Abstract
Omega-3 and n-6 fatty acids are biosynthetic precursors to lipid mediators with antinociceptive and pronociceptive properties. We conducted a randomized, single-blinded, parallel-group clinical trial to assess clinical and biochemical effects of targeted alteration in dietary n-3 and n-6 fatty acids for treatment of chronic headaches. After a 4-week preintervention phase, ambulatory patients with chronic daily headache undergoing usual care were randomized to 1 of 2 intensive, food-based 12-week dietary interventions: a high n-3 plus low n-6 (H3-L6) intervention, or a low n-6 (L6) intervention. Clinical outcomes included the Headache Impact Test (HIT-6, primary clinical outcome), Headache Days per month, and Headache Hours per day. Biochemical outcomes included the erythrocyte n-6 in highly unsaturated fatty acids (HUFA) score (primary biochemical outcome) and bioactive n-3 and n-6 derivatives. Fifty-six of 67 patients completed the intervention. Both groups achieved targeted intakes of n-3 and n-6 fatty acids. In intention-to-treat analysis, the H3-L6 intervention produced significantly greater improvement in the HIT-6 score (−7.5 vs −2.1; P < 0.001) and the number of Headache Days per month (−8.8 vs −4.0; P = 0.02), compared to the L6 group. The H3-L6 intervention also produced significantly greater reductions in Headache Hours per day (−4.6 vs −1.2; P = 0.01) and the n-6 in HUFA score (−21.0 vs −4.0%; P < 0.001), and greater increases in antinociceptive n-3 pathway markers 18-hydroxy-eicosapentaenoic acid (+118.4 vs +61.1%; P < 0.001) and 17-hydroxy-docosahexaenoic acid (+170.2 vs +27.2; P < 0.001). A dietary intervention increasing n-3 and reducing n-6 fatty acids reduced headache pain, altered antinociceptive lipid mediators, and improved quality-of-life in this population.
Keywords: Omega-3, Omega-6, Headache, Migraine, Clinical trial
1. Introduction
Chronic daily headache (CDH), defined here as the presence of headaches lasting 4 hours or more for 15 or more days per month over at least 3 months, is a heterogeneous group of debilitating chronic pain syndromes affecting an estimated 10 million adults in the United States [10,45]. Loss of work and medical expenses add up to billions of dollars per year [14,38]. The term CDH encompasses several primary headache types including chronic migraine and chronic tension-type headache [10,45], which together account for up to 40% of patients presenting to headache specialty clinics. Conventional treatment relies heavily on medications that often provide only partial or transient relief and can be associated with significant side effects and costs [6–8,31,57,59]. Many chronic headache patients continue to have frequent headaches and impaired quality of life despite taking numerous pain-related medications [47,50]. Given the incomplete effectiveness and potential side effects of many headache medications, it is essential to investigate novel mechanisms and alternative approaches to manage pain.
1.1. A role for diet in chronic pain?
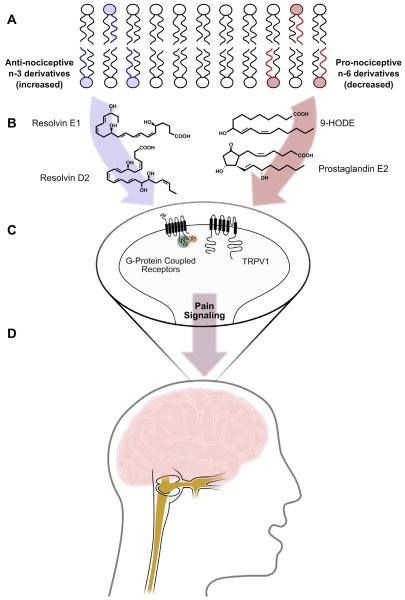
Omega-6 (n-6) and omega-3 (n-3) fatty acids regulate multiple pain-related biochemical pathways. As major components of vascular, immune, myelin, glial, and neuronal cell membranes [46], n-6 and n-3 fatty acids can be converted to lipid mediators with pro- or antinociceptive properties (eg, endovanilloids, eicosanoids, endocannabinoids, resolvins) [1,20,34–36,48,58,60]. In general, and with a few notable exceptions [52], lipid mediators derived from n-6 fatty acids have pronociceptive properties [1,2,19,20,22,35,36,56], while mediators derived from n-3 fatty acids have antinociceptive properties [32,34,48,58]. Therefore, dietary interventions with targeted alterations in n-6 and n-3 fatty acids may be able to reduce pain (Fig. 1). However, controlled dietary trials testing pain reduction and elucidating mechanisms of action in humans are lacking.
Fig. 1.
Proposed mechanisms linking targeted alterations in n-3 and n-6 fatty acids with pain reduction. This figure summarizes hypothetical mechanisms linking n-3 and n-6 fatty acids to pain reduction, including representative mediators measured in the present trial (9-HODE and Resolvin D2) as well as mediators that were not measured in this trial (Prostaglandin E2 and Resolvin E1). (A) Targeted dietary alterations increase the abundance of n-3 EPA and DHA (blue) and decrease the abundance of n-6 LA and AA (red) in fatty acid precursor pools, including the membranes of endothelial cells, platelets, immune cells, glia, myelin, and neurons. (B) These changes in precursor abundance alter the concentrations of n-3 and n-6 derived antinociceptive and pronociceptive mediators, including E- and D-series resolvins, maresins, prostaglandins, endovanilloids, and endocannabinoids. (C) Changes in the milieu of lipid mediators alter the activities of receptors involved in pain signaling, including the vanilloid receptor (TRPV1) and several G-protein coupled receptors (eg, E-prostanoid receptors, resolvin receptors, cannabinoid receptors). (D) Increases in antinociceptive mediators and decreases in pronociceptive mediators reduce pain signaling in the trigeminovascular system and central pain signaling pathways. EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; LA, linoleic acid; AA, arachidonic acid; 9-HODE, 9-hydroxy-octadecadienoic acid.
We hypothesized that hyperactive metabolism of n-6 linoleic (n-6 LA) and arachidonic (n-6 AA) acids, and insufficient metabolism of n-3 eicosapentaenoic (n-3 EPA) and docosahexaenoic (n-3 DHA) acids, contribute to headache pathogenesis. Therefore, we conducted a randomized, parallel-group clinical trial in which we assigned patients with CDH to either a high n-3 plus low n-6 (H3-L6) dietary intervention or a low n-6 (L6) intervention for 12 weeks to evaluate whether these targeted dietary alterations would 1) improve headache-related clinical outcomes; 2) increase n-3 and reduce n-6 fatty acids in erythrocytes; and 3) increase antinociceptive n-3 derivatives, and reduce pronociceptive n-6 derivatives.
2. Materials and methods
The trial protocol and detailed descriptions of the dietary interventions are reported elsewhere [29,41]. Briefly, adult men and women with any primary headache type meeting our CDH criteria of headaches ≥ 4 hours per day and ≥ 15 days per month for at least 3 months and a headache history of ≥ 2 years under the care of a physician for headache management, were recruited to participate in an outpatient dietary intervention trial (Supplementary Table 1). Patients with evidence of a secondary headache disorder attributed to head and/or neck trauma, cranial or cervical vascular disorder, nonvascular cranial disorder, substance use or withdrawal, infection, disorder of hemostasis, or headaches attributed to psychiatric disorder, were not eligible. Participants were enrolled by the study neurologist. All participants provided written informed consent after the nature and possible consequences of the trial were explained. During the 4-week preintervention run-in phase, participants continued their usual care and used an online daily headache diary to record headache characteristics and medication use. Upon completion of this run-in phase, participants were randomized to either the H3-L6 intervention or the L6 intervention, to be maintained for 12 weeks. Participants were advised to continue seeing their regular headache physician for usual care throughout the trial. The trial was conducted at the University of North Carolina at Chapel Hill (UNC) from April 2009 to November 2011 (ended when minimum acceptable sample size was met). Trial procedures were approved by the UNC Institutional Review Board. This trial is registered under ClinicalTrials.gov (NCT01157208).
2.1. Randomization and masking
Participants were randomized by the dietitian using an on-line, uneditable treatment assignment algorithm with a random permuted block design using a number sequence (1:1 allocation ratio) generated by a study programmer. Only the dietitian was unmasked by necessity at randomization in order to assign patients to their group and to administer the interventions. Participants were provided specific dietary advice and foods in accordance with their assigned intervention, and were masked to the nature and content of the other group’s intervention. All other investigators, study and laboratory staff, and each participant’s personal physician were masked to group assignment for the full duration of the trial.
2.2. Dietary interventions and nutrient intake assessment
The H3-L6 intervention was designed to reduce dietary n-6 LA, and concurrently increase dietary n-3 EPA and DHA. The L6 intervention was designed to reduce dietary n-6 LA, and maintain low n-3 EPA and DHA intakes typical of U.S. diets [4,29]. The interventions were designed to be equally credible and equivalent with respect to: 1) macronutrient and caloric intake; 2) the amounts of study foods provided; 3) interactions with the study dietitian and other investigators; and 4) the intensity and breadth of the dietary advice and intervention materials [29]. A registered dietitian provided intensive counseling at randomization and at regular 2-week intervals. Foods meeting nutrient targets were provided to participants sufficient for 2 meals and 2 snacks per day. Extensive intervention-specific Web-based materials were developed and made available to participants in order to reinforce dietitian advice and complement study food provision. Nutrient intakes were assessed for each participant using 6 unannounced telephone-administered 24-hour recalls – 3 during the 4-week run-in phase and 3 in the final 4 weeks of the intervention phase. Nutrient values were estimated using the Nutritional Data System for Research [43], which was updated to include the fatty acid contents of our laboratory-analyzed study foods to improve accuracy of intraintervention assessments [29].
2.3. Analysis of erythrocyte fatty acids
Fasting (>10 hours) blood was collected at the conclusion of the run-in phase, and after 4, 8, and 12 weeks of diet exposure, in EDTA tubes and immediately centrifuged at 2960 rpm for 15 minutes. Erythrocyte aliquots were prepared and stored at −80°C until analysis. Following Bligh/Dyer extraction [5], aliquots were heated at 100°C for 1 hour with methanol containing 14% boron trifluoride to generate fatty acidmethyl ester, which was then extracted into hexane and analyzed with a gas chromatography/flame ionization detector gas chromatograph (Agilent 6890; Agilent Technologies, Santa Clara, CA, USA) equipped with a 30-mDB-free fatty acid phase (DBFFAP) capillary column. Fatty acids were identified through comparison with a fatty acid methyl ester mixture (GLC-462). Two composite fatty acid indices [18,51] were calculated. The n-6 in HUFA score, the primary biochemical outcome, is equal to the proportion of n-6 fatty acids in total highly unsaturated fatty acids (HUFA). The n-3 index is equal to the sum of erythrocyte n-3 EPA and DHA.
2.4. Analysis of omega-3-derived antinociceptive mediators and pathway markers
The resolvin pathway precursors 18R/S-hydroxy-5Z,8Z,11Z,14Z,16E-eicosapentaenoic acid (18R/S-HEPE) and 17R/S-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid (17R/S-HDHA) derived from EPA and DHA, respectively, and 7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid (RvD2) derived from DHA, were analyzed as previously described [30]. Briefly, plasma (1 mL) and internal standard leukotriene B4-d4 (80 ng; Cayman Chemicals, Ann Arbor, MI, USA) were acidified to pH 3 with 0.25 M HCl. Samples were applied to solid-phase extraction cartridges (Bond Elut C18 500 mg; Agilent Technologies, Mulgrave, VIC, Australia) and washed in 15 mL 15% methanol, 15 mL double-distilled H2O, and 15 mL hexane. 18R/S-HEPE, 17R/S-HDHA, and RvD2 were eluted with 10 mL methyl formate, dried under nitrogen and then reconstituted in 100 μL 5 mmol/L ammonium acetate (pH = 9)/methanol (50/50; vol/vol) for analysis by liquid chromatography–tandem mass spectrometry using a Thermo Scientific TSQ QuantumUltra triple-quadrupole LC–MS system equipped with an electrospray ionization source (ESI) operated in the negative ion mode.
2.5. Analysis of omega-6-derived pronociceptive mediators
Omega-6 LA-derived 9- and 13R/S-hydroxy-octadecadienoic acid (9- and 13R/S-HODE), 9- and 13R/S-oxo-octadecadienoic acid (9- and 13R/S-oxoODE), and n-6 AA-derived 5-, 8-, 9-, 11-, 12-, and 15R/S-hydroxy-eicosatetraenoic acid (5-, 8-, 9-, 11-, 12-, and 15R/S-HETE) were analyzed as previously described [16,42]. Briefly, plasma (50 mL), internal standard [15(S)-HETE-d8 (Cayman Chemical), 10 mL of 1000 ng/mL], and sodium hydroxide were added to glass test tubes, overlaid with argon, and sealed. Lipids were hydrolyzed at 60°C under argon for 2 hours. Fatty acids were extracted twice by liquid/liquid extraction using hexane and hexane/isopropanol with 4 M acetic acid, with tubes capped under argon. The combined hexane layers were dried under nitrogen and then re-suspended in 200 mL 85% methanol/water (v/v). Fatty acid oxidation products were quantified using liquid chromatography online electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) (Waters 2690 Separations Module, Franklin, MA, USA and Quattro Ultima, Micromass, Manchester, UK).
2.6. Headache outcomes
The primary clinical outcome was the Headache Impact Test (HIT-6) [25], a commonly used measure of headache-related disability based on self-reported pain, social functioning, role functioning, vitality, cognitive functioning, and psychological distress [9,23]. Participants completed the HIT-6 and reported the number of headache days experienced during the last 4 weeks (Headache Days) immediately before randomization and on the final day of the 12-week intervention phase. In addition, participants were instructed to complete a daily Headache Diary [41] throughout the run-in phase and the dietary intervention phase (112 days total) recording hourly headache characteristics (rated as mild, moderate, or severe) and medication use. The following variables were derived from the Headache Diary: Headache Hours per day, Severe Headache Days (defined as ≥8 hours of mild headache or any moderate or severe headache), and medication use (vasoactive abortive, acute pain, adjunctive, preventive).
2.7. Other measures
The Borkovec and Nau credibility questionnaire [12] was completed after the initial in-depth dietitian counseling session to assess expectation for treatment success. Participants also reported demographics and completed the MIDAS (migraine disability assessment) [3], a validated 7-item measure of headache-related function. During the initial study visit, the study neurologist assessed the number and type of headache-related medications, and classified CDH as 1) chronic migraine according to International Headache Disorders-2 criteria [53]; 2) CDH with migraine features (eg, unilateral, pulsating, severe, sensory sensitivity, or aggravated by physical activity) but insufficient for a diagnosis of chronic migraine; or 3) CDH without migraine features.
2.8. Data analysis
Descriptive statistics were examined for all variables and, if necessary, steps were taken to account for nonnormal distributions. Statistical testing was 2-tailed at the 5% type I error, without adjustment for prespecified multiple comparisons. The erythrocyte and headache outcome analyses were prespecified. Analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) and Stata 12 (StataCorp LP, College Station, TX, USA.
We compared the effect of diet group assignment on postintervention headache-related outcomes (HIT-6, Headache Days per month, Headache Hours per day, Severe Headache Days) using analyses of covariance adjusted for the baseline values of each outcome. Missing follow-up values for HIT-6 and Headache Days per month (measured pre- and postintervention) were filled with baseline values in order to include all randomized participants in those analyses. For the outcomes that were recorded daily throughout the study (ie, Headache Hours per day and Severe Headache Days), we analyzed all available data without imputations using hierarchical models with either a Poisson or logistic distribution. For example, to model differences across time by diet group on the number of Headache Hours per day, we used subject-specific mixed-effects regression models with a Poisson distribution. Since we had 4 weeks of data prior to randomization, we used a linear spline variable to capture the trajectory of each individual’s baseline Headache Hours per day with their change in trajectory after randomization. Changes in Headache Hours per day were calculated based on model-predicted estimates for intervention days 1 and 80. In addition to the hierarchical model, we also used a Loess smoothing procedure to visualize the crude trajectory of average Headache Hours per day for each diet group.
For an exploratory analysis of changes in medication use by diet group, we classified medications into 3 broad categories: acute, preventive, or adjunctive (Supplementary Table 2). These variables were dichotomized (any vs no use per day) because specific medications and doses were not comparable between individuals. For each category, the proportion of subjects using any medication on each day after randomization was calculated using longitudinal logistic models clustering on subject ID. The percent change was calculated based on model-predicted estimates on intervention days 1 and 80. In an exploratory manner, we also examined longitudinal changes in vasoactive abortive medication use in each group, in the subset of participants using these medications (n = 37) with a subject-specific mixed-effects regression model (Poisson distribution).
2.9. Power
This was the first trial evaluating a low n-6 plus high n-3 dietary intervention for clinical pain reduction. Because clinical effect sizes were unknown, sample size calculations were based on biochemical data from rodents and human epidemiological studies [27,28]. An estimated 32 subjects per group were needed for 80% power to detect a between-group difference in the erythrocyte n-6 in HUFA score, with predicted difference of 0.5 and a maximum SD of 0.5.
3. Results
3.1. Demographics
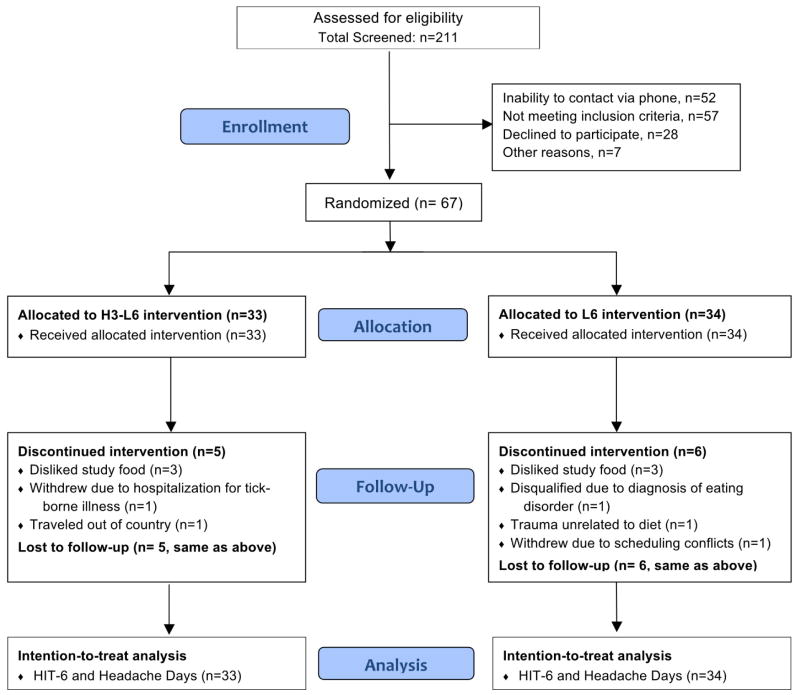
Sixty-seven subjects were randomized to either the H3-L6 intervention or the L6 intervention (Fig. 2). Of those, 56 (84%) completed the 12-week intervention phase (28 in each group). Demographic characteristics were comparable between groups (Table 1). Ninety-three percent had chronic migraine or CDH with migraine features. Participants reported taking an average of 6 different headache-related medications at the baseline interview. Expectation of benefit was moderate, with no between-group differences as measured by the Credibility Scale.
Fig. 2.
CONSORT trial profile.
Table 1.
Baseline demographic and clinical characteristics of 67 patients with chronic headaches.
| H3-L6 diet n = 33 |
L6 diet n = 34 |
|
|---|---|---|
| Age, years: Mean (SD) | 41 (13.4) | 42 (11.1) |
| Female, n (%) | 28 (84.8) | 30 (88.2) |
| White race, n (%) | 28 (84.8) | 30 (88.1) |
| Married, n (%) | 19 (57.6) | 19 (55.9) |
| Education, n (%) | ||
| High school | 2 (6.1) | 2 (6.2) |
| Attended college | 18 (54.5) | 17 (53.1) |
| Master’s degree or higher | 13 (40.6) | 13 (39.4) |
| Employment, n (%) | ||
| Employed/student | 26 (78.8) | 23 (69.7) |
| Retired/caretaker | 3 (9.1) | 3 (9.1) |
| Disabled/unemployed | 4 (12.1) | 7 (20.0) |
| Headache categorya, n (%) | ||
| Chronic migraine | 26 (78.8) | 24 (70.6) |
| CDH with migraine features | 6 (18.2) | 6 (17.6) |
| CDH without migraine features | 1 (3.0) | 4 (11.8) |
| Headaches managed by neurologist, n (%) | 24 (72.7) | 24 (70.6) |
| Age at first headache, median (IQR) | 15 (10) | 18 (18) |
| Patients reporting 2 or more headache-related medications, n (%) | ||
| Acute medicationsb | 31 (93.9) | 29 (85.3) |
| Preventive medicationsb | 13 (39.4) | 13 (38.2) |
| Adjunctive medicationsb | 8 (24.2) | 12 (35.3) |
| Total number of different headache-related medications reported, mean (SD) | 6.4 (3.4) | 5.6 (3.3) |
| MIDAS (Headache-related disability), Median (95% CI)c | 43 (28, 59) | 34 (24, 47) |
| Credibility Scale (Borkovec & Nau) (range 0–45), mean (SD) | 28.3 (5.5) | 27.1 (3.8) |
CDH, chronic daily headache; IQR, interquartile range; MIDAS, migraine disability assessment; CI, confidence interval.
Subjects classified as chronic migraine met International Headache Disorders-2 criteria. Subjects classified as CDH with migraine features had some characteristics of migraine (eg, unilateral, pulsating, severe, sensory sensitivity, or aggravated by physical activity) but did not meet all criteria needed for chronic migraine diagnosis. Subjects classified with CDH without migraine features had no evidence of migraine.
Headache medication categories are shown in Supplementary Table 2.
95% confidence interval around the median per binomial-based method in Stata 12.
3.2. Dietary and erythrocyte fatty acids
Pre- and postintervention nutrient intakes have been previously published [29] and are summarized in Table 2. The H3-L6 intervention group achieved the targeted reduction in dietary n-6 LA, and increases in n-3 EPA and DHA. The L6 intervention group achieved targeted reductions in dietary n-6 LA and AA, without alterations in n-3 fatty acids. Pre- and postintervention erythrocyte n-6 and n-3 fatty acids are shown in Table 2. Compared to baseline, both interventions produced significant reductions in the erythrocyte n-6 in HUFA score and significant increases in the n-3 Index. Both of these erythrocyte fatty acid indices changed significantly more in the H3-L6 group compared to the L6 group. The H3-L6 intervention also significantly reduced erythrocyte n-6 AA, while the L6 intervention had no effect on AA. Both interventions produced comparable, statistically significant reductions in erythrocyte LA.
Table 2.
Dietary and erythrocyte fatty acids at baseline and after 12-week intervention.
| H3-L6 intervention
|
P-valuea (within-group) | L6 intervention
|
P-valuea (within-group) | P-valueb (within-group) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre
|
Post
|
Pre
|
Post
|
||||||||
| Median | 25%–75% | Median | 25%–75% | Median | 25%–75% | Median | 25%–75% | ||||
| Dietary fatty acids (n = 55)d | |||||||||||
| n-6 Linoleic acid (en%) | 6.4 | (5.3, 7.4) | 2.5 | (2.2, 3.9) | <0.001 | 7.4 | (5.7, 9.6) | 2.4 | (2.0, 2.9) | <0.001 | |
| n-6 Arachidonic acid (mg) | 110c | (66, 176) | 114c | (69, 195) | 0.75 | 106c | (57, 159) | 48c | (18, 74) | <0.001 | <0.001 |
| n-3 EPA + DHA (mg) | 47c | (20, 71) | 1482c | (374, 2558) | <0.001 | 43c | (25, 73) | 76c | (19, 264) | 0.32 | <0.001 |
| Erythrocyte fatty acids (n = 52)d | |||||||||||
| n-6 Linoleic acid | 11.6 | (10.3, 12.6) | 10.2 | (8.8, 10.8) | <0.001 | 12.2 | (11.0, 13.0) | 10.5 | (9.5, 11.1) | <0.001 | |
| n-6 Arachidonic acid | 14.3 | (12.7, 15.2) | 12.3 | (11.6, 13.0) | 0.001 | 14.2 | (12.1, 15.0) | 13.1 | (12.7, 14.6) | 0.53 | |
| n-6 in HUFA score | 77.1 | (74.6, 78.3) | 60.9 | (57.8, 63.5) | <0.001 | 77.8 | (75.3, 80.5) | 74.6 | (71.2, 77.5) | <0.001 | <0.001 |
| EPA + DHA (n-3 index) | 4.3 | (3.6, 4.8) | 8.5 | (6.8, 9.7) | <0.001 | 3.7 | (2.9, 4.5) | 4.1 | (3.6, 6.2) | <0.001 | <0.001 |
H3-L6, high n-3, low n-6 dietary intervention; L6, low n-6 dietary intervention; n-6 in HUFA score, percentage of n-6 highly unsaturated fatty acids (HUFA) in total HUFA; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
P-values for within-group changes were calculated with the Wilcoxon signed-rank test.
P-values for effect of diet group assignment on postintervention fatty acids derived from analysis of covariance, adjusting for baseline values.
Chemical analyses of study-provided foods reduced the number of missing database values for arachidonic acid, EPA and DHA, which were not uniformly present for the preintervention phase.
Dietary analysis includes all subjects who completed the intervention and provided both pre- and postintervention 24-hour recalls (H3-L6, n = 27 and L6, n = 28). Erythrocyte analysis includes all subjects who completed the intervention and provided both pre- and postintervention samples (H3-L6, n = 25 and L6, n = 27). Blood draw was unsuccessful for 1 subject and 3 erythrocyte samples were lost prior to analyses. Erythrocyte fatty acids expressed as percent of total fatty acids.
3.3. Anti- and pronociceptive n-3 and n-6 derivatives
Pre- and postintervention antinociceptive mediators and pathway markers derived from n-3 fatty acids and pronociceptive mediators derived from n-6 fatty acids are shown in Table 3. Compared to baseline, both interventions significantly increased n-3 EPA- and DHA-derived resolvin pathway precursors 18R/S-hydroxy-eicosapentaenoic acid (18-HEPE) and 17R/S-hydroxy-docosahexaenoic acid (17-HDHA), and also significantly reduced a number of pronociceptive derivatives of both n-6 LA (eg, hydroxy-octadecadienoic acids [HODEs]) and n-6 AA (hydroxyeicosatetraenoic acids [HETEs]). Compared to the L6 intervention, the H3-L6 intervention produced significantly more pronounced increases in 18-HEPE and 17-HDHA. Reductions in HODEs and HETEs were comparable in the 2 groups.
Table 3.
Diet-induced changes in putative anti- and pronociceptive n-3 and n-6 metabolites.
| Changes pre post diet intervention
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H3-L6 (n = 27)a
|
L6 (n = 28)a
|
Between diets
|
||||||||||||
| Pre
|
Post
|
% Change (median) | P valueb | Pre
|
Post
|
% Change (median) | P valueb | Pre P value | Post P valuec | |||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||||||
| Omega-3 derived (pg/mL) | ||||||||||||||
| EPA-derived pathway marker (precursor for E-series resolvin biosynthesis) | ||||||||||||||
| 18-HEPE | 45.2 | 33.7, 66.5 | 98.7 | 70.6, 206.3 | +118 | <0.001 | 37.1 | 27.4, 69.3 | 59.8 | 41.3, 81.0 | +61 | 0.02 | 0.58 | <0.001 |
| DHA-derived pathway marker (precursor for D-series resolvin and protectin biosynthesis) | ||||||||||||||
| 17-HDHA | 64.3 | 47.4, 91.4 | 173.7 | 88.5, 355.8 | +170 | <0.001 | 57.1 | 30.2, 101.6 | 72.6 | 37.9, 125.2 | +27 | 0.01 | 0.31 | <0.001 |
| DHA derived mediator with antinociceptive actions | ||||||||||||||
| RvD2 | 35.4 | 18.2, 65.5 | 49.0 | 18.6, 102.4 | +39 | 0.15 | 30.7 | 15.5, 63.4 | 35.6 | 24.7, 77.7 | +16 | 0.12 | 0.45 | 0.60 |
| LA-derived mediators | ||||||||||||||
| 9-HODE | 272.3 | 190.8–369.5 | 226.7 | 161.4, 274.1 | −17 | <0.01 | 263.5 | 226.3, 341.4 | 225.0 | 188.0, 278.4 | −15 | 0.10 | 0.81 | 0.35 |
| 13-HODE | 262.3 | 206.7, 397.5 | 246.8 | 212.0, 284.5 | −6 | <0.01 | 289.9 | 232.6, 370.3 | 229.5 | 204.6, 302.8 | −21 | 0.03 | 0.83 | 0.41 |
| Total HODEs | 545.3 | 397.2, 772.0 | 478.6 | 375.0, 568.3 | −12 | <0.01 | 532.3 | 461.9, 753.6 | 463.4 | 385.4, 556.8 | −13 | 0.05 | 0.70 | 0.36 |
| 9-oxoODE | 152.5 | 132.7, 200.6 | 125.5 | 105.6, 185.3 | −18 | 187.8 | 160.3, 247.2 | 165.7 | 125.6, 214.1 | −12 | 0.16 | 0.03 | 0.11 | |
| 13-oxoODE | 248.9 | 193.7, 351.0 | 173.8 | 148.2, 255.7 | −30 | <0.01 | 272.4 | 232.5, 345.0 | 220.8 | 177.4, 287.4 | −19 | <0.01 | 0.09 | 0.35 |
| Total oxoODEs | 396.8 | 325.2, 523.6 | 307.1 | 264.3, 438.6 | −23 | <0.01 | 466.0 | 405.3, 590.2 | 386.0 | 317.2, 493.5 | −17 | 0.02 | 0.06 | 0.22 |
| Total OXLAMs | 939.0 | 768.0, 1310.0 | 774.1 | 691.4, 988.6 | −18 | <0.001 | 982.8 | 875.8, 1341.0 | 873.5 | 709.9, 1043.3 | −11 | 0.02 | 0.27 | 0.27 |
| Omega-6 derived (nM) | ||||||||||||||
| AA-derived mediators | ||||||||||||||
| 5-HETE | 72.3 | 47.6, 91.2 | 52.7 | 42.3, 63.9 | −27 | 0.001 | 68.1 | 46.4, 82.7 | 57.2 | 46.4, 69.7 | −16 | 0.10 | 0.44 | 0.13 |
| 8-HETE | 39.6 | 33.4, 47.3 | 31.1 | 24.2, 36.0 | −21 | 0.001 | 39.6 | 32.3, 45.0 | 32.6 | 28.8, 39.3 | −18 | 0.02 | 0.81 | 0.27 |
| 9-HETE | 54.7 | 42.5, 67.1 | 34.2 | 27.8, 45.4 | −38 | <0.001 | 48.6 | 37.9, 62.8 | 37.6 | 31.6, 43.1 | −23 | <0.01 | 0.38 | 0.24 |
| 11-HETE | 35.2 | 26.8, 39.7 | 24.6 | 20.0, 28.7 | −30 | 0.001 | 31.8 | 25.1, 37.1 | 27.1 | 23.5, 30.8 | −15 | 0.05 | 0.36 | 0.24 |
| 12-HETE | 46.3 | 36.8, 52.3 | 35.5 | 28.0, 41.2 | −23 | <0.01 | 43.2 | 34.9, 50.5 | 37.3 | 31.4, 43.1 | −14 | 0.06 | 0.53 | 0.39 |
| 15-HETE | 64.9 | 52.3, 77.7 | 53.4 | 41.3, 72.8 | −18 | 0.06 | 60.4 | 53.1, 70.7 | 55.2 | 49.6, 71.6 | −9 | 0.45 | 0.85 | 0.41 |
| Total HETEs | 309.3 | 249.9, 381.3 | 236.7 | 187.0, 276.8 | −23 | 0.001 | 281.9 | 224.2, 346.7 | 250.5 | 205.5, 293.2 | −11 | 0.04 | 0.52 | 0.25 |
EPA, eicosapentaenoic acid; 18-HEPE, 18R/S-hydroxy-5Z,8Z,11Z,14Z,16 Eeicosapentaenoic acid; DHA, docosahexaenoic acid; 17-HDHA, 17R/S-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoic acid; RVD2, 7S,16R,17S-trihydroxy-4Z,8E,10Z,12E,14E,19Z-docosahexaenoic acid; LA, linoleic acid; HODE, R/S-hydroxyoctadecadienoic acid; oxo-ODE, R/S-oxo-octadecadienoic acid; OXLAM, oxidized LA metabolites; AA, arachidonic acid; HETE, R/S-hydroxyeicosatetraenoic acid.
Analysis includes all subjects who completed the intervention and provided both pre- and postintervention samples (H3-L6, n = 27 and L6, n = 28). Blood draw was unsuccessful for one subject; one additional sample was lost prior to analysis of n-3 derivatives (n = 26).
P-values for pre to post changes were calculated with the Wilcoxon signed-rank test.
P-values for effect of diet group assignment on postintervention fatty acids derived from analysis of covariance, adjusting for baseline values.
3.4. Clinical headache-related outcomes
While both groups showed statistically significant improvements in clinical outcomes (Headache Days, HIT-6, Headache Hours, Severe Headache Days) compared to the preintervention phase (Table 4), the H3-L6 intervention produced significantly greater improvements in all 4 of these clinical outcomes compared to the L6 intervention (Table 4, Fig. 3). In intent-to-treat analyses, the H3-L6 intervention produced significantly greater improvements in the HIT-6 (−7.5 vs −2.1; P < 0.001) and Headache Days per month (−8.8 vs −4.0; P = 0.02) compared to the L6 group. The H3-L6 intervention also significantly reduced Headache Hours per day (−4.6 vs −1.2; P = 0.01) and the probability of experiencing a Severe Headache Day (−28% vs −8%; P = 0.02) compared to the L6 group. Between-group differences in Headache Hours per day became evident at 8 weeks of diet exposure (P = 0.04), and remained significant thereafter (P = 0.01).
Table 4.
Clinical effects of H3-L6 vs L6 interventions in chronic headache patients.
| H3-L6 intervention (n = 33) Mean (95% CI) |
L6 intervention (n = 34) Mean (95% CI) |
Between-group difference Mean (95% CI) |
Between-group P-valued | |
|---|---|---|---|---|
| Headache Impact Test (HIT-6)a | ||||
| Baseline | 61.0 (59.5, 62.5) | 60.6 (58.7, 62.6) | ||
| Follow-up | 53.5 (50.8, 56.2) | 58.5 (56.3, 60.8) | −5.4 (−8.2, −2.6) | <0.001 |
| Pre- to postchange | −7.5 (−4.9, −10.2) | −2.1 (−0.99, −3.2) | ||
| P-value c | <0.001 | <0.001 | ||
| Headache Days per montha | ||||
| Baseline | 23.3 (20.9, 25.8) | 23.2 (20.2, 25.8) | ||
| Follow-up | 14.5 (10.8, 18.2) | 19.0 (15.4, 22.7) | −4.8 (−8.9, −0.7) | 0.02 |
| Pre- to postchange | −8.8 (−5.4, −12.2) | −4.0 (−1.45, −6.55) | ||
| P-valuec | <0.01 | <0.01 | ||
| Headache Hours per dayb | ||||
| Baseline | 10.2 (8.4, 12.3) | 9.8 (8.1, 11.8) | ||
| Follow-up | 5.6 (4.3, 7.3) | 8.6 (7.0, 10.6) | −45% (−53%, −9%) | 0.01 |
| Pre- to postchange | −44% (−49%, −39%) | −13% (−18%, −8%) | ||
| P-valuec | <0.001 | <0.001 | ||
|
|
||||
| Percent (95% CI) | Percent (95% CI) | Percent (95% CI) | ||
| Probability of experiencing a Severe Headache dayb | ||||
| Baseline | 66 (55, 77) | 68 (58, 79) | ||
| Follow-up | 38 (24, 52) | 61 (47, 73) | −23 (−42, −4.1) | 0.02 |
| Pre- to postchange | −28 (−44, −13) | −7.8 (−15, −1) | ||
| P-value c | 0.02 | 0.03 | ||
CI, confidence interval.
Measured at baseline and at intervention end. Last values of each randomized subject brought forward for “intent-to-treat” analysis.
Measured daily throughout 4-week preintervention and 12-week intervention phase. Hierarchical linear models used all available data for each randomized participant.
Indicates paired sample t-test.
Between-group difference P-values are based on analysis of covariance comparing postintervention values controlling for baseline values.
Fig. 3.
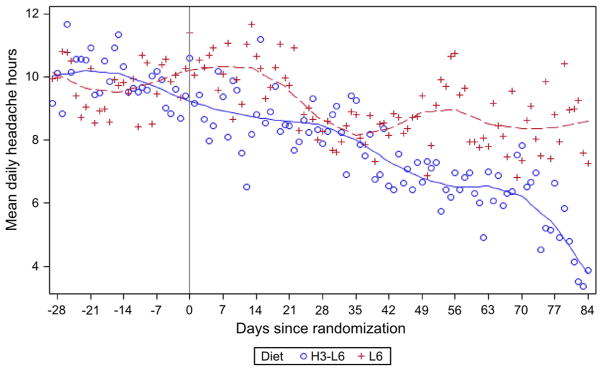
Mean daily Headache Hours by dietary intervention group. Graph depicts the average number of Headache Hours calculated for each day according to intervention group. A Loess smoothing procedure was employed to visualize trends. Participants in the 2 groups provided equivalent amounts of Headache Diary data after randomization (2145 total days of records in the L6 group and 2175 in the H3-L6 group).
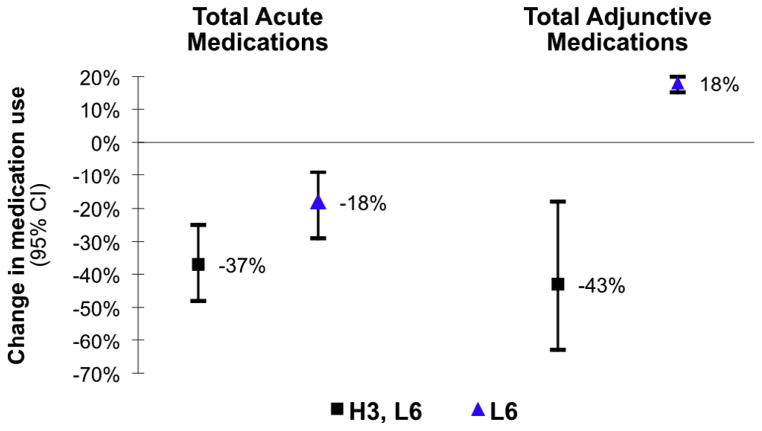
Compared to baseline, the proportion of subjects in the H3-L6 group who used any pain-related acute or adjunctive medication at 12 weeks was reduced by 37% and 43%, respectively (P < 0.01) (Fig. 4). Use of preventive medications did not change significantly during the intervention. In an exploratory analysis of the sub-sample who used vasoactive abortive medications (n = 37), there was a 33% reduction in use of these migraine-specific medications (95% confidence interval −52-0) in the H3-L6 group, with no change in the L6 group.
Fig. 4.
Change in medication use over the course of the intervention by diet group. Acute medications included vasoactive abortive medications, acute opioids, and nonsteroidal antiinflammatory drugs; Adjunctive medications included antiemetics, anxiolytics, sleep aids/hypnotics, and muscle relaxants. All changes depicted in Fig. 4 were significantly changed compared to baseline. There was no significant change in the use of Preventive medications (eg, anticonvulsants, antidepressants, vasoactive preventives, long-acting opiates) over time in either group.
4. Discussion
In this randomized trial, the combination of increasing dietary n-3 fatty acids with concurrent reduction in n-6 LA (the H3-L6 intervention) produced statistically significant, clinically relevant improvements in headache hours per day, severe headache days, and headache-related quality of life compared to baseline, and compared to the n-6-lowering (L6) intervention. Prior to the intervention, this chronic headache population averaged 23 headache days per month and 10 headache hours per day, despite using an average of 6 different headache-related medications per subject. Hence, the H3-L6 intervention provided clinical benefit in a population resistant to conventional pharmacological headache treatment.
This trial compared 2 active interventions that were both hypothesized to have antinociceptive effects. Both interventions were designed to be equally intensive and were perceived to be equally credible by participants. Therefore, improvements due to factors other than the dietary changes (eg, placebo effect, natural history of disease) are expected to affect both intervention groups equally. In this context, the significantly greater clinical improvement in the H3-L6 group compared to the L6 group reflects clinical benefit beyond the placebo effect.
Clinical improvements in the H3-L6 group were not due to increased use of pain medications. In fact, these improvements were observed despite significant reductions in the use of vasoactive abortive, total acute, and adjunctive medications compared to baseline. Improved headache outcomes with concurrent reduction in medication use may be important given potential side effects of many headache medications [6–8,31,57,59].
4.1. Investigating the biochemical mechanisms of pain reduction
Clinical improvements in the H3-L6 group were accompanied by significantly greater increases in erythrocyte n-3 EPA and DHA, and a more marked reduction in n-6 AA compared to the L6 group, which may help explain the significant between-group difference in pain reduction. The H3-L6 intervention also markedly increased the antinociceptive n-3 derivatives 18-HEPE and 17-HDHA (precursors to E- and D-series resolvins [40,54]) in circulation. Diet-induced increases in these and perhaps other antinociceptive n-3 derivatives that were not measured in the present trial, including maresins, protectins, and n-3 monoepoxides [32,34,48,58], may have contributed to the observed clinical improvement in the H3-L6 group.
Omega-6 AA, which was significantly reduced in erythrocytes by the H3-L6 but not the L6 intervention, is the biosynthetic precursor to a variety of pronociceptive and vasoactive lipid mediators implicated in headache pathogenesis [1,2,19,22,56]. Notably, n-6 AA is converted to 2-series prostaglandins by cyclooxygenase [1], the target of medications commonly administered for headache relief (nonsteroidal antiinflammatory drugs, aspirin). Thus, the observed pain reduction produced by the H3-L6 intervention may reflect a combination of reductions in n-6 AA and its pronociceptive derivatives and increases in n-3 EPA and DHA and their antinociceptive derivatives.
The H3-L6 intervention also significantly reduced numerous HODE and HETE derivatives of n-6 LA and n-6 AA, respectively, compared to baseline. Since HODEs and HETEs have putative pronociceptive properties by acting as endogenous ligands for the vanilloid (TRPV1) receptor channel (ie, endovanilloids) [20,35,36,56], these biochemical changes were hypothesized to reduce pain. However, because comparable reductions in a number of HODEs and HETEs occurred in both groups, these changes are unlikely to account for the significantly greater clinical benefit of the H3-L6 intervention.
4.2. Is dietary n-6 lowering necessary for antinociception?
The only randomized controlled trial to test n-3 supplementation in a migraine population showed no clinical benefit [37], despite providing a dose of EPA + DHA comparable to our H3-L6 intervention. A possible explanation for this discrepancy is that the dietary n-6-lowering component of our H3-L6 intervention may be necessary to produce maximal clinical benefit. Although the n-3 supplementation trial in migraineurs did not report biochemical outcomes [37], n-3 supplementation is known to increase circulating EPA + DHA and bioactive n-3 derivatives [30], and to reduce AA and bioactive AA derivatives. The H3-L6 intervention in the present trial differs from previous trials because it not only increased n-3 fatty acids, but also markedly reduced dietary n-6 LA, which competes with n-3 fatty acids for hepatic desaturation, tissue incorporation, and enzymatic conversion to bioactive derivatives [17,24,29,33,39].
4.3. Biochemical effects of the L6 intervention
The L6 intervention provides a rare opportunity to evaluate the biochemical effects of dietary n-6 lowering without altering dietary n-3 fatty acids in humans [29]. Although biochemical effects were less pronounced compared to the H3-L6 group, this L6 intervention did significantly alter erythrocyte fatty acids and their bioactive derivatives in a manner that we hypothesized would reduce pain. As expected, the L6 intervention reduced erythrocyte n-6 LA and a number of its pronociceptive derivatives compared to baseline. Unexpectedly, the L6 intervention also reduced a number of pronociceptive HETEs compared to baseline, despite no change in their precursor n-6 AA in erythrocytes. Importantly, the L6 intervention also increased erythrocyte EPA + DHA and their antinociceptive derivatives 18-HEPE and 17-HDHA compared to baseline. Since dietary n-3 fatty acids were not altered in this group, these increases in circulating n-3 fatty acids and their derivativeswere likely due to reduced metabolic competition with n-6 fatty acids [17,24,29,33,39].
These putative antinociceptive biochemical changes in the L6 group were accompanied by statistically significant (but modest) pain reduction compared to baseline. However, regression to the mean, the natural course of the disorder, and/or the placebo effect may have accounted for most or all of these clinical improvements [11]. Regardless of whether the L6 intervention provided any antinociceptive effects, the significantly greater clinical improvement seen in the H3-L6 group compared to the L6 group demonstrates clinical benefit beyond the placebo effect.
4.4. Strengths and limitations
A strength of the present trial was the use of an active intervention as a comparison group for the H3-L6 intervention. A common limitation of dietary trials is the use of a nonintervention control group in which subjects are simply advised to continue their habitual diets, without equivalent investigator interaction or food provision. Since the L6 intervention was equally intensive and perceived to be equally credible by participants, it likely provided a better control for the placebo effect than a nonintervention control. Nevertheless, the clinical effects of the H3-L6 intervention should also be evaluated in comparison to a control intervention providing habitual intakes of targeted dietary fatty acids. Care should be taken to ensure that such a control intervention is equally intensive and credible.
The combination of intensive dietary guidance and provision of study foods with laboratory-analyzed fatty acid composition allowed for greater control over nutrient intake compared to supplementation trials. Demonstration of adherence via dietary recalls and erythrocyte fatty acid analyses [29] is an improvement over a previous n-3 supplementation trial in migraineurs [37]. The demonstration of significant alterations in fatty acids and lipid mediators along the proposed causal chain linking diet to physical pain, with consistent directionality in both clinical and biochemical end points, is another strength of the present trial. In addition to the pre- and postintervention questionnaires, our use of longitudinal data from the daily Headache Diary allowed for a more detailed comparison of the clinical trajectory of the 2 groups. The erythrocyte fatty acid alterations and the downward trajectory in Headache Hours continued between intervention weeks 8 and 12 (Fig. 3), suggesting that continuation of the H3-L6 diet beyond 12 weeks might produce even more marked benefit.
This trial also had important limitations. Although there was sufficient power to detect between-group differences in the targeted biochemical and clinical outcomes, the present trial was relatively small and should be replicated in a larger trial. As in other outpatient diet trials, the targeted fatty acids could not be altered as independent variables. Therefore, it is possible that changes in other nutrients could have contributed to the favorable effects of the H3-L6 intervention. While the lipid mediators measured here have been linked to physical pain by plausible mechanisms, the present trial cannot establish whether changes in any specific mediator contributed to the observed clinical improvement. Diet-induced changes in n-3 and n-6 derivatives with vasoactive properties [2,15,21,49,55] in circulation may have contributed to headache reduction via direct actions on the vasculature. However, future trials are needed to establish whether comparable diet-induced biochemical alterations are possible in other tissues implicated in headache pathogenesis, such as trigeminal nerve, brain, and skeletal muscle.
4.5. Generalizability
Biochemical derangements targeted by the H3-L6 intervention have been implicated in several headache types [2,26] and numerous other pain syndromes [13,22,44]. Therefore, a logical next step would be to evaluate the efficacy of the H3-L6 intervention in a more homogeneous headache population such as chronic migraine or chronic tension-type headache, and in other populations with chronic pain. Since the present trial was conducted in a population with high n-6 LA and low n-3 EPA + DHA consumption, some caution should be used when extrapolating results to populations with different dietary characteristics. Finally, the magnitudes of the observed dietary changes and biochemical and clinical effects are not necessarily generalizable to populations with less severe or less frequent pain.
4.6. Conclusion
Targeted dietary manipulation of n-3 and n-6 fatty acids reduced pain and improved quality of life in this population with chronic headaches, and could represent a novel strategy for treating chronic pain in general. Future trials evaluating clinical efficacy and elucidating biochemical mechanisms in populations with chronic pain are warranted.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in the trial, and acknowledge the following individuals for their research assistance: Marjorie Busby for expertise with study design; Beth Fowler, Carol Carr, Regina McCoy, and Tim McCaskill for design and functionality of the study Website; Meg Mangan for 24-hour recall data collection and management; Jim Loewke and Duk Hyun for fatty acid analyses; and Sharon Majchrzak-Hong for data management support. This project was supported by the Mayday Fund (primary source); the UNC Research Fellowship in Complementary and Alternative Medicine (grant T32-AT003378, National Center for Complementary and Alternative Medicine [NCCAM], National Institutes of Health [NIH]); the North Carolina Clinical and Translational Sciences Institute (grant UL1RR025747, National Center for Research Resources [NCRR], NIH); the UNC Nutrition Obesity Research Center, CHAI Core (grant DK056350, National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK], NIH); and the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.pain.2013.07.028.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Conflict of interest statement
The authors declare that they have no competing interests. The authors alone are responsible for the content and writing of the article.
References
- 1.Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia. 2012;32:822–33. doi: 10.1177/0333102412451360. [DOI] [PubMed] [Google Scholar]
- 2.Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandins in migraine: update. Curr Opin Neurol. 2013;26:269–75. doi: 10.1097/WCO.0b013e328360864b. [DOI] [PubMed] [Google Scholar]
- 3.Bigal ME, Rapoport AM, Lipton RB, Tepper SJ, Sheftell FD. Assessment of migraine disability using the migraine disability assessment (MIDAS) questionnaire: a comparison of chronic migraine with episodic migraine. Headache. 2003;43:336–42. doi: 10.1046/j.1526-4610.2003.03068.x. [DOI] [PubMed] [Google Scholar]
- 4.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–62. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 6.Brown RT, Zuelsdorff M, Fleming M. Adverse effects and cognitive function among primary care patients taking opioids for chronic nonmalignant pain. J Opioid Manage. 2006;2:137–46. doi: 10.5055/jom.2006.0023. [DOI] [PubMed] [Google Scholar]
- 7.Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH, Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696–703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton A, Kornstein S, Prakash A, Mallinckrodt C, Wohlreich M. Changes in sexual functioning associated with duloxetine, escitalopram, and placebo in the treatment of patients with major depressive disorder. J Sex Med. 2007;4:917–29. doi: 10.1111/j.1743-6109.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 9.Coeytaux RR, Kaufman JS, Chao R, Mann JD, Devellis RF. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol. 2006;59:374–80. doi: 10.1016/j.jclinepi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Coeytaux RR, Linville JC. Chronic daily headache in a primary care population: prevalence and headache impact test scores. Headache. 2007;154:477–512. doi: 10.1111/j.1526-4610.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 11.Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: psychological and neurobiological mechanisms. PAIN®. 2013;154:511–4. doi: 10.1016/j.pain.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 13.Di Marzo V, Blumberg PM, Szallasi A. Endovanilloid signaling in pain. Curr Opin Neurobiol. 2002;12:372–9. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 14.Dodick DW. Chronic daily headache. N Engl J Med. 2006;354:158–65. doi: 10.1056/NEJMcp042897. [DOI] [PubMed] [Google Scholar]
- 15.Fang X, Kaduce TL, Spector AA. 13-(S)-hydroxyoctadecadienoic acid (13-HODE) incorporation and conversion to novel products by endothelial cells. J Lipid Res. 1999;40:699–707. [PubMed] [Google Scholar]
- 16.Feldstein AE, Lopez R, Tamimi TA-R, Yerian L, Chung Y-M, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–54. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friesen RW, Innis SM. Linoleic acid is associated with lower long-chain n-6 and n-3 fatty acids in red blood cell lipids of Canadian pregnant women. Am J Clin Nutr. 2010;91:23–31. doi: 10.3945/ajcn.2009.28206. [DOI] [PubMed] [Google Scholar]
- 18.Harris WS. The omega-3 index: from biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;11:411–7. doi: 10.1007/s11883-009-0062-2. [DOI] [PubMed] [Google Scholar]
- 19.Hwang SH, Wecksler AT, Wagner K, Hammock BD. Rationally designed multitarget agents against inflammation and pain. Curr Med Chem. 2013;20:1783–99. doi: 10.2174/0929867311320130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang SW, Cho H, Kwak J, Lee S-Y, Kang C-J, Jung J, Cho S, Min KH, Suh Y-G, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci. 2000;97:6155–60. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karanian JW, Kim HY, Salem N., Jr Inhibitory effects of n-6 and n-3 hydroxy fatty acids on thromboxane (U46619)-induced smooth muscle contraction. J Pharmacol Exp Ther. 1994;270:1105–9. [PubMed] [Google Scholar]
- 22.Kawabata A. Prostaglandin E2 and pain—an update. Biol Pharm Bull. 2011;34:1170–3. doi: 10.1248/bpb.34.1170. [DOI] [PubMed] [Google Scholar]
- 23.Kawata AK, Coeytaux RR, Devellis RF, Finkel AG, Mann JD, Kahn K. Psychometric properties of the HIT-6 among patients in a headache-specialty practice. Headache. 2005;45:638–43. doi: 10.1111/j.1526-4610.2005.05130.x. [DOI] [PubMed] [Google Scholar]
- 24.Kitson AP, Stroud CK, Stark KD. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010;45:209–24. doi: 10.1007/s11745-010-3391-6. [DOI] [PubMed] [Google Scholar]
- 25.Kosinski M, Bayliss MS, Bjorner JB, Ware JE, Jr, Garber WH, Batenhorst A, Cady R, Dahlöf CGH, Dowson A, Tepper S. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12:963–74. doi: 10.1023/a:1026119331193. [DOI] [PubMed] [Google Scholar]
- 26.Lampl C, Voelker M, Steiner TJ. Aspirin is first-line treatment for migraine and episodic tension-type headache regardless of headache intensity. Headache. 2012;52:48–56. doi: 10.1111/j.1526-4610.2011.01974.x. [DOI] [PubMed] [Google Scholar]
- 27.Lands WE, Libelt B, Morris A, Kramer NC, Prewitt TE, Bowen P, Schmeisser D, Davidson MH, Burns JH. Maintenance of lower proportions of (n - 6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n - 3) fatty acids. Biochim Biophys Acta. 1992;1180:147–62. doi: 10.1016/0925-4439(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 28.Lands WE, Morris A, Libelt B. Quantitative effects of dietary polyunsaturated fats on the composition of fatty acids in rat tissues. Lipids. 1990;25:505–16. doi: 10.1007/BF02537156. [DOI] [PubMed] [Google Scholar]
- 29.Macintosh BA, Ramsden CE, Faurot KR, Zamora D, Mangan M, Hibbeln JR, Mann JD. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. Br J Nutr. 2013;110:559–68. doi: 10.1017/S0007114512005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58:1476–84. doi: 10.1373/clinchem.2012.190199. [DOI] [PubMed] [Google Scholar]
- 31.Meng ID, Cao L. From migraine to chronic daily headache: the biological basis of headache transformation. Headache. 2007;47:1251–8. doi: 10.1111/j.1526-4610.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 32.Morisseau C, Inceoglu B, Schmelzer K, Tsai H-J, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–90. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura MT, Nara TY. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukotrienes Essent Fatty Acids. 2003;68:145–50. doi: 10.1016/s0952-3278(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 34.Park C-K, Xu Z-Z, Liu T, Lü N, Serhan CN, Ji R-R. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. 2011;31:18433–8. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–26. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci. 2009;106:18820–4. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradalier A, Bakouche P, Baudesson G, Delage A, Cornaille-Lafage G, Launay JM, Biason P. Failure of omega-3 polyunsaturated fatty acids in prevention of migraine: a double-blind study versus placebo. Cephalalgia. 2001;21:818–22. doi: 10.1046/j.1468-2982.2001.218240.x. [DOI] [PubMed] [Google Scholar]
- 38.Pryse-Phillips W. Assessment and management of disability in chronic daily headache. Curr Pain Headache Rep. 2005;9:53–8. doi: 10.1007/s11916-005-0075-7. [DOI] [PubMed] [Google Scholar]
- 39.Rahm JJ, Holman RT. Effect of linoleic acid upon the metabolism of linoleic acid. J Nutr. 1964;84:15–9. doi: 10.1093/jn/84.1.15. [DOI] [PubMed] [Google Scholar]
- 40.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol. 2012;189:1036–42. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsden CE, Mann JD, Faurot KR, Lynch C, Imam ST, MacIntosh BA, Hibbeln JR, Loewke J, Smith S, Coble R, Suchindran C, Gaylord SA. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials. 2011;12:97. doi: 10.1186/1745-6215-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung Y-M, Berk M, Douglas Mann J. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukotrienes Essent Fatty Acids. 2012;87:135–41. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–71. [PubMed] [Google Scholar]
- 44.Salat K, Moniczewski A, Librowski T. Transient receptor potential channels – emerging novel drug targets for the treatment of pain. Curr Med Chem. 2013;20:1409–36. doi: 10.2174/09298673113209990107. [DOI] [PubMed] [Google Scholar]
- 45.Saper JR. Chronic daily headache: transformational migraine, chronic migraine, and related disorders. Curr Neurol Neurosci Rep. 2008;8:100–7. doi: 10.1007/s11910-008-0017-y. [DOI] [PubMed] [Google Scholar]
- 46.Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24:69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 47.Schulman E. Refractory migraine – a review. Headache. 2013;53:599–613. doi: 10.1111/head.12047. [DOI] [PubMed] [Google Scholar]
- 48.Serhan CN, Dalli J, Karamnov S, Choi A, Park C-K, Xu Z-Z, Ji R-R, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–65. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siangjong L, Gauthier KM, Pfister SL, Smyth EM, Campbell WB. Endothelial 12(S)-HETE vasorelaxation is mediated by thromboxane receptor inhibition in mouse mesenteric arteries. Am J Physiol Heart Circ Physiol. 2013;304:H382–92. doi: 10.1152/ajpheart.00690.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53:427–36. doi: 10.1111/head.12074. [DOI] [PubMed] [Google Scholar]
- 51.Stark KD. The percentage of n-3 highly unsaturated fatty acids in total HUFA as a biomarker for omega-3 fatty acid status in tissues. Lipids. 2008;43:45–53. doi: 10.1007/s11745-007-3128-3. [DOI] [PubMed] [Google Scholar]
- 52.Sun T, Yu E, Yu L, Luo J, Li H, Fu Z. LipoxinA(4) induced antinociception and decreased expression of NF-κB and pro-inflammatory cytokines after chronic dorsal root ganglia compression in rats. Eur J Pain. 2012;16:18–27. doi: 10.1016/j.ejpain.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2) 2004;24:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 54.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–13. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wen H, Östman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. J Biol Chem. 2012;287:13868–76. doi: 10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whyte CA, Tepper SJ. Adverse effects of medications commonly used in the treatment of migraine. Expert Rev Neurother. 2009;9:1379–91. doi: 10.1586/ern.09.47. [DOI] [PubMed] [Google Scholar]
- 58.Xu Z-Z, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji R-R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–7. doi: 10.1038/nm.2123. 1p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yurekli VA, Akhan G, Kutluhan S, Uzar E, Koyuncuoglu HR, Gultekin F. The effect of sodium valproate on chronic daily headache and its subgroups. J Headache Pain. 2008;9:37–41. doi: 10.1007/s10194-008-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE. The role of endocannabinoids in pain modulation. Fundam Clin Pharmacol. 2013;27:64–80. doi: 10.1111/fcp.12008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.