Abstract
Purpose
To investigate the effect of a yearlong moderate-intensity aerobic exercise intervention on C-reactive protein (CRP), serum amyloid A (SAA), and interleukin 6 (IL-6) among overweight or obese postmenopausal women.
Methods
In a randomized controlled trial, 115 postmenopausal, overweight or obese, sedentary women, aged 50-75 years were randomized to an aerobic exercise intervention of moderate-intensity (60-75% observed maximal heart rate), for ≥45 min/day, 5 days/week (n=53), or to a 1 day/week stretching control (n=62), on an intent-to-treat basis. CRP, SAA, and IL-6 were measured at baseline, 3-months, and 12-months.
Results
From baseline to 12-months, CRP decreased 10% in exercisers and increased 12% in controls (p=0.01); no effects were observed for SAA and IL-6. Among participants at baseline who were obese (BMI≥30kg/m2) or had abdominal obesity (waist circumference (WC)≥88cm), exercise resulted in a more pronounced reduction in CRP (BMI≥30kg/m2: p=0.002; WC≥88cm: p<0.0001), borderline for SAA (BMI≥30kg/m2: p=0.08; WC≥88cm: p=0.04); no intervention effects were observed among women who did not have these characteristics. Overall, weight loss was minimal in the exercise intervention (~1.8kg). Linear trends were observed between CRP and 12-month changes in: aerobic fitness (ptrend = 0.006), exercise adherence (ptrend = 0.004), percentage body fat (ptrend = 0.002), body weight (ptrend = 0.002), waist circumference (ptrend = 0.02), and intra-abdominal fat (ptrend = 0.03).
Conclusion
A moderate-intensity exercise intervention reduced CRP over 12-months among women who were obese at baseline. These findings support the role of exercise in modulating inflammatory processes that are related to increased risk of chronic disease among obese women.
Keywords: overweight, inflammation, C-reactive protein, physical activity, randomized controlled trial, serum amyloid A
INTRODUCTION
Paragraph Number 1
C-reactive protein (CRP), serum amyloid A (SAA), and interleukin 6 (IL-6) are non-specific markers of chronic low-grade inflammation that correlate with adiposity, sedentary lifestyle, and low aerobic fitness (5,9,16,20,23). Observational studies have suggested that a reduction in chronic inflammation, often achieved through lifestyle modification, is associated with reduced risk of disease and mortality (1,22,25).
Paragraph Number 2
Randomized trials of combined exercise and dietary interventions have been shown to reduce CRP and IL-6 (3,7,14,26), while we are unaware of comparable studies that have examined the combined effects of exercise and dietary intervention on SAA. The effect of exercise alone on inflammatory markers is not clear (2,8,10,12,13,17,21), with a recent meta-analysis suggesting a non-significant 3% decrease in CRP from five studies with aerobic exercise interventions of eight weeks or longer (13). It is important to determine the independent effect of exercise on chronic inflammation in order to better define the public health role of physical activity, in the absence of weight loss or diet intervention, for primary disease prevention
Paragraph Number 3
With inconsistent evidence from observational studies and intervention trials, the role of exercise on reducing chronic inflammation remains unclear. Similarly, the potential for a mediating effect by weight loss is not well understood. In a randomized controlled trial of 115 previously sedentary postmenopausal, overweight or obese women, with high retention, we examined the effect of a one-year aerobic exercise intervention compared to a stretching control on CRP, SAA, and IL-6. We hypothesized that exercise intervention would result in decreased levels of inflammation, and this effect would be partly mediated by fat loss.
METHODS and PROCEDURES
Setting and Participants
Paragraph Number 4
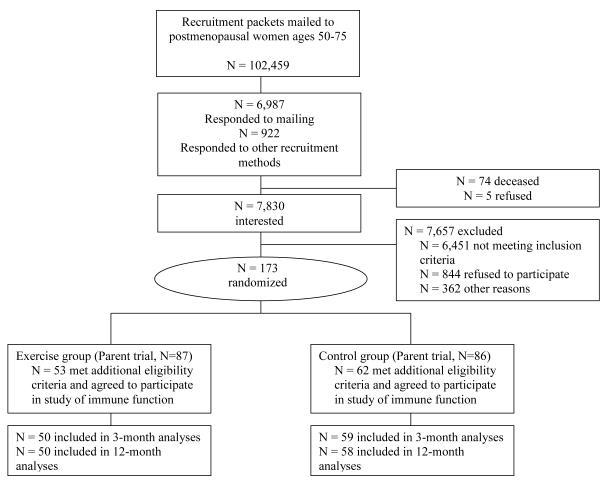
Participants (n = 115) were a subset of women (n = 173) recruited for an exercise intervention trial who met additional eligibility criteria for this study of immune function (4) and inflammation. For this study, 53 exercisers and 62 controls were eligible from the parent trial (Figure 1). Eligibility criteria included: age 50 – 75 years; body mass index (BMI) between 25 and 40 kg/m2 (or BMI 24.0–24.9 if body fat >33%); postmenopausal; not taking postmenopausal hormones; non-smoker; sedentary at baseline (<60 min/wk of moderate- and vigorous-intensity recreational activity and VO2max <25.0 ml·kg·min−1); alcohol consumption of fewer than two drinks per day; no personal history of invasive cancer, diabetes, cardiovascular disease, or asthma; no current serious allergies; no regular (≥two times/week) use of aspirin or other non-steroidal anti-inflammatory medications; and no use of corticosteroids or other medications known to affect immune function. Women were recruited through a combination of mass mailings and media placements, as described previously (24). Randomization was stratified by BMI (<27.5 or ≥27.5 kg/m2) to ensure equal numbers of heavier and lighter women in each study group. Approximately equal numbers of exercisers and controls were enrolled during each month of recruitment. All women provided written informed consent and all procedures were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board.
Figure 1.
Flow of participants through the trial.
Randomization and Exercise Intervention
Paragraph Number 5
The exercise intervention was designed to progress to at least 45 minutes of moderate-intensity exercise at a target heart rate of 60-75% observed maximal heart rate, 5 days per week, by the 8th week of the trial. Participants attended 3 supervised sessions per week at a study facility and exercised 2 days per week at home during months 1-3. During months 4-12, participants attended at least 1 session per week at a study facility and exercised 4 days per week either at home or at a facility. Participants wore Polar heart rate monitors during all exercise sessions.
Paragraph Number 6
VO2max was directly assessed at baseline (prior to randomization) and at 12-months in all participants by maximal-graded treadmill test. Exercise intervention participants kept daily activity logs of all sport and recreational activities of moderate-to-vigorous intensity (estimated to be ≥3 METS). For each exercise session, participants recorded the mode and duration of exercise, peak heart rate, and rating of perceived exertion. Activity logs were reviewed weekly by study staff to monitor compliance and to intervene when needed. Women randomized to the control group attended once-weekly 45-minute stretching sessions and were asked to not otherwise change exercise habits during the study. All participants were asked to maintain their usual diet.
Outcomes
Paragraph Number 7
At baseline (prior to randomization), 3-months, and 12-months, all women came to the University of Washington (UW) Medical Center for blood draws between 7.30-8.30am, after a 12h fast, under the following blood-draw criteria: no infection or symptoms of any infection for ≥7 days; adequate sleep (6-9h); no exercise or alcohol for 24h; no topical corticosteroids or aspirin for 48h; no systemic antihistamines or corticosteroids for one week; and no immunizations during the previous three weeks. All participants were contacted 1 week after blood draw to track recent illness; several reported being ill shortly after their blood draw and returned for a second blood draw when symptom free. Serum and plasma were collected and stored at −70°C. All inflammation marker assays were conducted at the UW Clinical Immunology Laboratory (MHW); participant samples from each of the three time-points were assayed in the same batch to avoid biased measurement from inter-assay variability.
C-reactive protein (CRP) and Serum amyloid A (SAA)
Paragraph Number 8
CRP and SAA were measured in serum by latex-enhanced nephelometry using high sensitivity assays on the Behring Nephelometer II analyzer (Dade-Behring Diagnostics, Deerfield, IL) with lower detection limits of 0.2 mg/L for CRP and 0.7 mg/L for SAA. Inter-assay coefficients of variation were 5-9% for CRP and 4-8% for SAA.
Interleukin 6 (IL-6)
Paragraph Number 9
IL-6 was assayed in duplicate in serum by an ultrasensitive solid-phase sandwich Enzyme Linked Immuno Sorbent Assay (ELISA) with the Biosource Human IL-6 Immunoassay kit (Biosource, Camarillo, CA).
Body composition and distribution
Paragraph Number 10
At baseline, 3-months, and 12-months, body weight to the nearest 0.1kg and height to the nearest 0.1cm were taken in duplicate and the average was used to compute BMI (kg/m2). At baseline and 12-months, waist circumference (WC) was measured to the nearest 0.1 cm in duplicate and averaged. Also at baseline and 12-months, total body fat and percentage body fat were analyzed by DXA (QDR 1500; Hologic, Waltham, MA); and intra-abdominal fat images were obtained via a 1-slice computed tomography at L4-5 (CT; model CT 9800 scanner; General Electric, Waukesha, WI), with coefficients of variation for both of 1.2%.
Other study measures
Paragraph Number 11
At baseline, 3-months, and 12-months, data were collected on demographic information, medical history, health habits, medication use, reproductive and bodyweight history, total energy intake (via 120-item self-administered food frequency questionnaire), and frequency, duration, and intensity of physical activity (via self-administered Minnesota Physical Activity Questionnaire).
Statistical Analyses
Paragraph Number 12
Intervention effects were assessed on the inflammation related outcomes at 3-months and 12-months post-randomization, based on comparisons between exercisers and controls as defined at randomization (i.e. intent-to-treat). Changes between groups were assessed with generalized estimating equations (GEE), which account for repeated observations on the same subjects over time. Baseline measures were included in each GEE model. In secondary analyses, we examined subgroup effects according to baseline values for BMI (above or below 30kg/m2) and waist circumference (above or below 88cm). Additional subgroup analyses were conducted for changes at 12-months in aerobic fitness (controls versus exercisers who increased VO2max by: <5%, 5-15% or >15%), exercise adherence (controls versus exercisers who performed physical activity an average of: <136 min/wk, 136-195 min/wk, or >195 min/wk), and 12-month changes in percentage body fat (controls versus exercisers who had: minimal percent fat loss (<0.1%) or gained percent body fat, lost between 0.1% and ≤ 2% body fat, or lost >2% body fat), body weight (controls versus exercisers who had: minimal weight loss (<0.5 kg) or gained body weight, lost between 0.5 and ≤ 3 kg body weight, or lost > 3 kg of body weight), waist circumference (controls versus exercisers who had: minimal reduction in WC (<0.5 cm) or gained WC, lost between 0.5 cm and ≤ 3 cm of WC, or reduced WC by > 3 cm) and intra-abdominal fat (controls versus exercisers who had: gained intra-abdominal fat or had minimal reduction in intra-abdominal fat (<2 cm2), decreased intra-abdominal fat by 2 to 8 cm2, or had decreased intra-abdominal fat by greater than 8 cm2). CRP, SAA, and IL-6 were non-normally distributed and were log-transformed for all analyses. Missing data were omitted from GEE analyses. All statistical tests were 2-sided. All analyses were performed with SAS software (version 9.1, SAS Institute, Cary, North Carolina, USA).
RESULTS
Paragraph Number 13
115 women were randomized, 53 to intervention and 62 to control. One exerciser did not have data for CRP and SAA at baseline and was excluded. Two participants (one exerciser, one control) were excluded from analyses because of unusually high CRP and SAA concentrations at one time point in the study (i.e. about 10-fold greater than their values at other time points in the study, suggesting an unreported or unknown acute inflammatory event). Of the 112 remaining participants at baseline (exercisers, n = 51; controls, n =61), 106 (95%) provided blood samples at 3-months (exercisers, n = 48; controls, n =58), and 104 (93%) provided blood samples at 12-months (exercisers, n = 47; controls, n = 57) (see Figure 1).
Paragraph Number 14
At baseline there were no significant differences between exercisers and controls (Table 1). Women were, on average, 60 years of age and most described themselves as non-Hispanic white. In line with the eligibility criteria, participants had an average BMI of 30 kg/m2, 47% body fat, and low aerobic fitness. Over 12-months, the intervention group participated in moderate activity an average of 3.8 days per week (SD: 1.3) for 166 minutes per week (SD: 76.1). At the end of study, exercisers increased aerobic fitness (VO2max) by 13.8% whereas controls experienced a 0.1% increase (between-group p-value at 12-months: <0.0001). Similarly, the exercise intervention resulted in decreased body weight versus control (exercise: −1.8kg; control: +0.3kg; p-value: 0.002), and decreased percentage body fat (exercise: −1.5%; control: +0.02%, p-value: <0.0001). There were no differences between or within intervention groups for total caloric intake throughout the trial (baseline values are shown in Table 1; 12-month values: exercisers 1618±621 kcal; controls 1684±720 kcal, all p-values ≥ 0.47).
Table 1.
Baseline characteristics of exercisers and stretchers.
| Exercisers (N=53) |
Controls (N=62) |
p-value | |
|---|---|---|---|
| mean ± SD or n | |||
| Age | 60.5 ± 7.0 | 60.9 ± 6.8 | 0.76 |
| Race/ ethnicity | |||
| American Indian | 0 | 1 | |
| African American | 1 | 1 | |
| Asian/ Pacific Islander | 3 | 2 | |
| Hispanic/ Latino | 0 | 2 | |
| Non-Hispanic white | 46 | 55 | |
| Other/ left blank | 3 | 1 | 0.50 |
| BMI (kg/m2) | 30.2 ± 4.0 | 30.4 ± 3.8 | 0.82 |
| <30 (n per subgroup) | 29 | 34 | |
| ≥30 (n per subgroup) | 24 | 28 | 0.99 |
| Waist circumference (cm) | 91.4 ± 11.2 | 93.0 ± 10.4 | 0.44 |
| <88 (n per subgroup) | 26 | 24 | |
| ≥88 (n per subgroup) | 27 | 38 | 0.26 |
| Intra-abdominal fat (cm2) | 132 ± 52 | 143 ± 50 | 0.29 |
| Body fat (%) | 47.2 ± 5.0 | 47.0 ± 4.4 | 0.88 |
|
Average calorie intake
(kcal/d) |
1581 ± 666 | 1734 ± 687 | 0.23 |
| VO2max (ml·kg·min−1) | 20.7 ± 3.7 | 20.6 ± 3.1 | 0.88 |
| Statin use | |||
| Yes | 5 | 2 | |
| No | 48 | 60 | 0.25 |
Main intervention effects
Paragraph Number 15
Exercisers statistically significantly decreased CRP relative to controls at 12-months (Table 2). SAA decreased among exercisers and increased slightly among controls, although the between-group difference was not statistically significant. IL-6 was not affected by the exercise intervention. No intervention effects were noted at 3-months. Results were similar when statin-users (n=7) were excluded.
Table 2.
Effect of exercise compared to stretching control on markers of inflammation: 3-month and 12-month comparisons.
|
Baseline
Geometric Mean |
3-months
Geometric Mean |
12-months
Geometric Mean |
||||||
|---|---|---|---|---|---|---|---|---|
| n | (95% CI) | n | (95% CI) | p-value ^ | n | (95% CI) | p-value # | |
| CRP (mg/L) | ||||||||
| Exercisers | 51 | 2.39 (1.85, 3.10) | 48 | 2.38 (1.87, 3.05) | 0.94 | 47 | 2.15 (1.66, 2.78) | 0.01 |
| Controls | 61 | 2.36 (1.88, 2.97) | 58 | 2.40 (1.90, 3.04) | 57 | 2.65 (2.09, 3.36) | ||
| SAA (mg/L) | ||||||||
| Exercisers | 51 | 4.87 (4.04, 5.87) | 48 | 4.70 (3.94, 5.60) | 0.96 | 47 | 4.57 (3.81, 5.48) | 0.57 |
| Controls | 61 | 5.11 (4.43, 5.90) | 58 | 4.88 (4.16, 5.72) | 57 | 5.29 (4.55, 6.16) | ||
| IL-6 (ng/L) | ||||||||
| Exercisers | 51 | 2.36 (1.82, 3.05) | 48 | 2.43 (1.89, 3.13) | 0.79 | 47 | 2.66 (2.02, 3.52) | 0.45 |
| Controls | 61 | 2.26 (1.80, 2.83) | 58 | 2.26 (1.80, 2.85) | 57 | 2.36 (1.88, 2.96) |
P-values indicate biomarker difference at 3-months comparing exerciser intervention to control, controlling for baseline values.
P-values indicate biomarker difference at 12-months comparing exerciser intervention to control, controlling for baseline values.
Stratified Results: Baseline BMI and waist circumference
Paragraph Number 16
The effect of exercise intervention on CRP was restricted to women who were obese at baseline (Table 3). Obese women (BMI ≥30 kg/m2) in the exercise group experienced a statistically significant and continuous decline in CRP over the 12-month trial (baseline CRP: 3.95 mg/L (95% CI: 2.87, 5.44); 3-month CRP: 3.65 mg/L (95% CI: 2.6-5.14); 12-month CRP: 3.16 mg/L (95% CI: 2.38, 4.2); whereas obese women in the control group experienced a moderate increase in CRP values over the same period (p-value for between group difference at 12-months: 0.002). Similar trends were noted for women with abdominal obesity (p-value: < 0.0001). BMI and WC at baseline were highly correlated (r = 0.82; p-value: < 0.0001) and both anthropometric measures were identically correlated with baseline CRP (both correlations = 0.42; and p-values < 0.0001), which explains these similar trends. Similar trends across BMI and WC were observed for SAA, although the effects only reached borderline statistical significance for the obese BMI group (p-value: 0.08).
Table 3.
Effect of a yearlong exercise intervention compared to a stretching control on markers of inflammation, stratified by baseline body mass index (BMI) and waist circumference (WC).
| Exercisers | Controls | p-value # | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n |
Baseline
Geometric Mean (95% CI) |
12 month
Geometric Mean (95% CI) |
n |
Baseline
Geometric Mean (95% CI) |
n |
12 month
Geometric Mean (95% CI) |
|||
| CRP (mg/L) | |||||||||
| BMI < 30 kg/m2 | 27 | 1.53 (1.12, 2.09) | 25 | 1.53 (1.06, 2.21) | 33 | 1.88 (1.40, 2.53) | 29 | 1.99 (1.45, 2.72) | 0.39 |
| BMI ≥ 30 kg/m2 | 24 | 3.95 (2.87, 5.44) | 22 | 3.16 (2.38, 4.20) | 28 | 3.09 (2.21, 4.32) | 28 | 3.57 (2.58, 4.94) | 0.002 |
| WC < 88 cm | 24 | 1.62 (1.16-2.26) | 22 | 1.73 (1.15, 2.60) | 23 | 1.50 (1.11, 2.03) | 22 | 1.65 (1.17, 2.34) | 0.93 |
| WC ≥ 88 cm | 27 | 3.38 (2.40-4.76) | 25 | 2.60 (1.91, 3.56) | 38 | 3.11 (2.33, 4.14) | 35 | 3.57 (2.69, 4.72) | <0.0001 |
| SAA (mg/L) | |||||||||
| BMI < 30 kg/m2 | 27 | 3.81 (3.14, 4.62) | 25 | 4.15 (3.26, 5.28) | 33 | 4.72 (3.95, 5.63) | 29 | 4.64 (3.82, 5.65) | 0.22 |
| BMI ≥ 30 kg/m2 | 24 | 6.43 (4.76, 8.68) | 22 | 5.09 (3.88, 6.69) | 28 | 5.62 (4.47, 7.07) | 28 | 6.05 (4.83, 7.58) | 0.08 |
| WC < 88 cm | 24 | 3.72 (3.02, 4.57) | 22 | 4.09 (3.07, 5.45) | 23 | 4.12 (3.38, 5.02) | 22 | 4.17 (3.33, 5.22) | 0.15 |
| WC ≥ 88 cm | 27 | 6.19 (4.70, 8.16) | 25 | 5.03 (4.01, 6.32) | 38 | 5.82 (4.84, 7.01) | 35 | 6.15 (5.09, 7.42) | 0.04 |
| IL-6 (ng/L) | |||||||||
| BMI < 30 kg/m2 | 27 | 2.02 (1.40, 2.92) | 25 | 2.49 (1.64, 3.77) | 33 | 2.15 (1.57, 2.95) | 29 | 2.03 (1.47, 2.81) | 0.12 |
| BMI ≥ 30 kg/m2 | 24 | 2.80 (1.97, 4.00) | 22 | 2.88 (2.00, 4.16) | 28 | 2.40 (1.73, 3.32) | 28 | 2.76 (2.02, 3.76) | 0.76 |
| WC < 88 cm | 24 | 2.17 (1.62, 2.90) | 22 | 2.60 (1.77, 3.81) | 23 | 1.84 (1.30, 2.61) | 22 | 1.69 (1.17, 2.45) | 0.13 |
| WC ≥ 88 cm | 27 | 2.54 (1.68, 3.85) | 25 | 2.72 (1.81, 4.10) | 38 | 2.56 (1.91, 3.42) | 35 | 2.91 (2.23, 3.80) | 0.75 |
P-value for difference in inflammation marker at 12-months in exercise intervention versus control within BMI/waist circumference strata, controlling for baseline. Note: 3-month data not shown for brevity (all p-values ≥ 0.38)
Intervention effects on CRP stratified by exercise adherence and fat loss
Paragraph Number 17
The exercise intervention group was stratified into subgroups based on: 12-month changes in aerobic fitness (percentage change in VO2max); average adherence to the exercise intervention (physical activity minutes per week); and 12-month changes in percentage body fat, body weight, waist circumference and intra-abdominal fat. As described above, among women in the control group CRP concentrations increased over the 12-month trial. Linear trends were observed between CRP and 12-month changes in VO2max (p-value: 0.006), exercise adherence (p-value: 0.004), body fat loss (p-value: 0.001), weight loss (p-value: 0.002), waist circumference loss (p-value: 0.02), and intra-abdominal fat loss (p-value: 0.03) (Table 4). No sub-group effects were noted for SAA or IL-6 (data not shown).
Table 4.
Effect of exercise on CRP from baseline to 12-months: controls versus exercisers stratified by changes in aerobic fitness, exercise adherence, adiposity, and adipose tissue distribution.
| CRP (mg/L) | ||||||
|---|---|---|---|---|---|---|
|
Baseline
Geometric Mean |
12 month Geometric Mean |
|||||
| n | (95% CI) | n | (95% CI) | p-value § | ptrend ‡ | |
| Controls * | 61 | 2.36 (1.88, 2.97) | 57 | 2.65 (2.09, 3.36) | ||
| Exercisers – Subgroups: | ||||||
| Change in Aerobic Fitness | ||||||
| Gained < 5% VO2max | 20 | 2.43 (1.65, 3.58) | 16 | 2.46 (1.62, 3.74) | 0.62 | |
| Gained 5-15% VO2max | 14 | 2.73 (1.42, 5.24) | 14 | 1.92 (1.03, 3.57) | 0.0006 | |
| Gained > 15% VO2max | 17 | 2.10 (1.48, 2.98) | 17 | 2.08 (1.51, 2.87) | 0.11 | 0.006 |
| Exercise Adherence | ||||||
| Exercised < 136 min/wk | 15 | 2.57 (1.54, 4.29) | 13 | 2.73 (1.43, 5.19) | 0.48 | |
| Exercised 136-195 min/wk | 21 | 2.60 (1.69, 4.01) | 19 | 2.19 (1.49, 3.22) | 0.06 | |
| Exercised > 195 min/wk | 15 | 1.98 (1.34, 2.93) | 15 | 1.71 (1.24, 2.35) | 0.02 | 0.004 |
| Change in total percentage body fat | ||||||
| No change or gained fat | 16 | 2.48 (1.54, 4.01) | 12 | 2.54 (1.49, 4.33) | 0.71 | |
| Body fat decreased ≤ 2% | 17 | 2.85 (1.84, 4.42) | 17 | 2.53 (1.56, 4.10) | 0.12 | |
| Body fat decreased > 2% | 18 | 1.96 (1.27, 3.02) | 18 | 1.65 (1.18, 2.29) | 0.002 | 0.001 |
| Change in body weight (kg) | ||||||
| No change or gained body weight | 20 | 2.19 (1.47, 3.26) | 16 | 2.31 (1.49, 3.56) | 0.84 | |
| Body weight decreased ≤ 3kg | 18 | 2.28 (1.49, 3.49) | 18 | 1.80 (1.15, 2.81) | 0.004 | |
| Body weight decreased > 3kg | 13 | 2.92 (1.64, 5.19) | 13 | 2.53 (1.61, 3.97) | 0.04 | 0.002 |
| Change in waist circumference (cm) | ||||||
| No change or increased waist circumference | 21 | 2.11 (1.44, 3.09) | 17 | 1.92 (1.34, 2.77) | 0.16 | |
| Waist circumference decreased ≤ 3cm | 14 | 2.37 (1.36, 4.12) | 14 | 2.23 (1.32, 3.76) | 0.08 | |
| Waist circumference decreased > 3cm | 16 | 2.85 (1.82, 4.47) | 16 | 2.34 (1.45, 3.79) | 0.06 | 0.02 |
| Change in intra-abdominal fat (cm2) | ||||||
| No change or increased intra-abdominal fat | 17 | 2.18 (1.41, 3.37) | 13 | 1.86 (1.26, 2.75) | 0.03 | |
| Intra-abdominal fat decreased ≤ 8 cm2 | 18 | 2.51 (1.56, 4.04) | 18 | 2.25 (1.40, 3.62) | 0.14 | |
| Intra-abdominal fat decreased > 8 cm2 | 16 | 2.50 (1.61, 3.90) | 16 | 2.29 (1.47, 3.59) | 0.07 | 0.03 |
Referent group.
P-value comparing C-reactive protein at twelve months among sub-strata of exercisers to control group, controlling for baseline C-reactive protein and age.
P-value for trend test of baseline-to-12-month difference across groups (control, low, medium, high), controlling for baseline C-reactive protein and age.
DISCUSSION
Paragraph Number 18
This study demonstrated that a yearlong aerobic exercise intervention compared to a stretching control program reduced CRP among previously sedentary obese postmenopausal women. The obese women in this study had clinically ‘high’ average CRP values according to established criteria (19) and, despite decreased CRP after 3- and 12-months of exercise intervention, their CRP values remained high throughout the trial. Future studies should assess exercise interventions of longer duration and greater intensity to determine the dose of exercise or fat loss needed to reduce CRP further. Although the CRP values in this study remained relatively high despite an exercise intervention effect, obese exercisers experienced a decrease in CRP of an average of 1.59mg/L over 12-months, which may have public-health relevance in that a recent prospective study reported that each 1.02 mg/L increase in CRP was associated with about a 35% increase (95% CI: 1.05-1.74) in colon cancer risk (6). Similarly, a recent cohort study identified a 29% increase (95% CI: 1.07-1.55) in coronary heart disease risk for a 1 unit increase in log CRP (11).
Paragraph Number 19
Our stratified analyses showed that only those exercisers who decreased body fat by 2% or greater experienced a statistically significant reduction in CRP versus controls, suggesting that the exercise effect is at least partially dependent on fat loss. Moreover, the exercisers who lost more body weight/body fat were largely the same exercisers who were most adherent to the exercise intervention (χ2=10.34, p-value: 0.03). And because weight/fat loss was generally minimal in this trial, this suggests that exercise, in the absence of caloric restriction, is sufficient to reduce CRP among obese women. Two previous multi-arm randomized controlled trials have offered valuable perspective concerning the role of exercise and diet interventions (both alone and in combination) on biomarkers of inflammation (18,27). Among a group of 316 community dwelling older adults with movement limitations, diet-alone (but not exercise-alone or diet-and-exercise) favorably reduced CRP and IL-6 (18). Among a study population of 34 sedentary, overweight or obese, postmenopausal women (that is, a population that is more comparable to the current study), a six month exercise-and-diet intervention, but not diet alone, decreased CRP and IL-6 (27). Given the somewhat discrepant findings and small number of studies on this topic, more multi-arm trials seem justified to better discern the individual and combined effects of diet and exercise on inflammation.
Paragraph Number 20
A recent meta-analysis of five randomized controlled trials summarized the effect of aerobic exercise-alone on CRP (13). The studies included in the meta-analysis had intervention durations of 8 weeks to 6 years and were undertaken in varied populations, including individuals with rheumatoid arthritis (2), postmenopausal breast cancer survivors (8), elderly men and women (10), overweight men and women (17), and middle-aged men (21). Overall, a statistically non-significant 3% decrease in CRP was reported; however, the majority of the individual studies suggested a trend towards exercise-driven reduction of CRP(8,10,21). All but one(21) of these previous trials had considerably shorter intervention durations than the current study and, given that our three-month analyses suggested no exercise effect, the collective evidence suggests that longer periods of exercise are required to reduce CRP.
Paragraph Number 21
The six year trial by Rauramaa and colleagues (21) showed a modest trend towards reduction in CRP; compared to the women in the current study, however, those male participants were considerably leaner, more aerobically fit, and had lower baseline CRP values. Consistent with the present study, a more recent trial (not included in the above meta-analysis) suggested that six months of exercise alone statistically significantly reduced CRP among obese patients with type 2 diabetes mellitus despite minimal changes in body weight (12). Similarly, a recent randomized trial of 10-months of aerobic exercise versus flexibility/strength training reduced CRP, IL-6, and IL-18 among older men and women (15). From these data, it appears that exercise is most beneficial, in terms of reducing chronic inflammation, among those persons with relatively high levels of baseline inflammation.
Paragraph Number 22
The specific pathways through which exercise may decrease CRP are not established, but several plausible mechanisms exist. One mechanism is through regulation of interleukins and related cytokines, including IL-6 and tumor necrosis factor-α (TNF-α), both of which are released by adipose tissue (as well as by peripheral blood mononuclear cells) and the former stimulates hepatic release of CRP. Because our study showed no effect of exercise on serum IL-6, our data do not support the direct role of circulating IL-6 on CRP reduction.
Paragraph Number 23
Strengths of the current study include: the relatively long duration of exercise intervention; good adherence and high retention rates; effective aerobic exercise intervention as demonstrated by gold-standard measures of cardiorespiratory fitness; and a randomized controlled trial design. Limitations that should be considered when interpreting these data include the strict inclusion criteria that preclude generalization to other populations and the narrow range of inflammatory markers selected. Secondly, the intervention was not intended to induce weight loss, and we cannot therefore assess the effect of substantial weight loss, with or without exercise, on chronic inflammation. Lack of racial diversity is another limitation of the current study that should be addressed in future intervention trials; in cross-sectional data LaMonte and colleagues (16) showed that cardiorespiratory fitness was correlated with CRP among Caucasian and Native American, but not African American, women.
Paragraph Number 24
In conclusion, a 12-month aerobic-exercise intervention resulted in improved aerobic fitness and reduced CRP, despite relatively little fat loss. CRP reduction was limited to women who were obese at baseline, a group with clinically high CRP values.
Acknowledgments and Grant Support
This study was supported by research grants from the National Institutes of Health (CA 69334, DK 02860, DK 035816). Dr P Campbell was Research Fellow of the Canadian Cancer Society through an award from the National Cancer Institute of Canada and also supported by a NIH Transdisciplinary Research on Energetics and Cancer Postdoctoral Fellowship (NCI U54 CA116847). Dr K Campbell was supported through a Research Fellow Award from the Canadian Institutes for Health Research. The sponsors had no role on: the design or conduct of the study, the collection, the management, the analyses, the interpretation of the data, or on preparing, reviewing, or approving the manuscript.
Footnotes
Disclaimer: The results of this study do not constitute endorsement by the ACSM.
Disclosure Statement The authors have no conflicts to disclose.
References
- 1.Alley DE, Crimmins E, Bandeen-Roche K, Guralnik J, Ferrucci L. Three-year change in inflammatory markers in elderly people and mortality: the Invecchiare in Chianti study. J Am Geriatr Soc. 2007;55(11):1801–7. doi: 10.1111/j.1532-5415.2007.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baslund B, Lyngberg K, Andersen V, et al. Effect of 8 wk of bicycle training on the immune system of patients with rheumatoid arthritis. J Appl Physiol. 1993;75(4):1691–5. doi: 10.1152/jappl.1993.75.4.1691. [DOI] [PubMed] [Google Scholar]
- 3.Bo S, Ciccone G, Baldi C, et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J Gen Intern Med. 2007;22(12):1695–703. doi: 10.1007/s11606-007-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell PT, Wener MH, Sorensen B, et al. Effect of exercise on in vitro immune function: a 12-month randomized, controlled trial among postmenopausal women. J Appl Physiol. 2008;104(6):1648–55. doi: 10.1152/japplphysiol.01349.2007. [DOI] [PubMed] [Google Scholar]
- 5.Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22(11):1869–76. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- 6.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. Jama. 2004;291(5):585–90. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- 7.Esposito K, Pontillo A, Di Palo, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Jama. 2003;289(14):1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 8.Fairey AS, Courneya KS, Field CJ, Mackey JR. Physical exercise and immune system function in cancer survivors: a comprehensive review and future directions. Cancer. 2002;94(2):539–51. doi: 10.1002/cncr.10244. [DOI] [PubMed] [Google Scholar]
- 9.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153(3):242–50. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 10.Hammett CJ, Oxenham HC, Baldi JC, et al. Effect of six months’ exercise training on C-reactive protein levels in healthy elderly subjects. J Am Coll Cardiol. 2004;44(12):2411–3. doi: 10.1016/j.jacc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 11.Hung J, Knuiman MW, Divitini ML, Langton PE, Chapman CL, Beilby JP. C-reactive protein and interleukin-18 levels in relation to coronary heart disease: prospective cohort study from Busselton Western Australia. Heart Lung Circ. 2008;17(2):90–5. doi: 10.1016/j.hlc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Kadoglou NP, Iliadis F, Angelopoulou N, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14(6):837–43. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 13.Kelley GA, Kelley KS. Effects of aerobic exercise on C-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism. 2006;55(11):1500–7. doi: 10.1016/j.metabol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Shin YO, Bae JS, et al. Beneficial effects of cardiac rehabilitation and exercise after percutaneous coronary intervention on hsCRP and inflammatory cytokines in CAD patients. Pflugers Arch. 2008;455(6):1081–8. doi: 10.1007/s00424-007-0356-6. [DOI] [PubMed] [Google Scholar]
- 15.Kohut ML, McCann DA, Russell DW, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20(3):201–9. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 16.LaMonte MJ, Durstine JL, Yanowitz FG, et al. Cardiorespiratory fitness and C-reactive protein among a tri-ethnic sample of women. Circulation. 2002;106(4):403–6. doi: 10.1161/01.cir.0000025425.20606.69. [DOI] [PubMed] [Google Scholar]
- 17.Marcell TJ, McAuley KA, Traustadottir T, Reaven PD. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54(4):533–41. doi: 10.1016/j.metabol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79(4):544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 19.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 20.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obes Res. 2003;11(9):1055–64. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 21.Rauramaa R, Halonen P, Vaisanen SB, et al. Effects of aerobic physical exercise on inflammation and atherosclerosis in men: the DNASCO Study: a six-year randomized, controlled trial. Ann Intern Med. 2004;140(12):1007–14. doi: 10.7326/0003-4819-140-12-200406150-00010. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 23.Rothenbacher D, Hoffmeister A, Brenner H, Koenig W. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med. 2003;163(10):1200–5. doi: 10.1001/archinte.163.10.1200. [DOI] [PubMed] [Google Scholar]
- 24.Tworoger SS, Yasui Y, Ulrich CM, et al. Mailing strategies and recruitment into an intervention trial of the exercise effect on breast cancer biomarkers. Cancer Epidemiol Biomarkers Prev. 2002;11(1):73–7. [PubMed] [Google Scholar]
- 25.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery Study. Circulation. 2005;112(7):976–83. doi: 10.1161/CIRCULATIONAHA.104.513085. [DOI] [PubMed] [Google Scholar]
- 26.Villareal DT, Miller BV, 3rd, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84(6):1317–23. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 27.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89(4):1739–46. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]