Abstract
Rationale
Sarcoidosis is a granulomatous disease of unknown etiology. Many patients with sarcoidosis demonstrate antigen-specific immunity to mycobacterial virulence factors. Th-17 cells are crucial to the immune response in granulomatous inflammation, and have recently been shown to be present in greater numbers in the peripheral blood and bronchoalveolar lavage (BAL) fluid (BALF) of sarcoidosis patients than healthy controls. It is unclear whether Th-17 cells in sarcoidosis are specific for mycobacterial antigens, or whether they have similar functionality to control Th-17 cells.
Methods
Flow cytometry was used to determine the numbers of Th-17 cells present in the peripheral blood and BALF of patients with sarcoidosis, the percentage of Th-17 cells that were specific to the mycobacterial virulence factor ESAT-6, and as well as to assess IFN-γ expression in Th-17 cells following polyclonal stimulation.
Results
Patients with sarcoidosis had greater numbers of Th-17 cells in the peripheral blood and BALF than controls and produced significantly more extracellular IL-17A (p=0.03 and p=0.02, respectively). ESAT-6 specific Th-17 cells were present in both peripheral blood and BALF of sarcoidosis patients (p<0.001 and p=0.03, respectively). After polyclonal stimulation, Th-17 cells from sarcoidosis patients produced less IFN-γ than healthy controls.
Conclusions
Patients with sarcoidosis have mycobacterial antigen-specific Th-17 cells peripherally and in sites of active sarcoidosis involvement. Despite the Th1 immunophenotype of sarcoidosis immunology, the Th-17 cells have reduced IFN-γ expression, compared to healthy controls. This reduction in immunity may contribute to sarcoidosis pathogenesis.
Keywords: Sarcoidosis, Th-17, mycobacterial ESAT-6
Introduction
Sarcoidosis is an idiopathic granulomatous disease defined by the presence of localized collections of activated macrophages and predominantly Th-1 CD4+ lymphocytes in affected tissues [1]. While nearly any organ may be affected, most deaths are due to progressive pulmonary disease [2]. Though most of the CD4+ cells within the sarcoidosis granuloma have a Th-1 immunophenotype, Th-17 CD4+ cells, producing IL-17A, are also present in sarcoidosis granulomas; it has been reported that these cells contribute to sarcoidosis fibrotic disease [3]. Th-17 CD4+ lymphocytes are not the only potential source of IL-17A; T cells expressing the γδ T-cell receptor produce IL-17A [4, 5] and γδ T-cell-derived IL-17A has been shown to be necessary for mature granuloma formation and sequestration in the lung [6, 7].
Multiple studies from independent laboratories have shown that mycobacterial antigens are frequently targets of the CD4+ T cell response in sarcoidosis peripheral blood mononuclear cells (PBMC) and bronchoalveolar lavage fluid (BALF) [8–10]. It is unclear whether the Th-17 response in sarcoidosis patients is mycobacterial antigen-specific and if they possess normal expression of IFN-γ upon T cell receptor (TCR) stimulation. This distinction may have important diagnostic and pathologic implications, as Th-17 cells have been shown to be involved in progression of fibrotic disease in an Italian cohort (7). Here we report that mycobacterial antigen-specific Th-17 responses are present in the periphery and in BALF of sarcoidosis subjects. Additionally, we report that sarcoidosis Th17 cells have reduced expression of IFN-γ following TCR stimulation.
Methods
Subjects and Samples
The study was approved by the Vanderbilt, Cleveland Clinic and University of Vermont Institutional Review Boards, and informed consent was obtained from each study participant. Peripheral blood mononuclear cells (PBMC) were collected from 37 sarcoidosis subjects and 14 healthy controls (Table I). Thirty-four sarcoidosis subjects underwent tuberculin skin testing at the discretion of their primary care physician; all were PPD negative. The healthy control subjects were not screened for latent tuberculosis infection by the placement of PPD. Bronchoalveolvar lavage fluid (BALF) was collected from 10 sarcoidosis subjects and five disease controls (Table II). Bacterial culture is the gold standard for ruling out tuberculosis; all of the sarcoidosis and control BAL specimens were culture negative for mycobacterial infection.
Table I.
Demographics of sarcoidosis and control population
| Sarcoidosis (37) | Healthy Control (14) | P value | |
|---|---|---|---|
| Sex (M/F) | 11/26 | 2/12 | NS |
| Age (yrs) | 51.7±9.4 | 42.4±11.4 | NS |
| Race (C/AA) | 23/14 | 7/7 | NS |
| Site of involvement | 19 Lung/18 Skin | NA | NA |
| Scadding Stagea 0/I/II/III/IV | 0/1/6/2/1 | NA | NA |
| Immunosuppression (Yes/No) | 11/26 | NA | NA |
M Male, F Female, Yrs years, C Caucasian, AA African American
Chest X-rays at the time of study entry were not available on all pulmonary subjects
Table II.
Sarcoidosis and control BAL demographics and immunophenotype
| Diagnosis | Race | Sex | Scadding stage | Extrapulmonary disease | Imunosuppression | Macrophages | Lymphocytes | PMN | Eos | CD4/CD8 ratio | Th17 cell frequency | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sarcoidosis 1 | Sarcoidosis | AA | M | 3 | No | No | 79 | 22 | 0 | 0 | 1.23 | 1.17 |
| Sarcoidosis 2 | Sarcoidosis | C | M | 1 | No | No | 92 | 4 | 4 | 0 | ND | 1.13 |
| Sarcoidosis 3 | Sarcoidosis | C | M | 2 | No | No | 97 | 2 | 1 | 0 | 0.57 | 2.37 |
| Sarcoidosis 4 | Sarcoidosis | AA | F | 1 | Ocular | no | 84 | 16 | 0 | 0 | 4.94 | 0.99 |
| Sarcoidosis 5 | Sarcoidosis | AA | M | 1 | CNS | Azathioprine | 96 | 3 | 1 | 0 | 1.29 | 0.44 |
| Sarcoidosis 6 | Sarcoidosis | C | F | 1 | No | No | 83 | 15 | 1 | 1 | 6.08 | 0.23 |
| Sarcoidosis 7 | Sarcoidosis | C | F | 1 | CNS | No | 86 | 1 | 2 | 0 | 3.32 | 0.08 |
| Sarcoidosis 8 | Sarcoidosis | C | M | 1 | No | ND | 50 | 43 | 6 | 1 | 22 | 0.6 |
| Sarcoidosis 9 | Sarcoidosis | C | F | 3 | Cardiac | ND | 80 | 1 | 10 | 0 | ND | 0.8 |
| Sarcoidosis 10 | Sarcoidosis Alveolar | C | F | 2 | No | No | 4 | 4 | 89 | 3 | ND | 1.11 |
| Control 1 | Hemorrhage | C | M | No | 87 | 13 | 0 | 0 | 1.45 | 0.28 | ||
| Control 2 | Hemosiderosis | C | F | No | 91 | 2 | 1 | 0 | 0.4 | 0.35 | ||
| Control 3 | Undefined ILD | C | M | Prednisone | 85 | 2 | 13 | 0 | 0.32 | 0.22 | ||
| Control 4 | RBILD | C | M | No | 100 | 0 | 0 | 0 | 0.19 | 0.39 | ||
| Control 5 | RBILD | C | F | No | 99 | 0 | 1 | 0 | 0.18 | 0.34 |
AA African-American, C Causasian, M Male, ILD Interstitial Lund Disease, RBILD Right Brochiolitis ILD, ND Not determined, CNS Central nervous System
RNA Purification and Quantitative Real-Time PCR for RORγt Expression
CD4+ T cells were sorted from sarcoidosis and healthy control PBMC. Total cellular RNA was extracted from 1×106 sarcoid CD4+ T cells using RNeasy Mini Kit (Qiagen, Germany), according to the manufacturer's protocol. First strand complementary DNA (cDNA) was generated from 400 ng total RNA using oligo-dT primer and the AMV reverse transcriptase (Reverse Transcription System, Promega Corporation, Madison, WI, USA). Quantitative real-time PCR amplification reactions were carried out in a StepOnePlus Real Time PCR System (Applied Biosystems, Foster City, CA) in a 10 μl volume. SYBR Green PCR Master Mix was purchased from Applied Biosystems (P/N 4309155), containing AmpliTaq Gold DNA Polymerase and optimized buffer components. A fraction of 5 μM primers and 2 μl of cDNA were added to SYBR Green master mix to make a final 10 μl reaction volume. Human β-actin and gylceraldehype-3-phosphate dehydrogenase (GAPDH) were used as housekeeping genes. The primers used for RORγt amplifications were: 1) RORγt: Forward 5'- ATg TCCCgAgATgCTgTCAAgTTC -3', Reverse 5'- gTCgTCCCTCTgCTTCTTg -3'. β actin: Forward 5'-ATCATGTTTGAGACCTTCAAC; Reverse 5'-CAGGAAGGAAGGCTGGAAGAG and HuGAPDH: FORWARD 5'-AATCCCATCACCATCTTCCA 3'; REVERSE 5'-TGGACTCCACGACGTACTCA3'; PCR reactions were performed under the following conditions: 10 min denaturation at 95 °C followed by 95 °C 15 s, 60 °C 1 min cycled 50 times. Each quantitative target was amplified in duplicate sample. A sample containing no template served as the negative control for each master mix; three standard curves were generated for RORγt, β-actin, and GADPH using total T cell cDNA in a serial dilution 1:1, 1:5, 1:25, and 1:125. The relative amounts of mRNA were determined by comparison with standard curves. Each sample result was normalized for β-actin and GADPH expression. To distinguish specific amplicons from non-specific amplifications, a dissociation curve was generated.
Cytometric Bead Array for Extracellular IL-17A Production
For the extracellular cytokine assay, CD4+ Tcells were isolated from PBMC using Dynabeads CD4+ isolation kit according to the manufacturer's protocol (Invitrogen). Sorted CD4+ T cells were>95 % pure as measured by CD4+ expression via flow cytometry. 2×105 CD4+ T cells were then incubated in RPMI 1640 supplemented medium with anti-CD28 and anti-CD3 Abs (1 μg/ml each; BD Biosciences) at 37 °C under 5 % CO2 for 24 h. Supernatants were then collected and analyzed for extracellular cytokine by using cytokine bead array according to the manufacturer's instructions (BD Biosciences).
Flow Cytometry
Intracellular staining was performed in order to identify IL-17A and IFN-γ secreting CD4+ T cells in response to polyclonal and mycobacterial ESAT-6 stimulation. For the intracellular cytokine assay, 5×105 PBMC or BAL cells were incubated in RPMI 1640 supplemented medium with or without antigen (ESAT-6 peptide NNALQNLARTI-SEAG [20 μg/ml]) and the anti-CD28 and anti-CD3 Abs (1 μg/ml each; BD Biosciences) at 37 °C under 5 % CO2 for 30 min before addition of BD GolgiStop (BD Biosciences). After 6 h of stimulation, the cells were then harvested and washed for surface and extracellular staining to CD3, CD4, TCR γδ (peripheral blood only), IL-23R (peripheral blood only), and intracellular IL-17A, IFN-γ, and FoxP3. All antibodies were from BD Biosciences, excluding FoxP3 (eBioscience). Extracellular staining was performed at 4 °C for 30 min. After washing, fixation, and permeabilization, using a Fix & Perm Kit according to the manufacturer's instructions (BD Biosciences), intracellular antibodies were added at 4 °C for 45 min. Cells were washed and analyzed via flow cytometry. Cells were stained with Fixable Viability Dye eFluor450 (eBioscience) to gate on live cells. For the gating strategy, lymphocytes were gated by FSC/SSC, then doublets were gated out, then dead cells, then cells were analyzed for CD3 and SSC. CD3+ cells were gated and subsequently analyzed for CD4 expression. CD3+CD4+ cells were gated and analyzed for surface expression of TCR γδ, IL-23R, and intracellular IL-17A and IFN-γ. For some analyses, expression of surface and intracellular markers was measured on CD3+CD4+IL-17A+cells. All flow cytometry experiments were acquired with a FACSCalibur or LSR-II flow cytometer (BD Biosciences). Analysis was performed using FlowJo software (Tree Star). A minimum of 200,000 events were acquired for each sample, and 5,000 events in all subgates to ensure adequate numbers for statistical analysis. The cytokine frequencies were defined as the subject's percentage of stimulated CD3+ CD4+ T cells minus their unstimulated background frequency. A response was considered positive when the frequency of recognition was at least twice that of background.
Statistical Analysis
All statistical tests were performed using a Mann–Whitney U test. Differences were considered significant with P values less than 0.05.
Results
Subjects
The sarcoidosis subjects met American Thoracic Society/World Association of Sarcoidosis and Other Granulomatous Diseases (ATS/WASOG) criteria for diagnosis of sarcoidosis [11]. Of the 37 sarcoidosis patients, 38 % were African-Americans; 70 % of the subjects were female. The median age was 51.7 years. Of the 14 healthy control subjects, 50 % were African American; 86 % were female. The median age was 42.7 years. There was no significant difference according to age, race or sex among the sarcoidosis or healthy control subjects (Table I).
Patients with Sarcoidosis have a Greater Frequency of Th-17 Cells in the Peripheral Blood Compared to Healthy Controls
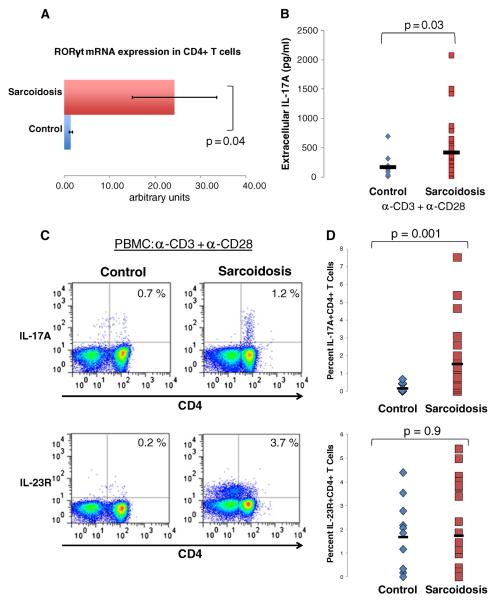
We began by comparing the numbers of Th-17 CD4+ T cells present in sarcoidosis patients to healthy controls. CD4+ T cells were isolated from PBMC from healthy controls and sarcoidosis patients. We assessed for the master transcription factor, retinoic acid-related receptor (RORγt), which has a central role in Th17 cell development. Real-time PCR (RT-PCR) evaluation of RORγt revealed higher expression in CD4+ T cells from sarcoidosis patients (29.3±9.3) than in CD4+ T cells from healthy controls (0.75±.33) (p=0.04) (Fig. 1a). Moreover, extracellular IL-17A release in sorted CD4+ T cells was higher in patients with sarcoidosis (421±507 pg/ml) than healthy controls (170±198 pg/ml) (p=0.03) (Fig. 1b) following polyclonal stimulation with anti-CD3 and anti-CD28 antibodies. Finally, we stimulated total PBMC to assess intracellular production of IL-17A and surface expression of IL-23R on CD4+ T cells of sarcoidosis patients and healthy controls. The frequency of CD4+ T cells expressing IL-17A was greater in patients with sarcoidosis (1.5 %±1.86 %) than healthy controls (0.173 %±0.235 %) (p=0.001) (Fig. 1c). Sarcoidosis CD4+ T cells expressed similar levels of IL-23R as compared to healthy controls (p=0.9) (Fig. 1d).
Fig. 1. Patients with sarcoidosis have a greater frequency of Th-17 cells in the peripheral blood compared to healthy controls.
a Analysis of the mRNA expression of the master transcription factor retinoic acid-related orphan receptor RORγt from CD4+ T cells isolated from the peripheral blood of healthy controls, (n=6) and sarcoidosis patients (n=10). b CBA analysis of IL-17A production from polyclonally activated CD4+ T cells isolated from the peripheral blood of healthy controls (diamonds, n=11) and patients with sarcoidosis (squares, n=28). c Representative plots of flow cytometric detection of intracellular IL-17A and surface expression of IL-23R on CD4+ T cells from polyclonally activated PBMC. d Frequencies of IL-17A+CD4+ and IL-23R+CD4+ from polyclonally activated PBMC of healthy controls (diamonds, n=11) and patients with sarcoidosis (square, n=25). P values are indicated
Patients with Sarcoidosis have a Greater Frequency of IL-17A+and IFN-γ+TCR γδ Cells in the Peripheral Blood Compared to Healthy Controls
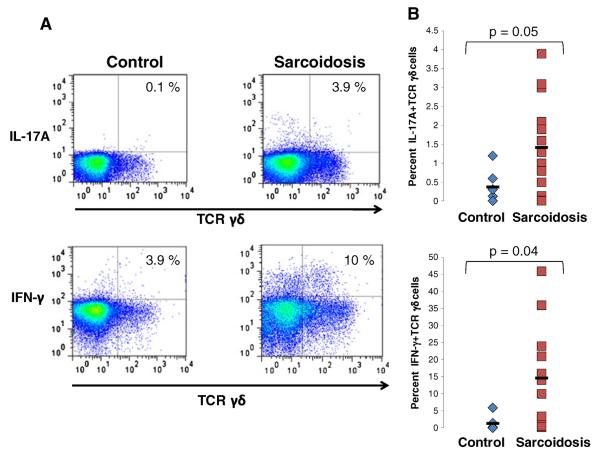
In addition to Th-17 cells, TCR γδ cells are known to produce IL-17. Though it has previously been reported that patients with sarcoidosis have increased numbers of TCR γδ cells in the peripheral blood and BALF [12, 13] it is has not been demonstrated whether these cells produce IL-17. Therefore we assessed IL-17 production of peripheral blood TCR γδ cells from sarcoidosis patients. We found a higher frequency of IL-17A+TCR γδ cells in the peripheral blood of patients with sarcoidosis as compared to healthy controls (p=0.05) (Fig. 2a and b). Additionally, we found significantly more IFN-γ+TCR γδ cells in sarcoidosis patients compared to healthy controls (p=0.04) (Fig. 2b).
Fig. 2. Patients with sarcoidosis have a greater frequency of IL-17A+and IFN-γ+TCR γδ cells in the peripheral blood as compared to healthy controls.
a Representative plots of intracellular IL-17A and IFN-γ in TCR γδ cells from polyclonally activated PBMC of healthy controls and patients with sarcoidosis. b Frequencies of IL-17A+TCR γδ+and IFN-γ+TCR γδ+from polyclonally activated PBMC of healthy controls (diamonds, n=9) and patients with sarcoidosis (squares, n=16). P values are indicated
Patients with Sarcoidosis Exhibit ESAT-6 Specific Th-1/Th-17 Responses
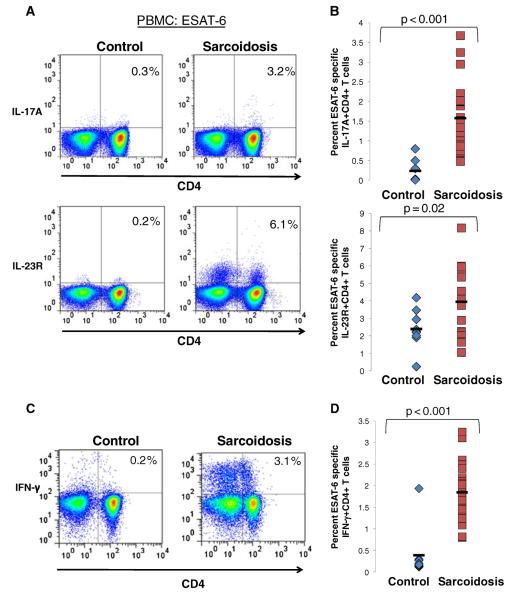
Prior reports of antigen-specific Th-1 CD4+ responses in sarcoidosis patients prompted us to investigate whether mycobacterial ESAT-6 specific Th-17 responses were also present systemically and in bronchoalveolar lavage. PBMC were stimulated with ESAT-6, then CD4+ T cell responses were measured. While no increase in IL-17A expression above baseline levels was seen in CD4+ T cells from healthy controls, following ESAT-6 stimulation (0.3 %±0.3 %), there was a robust expression of IL-17A in ESAT-6 stimulated CD4+ T cells from sarcoidosis subjects (1.8 %±0.9 %) (p<0.001) (Fig. 3a and b). Additionally, sarcoidosis ESAT-6 stimulated CD4+ T cells had a greater frequency of IL-23R+ cells (3.9 %±2.1 %) than healthy controls (2.2 %±1.7 %) (p=0.02) (Fig. 3a and b). Similar to previous reports, CD4+ Tcells from the peripheral blood of sarcoidosis patients produced significantly more IFN-γ (1.9 %±0.9 %) in response to ESAT-6 stimulation than healthy controls (0.4 %±0.7 %) (p<0.001) (Fig. 3c and d).
Fig. 3. Patients with sarcoidosis exhibit an ESAT-6 specific Th-1/Th-17 response.
a Representative plots of flow cytometric detection of production of intracellular IL-17A, surface expression of IL-23R and production of intracellular IFN-γ in CD4+ T cells from ESAT-6 stimulated PBMC of healthy controls and patients with sarcoidosis. b Frequencies of IL-17A+CD4+ and IL-23R+CD4+ from ESAT-6 stimulated PBMC of healthy controls (diamonds, n=9) and patients with sarcoidosis (squares, n=24). c Representative plots of flow cytometric detection of production of intracellular IFN-γ in CD4+ T cells from ESAT-6 stimulated PBMC of healthy controls and patients with sarcoidosis. d Frequencies of CD4+ T cells expressing IFN-γ from ESAT-6 stimulated PBMC of healthy controls (diamonds, n=9) and patients with sarcoidosis (squares, n=24). P values are indicated
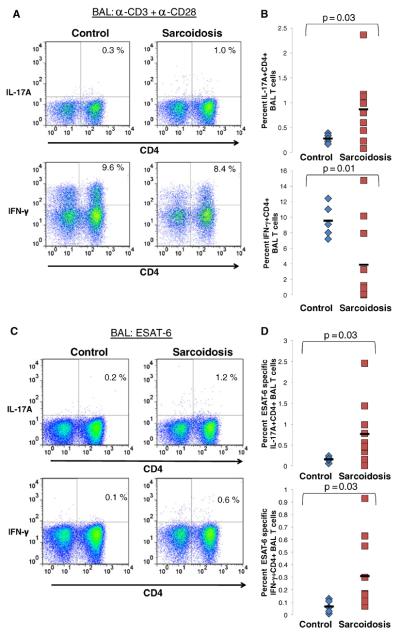
To assess an active site of sarcoidosis involvement, BAL was collected and analyzed for Th-17 responses to both polyclonal and mycobacterial stimulation among sarcoidosis and control subjects. There was no correlation with the degree of alveolitis and Th-17 cell frequency within the subjects (Table II). None of the subjects had evidence of infection. Sarcoidosis patients demonstrated increased production of IL-17A as compared to disease controls (p=0.03) (Fig. 4a). One of the sarcoidosis patient (Sarcoidosis 10) had significant neutrophilia within the BAL, which could reflect IL-17A expression. The results were unchanged whether this subject was included or not. Conversely, BAL CD4+ T cells produced significantly less IFN-γ as compared to disease controls (p=0.01) (Fig. 4b). Furthermore, mycobacterial ESAT-6 stimulation induced both IL-17A and IFN-γ from sarcoidosis CD4+ T cells (p=0.03; p=0.03, respectively) (Fig. 4c and d).
Fig. 4. Sarcoidosis BAL cells exhibit an ESAT-6 specific Th-1/Th-17 response.
a Representative plots of flow cytometric detection of production of intracellular IL-17A and production of intracellular IFN-γ in BAL CD4+ T cells from polyclonally activated BAL cells of disease controls and patients with sarcoidosis. b Frequencies of IL-17A+CD4+ and IFN-γ+ CD4+ T cells from polyclonally activated BAL of disease controls (diamonds, n=5) and patients with sarcoidosis (square, n=10). c Representative plots of flow cytometric detection of production of intracellular IL-17A and production of intracellular IFN-γ in BAL CD4+ T cells from ESAT-6 stimulated BAL cells of disease controls and patients with sarcoidosis. d Frequencies of IL-17A+CD4+ and IFN-γ+CD4+ from ESAT-6 stimulated BAL cells of disease controls (diamonds, n=5) and patients with sarcoidosis (squares, n=10). P values are indicated
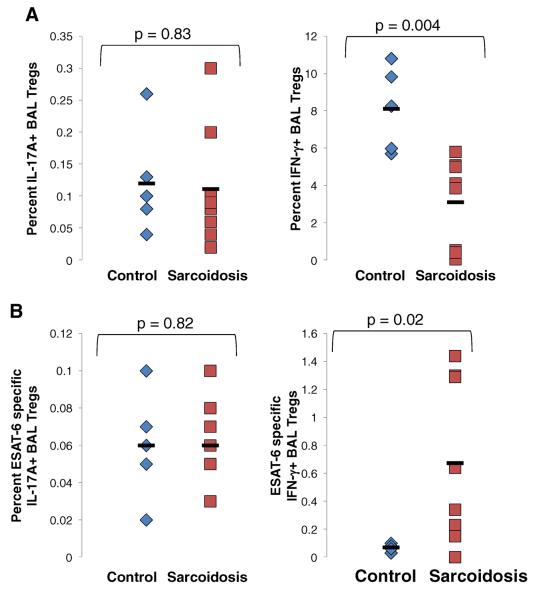
Prior reports have indicated that regulatory T cells (Tregs) may also produce IL-17A [14]. Therefore BAL CD4+ T cells were subgated into Tregs by high FoxP3 expression and analyzed for IL-17A production. Tregs did not produce significant amounts of IL-17A upon polyclonal or mycobacterial ESAT-6 stimulation (P=0.8) (Figure (Fig. 5a and b). However, sarcoidosis Tregs produced increased IFN-γ upon ESAT-6 stimulation as compared to disease controls (p=0.02) (Fig. 5b), while control Tregs produced significantly more IFN-γ upon polyclonal stimulation (p=0.004) (Fig. 5a).
Fig. 5. Sarcoidosis BAL Tregs do not produce IL-17.
a Frequencies of IL-17A+CD4+FoxP3+ and IFN-γ+CD4+FoxP3+ cells from polyclonally activated BAL of disease controls (diamonds, n=5) and patients with sarcoidosis (square, n=10). FoxP3 used a marker for Tregs. b Frequencies of IL-17A+CD4+FoxP3+ and IFN-γ+CD4+FoxP3+ cells from ESAT-6 stimulated BAL from disease controls (diamonds, n=5) and patients with sarcoidosis (squares, n=10). P values are indicated
Th-17 Cells Demonstrate Reduced IFN-γ Expression
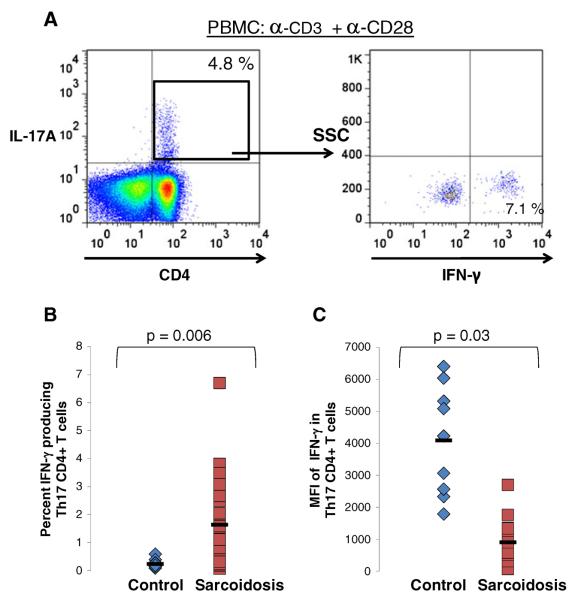
The effectiveness of the Th-1 immune response in granulomatous diseases such as tuberculosis and coccidiomycosis is an important component to disease resolution or progression [15, 16]. Though patients with sarcoidosis develop inflammation at sites of active involvement, an anergic phenotype characterized by reduced recognition of recall antigens dominates peripherally [17]. Proposed mechanisms to explain this anergic phenotype include increased numbers of regulatory T cells (Tregs) [18], defective CD4+ T cell signaling [19], and impaired dendritic cell function [20]. To our knowledge no studies have examined IFN-γ expression in Th-17 cells in sarcoidosis. We stimulated PBMC with anti-CD3 and anti-CD28 for 6 h then measured the dual expression of IL-17A and IFN-γ in CD4+ T cells. Though patients with sarcoidosis had higher total numbers of CD4+IL-17A+IFN-γ+cells than controls (p=0.006) (Fig. 6a/b), the median fluorescent intensity (MFI) of IFN-γ expression in the CD4+IL-17A+cells was significantly lower in sarcoidosis patients (p=0.03) (Fig. 6c), indicating that sarcoidosis CD4+IL-17A+cells produced less IFN-γ per cell.
Fig. 6. Sarcoidosis Th-17 cells demonstrate reduced IFNγ expression.
a Representative gating strategy used for identification of the IFN-γ producing CD4+IL-17A+cells. b Percent of Th-17 cells producing IFN-γ following polyclonal activation of PBMC from healthy controls (triangles, n=9) and sarcoidosis patients (squares, n=24). c Median fluorescent intensity (MFI) of IFN-γ expression on CD4+IL-17A+cells following polyclonal activation of PBMC of healthy controls (triangles, n=9) and sarcoidosis patients (circles, n=24). P values are indicated
Discussion
T cells, specifically CD4+ T cells, are core contributors to adaptive immunity within sarcoidosis subjects; sarcoidosis Th17 cells have been associated with sarcoidosis pathogenesis. This is the first report of mycobacterial ESAT-6-specific Th-17 cells within sarcoidosis periphery and BALF. In addition, this study demonstrates for the first time that peripheral blood Th-17 cells demonstrate reduced IFNγ expression.
Th-17 cells produce IL-17A [21], IL-17F [22], IL-21 [23, 24] and IL-22 [25] and mediate recruitment of CXCR3+ T cells to the lung by production of the chemokines CXCL9, CXCL10, and CXCL11 [26]. In tuberculosis models, Th-17 cells coordinate a rapid protective immune response via neutrophil and macrophage recruitment and aid in tissue repair [27]. Antigen specific Th-17 cells have been described in other chronic lung diseases, such as tularemia and tuberculosis, and are associated with protective functions [28, 29]. ESAT-6 antigen-specific Th-17 cells have been demonstrated to provide surveillance function within tuberculosis peripheral tissues such that when mycobacterial antigens are detected, they respond rapidly and recruit other effector recall responses, which effectively stop pathogen growth [26]. Mass spectrometry analysis revealed signals consistent with ESAT-6 in sarcoidosis granulomas [30]. The presence of ESAT-6 specific Th17 cells within sarcoidosis BAL (Fig. 4) suggests that antigen-specific Th17 immune responses are present within a site of active sarcoidosis involvement.
Mycobacterial antigens or signals consistent with mycobacterial antigens have been detected within sarcoidosis granulomas [30, 31]. The detection of antigen-specific Th17 cells may reflect immune responses against the antigens; the clearance of these antigens leads to clinical recovery, and down-regulation of the antigen-specific Th17 cells. In addition, inferior expression of IFN-γ from Th17 and Th1 CD4+ T cells Figs. 4 and 6) could contribute to sarcoidosis pathology by ineffective activation of macrophages or poor recruitment of effector cells, which also leads to microbial antigen persistence. In the murine model of hypersensitivity pneumonitis, chronic inhaled microbial antigen leads to the development of pulmonary fibrosis. Decreased collagen content is halted in the presence of Th17 cells through their capacity to eliminate infectious antigens [32]. It is possible that reduced secretion of IFN-γ by Th17 cells inhibit the capacity of Th17 cells to clear foreign antigen, leading to pulmonary fibrosis.
The observation that reduced IFN-γ expression in Th-17 cells, from sarcoidosis peripheral blood and BAL following TCR stimulation is noteworthy (Fig. 6), as this could be an important contributor to sarcoidosis immunopathogenesis. Adequate expression of IFN-γ is important for macrophage activation and cellular cytotoxicity [33]. Inferior production of IFN-γ in Th17 cells has been previously reported in tuberculosis subjects co-infected with filariasis [34]. This reduced expression is associated with inhibitory effects on protective immunity against microbial virulence factors. We noted restoration of the T cell response with IFN-γ expression similar to healthy controls among sarcoidosis subjects who spontaneously resolved their disease; subjects with progressive disease continued to have diminished IFN-γ expression (manuscript under review). IFN-γ is a key mediator in the adaptive immune response. The observation of reduced expression in Th1 and Th17 cells suggests that while sarcoidosis immunopathogenesis is characterized by enhanced Th1 immune responses, IFN-γ expression following TCR stimulation is inferior to healthy controls. The expression of IFN-γ may not be sufficient to support immunologic clearance of antigenic perpetuators of sarcoidosis pathogenesis.
There was increased production of IFN-γ in the sarcoidosis IL-17A+TCR γδ cells, compared to healthy controls (Fig. 2). This demonstrates that not every IL-17 secreting cell population demonstrates reduced IFN- expression upon TCR stimulation. This population of IL-17 secreting cells has been noted to be important protectors against collagen deposition in the murine lung model of hypersensitivity pneumonitis [35]. This was the smaller population of IL-17 secreting cells within sarcoidosis PBMC. Future studies to assess for distinctions in the IL-17 secreting populations of sarcoidosis subjects with and without fibrosis is warranted, as it could provide new therapeutic targets. We will also determine if distinctions in the effector memory Th17 population are present between sarcoidosis and control subjects.
Overall, these studies enhance current understanding of sarcoidosis Th17 cells by demonstrating their antigen-specific nature with sarcoidosis BAL, as well as the reduced cytokine expression. Further investigation of the mediators of IFN-γ expression within Th-17 cells and Th1 CD4+ T cells is necessary in order to better understand their contribution to sarcoidosis pulmonary fibrosis and clinical outcome.
Acknowledgments
We thank the sarcoidosis patients who participated in this study. We also thank the following agencies for supporting this research: National Institutes of Health K01HL103179-01 and Foundation for Sarcoidosis Research grant to K.R., T32HL069765-08 to B.R., T35 HL090555 to K.P., T32HL094296 to T.T., and RO1-HL83839, MO1 RR-00095, The Eliassen Foundation, The Pierce Foundation, 1 U01 HL112694-01 and Vanderbilt CTSA grant 1 UL1 RR024975 to W.P.D.
References
- 1.Zissel G, Prasse A, Muller-Quernheim J. Immunologic response of sarcoidosis. Semin Respir Crit Care Med. 2010;31(4):390–403. doi: 10.1055/s-0030-1262208. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Winget DB, Bowen EH, Lower EE. Predicting respiratory failure in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14(2):154–8. [PubMed] [Google Scholar]
- 3.Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66(2):144–50. doi: 10.1136/thx.2010.140319. [DOI] [PubMed] [Google Scholar]
- 4.Fenoglio D, Poggi A, Catellani S, Battaglia F, Ferrera A, Setti M, et al. Vdelta1 T lymphocytes producing IFN-gamma and IL-17 are expanded in HIV-1-infected patients and respond to Candida albicans. Blood. 2009;113(26):6611–8. doi: 10.1182/blood-2009-01-198028. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Usui T, Kobayashi S, Iguchi-Hashimoto M, Ito H, Yoshitomi H, et al. Gamma/delta T cells are the predominant source of interleukin-17 in affected joints in collagen-induced arthritis, but not in rheumatoid arthritis. Arthritis Rheum. 2009;60(8):2294–303. doi: 10.1002/art.24687. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto YY, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol. 2010;184(8):4414–22. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 7.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178(6):3786–96. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 8.Drake WP, Dhason MS, Nadaf M, Shepherd BE, Vadivelu S, Hajizadeh R, et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007;75(1):527–30. doi: 10.1128/IAI.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen ES, Wahlstrom J, Song Z, Willett MH, Wiken M, Yung RC, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181(12):8784–96. doi: 10.4049/jimmunol.181.12.8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oswald-Richter KA, Culver DA, Hawkins C, Hajizadeh R, Abraham S, Shepherd BE, et al. Cellular responses to mycobacterial antigens are present in bronchoalveolar lavage fluid used in the diagnosis of sarcoidosis. Infect Immun. 2009;77(9):3740–8. doi: 10.1128/IAI.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du BR, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149–73. [PubMed] [Google Scholar]
- 12.Raulf M, Liebers V, Steppert C, Baur X. Increased gamma/delta-positive T-cells in blood and bronchoalveolar lavage of patients with sarcoidosis and hypersensitivity pneumonitis. Eur Respir J. 1994;7(1):140–7. doi: 10.1183/09031936.94.07010140. [DOI] [PubMed] [Google Scholar]
- 13.Shigehara K, Shijubo N, Nakanishi F, Hirasawa M, Inuzuka M, Ohmichi M, et al. Circulating gamma delta-T-cell-receptor-positive lymphocytes in sarcoidosis. Respiration. 1995;62(2):84–8. doi: 10.1159/000196397. [DOI] [PubMed] [Google Scholar]
- 14.Raffin C, Raimbaud I, Valmori D, Ayyoub M. Ex vivo IL-1 receptor type I expression in human CD4+ T cells identifies an early intermediate in the differentiation of Th17 from FOXP3+ naive regulatory T cells. J Immunol. 2011;187(10):5196–202. doi: 10.4049/jimmunol.1101742. [DOI] [PubMed] [Google Scholar]
- 15.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105(9):1317–25. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borchers AT, Gershwin ME. The immune response in Coccidioidomycosis. Autoimmun Rev. 2010;10(2):94–102. doi: 10.1016/j.autrev.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Lecossier D, Valeyre D, Loiseau A, Cadranel J, Tazi A, Battesti JP, et al. Antigen-induced proliferative response of lavage and blood T lymphocytes. Comparison of cells from normal subjects and patients with sarcoidosis. Am Rev Respir Dis. 1991;144(4):861–8. doi: 10.1164/ajrccm/144.4.861. [DOI] [PubMed] [Google Scholar]
- 18.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203(2):359–70. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee NS, Barber L, Kanchwala A, Childs CJ, Kataria YP, Judson MA, et al. Low levels of NF-kappaB/p65 mark anergic CD4+ T cells and correlate with disease severity in sarcoidosis. Clin Vaccine Immunol. 2011;18(2):223–34. doi: 10.1128/CVI.00469-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew S, Bauer KL, Fischoeder A, Bhardwaj N, Oliver SJ. The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol. 2008;181(1):746–55. doi: 10.4049/jimmunol.181.1.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18(3):349–56. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204(1):161–70. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 24.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19(6):362–71. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271–9. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 27.McAleer JP, Kolls JK. Mechanisms controlling Th17 cytokine expression and host defense. J Leukoc Biol. 2011;90(2):263–70. doi: 10.1189/jlb.0211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31(5):799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41(2):79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oswald-Richter KA, Beachboard DC, Seeley EH, Abraham S, Shepherd BE, Jenkins CA, et al. Dual Analysis for Mycobacteria and Propionibacteria in Sarcoidosis BAL. J Clin Immunol. 2012 May 3; doi: 10.1007/s10875-012-9700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201(5):755–67. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonian PL, Roark CL, Wehrmann F, Lanham AM, Born WK, O'Brien RL, et al. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J Immunol. 2009;182(10):6540–9. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazarevic V, Glimcher LH. T-bet in disease. Nat Immunol. 2011;12(7):597–606. doi: 10.1038/ni.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babu S, Bhat SQ, Kumar NP, Jayantasri S, Rukmani S, Kumaran P, et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J Infect Dis. 2009;200(2):288–98. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. Gammadelta T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207(10):2239–53. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]