0282(16)46401-0. [DOI] [PubMed] [Google Scholar]
- 47.Paduch DA. Testicular cancer and male infertility. Current opinion in urology. 2006;16:419–427. doi: 10.1097/01.mou.0000250282.37366.d2. doi: 10.1097/01.mou.0000250282.37366.d2. [DOI] [PubMed] [Google Scholar]
- 48.Winters SJ, Troen P. A reexamination of pulsatile luteinizing hormone secretion in primary testicular failure. The Journal of clinical endocrinology and metabolism. 1983;57:432–435. doi: 10.1210/jcem-57-2-432. [DOI] [PubMed] [Google Scholar]