Tissue plasminogen activator (tPA) is effective for treatment of ischemic stroke, but its efficacy is limited by a relatively narrow window of safety and its safety is limited by risk for increased bleeding and related toxicities [1, 2]. Activated protein C (APC) is neuroprotective in preclinical ischemic stroke models [3–5]. Remarkably, APC, in spite of having intrinsic anticoagulant activity, reduces tPA-induced bleeding and neurotoxicity primarily due to its ability to initiate cell signaling that provides anti-inflammatory and anti-apoptotic activities and stabilizes vascular endothelial barriers, thereby reducing leakage [3–9]. APC activities comprise both anticoagulant actions as well as beneficial cytoprotective actions on cells [5, 9]. The pharmacologic utility of APC for ischemic stroke might be diminished by the potentially adverse side effect of serious bleeding due to APC's anticoagulant activity. However, this theoretical risk can be greatly reduced by employing genetically engineered variants of recombinant APC that have markedly reduced anticoagulant activity (< 10 %) but preserved cytoprotective and anti-inflammatory activities compared to wild type (wt) APC [9–12]. The APC variant, 3K3A-APC in which the three lysine residues 191–193 are replaced by three alanine residues, is neuroprotective in stroke models and is the subject of current efforts for translating basic and preclinical research to a clinically novel biologic for ischemic stroke therapy [12, 13].
Since tissue plasminogen activator (tPA) therapy is useful for ischemic stroke, the potential utility of combination therapy using both tPA and 3K3A-APC [6, 7, 12, 13] raise questions about the effects of one agent on the biologic activities of the other agent. Thus, here we report studies of the effects of tPA on the anticoagulant activity of 3K3A-APC using activated partial thromboplastin time (APTT) clotting assay and studies of the effects of 3K3A-APC on the fibrinolytic activity of tPA using in vitro clot lysis assays [10].
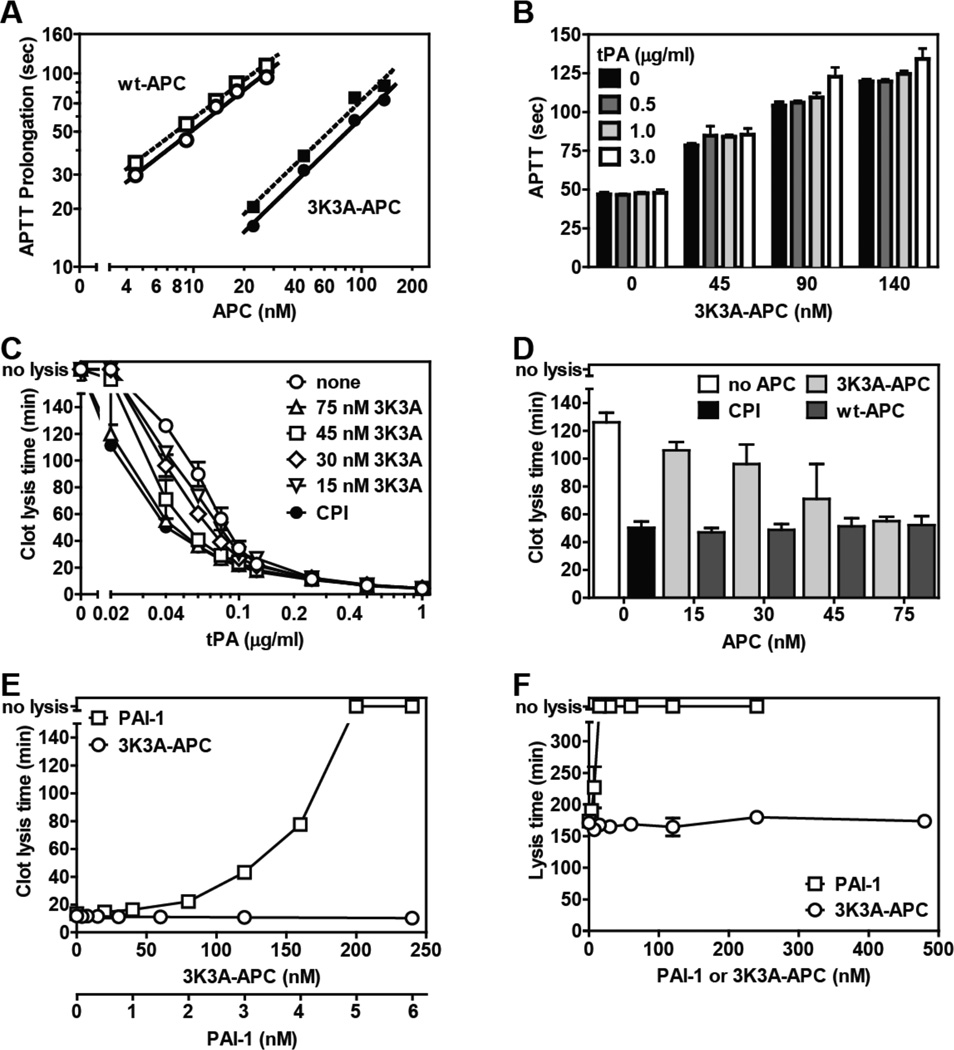
The anticoagulant potency of plasma-derived wild type (wt)-APC and of the 3K3A-APC variant in the presence or absence of 3.0 µg/ml of tPA was assessed as clotting time prolongation (Fig. 1A). The three mutations in the 3K3A-APC variant caused loss of approximately 90% of anticoagulant activity in the variant compared to plasma-derived wt APC, as expected [13], and overall, tPA had no effect or a very minor effect on the anticoagulant activity of 3K3A-APC. When tPA at 0.5 and 1.0 µg/ml was also studied, there was no significant effect of tPA on the anticoagulant activity of 3K3A-APC (Fig. 1B). At the highest tPA concentration (3.0 µg/ml) in presence of 90 and 140 nM 3K3A-APC, several additional seconds of APTT prolongation were seen. Thus, tPA is unlikely to provide significant prolongation of the APTT in the presence of 3K3A-APC at levels of < 140 nM.
Figure. Reciprocal effects of tPA on 3K3A-APC’s anticoagulant activity and of 3K3A-APC on tPA’s fibrinolytic activity.
(A) Prolongation of the APTT (APTT-XL, Pacific Haemostasis) beyond the baseline reference clotting time of 47 sec was determined in protein C depleted plasma (Affinity Biologicals) for plasma-derived wt APC (Enzyme Research Laboratories) (open symbols) and for 3K3A-APC (ZZBiotech LLC) (solid symbols) when tPA (Innovative Research) was present at 0 (○,●) or 3.0 µg/ml (□,■). (B) The influence of tPA on the anticoagulant activity of 3K3A-APC was determined at the indicated tPA concentrations. (C) The effect of 3K3A-APC on tPA’s (Alteplase, Genentech) fibrinolytic activity was determined in clot lysis assays using thrombininduced clot formation and tPA-induced fibrinolysis in normal plasma (George King, Overland KS) as described [10]. CPI (10 µg/ml) was used to inhibit active TAFI activity where indicated (●). (D) Comparison of the profibrinolytic effect of recombinant wt-APC [10] and 3K3A-APC at 0.04 µg/ml tPA. (E) The ability of PAI-1 (stable mutant I91L, Molecular Innovations) and of 3K3A-APC to inhibit clot lysis induced by tPA (0.25 µg/ml) in the presence of CPI (10 µg/ml) which neutralizes TAFI activity. (F) The effect of 3K3A-APC or PAI-1 on tPA-mediated lysis of aged clots. Clots consisting of 50% normal plasma, 4 pM tissue factor (Innovin, Dade), phospholipids (10 µM, PC/PS/PE (60%/20%/20%)), 17 mM CaCl2 in HBS/BSA were aged for 2 hr after which 1.0 µg/ml tPA and 3K3A-APC were added to the surface of the clot to induce lysis. Data points represent mean values from n ≥ 3 experiments). “no lysis” on the y-axis indicates no clot lysis was observed.
When human normal pooled plasma that contained 3K3A-APC and tPA (Alteplase) at varying concentrations was clotted by addition of thrombin and then clot lysis was monitored over several hours, the clot lysis time was dose-dependently shortened by tPA (Fig. 1C). The presence of 15–75 nM 3K3A-APC dose-dependently enhanced the ability of tPA to lyse fibrin clots at low tPA concentrations but had no effect on tPA fibrinolytic activity at higher tPA concentrations. Notably, at 75 nM 3K3A-APC, the observed clot lysis time was the same as that observed in the presence of 15 nM wt-APC or when the TAFI inhibitor, carboxypeptidase inhibitor from potato tuber (CPI) was present in the absence of 3K3A-APC (Fig. 1D). Activated TAFI is a plasma carboxypeptidase that proteolytically releases C-terminal lysine residues from fibrin, thereby inhibiting Lys-dependent fibrinolytic mechanisms [14]. It is likely that the residual anticoagulant activity of 3K3A-APC can reduce generation of thrombin which thereby can affect activation of TAFI that inhibits fibrinolysis [14]. By neutralizing active TAFI, the use of CPI thereby permits determination of the direct effect of 3K3A-APC on the fibrinolytic effects of tPA without any confounding effects of thrombin-generated activated TAFI.
Consistent with data above, in the presence of CPI that inhibits TAFI, no profibrinolytic or antifibrinolytic effects of 3K3A-APC at levels up to 240 nM were observed (Fig. 1E). To confirm that the fibrinolytic activity of tPA used in these assays was susceptible to inhibition by a known clot lysis inhibitor such as PAI-1, the ability of PAI-1 (0–6 nM) to inhibit clot lysis induced by tPA was compared to the effects of 3K3A-APC (0–240 nM) in the presence of CPI (10 µg/ml) which neutralizes TAFI activity. Clot lysis assays showed that, indeed, there was dose-dependent inhibition of tPA fibrinolytic activity by PAI-1 between 2 and 5 nM, with complete inhibition of tPA-induced clot lysis at higher PAI-1 levels (Fig. 1E). It is concluded that there is not a direct effect of 3K3A-APC on the fibrinolytic activity of tPA and that the profibrinolytic effect of 3K3A-APC seen in this assay protocol was due to a TAFI dependent pathway, as previously described [10, 14]. To define potential effects of 3K3A-APC on tPA’s fibrinolytic activity on preformed clots, we employed turbidimetric assays using aged clots where tPA and 3K3A-APC or PAI-1 were added to the clot surface 2 hr after clot formation. First, the assay using aged clots was characterized for tPA’s fibrinolytic activity. Addition of tPA to the surface of preformed, aged clots dose-dependently promoted clot lysis (data not shown), although notably higher concentrations of tPA were needed to promote lysis on surface of aged clots compared to clot lysis where tPA was incorporated into the forming clot. PAI-1 dose-dependently inhibited tPA lysis of aged clots and completely inhibited clot lysis when present at > 20 nM. In contrast, 3K3A-APC showed no inhibition, even at very high levels up to 480 nM (Fig. 1F). For aged clots, the potentially confounding effects of TAFI were not observed because the half-life of activated TAFI is ~ 8–10 min such that at 2 hr after clot formation, active TAFI activity has almost entirely spontaneously decayed and thus any TAFI effects, if present, have similarly and uniformly affected each clot in the study.
In summary, in these studies using traditional in vitro clot lysis assays, there were no deleterious effects of high levels of 3K3A-APC on the fibrinolytic activity of tPA. Thus, in considering combination therapy using tPA and 3K3A-APC for ischemic stroke, the findings here are reassuring in that they fail to give rise to any concerns about undesirable effects of 3K3A-APC on tPA’s thrombolytic actions.
Acknowledgments
Financial Support: This work was supported in part by NIH grants HL063290 (JHG), HL104165 (LOM), and HL052246 (BVZ).
JH Griffin and LO Mosnier are co-inventors for intellectual property owned by The Scripps Research Institute that is related to the subject of this report. TP Davis and JH Griffin are consultants for ZZBiotech LLC. BV Zlokovic is cofounder of ZZBiotech LLC.
Footnotes
Author Contributions
L.O. Mosnier, J. A. Fernández, and J.H. Griffin designed experiments. L.O. Mosnier and J. A. Fernández conducted experiments. T.P. Davis and B.V. Zlokovic contributed unique reagents. L.O. Mosnier and J.H. Griffin wrote the manuscript.
Conflicts of Interest
Other authors have nothing to declare.
References
- 1.Lyden PD, Lees KR, Davis SM. Alteplase for acute stroke revisited: the first 10 years. Lancet Neurology. 2006;5:722–724. doi: 10.1016/S1474-4422(06)70530-0. [DOI] [PubMed] [Google Scholar]
- 2.Wechsler LR, Jovin TG. Intravenous recombinant tissue-type plasminogen activator in the extended time window and the US Food and Drμg Administration: confused about the time. Stroke. 2012;43:2517–2519. doi: 10.1161/STROKEAHA.112.670554. [DOI] [PubMed] [Google Scholar]
- 3.Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–1805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 4.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 5.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 7.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, LaRue B, Griffin JH, Chopp M, Zlokovic BV. Activated protein C inhibits tissue plasminogen activator induced brain hemorrhage. Nat Med. 2006;12:278–285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 8.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 9.Mosnier LO, Zlokovic BV, Griffin JH. Mosnier The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 10.Mosnier LO, Yang XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activated fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- 11.Guo H, Singh I, Wang Y, Deane R, Barrett T, Fernández JA, Chow N, Griffin JH, Zlokovic BV. Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur J Neurosci. 2009;29:1119–1130. doi: 10.1111/j.1460-9568.2009.06664.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Wang Y, Zhang Z, Chow N, Davis TP, Griffin JH, Chopp M, Zlokovic BV. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke. 2012;43:2444–2449. doi: 10.1161/STROKEAHA.112.658997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams PD, Zlokovic BV, Griffin JH, Pryor KE, Davis TP. Preclinical safety and pharmacokinetic profile of 3K3A-APC, a novel, modified activated protein C for ischemic stroke. Curr Pharm Des. 2012;18:4215–4222. doi: 10.2174/138161212802430413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosnier LO, Bouma BN. Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Atheroscl Thromb Vasc Biol. 2006;26:2445–2453. doi: 10.1161/01.ATV.0000244680.14653.9a. [DOI] [PubMed] [Google Scholar]