Abstract
Two classical antifolates, a 2,4-diamino-5-substituted furo[2,3-d]pyrimidine and a 2-amino-4-oxo-6-substituted pyrrolo[2,3-d]pyrimidine, were synthesized as potential inhibitors of dihydrofolate reductase (DHFR) and thymidylate synthase (TS). The syntheses were accomplished by condensation of 2,6-diamino-3(H)-4-oxo-pyrimidine with α-chloro-ketone 21 to afford two key intermediates 23 and 24, followed by hydrolysis, coupling with l-glutamate diethyl ester and saponification of the diethyl ester to afford the classical antifolates 13 and 14. Compounds 13 and 14 with a single carbon atom bridge are both substrates for folylpoly-γ-glutamate synthetase (FPGS), the enzyme responsible for forming critical poly-γ-glutamate antifolate metabolites with increased potency and/or increased cell retention. Compound 14 is a highly efficient FPGS substrate demonstrating that 2,4-diamino-5-substituted furo[2,3-d]pyrimidines are important lead structures for the design of antifolates with FPGS substrate activity. It retains inhibitory potency for DHFR and TS compared to the two atom bridged analog 5. Compound 13 is a poor inhibitor of purified DHFR and TS, and both 13 and 14 are poor inhibitors of the growth of CCRF-CEM human leukemia cells in culture, indicating that single carbon bridged compounds in these series though conducive to FPGS substrate activity were not potent inhibitors.
Keywords: Pyrrolo[2,3-d]pyrimidines; Antifolates; Dihydrofolate reductase
1. Introduction
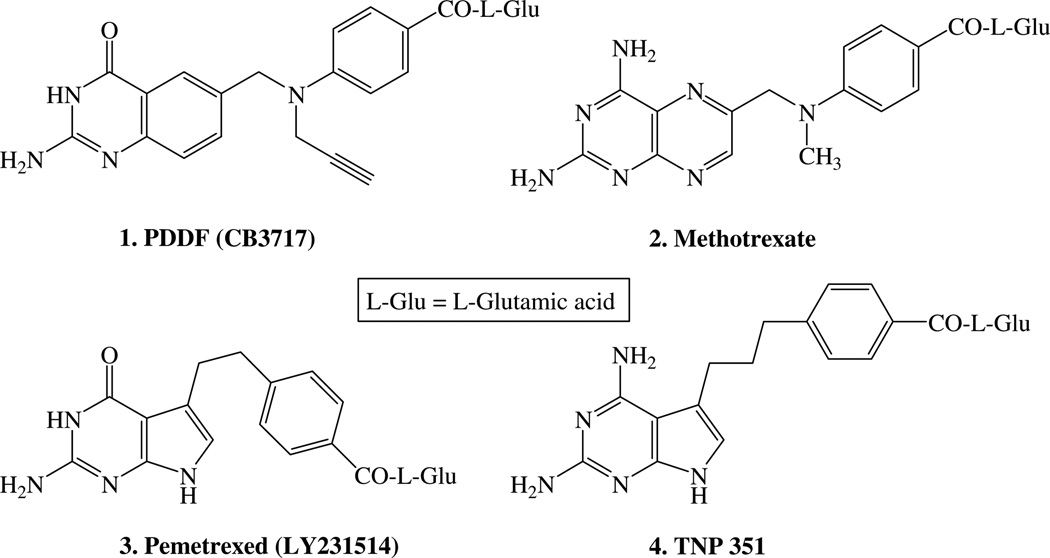
Folate metabolism is an attractive chemotherapeutic target since it plays a crucial role in the biosynthesis of nucleic acid precursors.2 Tetrahydrofolate (FH4), the key component of folate metabolism, serves as a cofactor to carry one-carbon units that are important in physiological pathways. FH4 is formed by the NADPH-dependent reduction of 7,8-dihydrofolate (FH2) by the enzyme dihydrofolate reductase (DHFR).3 Thymidylate synthase (TS) is a crucial enzyme that catalyzes the conversion of 2′-deoxyuridine-5′-monophosphate (dUMP) to 2′-deoxythymidine-5′-monophosphate (dTMP) utilizing the cofactor 5,10-methylenetetrahydrofolate (5,10-CH2FH4) as the source of the one carbon as well as the reductant. This is the only de novo synthesis of dTMP, and hence, TS and DHFR play a pivotal role in DNA biosynthesis and cell replication.4 Antifolate inhibitors of TS and DHFR have found clinical utility as antitumor, antibacterial, and antiprotozoan agents.2 Several classical antifolates including N10-propargyl-5,8-dideazafolate (1, PDDF),5 methotrexate (2, MTX), and pemetrexed (3) have been evaluated as antitumor agents.5–7 (Fig. 1).
Figure 1.
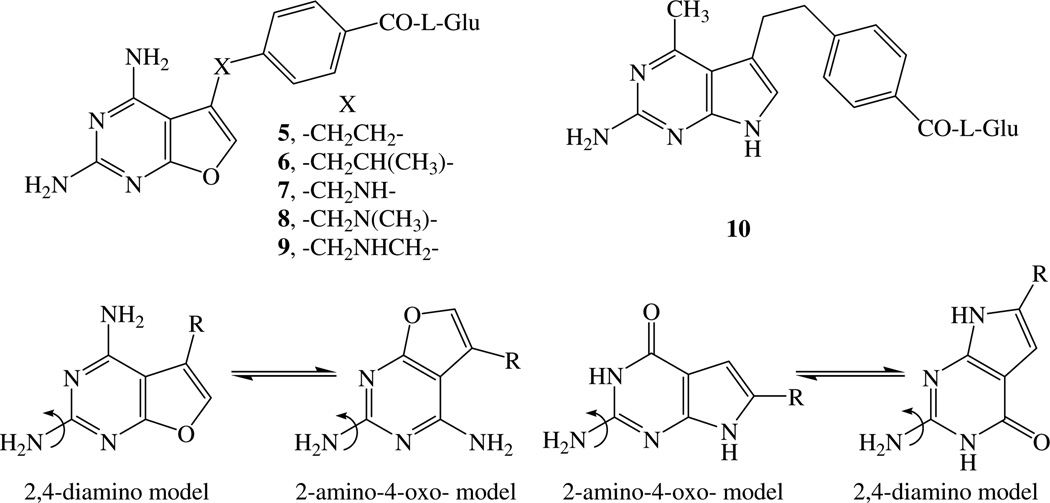
Both 1 PDDF and 2 MTX are 6-6 bicyclic classical antifolates. Recently, some 6-5 bicyclic pyrrolo[2,3-d]pyrimidines such as compounds 3 and 4 TNP3518,9 were also reported as potent antifolates. Gangjee et al.10–12 reported a series of novel furo[2,3-d]pyrimidines 5–9 as potent antifolates. A 2,4-diamino-substituted pyrimidine ring is considered important for potent DHFR inhibition, while a 2-substituted-4-oxopyrimidine ring is considered important for TS inhibition.2,13,14 Gangjee et al.10,11 suggested a dual DHFR–TS inhibitor model and proposed two binding modes for the 2-amino-4-oxo-pyrrolo[2,3-d]pyrimidine system as well as the 2,4-diamino-furo[2,3-d]pyrimidine (Fig. 2), Gangjee et al.15 demonstrated that compound 10, a 4-methyl analog of compound 3, binds in the alternate mode to DHFR.
Figure 2.
Since dual inhibitors can act two different sites (TS and DHFR), such inhibitors would afford dual mechanism of action in a single agent without the pharmacokinetic disadvantages of two separate agents. Thus, it was of interest to develop additional dual DHFR–TS inhibitors as antitumor agents. Gangjee et al.10–12 reported the synthesis of novel, classical 2,4-diamino-5-substitutedfuro[2,3-d]pyrimidines 5–9 as antifolates which have a two-, or three-atom bridge connecting the furo[2,3-d]pyrimidine ring system to the benzoyl-l-glutamic acid (l-Glu). Compounds 5–8 showed moderate to high potency against DHFR (IC50 = 1.0 × 10−6 to 1.0 × 10−8 M), whereas compound 9 was less active against DHFR (IC50 = 1.0 × 10−5 M). Compound 10 however showed excellent dual DHFR–TS inhibitory activities.15
Some of these analogs were also significantly cytotoxic to the growth of tumor cells in culture (EC50 = 1.0 × 10−7 to 1.0 × 10−8 M). This cytotoxicity was attributed to the efficient poly(γ-glutamylation) by the enzyme folypoly-γ-glutamate synthetase (FPGS). Polyglutamylation via FPGS is an important mechanism for trapping folates and classical antifolates within the cell, thus maintaining high intracellular concentrations which allow for increased antitumor activity. In addition, for some folate-dependent enzymes such as TS, polyglutamylation can also afford enhanced enzyme inhibition.16
Classical single-carbon bridged 2,4-diamino-5-substituted- furo[2,3-d]pyrimidines have not been explored in the literature as potential antifolates. Several single-atom bridged antifolates such as pyrrolo[2,3-d]pyrimidines (11 and 12) (Fig. 3) have been reported as potent inhibitors of TS and DHFR.17,18 These analogs demonstrated that a one-atom bridge in 6-5 bicyclic systems can provide potent inhibitory activity. In order to allow for a direct comparison of furo[2,3-d]pyrimidines with other reported 6-5 ring fused classical antifolates, and as part of a structure–activity relationship study on the nature and length of the bridge of classical 2,4-diamino-5-substituted-furo[2,3-d]pyrimidine antifolates, we designed and synthesized single-carbon atom bridged classical 2,4-diamino-5-substituted furo[2,3-d]pyrimidine 14 (Fig. 3). Compound 14 is a truncated analog of the classical two-carbon bridged 2,4-diamino-5-substituted-furo[2,3-d]pyrimidine parent compound 5.
Figure 3.
The synthetic methodology adopted for compound 14 also afforded the 2-amino-4-oxo-6-substituted-pyrrolo[2,3-d]pyrimidine intermediate for the synthesis of compound 13. Thus it was also of interest to synthesize the classical one-carbon bridged 2-amino-4-oxo-6-substituted pyrrolo[2,3-d]pyrimidine analog, compound 13 (Fig. 3), to determine the effect on biological activity of moving the substituent from the 5-position as in 11 to the 6-position in 13.
2. Results and discussion
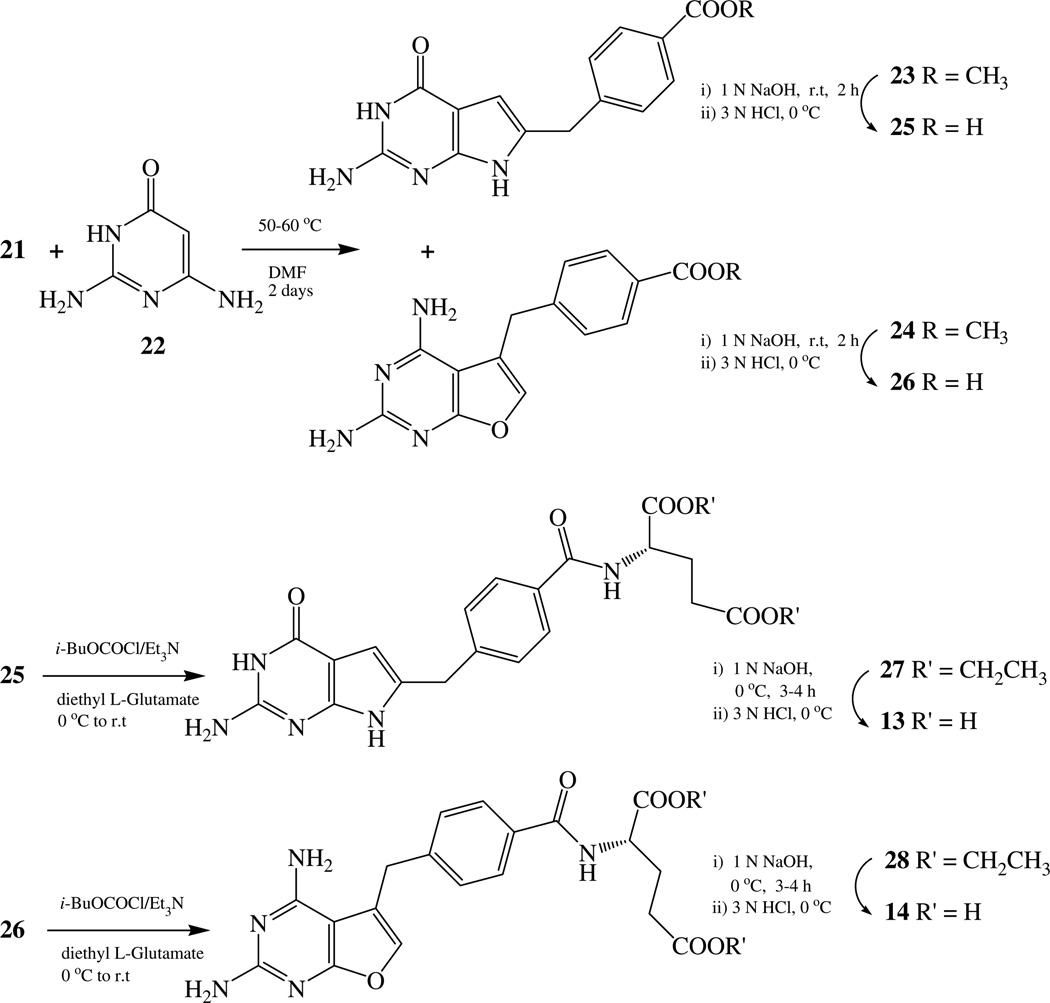
It was anticipated that condensation of 2,6-diamino-3(H)-4-oxo-pyrimidine 22 with an appropriate α-chloro ketone 21 could form both the 2-amino-4-oxo-6-substituted pyrrolo[2,3-d]pyrimidine 23 and the 2,4-diamino-5-substituted-furo[2,3-d]pyrimidine 24 in one step.19 For the synthesis of the α-halo ketone 21 from the corresponding α-diazomethane,20 the corresponding [4-(methoxycarbonyl)phenyl]acetic acid 18 was required. The literature provides several methods for the synthesis of 18.21,22 We elected to use the Arndt-Eistert rearrangement to homologate the carbon chain from the diazoketone 17 to 18 starting from commercially available mono-methyl terephthalate 15.23 Thus 15 was refluxed in thionyl chloride and benzene to give the acid chloride 16, which was converted to diazoketone 17 after being treated with ethereal diazomethane.24 Compound 17 was converted to the acid 18 via an Arndt-Eistert rearrangement. Conversion of the acid 18 to the corresponding acid chloride 19 followed by treatment with ethereal diazomethane gave the diazoketone 20, which was immediately treated with concentrated HCl to afford the α-chloro ketone 21 (Scheme 1).25
Scheme 1.
With key intermediate 21 in hand, several variations were attempted to optimize the annulation of 21 with 22 using different solvents and temperature conditions both with and without bases (Scheme 2). The reaction was finally optimized at 50–60 °C for 2 days (Scheme 2). Compound 21 condensed with 2,6-diamino-3(H)-4-oxo-6-pyrimidine 22 to afford a mixture of two products: 2-amino-3(H)-4-oxo-6-substituted-pyrrolo[2,3-d]pyrimidine 23 and 2,4-diamino-5-substituted-furo[2,3-d]pyrimidine 24. The mixture was separated via column chromatography to give 33% and 27% yields of 23 and 24, respectively. Hydrolysis of 23 and 24 afforded the corresponding acids 25 and 26, respectively, which were coupled with diethyl l-glutamate using isobutyl chloroformate to form the corresponding amides 27 and 28 in 65% and 60% yield, respectively. Hydrolysis of compounds 27 and 28 at 0 °C with 1N NaOH gave the target compounds 13 (56%) and 14 (60%) (Scheme 2).26
Scheme 2.
Compounds 13 and 14 were evaluated as inhibitors of Escherichia coli (ec), Toxoplasma gondii (tg), and recombinant human (rh) DHFR, and also as inhibitors of ecTS and rhTS. The inhibitory potencies (IC50) are reported in Table 1 and compared with previously reported values for compounds 5–8, MTX and PDDF, a first generation classical TS inhibitor. Compound 14 had reasonably potent DHFR and TS inhibitory activity compared with the parent two-carbon bridged furo[2,3-d]pyrimidine 5. However, the 6-substituted single-carbon bridged pyrrolo[2,3-d]pyrimidine 13 was devoid of DHFR or TS inhibitory activity. This indicated that the 5-substituted single-atom bridged 2,4-diaminofuro[2,3-d]pyrimidine retains inhibitory activity for DHFR at similar levels comparable with other two-atom bridged 2,4-diamino-furo[2,3-d]pyrimidines. However, the 6-substituted single-atom bridged 2-amino-4-oxo-pyrrolo[2,3-d]pyrimidine is not conductive to DHFR or TS inhibition.
Table 1.
Inhibitory concentration (IC50 in µM) against isolated DHFR and TSa
2.1. In vitro human tumor cell growth inhibition
Growth inhibitory potency of 13 and 14 were compared to that of MTX in a continuous exposure against the CCRF-CEM human lymphoblastic leukemia and a series of MTX-resistant sublines in culture during continuous exposure (Table 2). Compounds 13 and 14 were both at least 1250-fold less potent than MTX as growth inhibitors of CCRF-CEM. The MTX-resistant sublines also showed low potency of these two drugs demonstrating that none of the common mechanisms of MTX-resistance show collateral sensitivity to either agent.
Table 2.
Growth inhibition of parental CCRF-CEM human leukemia cells and sub-lines with single, defined mechanisms of MTX resistance during continuous (0–120 h) exposure to MTX, 13, or 14
| Drug | EC50 (nM) | |||
|---|---|---|---|---|
| CCRF-CEM | R1a (↑ DHFR) | R2b (⇓ Uptake) | R30dmc (⇓ Glun) | |
| MTX | 12.7 ± 3.3 | 915 ± 285 | 2600 ± 100 | 13.1 ± 3.4 |
| 13 | ≈18,000 | ≥15,000 | >10,000 | >20,000 |
| 14 | ≈16,000 | >20,000 | >10,000 | ≈20,000 |
Values presented are average ± SD for n > 2 or average ± range for n = 2.
CCRF-CEM subline resistant to MTX solely as a result of a 20-fold increase in wild-type DHFR protein and activity.27
CCRF-CEM subline resistant as a result of decreased uptake of MTX.28
CCRF-CEM subline resistant to MTX solely as a result of decreased polyglutamylation; this cell line has 1% of the FPGS specific activity (measured with MTX as the folate substrate) of parental CCRF-CEM.29
2.2. FPGS substrate activity
Since these structures contain the intrinsic glutamic acid residue of ‘classical’ antifolates, they were evaluated in vitro as substrates for recombinant human folylpolyglutamate synthetase (FPGS) and compared to AMT, a good substrate for FPGS. It is possible that poor metabolism to polyglutamate metabolites contributes to their low growth inhibitory potency. The data (Table 3) show that both 13 and 14 are substrates for human FPGS, however they differ markedly in their efficiency. Compound 14 has both a lower Km and Vmax than AMT, but is overall about 2-fold more efficient than AMT. In contrast, although the Km of 13 is near that of AMT, it has a very diminished Vmax and is 10-fold less efficient a substrate than AMT. Thus, the low growth inhibitory potency of 13 may be attributed, in part, to low metabolism to polyglutamates, but this is an unlikely explanation for the low activity of 14.
Table 3.
Activity of folate analogs as substrates for recombinant human FPGSa
| Substrate | Km (µM) | Vmax, relb | Vmax, rel/Km | n |
|---|---|---|---|---|
| AMT | 4.4 ± 1.1 | 1.00 | 0.23 | 6 |
| 13 | 4.7 ± 0.4 | 0.11 ± 0 | 0.023 | 3 |
| 14 | 1.6 ± 0.2 | 0.74 ± 0 | 0.48 | 2 |
FPGS substrate activity was determined as described in Section 3 at 2 mM l-[3H]glutamate. Values presented are the average ± SD if n ≥ 3 and are average ± range for n = 2.
Vmax, rel is calculated based on the apparent Vmax of a substrate relative to the apparent Vmax of AMT within the same experiment.
Compound 14 is the one carbon-bridge analog of the two carbon-bridge compound 5 described previously.11 Although polyglutamates of 5 are clearly involved in its mechanism of action, 14, which contains the shorter bridge, is a 7-fold more efficient human FPGS substrate than 5. This indicates that the shorter length of the bridge is more conducive to FPGS substrate activity. However, this activity does not translate into greater tumor growth inhibitory potency since 5 is >34-fold more potent as an inhibitor of CCRF-CEM cell growth in continuous exposure. The mechanism of action of 5 appears to involve DHFR inhibition;11 because of its low potency, however, the target of 14 could not be elucidated. The biological activity data with 13 and 14 suggest that one-carbon bridges are too short to allow one or more key determinants of antitumor activity in these two classes of agents.
In summary, truncation of the two-carbon bridge of 2,4-diamino-furo[2,3-d]pyrimidine to a single carbon leads to an enhanced efficiency for FPGS, a slight decrease in DHFR and TS inhibitory activities but a significant loss of cytotoxicity to CCRF-CEM cells in culture compared to the two-carbon bridged analog, thus indicating that the distance between the pyrimidine ring and the side chain l-glutamic acid in furo[2,3-d]pyrimidines, though not detrimental for FPGS activity, is important for activity against the growth of tumor cells in culture. In addition, 6-substituted pyrrolo[2,3-d]pyrimidines with a one-carbon atom bridge are substrates for FPGS, they are essentially inactive, however, as antifolates compared to their 5-substituted regioisomers indicating that the position of attachment to the heterocycle is also important for TS and/or DHFR inhibitory activity.
3. Experimental
Melting points were determined on a Mel-Temp II melting point apparatus with FLUKE 51 K/J electronic thermometer and are uncorrected. Nuclear magnetic resonance spectra for proton (1H) were recorded on a Bruker WH-300 (300 MHz) spectrometer. Chemical shift values are expressed in ppm (parts per million) relative to tetramethylsilane as internal standard; s = singlet, d = double, t = triplet, q = quartet, m = multiplet, br = broad singlet. The relative integrals of peak areas agreed with those expected for the assigned structures. High-resolution mass spectra (HRMS), using Electron Impact (EI), were recorded on a VG AUTOSPEC (Fisons Instruments) micromass (EBE Geometry) double focusing mass spectrometer. Thin-layer chromatography (TLC) was performed on POLYGRAM Sil G/UV254 silica gel plates with fluorescent indicator, and the spots were visualized under 254 and 366 nm illumination. Proportions of solvents used for TLC were by volume. Column chromatography was performed on 230–400 mesh silica gel purchased from Aldrich Chemical Co., Milwaukee, WI. All evaporations were carried out in vacuo with a rotary evaporator. Analytical samples were dried in vacuo (0.2 mmHg) in an Abderhalden drying apparatus over P2O5 at 75–110 °C. Elemental analysis was performed by Altlantic Microlabs, Norcross, GA. Element compositions are within ±0.4% of calculated values. Fractional moles of water or organic solvents frequently found in some analytical samples could not be prevented despite 24–48 h of drying in vacuo and were confirmed where possible by their presence in the 1H NMR spectra. All solvents and chemicals were purchased from Aldrich Chemical Co. and Fisher Scientific and were used as received.
3.1. [4-(Methoxycarbonyl)phenyl]acetic acid (18)
Mono-methyl terephthalate 15 (2 g, 5 mmol) was dissolved in benzene (10 mL) and thionyl chloride (10 mL) was added. The resulting reaction mixture was refluxed for 1 h. After removing the volatiles, the crude acyl acid chloride 16 was dissolved in anhydrous ethyl ether, and then added dropwise at 0 °C to a solution of diazomethane (made from N-methyl-N-nitrosourea) and triethyl amine (1.5 mL) in ether. The reaction was maintained for 16 h at room temperature, excess diazomethane was decomposed with l mL of acetic acid, and the salt was filtered and the solution evaporated to dryness to afford 17. To a stirred suspension of silver acetate (1.2 g) in 30 mL of water was added a solution of the diazo compound 17 in 30 mL of 1,4-dioxane. The reaction mixture was heated to reflux for 1.5 h, and then l.2 g of sodium carbonate was added. The solid obtained was filtered and extracted with chloroform (3× 25 mL). The combined chloroform extract was dried (Na2SO4), filtered, and the solvent was evaporated under reduced pressure. The residue was recrystallized from ethyl acetate to afford white needles of 18 (910 mg). The yield over three steps was 43%; mp: 107–110 °C (lit. 110–113 °C);21 TLC: Rf = 0.29 (hexane/EtOAc = 3:1); 1H NMR (DMSO-d6): δ 3.69 (s, 2H, CH2), 3.82 (s, 3H, OCH3), and 7.32–8.11 (dd, 4H, Ar-H). This compound was used directly for the next step without further purification.
3.2. Methyl 4-(3-chloro-2-oxopropyl)benzoate (21)
A solution of 4-methoxycarbonylphenyl acetic acid 18 (1.0 g, 5 mmol) in 5 mL of benzene and 5 mL of thionyl chloride was refluxed for 1 h and the solvents evaporated under reduced pressure. The resulting acid chloride 19 was dissolved in 8 mL of dry ether and added drop-wise to 30–40 mL of ethereal diazomethane (about 13 mmol, from 20 mmol of N-methyl-N-nitrosourea) at 0–5 °C. After 1 h at room temperature, concentrated HCl was added dropwise to the solution and then heated to reflux at 70–80 °C for 1 h. After cooling to room temperature, the ether layer was separated out. The organic layer was washed with water, saturated sodium bicarbonate solution, water, and dried (Na2SO4). The dried solvent was evaporated under reduced pressure and the residue was loaded on a silica gel column (15 × 150 mm) and eluted with hexane/EtOAc = 5:1. The desired fraction was pooled and evaporated to afford 21 (640 mg), yield over three steps 57%; mp: 67.5–70 °C; TLC: Rf = 0.69 (hexane/EtOAc = 3:1); 1H NMR (DMSO-d6): δ 3.84 (s, 3H, OCH3), 3.99 (s, 2H, CH2), 4.66 (s, 2H, CH2), and 7.23–7.99 (dd, 4H, Ar-H). This compound was used directly for the next step without further purification.
3.3. Methyl 4-[(2-amino-4-oxo-4,7-dihydro-3H-pyrrolo [2,3-d]pyrimidin-6-yl)methyl]benzoate (23) and methyl 4-[(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)methyl]benzoate (24)
Methyl 4-(3-chloro-2-oxopropyl)benzoate 21 (610 mg, 1.7 mmol) was placed in a 50 mL flask, and 2,6-diaminopyrimidin-4-one 22 (214 mg, 1.7 mmol) and 5 mL of DMF were added. The reaction mixture was stirred at 50–60 °C for 2 days. TLC showed two major products: Rf = 0.88 and 0.78 (CHCl3/MeOH = 5:1). To the reaction mixture was added 1 g of silica gel and evaporated to dryness under reduced pressure to form a plug. This plug was loaded on the top of a silica gel column (45 × 150 mm) and eluted with 4% methanol in chloroform. The desired fractions containing the product (Rf = 0.88) were pooled and evaporated to afford 220 mg of an off-white solid 24 (27%); mp: 200–222 °C. 1H NMR (DMSO-d6): δ 3.82 (s, 3H, OCH3), 4.09 (s, 2H, CH2), 6.02 (s, 2H, NH2), 6.25 (s, 2H, NH2), 7.07 (s, 1H, CH), and 7.39–7.91 (dd, 4H, Ar-H). Anal. Calcd for C15H14N4O3: C, 60.40; H, 4.73; N, 18.78. Found: C, 60.32; H, 4.85; N, 18.57.
Changing the eluent to 5% methanol in chloroform afforded fractions containing the desired product (Rf = 0.78). These fractions were pooled and evaporated to afford 270 mg of an off-yellow solid 23 (33%); mp: 237–241 °C; 1H NMR (DMSO-d6): δ 3.90 (s, 3H, OCH3), 3.96 (s, 2H, CH2), 5.91 (s, 1H, CH), 6.01 (s, 2H, NH2), 7.35–7.89 (dd, 4H, Ar-H), 10.18 (s, 1H, NH) and 10.97 (s, 1H, NH). Anal. Calcd for C15H14N4O3·0.5H2O: C, 58.63; H, 4.92; N, 18.23. Found: C, 58.30; H, 4.65; N, 18.03.
4. General procedure for the synthesis of 25 and 26
To a solution of 23 or 24 (250 mg, 0.8 mmol) in 10 mL of ethanol was added aqueous 1 N NaOH and the mixture stirred at 80 °C for 10 h. TLC (CHCl3/MeOH = 5:1) showed the disappearance of the starting material (Rf = 0.78 or 0.88) and formation of one major spot at the origin. The solution was evaporated to dryness under reduced pressure, and the sodium salt was dissolved in 10 mL of water and carefully acidified to pH 4 with dropwise addition of 3 N HCl. The resulting suspension was left at 0 °C for 2 h and filtered. The residue was washed with cold water, acetone, and, ether and dried in vacuum to afford the free acid.
4.1. 4-[(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)methyl]benzoic acid (25)
This compound was synthesized in 91% yield using the general procedure starting from 23; mp: 270 °C (dec); 1H NMR (DMSO-d6): δ 3.89 (s, 2H, CH2), 5.90 (s, 1H, CH), 6.02 (s, 2H, NH2), 7.33–7.87 (dd, 4H, Ar-H), 10.18 (s, 1H, NH), 10.96 (s, 1H, NH) and 12.85 (s, 1H, COOH). This compound was used directly for the next step without further purification.
4.2. 4-[(2, 4-Diaminofuro[2,3-d]pyrimidin-5-yl)methyl] benzoic acid (26)
This compound was synthesized in 85% yield using the general procedure starting from 24; mp: >250 °C (dec); 1H NMR (DMSO-d6): δ 4.08 (s, 2H, CH2), 6.05 (s, 2H, NH2), 6.28 (s, 2H, NH2), 7.07 (s, 1H, CH), 7.36–8.00 (dd, 4H, Ar-H) and 12.90 (s, 1H, COOH). This compound was used directly for the next step without further purification.
5. General procedure for the synthesis of 27 and 28
To a suspension of the acid 25 or 26 (150 mg, 0.5 mmol) in anhydrous DMF (10 mL) under nitrogen was added triethylamine (0.21 mL, 1.5 mmol) and the suspension heated to form a solution. The solution was cooled to 0 °C and isobutyl chloroformate (0.13 mL, 1 mmol) was added, followed 15 min later by diethyl l-glutamate hydrochloride (180 mg, 0.75 mmol) and immediately followed by triethylamine (0.21 mL, 1.5 mmol). The reaction mixture was warmed slowly to room temperature and stirred for 12 h. At this time the reaction mixture was cooled to 0 °C and the activation steps described above were repeated using triethylamine (0.11 mL, 0.75 mmol), followed by isobutyl chloroformate (0.065 mL, 0.5 mmol). After stirring for 15 min at 0 °C, diethyl l-glutamate hydrochloride (90 mg, 0.38 mmol) was added followed immediately by triethylamine (0.11 mL, 0.75 mmol). The reaction mixture was stirred for 24 h at room temperature. TLC (CHCl3/ MeOH = 5:1) showed a new major spot was formed. The reaction mixture was evaporated to dryness under reduced pressure. The residue was suspended in water and the pH adjusted to 8 with ammonium hydroxide and stirred for 30 min. The suspension was filtered and the residue washed well with water, air dried, and dissolved in methanol. To this solution was added 500 mg of silica gel, the solvent was evaporated and the residue dried under reduced pressure to form a plug. The dry plug was loaded on a silica gel column (15 × 150 mm) and eluted using 2% methanol in chloroform. The fractions containing the desired product were pooled and the solvent evaporated to give the desired compounds.
5.1. Diethyl N-{4-[(2-amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)methyl] benzoyl}-l-glutamate (27)
This compound was synthesized in 65% yield using the general procedure starting from 25; mp: 156–158 °C; TLC: Rf = 0.48 (CHCl3/MeOH = 5:1); 1H NMR (DMSO-d6): δ 1.12–1.32 (m, 6H, 2× OCH2CH3), 2.00–2.44 (m, 4H, 2× CH2), 3.88 (s, 2H, CH2), 3.99–4.12 (m, 4H, 2× OCH2CH3), 4.41–4.50 (m, 1H, CH), 5.86 (s, 1H, CH), 6.06 (s, 2H, NH2), 7.32–7.81 (dd, 4H, Ar-H), 8.65 (d, 1H, NH), 10.21 (s, 1H, NH) and 10.96 (s, 1H, NH2). Anal. Calcd for C23H27N5O6: C, 58.84; H, 5.80; N, 14.92. Found: C, 58.49; H, 6.02; N, 15.05.
5.2. Diethyl N-{4-[(2,4-diaminofuro[2,3-d]pyrimidin-5-yl)methyl]benzoyl}-l-glutamate (28)
This compound was synthesized in 60% yield using the general procedure starting from 26; mp: 140–142 °C. Rf = 0.59 (CHCl3/MeOH = 5:1); 1H NMR (DMSO-d6): δ 1.13–1.21 (m, 6H, 2× OCH2CH3), 2.00–2.50 (m, 4H, 2× CH2), 3.69 (s, 2H, CH2), 4.02–4.10 (m, 4H, 2× OCH2CH3), 4.34–4.45 (m, 1H, CH), 6.04 (s, 2H, NH2), 6.21 (s, 2H, NH2), 7.05 (s, 1H, CH), 7.35–7.84 (dd, 4H, Ar-H) and 8.69 (d, 1H, NH). Anal. Calcd for C23H27N5O6·0.3H2O: C, 58.18; H, 5.75; N, 14.75. Found: C, 58.25; H, 5.93; N, 15.02.
6. General procedure for the synthesis of 13 and 14
To a solution of 27 or 28 (120 mg, 0.25 mmol) in methanol (5 mL) was added 1 N NaOH (2 mL) at 0 °C and mixture stirred at room temperature for 3–4 h. TLC (CHCl3/MeOH = 5:1) showed the disappearance of the starting material (Rf 0.48 or 0.59) and the formation of one major spot at the origin. The methanol was evaporated under reduced pressure, the residue was dissolved in water (5 mL), and the solution was cooled to 0 °C and acidified carefully to pH 4 with dropwise addition of 3 N HCl. The suspension was left at 0 °C for 24 h and filtered. The residue was washed well with cold water. The solid was recrystallized from methanol, filtered, washed with ether, and dried well in vacuum.
6.1. N-{4-[(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-6-yl)methyl]benzoyl}-l-glutamic acid (13)
This compound was synthesized in 56% yield using the general procedure starting from 27; mp: 192–194.5 °C; 1H NMR (DMSO-d6): δ 1.94–2.49 (m, 4H, 2× CH2), 3.88 (s, 2H, CH2), 4.37 (s, 1H, CH), 5.87 (s, 1H, CH), 6.00 (s, 2H, NH2), 7.32–7.81 (dd, 4H, Ar-H), 8.55 (d, 1H, NH), 10.16 (s, 1H, NH), 10.96 (s, 1H, NH) and 12.41 (s, br, 2H, 2× COOH). Anal. Calcd for C19H19N5O6·1.8H2O: C, 51.19; H, 5.11; N, 15.71. Found: C, 51.02; H, 4.82; N, 15.75.
6.2. N-{4-[(2,4-Diaminofuro[2,3-d]pyrimidin-5-yl)methyl] benzoyl}-l-glutamic acid (14)
This compound was synthesized from 28 in 60% yield; mp: 167.5–170.5 °C; 1H NMR (DMSO-d6): δ 1.89–2.36 (m, 4H, 2× CH2), 4.07 (s, 2H, CH2), 4.38 (m, 1H, CH), 6.06 (s, 2H, NH2), 6.29 (s, 2H, NH2), 7.04 (s, 1H, CH), 7.35–7.83 (dd, 4H, Ar-H), 8.56 (1H, NH), 12.35 (s, br, 2H, 2× COOH). Anal. Calcd for C19H19N5O6·H2O·0.2CH3OH: C, 52.67; H, 5.02; N, 16.00. Found: C, 52.84; H, 4.77; N, 15.64.
6.2.1. Drug preparation
Drug solutions were standardized using extinction coefficients. Extinction coefficients were determined for 13 (pH 1, λmax 227 nm (22,900); pH 7, λmax 255 nm (19,200); pH 13, λmax 248 nm (19,300)) and for 14 (pH 1, λmax−1 248 nm (20,300), λmax−2 301 nm (7,900); pH 7, λmax 247 nm (20,400); pH 13, λmax 248 nm (20,200)) and the extinction coefficients for methotrexate (MTX), a gift of Immunex (Seattle, WA), were from the literature.30 Aminopterin was purchased from Sigma Chemical Co. (St. Louis, MO). Other chemicals and reagents were of reagent grade or higher.
6.2.2. Cell lines and methods for measuring growth inhibitory potency
Cell lines were verified to be negative for Mycoplasma contamination (Mycoplasma Plus PCR primers, Stratagene, La Jolla, CA). The human T-lymphoblastic leukemia cell line CCRF-CEM31 and its MTX-resistant sublines R1,27 R2,28 and R30dm29 were cultured as described.29 R1 expresses 20-fold elevated levels of dihydrofolate reductase (DHFR), the target enzyme of MTX. R2 has dramatically reduced MTX uptake, but normal levels of MTX-sensitive DHFR. R30dm expresses 1% of the folylpolyglutamate synthetase (FPGS) activity of CCRF-CEM and is resistant to short-term, but not continuous, MTX exposure; however, R30dm is cross-resistant in continuous exposure to antifolates that require polyglutamylation to form potent inhibitors. Growth inhibition of all cell lines by continuous drug exposure was assayed as described.29,32 EC50 values (drug concentration effective at inhibiting cell growth by 50%) were determined visually from plots of percent growth relative to a solvent-treated control culture versus the logarithm of drug concentration.
6.2.3. Folylpolyglutamate synthetase (FPGS) purification and assay
Recombinant human cytosolic FPGS was purified and assayed as described previously.33 Both 13 (80% recovery) and 14 (89% recovery) were themselves recovered during the standard assay procedure, thus suggesting that their polyglutamate products would be quantitatively recovered. Kinetic constants were determined by the hyperbolic curve fitting subroutine of SigmaPlot (Jandel) or Kaleidagraph (Synergy Software) using a ≥10-fold range of substrate concentration. Activity was linear with respect to time at the highest and lowest substrate concentrations tested. Assays contained ≈400 U of FPGS activity; one unit of FPGS catalyzes incorporation of 1 pmol of [3H]glutamate/h. Because Vmax/Km values for 13 and 14 were low, the assays to determine kinetic constants were modified to include 2 mM l-[3H]glutamate, instead of the standard 4 mM. The resulting lower background allowed quantitation at lower levels of product synthesis. TheKm value forAMTwas the same whether 2 or 4 mM glutamate was used (data not shown).
Acknowledgments
This work was supported, in part, by CA 89300 (A.G.), AI 44661 (A.G.), CA 43500 (J.J.M.), CA 16056 (J.J.M.), CA 10914 (R.L.K.), and Roswell Park Cancer Institute Core Grant CA16065 from the NCI. The authors thank Mr. William Haile for performing growth inhibition studies and FPGS activity assays.
Footnotes
See Ref. 1.
References and notes
- 1.Presented in part in the 12th International Symposium on Pteridine and Folates; National Institutes of Health; June 17–22, 2001; Bethesda, Maryland. [Google Scholar]
- 2.(a) Gangjee A, Elzein E, Kothare M, Vasudevan A. Curr. Pharm. Des. 1996;2:263. [Google Scholar]; (b) Berman EF, Werbel LM. J. Med. Chem. 1991;34:479. doi: 10.1021/jm00106a001. [DOI] [PubMed] [Google Scholar]; (c) Jackson RC. In: Antifolate Drugs in Cancer Therapy. Jackman AL, editor. Totowa, NJ: Humana Press; 1999. pp. 1–12. [Google Scholar]
- 3.Schnell JR, Dyson HJ, Wright PE. Annu. Rev. Biophys. Biomol. Struct. 2004;33:119. doi: 10.1146/annurev.biophys.33.110502.133613. [DOI] [PubMed] [Google Scholar]
- 4.Carreras CW, Santi DV. Annu. Rev. Biochem. 1975;64:721. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 5.Jones TR, Calvert H, Jackman AL, Brown SJ, Harrap KR. Eur. J. Med. Chem. 1981;17:11. doi: 10.1016/0014-2964(81)90206-1. [DOI] [PubMed] [Google Scholar]
- 6.Walling J. Investig. New Drugs. 2006;24:37. doi: 10.1007/s10637-005-4541-1. [DOI] [PubMed] [Google Scholar]
- 7.Kisliuk RL, Gaumont Y, Powers JF, Thorndike J, Nair MG, Piper JR. Synergistic growth inhibition by combinations of antifolates. In: Picciano MF, Stokstad ELR, Gregory JF III, editors. Evaluation of Folate Metabolism in Health and Disease. New York: Alan R. Liss; 1990. pp. 79–89. [Google Scholar]
- 8.Taylor EC, Kuhnt D, Shih C, Rinzel SM, Grindey GB, Barredo J, Lannatipour M, Moran RA. J. Med. Chem. 1992;35:4450. doi: 10.1021/jm00101a023. [DOI] [PubMed] [Google Scholar]
- 9.Miwa T, Hitaka T, Akimoto H, Nomura H. J. Med. Chem. 1991;34:555. doi: 10.1021/jm00106a012. [DOI] [PubMed] [Google Scholar]
- 10.Gangjee A, Devraj R, McGuire JJ, Kisliuk RL. J. Med. Chem. 1995;38:3798. doi: 10.1021/jm00019a009. [DOI] [PubMed] [Google Scholar]
- 11.Gangjee A, Devraj R, McGuire J, Kisliuk RL, Queener SF, Barrows LR. J. Med. Chem. 1994;37:1169. doi: 10.1021/jm00034a015. [DOI] [PubMed] [Google Scholar]
- 12.Gangjee A, Zeng Y, McGuire JJ, Kisliuk RL. J. Med. Chem. 2000;43:3125. doi: 10.1021/jm000130i. [DOI] [PubMed] [Google Scholar]
- 13.Takimoto CH. Semin. Oncol. 1997;24:S18. [PubMed] [Google Scholar]
- 14.Rosowsky A. Prog. Med. Chem. 1989;26:1. doi: 10.1016/s0079-6468(08)70241-8. [DOI] [PubMed] [Google Scholar]
- 15.Gangjee A, Yu J, McGuire JJ, Cody V, Galitsky N, Kisliuk RL, Queener SF. J. Med. Chem. 2000;43:3837. doi: 10.1021/jm000200l. [DOI] [PubMed] [Google Scholar]
- 16.Barredo J, Moran RG. Mol. Pharmacol. 1992;42:687. [PubMed] [Google Scholar]
- 17.Aso K, Imai Y, Yakishige K, Ootsu K, Akimoto H. Chem. Pharm. Bull. Jpn. 2001;49:1280. doi: 10.1248/cpb.49.1280. [DOI] [PubMed] [Google Scholar]
- 18.Gangjee A, Devraj R, McGuire JJ, Kisliuk RL. J. Med. Chem. 1995;38:4495. doi: 10.1021/jm00022a015. [DOI] [PubMed] [Google Scholar]
- 19.Secrist JA, III, Liu PS. J. Org. Chem. 1978;43:3937. [Google Scholar]
- 20.Gangjee A, Yang J, Ihnat MA, Kamat S. Bioorg. Med. Chem. 2003;11:5155. doi: 10.1016/j.bmc.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Grell W, Hurnaus R, Griss G, Sauter R, Rupprecht E, Mark M, Luger P, Nar H, Wittneben H, Mueller P. J. Med. Chem. 1998;41:5219. doi: 10.1021/jm9810349. [DOI] [PubMed] [Google Scholar]
- 22.Giroux A, Nadeau C, Han Y. Tetrahedron Lett. 2000;41:7601. [Google Scholar]
- 23.Banztti C, Carfagna N, Commisso R, Heidempergher F, Pegrassi L, Melloni P. J. Med. Chem. 1988;31:1466. doi: 10.1021/jm00402a036. [DOI] [PubMed] [Google Scholar]
- 24.Danhesier RL, Brisbois RG, Kowalczyk JJ, Miller RF. J. Am. Chem. Soc. 1990;112:3093. [Google Scholar]
- 25.DeGraw JI, Tsakotellis P, Kisliuk RL, Gaumont Y. J. Heterocycl. Chem. 1971;10:105. doi: 10.1021/jm00285a007. [DOI] [PubMed] [Google Scholar]
- 26.Gangjee A, Mavandadi F, Queener SF. J. Med. Chem. 1997;40:1173. doi: 10.1021/jm960717q. [DOI] [PubMed] [Google Scholar]
- 27.Mini E, Srimatkandada S, Medina WD, Moroson BA, Carman MD, Bertino JR. Cancer Res. 1985;45:317. [PubMed] [Google Scholar]
- 28.Rosowsky A, Lazarus H, Yuan GC, Beltz WR, Mangini L, Abelson HT, Modest EJ, Frei E., III Biochem. Pharmacol. 1980;29:648. doi: 10.1016/0006-2952(80)90391-3. [DOI] [PubMed] [Google Scholar]
- 29.McCloskey DE, McGuire JJ, Russell CA, Rowan BG, Bertino JR, Pizzorno G, Mini E. J. Biol. Chem. 1991;266:6181. [PubMed] [Google Scholar]
- 30.Blakley RL. The Biochemistry of Folic Acid and Related Pteridines. Amsterdam: Elsevier; 1969. p. 569. [Google Scholar]
- 31.Foley GF, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE. Cancer. 1965;18:522. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.McGuire JJ, Magee KJ, Russell CA, Canestrari JM. Oncol. Res. 1997;9:139. [PubMed] [Google Scholar]
- 33.Gangjee A, Yu J, Kisliuk RL, Haile WH, Sobrero G, McGuire JJ. J. Med. Chem. 2003;46:591. doi: 10.1021/jm0203534. [DOI] [PubMed] [Google Scholar]