Abstract
Both 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203) and 5-fluoro-2-(3,4-dimethoxyphenyl)-benzothiazole (GW 610) contain the benzothiazole pharmacophore and possess potent and selective in vitro antitumor properties. Prior studies suggested the involvement of cytochrome P450 (P450) 1A1 and 2W1-mediated bioactivation in the antitumor activities and P450 2S1-mediated deactivation of 5F 203 and GW 610. In the present study, the biotransformation pathways of 5F 203 and GW 610 by P450s 1A1, 2W1, and 2S1 were investigated and the catalytic parameters of P450 1A1- and 2W1-catalyzed oxidation were determined in steady-state kinetic studies. The oxidations of 5F 203 catalyzed by P450s 1A1 and 2W1 yielded different products, and formation of a hydroxylamine was observed for the first time in the latter process. Liquid chromatography-mass spectrometry (LC-MS) analysis with the synthetic hydroxylamine and also a P450 2W1/5F 203 incubation mixture indicated the formation of dGuo adduct via a putative nitrenium intermediate. P450 2W1-catalyzed oxidation of GW 610 was 5-fold more efficient than the P450 1A1-catalyzed reaction. GW 610 underwent a two-step oxidation process catalyzed by P450 1A1 or 2W1: a regiospecific O-demethylation and a further hydroxylation. Glutathione (GSH) conjugates of 5F 203 and GW 610, presumably through a quninoneimine and a 1,2-quinone intermediate, respectively, were detected. These results demonstrate that human P450s 1A1 and 2W1 mediate 5F 203 and GW 610 bioactivation to reactive intermediates and lead to GSH conjugates and a dGuo adduct, which may account for the antitumor activities of 5F 203 and GW 610 and also be involved in cell toxicity. P450 2S1 can catalyze the reduction of the hydroxylamine to the amine 5F 203 under anaerobic conditions and, to a lesser extent, under aerobic conditions, thus attenuating the anticancer activity.
INTRODUCTION
P450 enzymes catalyze oxidation and reduction of a variety of drugs, carcinogens, steroids, lipids, and other organic molecules.1, 2 The Human Genome Project has identified 57 P450 genes.3 About one-fourth are still classified as “orphan” P450s because their functions remain largely unknown.4 P450s 2W1 and 2S1 remain in this category, and a number of carcinogens were shown to be activated by P450 2W1 but not P450 2S1 when heterologously expressed in this laboratory.5 The only repeatedly demonstrated reaction of P450 2S1 to date is the reduction of a di-N-oxide, AQ4N, to its amino derivative AQ4 [1,4-bis([2-(dimethylamino)ethyl]amino)-5,8-dehydroxyanthracene-9,10-dione] under anaerobic conditions.6, 7 P450 2W1 has been found to be overexpressed in tumor cells, especially in colon tumor cells, with only low or undetectable expression levels in other tissues.8, 9 Prior studies have also reported that the expression level of P450 2W1 is an independent prognostic factor in colon cancer, with high expression levels associated with poor clinical outcome.10 The functions of these two P450s are still not well understood, although P450 2W1 has been shown to have activity towards some drugs and lysophospholipids.5, footnote a
In the screening of 2-arylbenzothiazoles for anticancer activity, a number of molecules have been discovered with novel anticancer mechanisms, 5F 203 and GW 610 being two promising drug candidates.11 5F 203 showed antiproliferative potency in sensitive human breast and ovarian cancer cell lines (GI50 < 1 nM for MCF-7, MDA 468, and IGROV-1 cells) and emerged as a lead compound, currently in phase 1 clinical trials in the United Kingdom.12, 13 The results of co-incubation of 2-4(-amino-3-methyl-phenyl)benzothiazole (DF 203, an anticancer agent discovered earlier, yielding inactive metabolites that led to the discovery of 5F 20312) with α-naphthoflavone, a P450 1A1 inhibitor, showed inhibition of anticancer activity and revealed a role for P450 1A1 in the anticancer activity of the benzothiazoles.14 The involvement of P450 1A1 was reported for 5F 203 bioactivation (to generate reactive intermediates to form protein and DNA adducts) in the antitumor process within sensitive cells.13, 15 P450 1A1 is expressed in fetal liver and in some extrahepatic adult human tissues but not at appreciable levels in adult liver.16, 17 5F 203 acts as both a substrate and inducer of P450 1A1, the latter process involving binding to the aryl hydrocarbon receptor.18
Although P450 1A1 has been shown to be involved in the bioactivation of 5F 203, the products are still uncharacterized. In a prior study of the metabolism of 5F 203 in human B-lymphoblastoid cells expressing P450 1A1, 1B1, and 1A2, respectively,13 P450 1A1 exhibited the highest level of metabolic capacity. However, no chemical structures of the metabolites were determined.
More recently GW 610, a dimethoxyphenyl benzothiazole derivative, was discovered to exhibit potent (GI50 < 0.1 nM) and selective in vitro inhibitory activity against colon, lung, and breast cancer cell lines.19 Even less information has been reported on the metabolism of GW 610. GW 610,19 like 5F 203,18 can induce P450 1A1 in breast cancer cell lines (MCF-7 and MDA 468). However, P450 1A1 was neither highly expressed nor could be induced in the sensitive colon cancer cell lines KM12 and HCC. The conclusion was reached that the antitumor properties of GW 610 were unlikely to depend upon P450 1A1-mediated bioactivation alone. Demethylation was proposed as an initial mechanistic step in the antitumor process,11 although no evidence was provided.
The suggestion has been made that P450 1A1-mediated metabolism of 5F 203 and GW 610 is involved in their anticancer activities.20 In addition, P450 2W1-mediated bioactivation was concluded to be essential for GW 610 activation in colon cancer cells.20 In contrast, 5F 203 and GW 610 showed enhanced growth inhibition effects in P450 2S1 knock-down cancer cells, i.e., P450 2S1 deactivated these two anticancer molecules.20
The biotransformation of GW 610 and 5F 203 by P450 1A1, 2W1, and 2S1 plays a critical role in their potent and selective antitumor properties, as well as in any toxicity associated with these molecules. Knowledge of the biotransformation pathways is important in understanding the antitumor activity and improvement of drug efficacy. In this article, we report results on the biotransformation pathways of GW 610 and 5F 203 mediated by P450s 1A1, 2W1, and 2S1, characterization of the major oxidation products, and evidence for GSH conjugates and dGuo adduct formation from the reactive products.
EXPERIMENTAL PROCEDURES
All commercially obtained solvents and other chemicals were used directly without further purification. 2-Amino-5-fluorobenzothiazole and 2-phenylbenzothiazole were purchased from Alfa Aesar (Ward Hill, MA). 3-Methyl-4-nitrobenzoic acid was purchased from Sigma Aldrich (St. Louis, MO). 1-D and 2-D NMR spectroscopy data were obtained with 400 or 600 MHz Bruker NMR spectrometers, in the solvents noted, in the Vanderbilt facility. UV spectra were obtained (on-line) with a Waters Acquity UPLC system equipped with a photodiode array detector (Waters, Milford, MA).
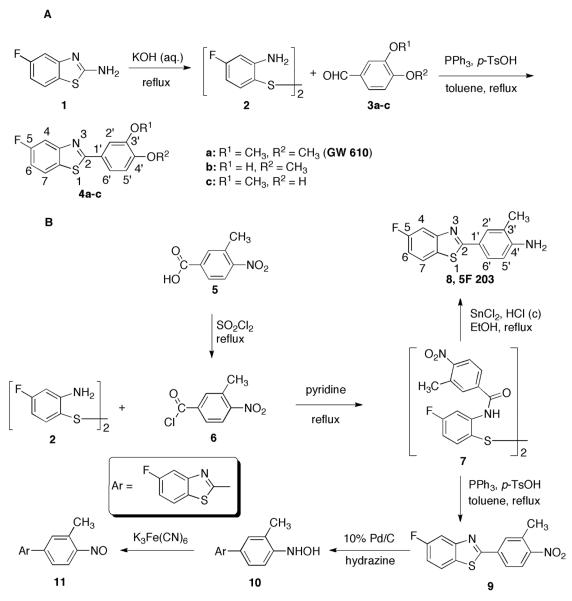
Chemical Synthesis (Scheme 1)
Scheme 1.
Synthesis of GW 610, 5F 203, and their putative metabolites
Bis(aminofluorophenyl) Disulfide (2). The title compound was prepared using a known procedure.12 2-Amino-5-fluorobenzothiazole (1) (0.125 g, 0.74 mmol) was added to a solution of KOH (0.625 g) in H2O (4 mL), and the resulting mixture was heated to reflux. After 3 h, a clear solution was formed and the solution was stirred under reflux for another 2 h. The solution was cooled to room temperature and acidified to pH 6 by the addition of CH3CO2H. H2O (1 mL) was added to the mixture, which was then stirred overnight. The solid precipitate was collected and purified by flash column chromatography on silica gel (CH2Cl2:hexanes, 3:1, v/v). 2 was obtained as a pale yellow solid (0.077 g, 73% yield). 1H NMR (400 MHz, CDCl3): δ 7.05 (dd, J = 8.5, 6.5 Hz, 2H, H-6), 6.41 (dd, J = 10.5, 2.6 Hz, 2H, H-3), 6.29 (td, J = 8.5, 2.6 Hz, 2H, H-5), 4.47 (br s, 4H, NH2).
General Method for the Synthesis of GW 610 and Its Mono-demethylated Analogs, 4a-c. 4a-c were synthesized by using a previously reported method.11 Each disubstituted benzaldehyde (3a-c; 0.20 mmol), p-toluenesulfonic acid monohydrate (0.040 mmol), and triphenylphosphine (0.20 mmol) were added to a solution of 2 in toluene (5 mL). The resulting mixture was stirred and heated under reflux for 24 h. After each mixture was cooled to room temperature, the solvent was removed in vacuo. Flash chromatography (CH2Cl2, 100%) afforded corresponding 4a-c as white solids in yields of 68–87%.
5-Fluoro-2-(3,4-dimethoxyphenyl)-benzothiazole (4a, GW 610) was prepared from 2 and veratraldehyde (68% yield): UV λmax 331 nm; 1H NMR (400 MHz, CDCl3): δ 7.80 (dd, J = 8.8, 5.1 Hz, 1H, H-7), 7.73 (dd, J = 9.6, 2.3 Hz, 1H, H-4), 7.72 (d, J = 2.3 Hz, 1H, H-2'), 7.60 (dd, J = 8.4, 2.1 Hz, 1H, H-6'), 7.14 (td, J = 8.8, 2.5 Hz, 1H, H-6), 6.96 (d, J = 8.4 Hz, 1H, H-5'), 4.03 (s, 3H, OCH3-3'), 3.97 (s, 3H, OCH3-4'). HRMS m/z calcd for C15H13FNO2S (MH+), 290.0646; found 290.0643.
5-Fluoro-2-(3-hydroxy-4-methoxyphenyl)-benzothiazole (4b) was prepared from 2 and 3-hydroxy-4-dimethoxybenzaldehyde (87% yield): UV λmax 331 nm; 1H NMR (400 MHz, CDCl3): δ 7.79 (dd, J = 8.8, 5.1 Hz, 1H, H-7), 7.72 (dd, J = 9.6, 2.5 Hz, 1H, H-4), 7.65 (m, 2H, H-2', H-6'), 7.13 (td, J = 8.8, 2.5 Hz, 1H, H-6), 6.95 (d, J = 9.0 Hz, 1H, H-5'), 5.73 (br s, 1H, OH-3'), 3.98 (s, 3H, OCH3-4'). HRMS m/z calcd for C14H11FNO2S (MH+), 276.0489; found 276.0487.
5-Fluoro-2-(4-hydroxy-3-methoxyphenyl)-benzothiazole (4c) was prepared from 2 and vanillin (83% yield): UV λmax 331 nm; 1H NMR (400 MHz, CDCl3): δ 7.79 (dd, J = 8.8, 5.1 Hz, 1H, H-7), 7.72 (d, J = 2.1 Hz, 1H, H-2'), 7.71 (dd, J = 10.2, 2.1 Hz, 1H, H-4), 7.53 (dd, J = 8.3, 2.0 Hz, 1H, H-6'), 7.13 (td, J = 8.8, 2.5 Hz, 1H, H-6), 7.01 (d, J = 8.3 Hz, 1H, H-5'), 5.95 (br s, 1H, OH-4'), 4.04 (s, 3H, OCH3-3'). HRMS m/z calcd for C14H11FNO2S (MH+), 276.0489; found 276.0479.
3-Methyl-4-nitrobenzoyl Chloride (6). 3-Methyl-4-nitrobenzoic acid (0.60 g), thionyl chloride (3 mL), and DMF (0.05 mL) were heated under reflux for 4 h, cooled, and concentrated in vacuo. The residue was slurried twice with hexanes and concentrated to yield 6 as a yellow solid, which was used immediately to the next reaction.
Bis[4-fluoro-2-(3-methyl-4-nitrobenzoyl)aminophenyl] Difulfide (7) was synthesized as reported previously.12 Compound 6 (25 mg, 0.125 mmol) was added to a solution of 2 (15 mg, 0.053 mmol) in anhydrous pyridine (3 mL). The reaction mixture was stirred and heated under reflux for 30 min and then poured into H2O (15 mL). The precipitate was collected and washed with H2O to afford 7 as a pale yellow solid (12 mg, 75%). 1H NMR (400 MHz, CDCl3): δ 8.87 (brs, 2H, NH), 8.18 (dd, J = 11.0, 2.8 Hz, 2H, H-3), 8.01 (d, J = 8.4 Hz, 2H, H-5'), 7.73 (s, 2H, H-2'), 7.53 (dd, J = 8.4, 1.8 Hz, 2H, H-6'), 7.47 (dd, J = 8.6, 6.2 Hz, 2H, H-6), 6.67 (td, J = 8.1, 2.8 Hz, 2H, H-5), 2.68 (s, 6H, CH3).
2-(4-Amino-3-methylphenyl)-5-fluorobenzothiazole (8, 5F 203) was synthesized as reported elsewhere.12 To a solution of conc. HCl (1 mL), C2H5OH (2 mL), and H2O (0.2 mL) was added the disulfide 7 (12 mg) and SnCl2•2H2O (80 mg). The resulting mixture was stirred and heated under reflux for 15 h, allowed to cool, and then H2O (7.5 mL) and NaOH (0.40 g) were added slowly. The mixture was stirred for 1 h, after which the precipitate was collected and washed with H2O (5 mL). Flash chromatography (CH2Cl2, 100%) afforded 8 as a pale yellow solid (6.9 mg, 68%). UV λmax 347 nm; 1H NMR (400 MHz, CDCl3): δ 7.83 (s, 1H, H-2'), 7.79–7.72 (m, 2H, H-7, H-6'), 7.69 (dd, J = 9.7, 2.5 Hz, 1H, H-4), 7.09 (td, J = 8.8, 2.5 Hz, 1H, H-6), 6.73 (d, J = 8.3 Hz, 1H, H-5'), 2.25 (s, 3H, CH3). HRMS m/z calcd for C14H12FN2S (MH+), 259.0700; found 259.0689.
2-(3-Methyl-4-nitrophenyl)-5-fluorobenzothiazole (9). To a solution of the disulfide 7 (29 mg, 0.048 mmol) in toluene (3 mL) was added triphenylphosphine (12.7 mg, 0.048 mmol) and p-toluenesulfonic acid monohydrate (1.9 mg, 9.7 μmol). The resulting mixture was heated under reflux for 24 h. The solvent was removed in vacuo, and flash column chromatography gave 9 as a pale yellow solid (23 mg, 83%). UV λmax 323 nm; 1H NMR (400 MHz, CDCl3): δ 8.10 (d, J = 8.6 Hz, 1H, H-5'), 8.09 (s, 1H, H-2'), 8.02 (dd, J = 8.4, 1.8 Hz, 1H, H-6'), 7.88 (dd, J = 8.8, 5.0 Hz, 1H, H-7), 7.80 (dd, J = 9.3, 2.5 Hz, 1H, H-4), 7.22 (td, J = 8.8, 2.5 Hz, 1H, H-6), 2.72 (s, 3H, CH3). HRMS m/z calcd for C14H10FN2O2S (MH+), 289.0442; found 289.0429.
2-(4-Hydroxylamino-3-methylphenyl)-5-fluorobenzothiazole (10) and 2-(3-methyl-4-nitrosophenyl)-5-fluorobenzothiazole (11). To a solution of 9 (1.4 mg, 4.9 μmol) in THF (3 mL) was added Pd/C (10%, catalyst) and hydrazine hydrate (20 μL) under Ar.21 The reaction mixture was stirred at room temperature. After 2 h, the reaction was complete. The solvent was removed in vacuo, and the residue was purified by flash chromatography to afford 10 as a yellow solid (1.1 mg, 83%) (note: the solubility of 10 in CHCl3 and CH3OH is very poor). UV λmax 339 nm; 1H NMR (400 MHz, DMSO-d6): δ 8.68 (s, 1H), 8.57 (s, 1H), 8.11 (dd, J = 8.7, 5.4 Hz, 1H, H-7), 7.85 (d, J = 8.5 Hz, 1H, H-6'), 7.80 (dd, J = 8.8, 2.4 Hz, 1H, H-4), 7.75 (s, 1H, H-2'), 7.31 (td, J = 8.8, 2.4 Hz, 1H, H-6), 7.20 (d, J = 8.4, 1H, H-5'), 2.19 (s, 3H, H3). HRMS m/z calcd for C14H12FN2OS (MH+), 275.0649; found 275.0642.
Compound 10 was then dissolved in C2H5OH (100 μL), to which was added a solution of K3Fe(CN)6 (20 mg, 60 μmol).22 The resulting mixture was stirred at room temperature for 3 h and then diluted with H2O (2 mL). The white precipitate (11) was collected by filtration. UV λmax 362 nm; HRMS m/z calcd for C14H10FN2OS (MH+), 273.0492; found 273.0496.
Enzyme Preparations. Human P450s 1A1 and 2W1 were co-expressed with NADPH-P450 reductase and P450s 2S1 and 2W1 were expressed in Escherichia coli membranes as previously described.5, 23 Human P450 2S1 and 2W1 were purified as described.5 Human liver microsomes were prepared from ten randomly chosen human liver donor samples using previously reported procedures.24
Incubation Conditions. A typical incubation mixture for qualitative studies contained P450 enzyme (0.3–0.8 μM) in bacterial membranes or liver microsomes, an NADPH-generating system (10 mM glucose 6-phosphate, 0.5 mM NADP+, and 4 μg mL−1 yeast glucose 6-phosphate dehydrogenase), and the substrate (100 μM) in 0.10 M potassium phosphate buffer (pH 7.4).24 When a purified P450 was used, it was generally reconstituted with a 2-fold excess of NADPH-P450 reductase and 30 μM L-α-dilauroy-sn-glycero-3-phosphocholine.55,7,24, footnote a The incubations were performed at 37 °C for 30–60 min. CH2Cl2 (2-fold volume) was added to terminate the reaction, and the resulting mixture was separated by centrifugation at 2 × 103 g for 5 min. The organic layer was transferred and dried under an N2 stream. The residue was dissolved in CH3CN for HPLC and LC-MS analysis.
Large-scale reactions (total volume of 50–100 mL) were performed in order to obtain sufficient amounts of metabolites for NMR analysis. The incubation conditions were the same as in the qualitative studies except that catalase (4 μg mL−1, Sigma Aldrich, from bovine erythrocytes, dialyzed before use to remove thymol) was also present in the mixture. The reactions were terminated by addition of CH2Cl2 (2-fold volume). The resulting mixtures were centrifuged at 2 × 103 g for 5 min, and the lower layer of each was concentrated using a rotary evaporator. A minimum amount of CH3CN was added to dissolve the residue, which was subsequently subjected to preparative HPLC isolation (vide infra).
Anaerobic Incubations. Anaerobic reduction experiments were performed in Thunberg tubes, with the samples deaerated with the use of a manifold, using alternating cycles of argon and vacuum (20 mm Hg) (five cycles). A typical 500 μL incubation mixture contained 0.5 μM P450 2S1, 1.0 μM NADPH-P450 reductase, 160 μM L-α-1,2-dilauoryl-sn-glycero-3-phosphocholine, 100 mM potassium phosphate buffer (pH 7.4), and 100 μM 10 (from a freshly prepared 10 mM DMSO stock). After 5 min preincubation, an NADPH-generating system was added from the neck of the tube under anaerobic conditions. The reactions were incubated at 37 °C for 45 min, terminated by the addition of 1 mL of CH3CN, and analyzed by HPLC.
Steady-state Kinetic Measurements of P450 Reactions. The incubation conditions for kinetic studies were similar to those employed in the qualitative studies. The concentrations of P450 and the NADPH-generating system remained the same. Reactions with a series of different substrate concentrations ranging from 0 to 200 μM were carried out, in duplicate, at 37 °C for 10 and 15 min for GW 610 with P450s 2W1 and 1A1, respectively, and for 15 and 25 min for 5F 203 with P450s 2W1 and 1A1, respectively. After the reactions were quenched with CH2Cl2 (2-fold volume), a small amount of internal standard (5-fluoro-2-(3-hydroxy-4-methoxyphenyl)-benzothiazole, 4b, 5 μL, 0.1 mM for GW 610 reactions; 2-phenylbenzothiazole, 12, 5 μL, 0.1 mM for 5F 203 reactions) was added to each reaction mixture. The mixture was centrifuged at 2 × 103 g for 5 min, and the organic layer was transferred and dried under an N2 stream. The residue was dissolved in CH3CN for HPLC analysis, from which the P450 enzyme kinetic data (Km and kcat) were calculated using nonlinear regression analysis (Prism, GraphPad Software, La Jolla, CA).
GSH Adduct Formation from GW 610 and 5F 203 Oxidation Products. A typical incubation mixture (total volume of 0.50 mL) contained P450 enzyme (2W1, 1A1, or microsomes, 0.50 μM) in bacterial membranes or liver microsomes, an NADPH-generating system (10 mM glucose 6-phosphate, 0.5 mM NADP+, 4 μg mL−1 yeast glucose 6-phosphate dehydrogenase), catalase (4 μg mL−1), GSH (5 mM), and the substrate (100 μM) in 0.10 M potassium phosphate buffer (pH 7.4). The incubations were performed at 37 °C for 12–15 h. CH3CN (2-fold volume of incubation mixture) was added to terminate the reaction, and the supernatant was analyzed by LC-MS.
Formation of dGuo Adduct with Synthetic 10 and P450 2W1-catalyzed Oxidation of 5F 203
With Synthetic 10. To a freshly prepared solution of 10 in CH2Cl2 (10 μM, 1.0 mL), 20 μL of Ac2O was added. The mixture was swirled for 5 min, the solvent was removed in vacuo, and 30 μL of CH3CN was added. The CH3CN solution (5 μL) was added to a solution of 100 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM EDTA (245 μL), followed by the addition of aqueous dGuo (250 μL of a 10 mM solution). The resulting mixture was incubated at 37 °C under argon for 2 h and analyzed by LC-MS.
With the P450 2W1-catalyzed Oxidation of 5F 203. 5F 203 was incubated with P450 2W1 membranes under the usual incubation conditions at 37 °C for 45 min (total volume, 0.5 mL), the reaction was quenched by the addition of 1 mL of CH2Cl2 and mixed with a vortex device, and the organic layer was transferred. Ac2O (20 μL) was added to the CH2Cl2 solution, which was swirled for 5 min and dried under an N2 stream. CH3CN (5 μL) and 100 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM EDTA (245 μL) were added to the residue. The contents were mixed with a vortex device and added to a 10 mM aqueous solution of dGuo (250 μL). The resulting mixture was incubated at 37 °C under argon for 2 h and analyzed by LC-MS.
HPLC-UV Assays. HPLC analysis was performed on a Waters Acquity UPLC system equipped with two pumps, an autosampler, and a photodiode array detector (Waters, Milford, MA). Samples for qualitative and kinetic studies were injected onto an octadecylsilane (C18) reversed-phase column (5 μm, 2.1 mm × 250 mm, Hypersil GOLD, Thermo Scientific, Odessa, TX). The column was maintained at room temperature. The eluting solvents used were mobile phase A (CH3CN: H2O, 5:95, 0.5% HCO2H, v/v) and mobile phase B (CH3CN: H2O, 95:5, 0.5% HCO2H, v/v). The flow rate was 0.5 mL min−1. The gradient conditions for all of the qualitative and kinetic studies (except the kinetic analysis of the transformation of GW 610) started from 5% B (v/v) during the first 2 min (0–2 min), and was increased to 100% B over 5 min (2–7 min), maintained at that level for 4.5 min (7–11.5 min), and then returned to 5% B (v/v) over 0.5 min (11.5–12 min) before equilibration (12–15 min). 5-Fluoro-2-(3-hydroxy-4-methoxyphenyl)-benzothiazole (4b) was used as the internal standard in the kinetic analysis of the oxidation of GW 610. Separation of the products, internal standard, and parent compound was achieved using gradient conditions as follows: 30% B (v/v) during the first 2 min (0–2 min), increased to 65% B (v/v) over 10 min (2–12 min), then decreased to 30% B (v/v) over 1 min (12–13 min) before equilibration (13–15 min). Some products were identified by comparison with their retention times, UV spectra, and LC-MS with corresponding reference compounds (vide infra).
Isolation of metabolites was performed using a Hitachi L-7100 HPLC system (Tokyo, Japan) equipped with a single pump and UV detector (LDC Spectro Monitor III Model 1204D, Thermo). Samples (typical injection 100–150 μL) were injected onto a semi-preparative octadecylsilane (C18) reversed-phase column (5 μm, 10 mm × 250 mm, Prodigy, Phenomenex, Torrance, CA). The column was maintained at room temperature. The eluting solvents used were the same as used for analytical HPLC analysis, mobile phase A (CH3CN: H2O, 5:95, 0.5% HCO2H, v/v) and mobile phase B (CH3CN: H2O, 95:5, 0.5% HCO2H, v/v). The flow rate was 4 mL min−1. Isocratic elution (65% B, v/v) was used for isolation of 4c'. The gradient for isolation of 8a and 8b started from 60% B (v/v), was increased to 70% B (v/v) over 10 min (0–10 min), and then returned to 60% B (v/v) in 2 min (10–12 min) before equilibration (12–20 min). The fractions containing products of interest were collected, and CH3CN was removed under a stream of N2. The resulting mixture was extracted with CH2Cl2 three times, and the combined organic layers were dried over Na2SO4. CH2Cl2 was removed under a stream of N2, and the residue was dissolved in CDCl3 for NMR analysis.
LC-MS Analysis. LC/MS and LC/MS/MS analyses were performed on Finnigan LTQ ion trap mass spectrometers (Thermo Fisher Scientific, San Jose, CA). The samples were analyzed in the electrospray ionization (ESI) positive ion mode. A Waters Acquity UPLC system was coupled with the mass spectrometer. An octadecylsilane (C18) reversed-phase column (5 μm, 2.1 mm × 250 mm, Hypersil GOLD, Thermo Scientific, Odessa, TX) was used and was maintained at room temperature in the analyses. The eluting solvents used were mobile phase A (CH3CN: H2O, 5:95, 0.5% HCO2H, v/v) and mobile phase B (CH3CN: H2O, 95:5, 0.5% HCO2H, v/v). The flow rate was 0.5 mL min−1. The gradient conditions were the same as described above: 5% B (v/v) during the first 2 min (0–2 min), and increased to 100% B (v/v) in 5 min (2–7 min), maintained at that level for 4.5 min (7–11.5 min), and then returned to 5% B (v/v) in 0.5 min (11.5–12 min) before equilibration (12–15 min). MS conditions (N2 gas flow, spray current, voltages, etc.) were tuned to give maximum sensitivity for the parent compound.
LC-HRMS was performed on a Waters Acquity UPLC system coupled with a Waters Synapt hybrid quadropole/OA-TOF mass spectrometer (Waters, Milford, MA). The samples were analyzed in the ESI positive ion mode. The MS conditions were tuned to give maximum sensitivity for the parent compound. The LC conditions were the same as in the LC-MS studies (vide supra).
RESULTS
Incubations of 2-Phenylbenzothiazole, GW 610, and 5F 203 with P450 2W1 and 2S1: Preliminary Assays. No products were observed following the incubation of 2-phenylbenzothiazole with reconstituted systems containing recombinant human P450 2W1 or 2S1. In addition, no products were observed by HPLC-UV following the incubation of either GW 610 or 5F 203 with P450 2S1. Products were formed in the incubation of GW 610 or 5F 203 with P450 2W1, as judged by HPLC-UV assays.
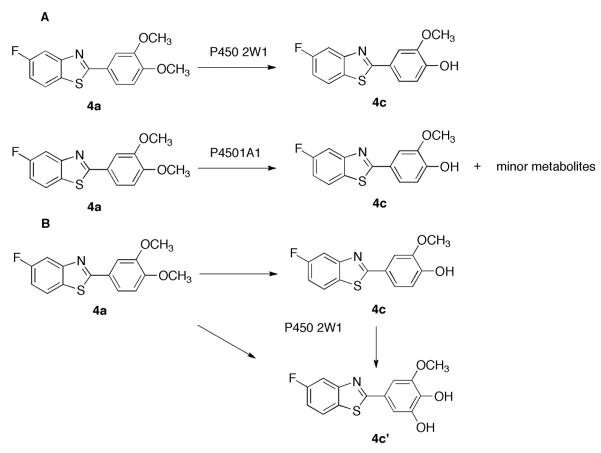
Hydroxylation and Demethylation of GW 610 by P450s 2W1 and 1A1. An HPLC peak appeared at retention time (tR) 6.05 min in the incubation of GW 610 (tR 6.55 min) with P450 2W1 (Figure 1B). Further LC-MS analysis showed that the peak had m/z 276 (Figure S9 in the Supporting Information) possibly corresponding to a demethylated product. In order to determine the structure of the product, two chemicals were synthesized as potential products (4b and 4c, Scheme 1A). Comparison of synthetic 4b and 4c with the incubation mixture using HPLC showed co-chromatography with 4c (tR 6.05 min, Figure 1C), whereas 4b was eluted slightly earlier (tR 5.98 min, Figure 1D) under the same conditions. The tR 6.05 min peak was also the predominant peak obtained from the incubation of GW 610 with P450 1A1 (Figure 1A).
Figure 1.

HPLC chromatograms of GW 610 oxidation products formed with P450s 1A1 and 2W1. (A) Incubation products of GW 610 formed with P450 1A1 (45 min). (B) Incubation products of GW 610 formed with P450 2W1 (45 min). (C) Chemically synthesized 4c. (D) Synthetic 4b. (E) Incubation products of GW 610 formed with P450 2W1 (60 min). (F) Incubation products of 4c formed with P450 2W1 (60 min).
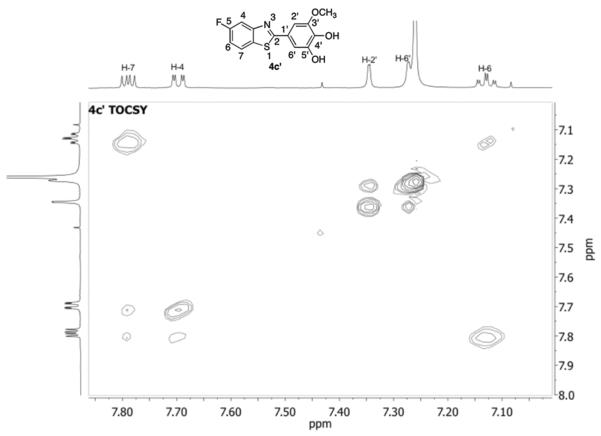
Using more P450 2W1 and a longer incubation time, a new peak appeared at tR 5.55 min (Figure 1E). In a separate experiment, 4c was incubated with P450 2W1 and also yielded the tR 5.55 min peak as a single product (Figure 1F). LC-MS analysis indicated that the peak had an m/z of 292 (MH+, Figure S9), 2 Da greater than GW 610 and 16 Da greater than 4c, suggesting that it might be a secondary oxidation metabolite. A sufficient amount of 4c' was obtained from a large-scale incubation of 4c with P450 2W1 for NMR analysis. In the 1-D 1H NMR spectrum of 4c' (Figure S2), one high field proton signal disappeared in the aromatic region and the two doublet proton signal (in 4c) was replaced with two singlet proton signals (in 4c' at 7.27 and 7.34 ppm), suggesting substitution at 5' position. The proton coupling pattern on the fluorine-containing ring was unchanged (2-D TOCSY results, Figure 2), supporting this conclusion. 4c' was assigned the structure shown in Scheme 2, based on both the LC-MS and NMR.
Figure 2.
TOCSY spectrum of 4c' in CDCl3 (600 MHz).
Scheme 2.
P450 2W1, P450 1A1, and microsomal GW 610 reactions
Oxidation of 5F 203 by P450 2W1, 1A1, and human liver microsomes. Two product peaks appeared at tR 5.45 min (8a, UV λmax 343 nm) and 6.02 min (10, UV λmax 339 nm) following incubation of 5F 203 (tR 6.31 min, UV λmax 347 nm) with P450 2W1 (Figure S7A). LC-MS results showed that both peaks had an m/z 275 ion ([MH]+), 16 Da greater than the parent compound 5F 203, very likely corresponding to oxidation products. Two product peaks also appeared at tR 5.45 min (8a, UV λmax 343 nm) (which was present in the P450 2W1 oxidation as well) and tR 5.80 min (8b, UV λmax 356 nm) from the incubation of 5F 203 (tR 6.31 min) with P450 1A1 (Figure S7B). Interestingly, the HPLC results for the incubation of 5F 203 with human liver microsomes showed all three major products (8a, 8b, and 10). LC-MS showed that all three peaks had m/z 275. Arylamines are known to undergo oxidation to form hydroxylamines and nitroso and nitro compounds,25 in addition to hydroxylation on the aromatic rings. The hydroxylamine (10), nitroso (11), and nitro (9) derivatives of 5F 203 were readily synthesized. The tR values of 10, 11, and 9 were 6.04, 7.43, and 7.11 min, respectively, under the same conditions. The tR and the UV spectrum of synthetic 10 were identical to those of oxidation product 10 found in the 5F 203 incubation with P450 2W1. A mass signal (m/z 273, [MH]+) corresponding to the nitroso derivative (11) was observed, consistent with the tR (7.49 min) in LC-MS. However, no signal for the nitro derivative (9) was detected. Compound 10 was also detected following incubation of 5F 203 with P450 1A1, and 8b was detected as well in incubations of 5F 203 with P450 2W1, respectively, both as minor products (Figure S10). A large-scale incubation of 5F 203 with human liver microsomes provided sufficient amounts of 8a and 8b for NMR spectra (the instability and poor solubility of 10 in common organic solvents (eg. CHCl3, CH2Cl2 and CH3OH) complicated the use of NMR for comparison). In the 1-D 1H-NMR of 8a (Figure S3), the retention of the two pairs of doublet signals at δ 7.84 and 6.76 ppm and the disappearance of a singlet proton signal clearly indicated the presence of a non-hydrogen substitutent at 2' postion. Additionally, the homonuclear COSY NMR spectrum of 8a (Figure S9) showed an unambiguous coupling relationship between the two pairs of doublet signals at δ 7.84 and 6.76 ppm, providing further evidence for 2'-substitution. Given the LC-MS results, the structure 8a was proposed, i.e. a 2'-hydroxy derivative of 5F 203 (Scheme 3). In the 1H NMR spectrum of 8b (Figure S4), the proton signal splitting pattern was similar to that of 4c', where one high field aromatic proton signal disappeared and two singlet proton signals replaced the original doublets in the precursors. Based on the LC-MS and NMR data available, the structure 8b was assigned, i.e. the 5'-hydroxy product of 5F 203 (Scheme 3).
Scheme 3.

P450 2W1, P450 1A1, and microsomal 5F 203 reactions
Steady-state Kinetic Data for P450 2W1 and 1A1 Oxidation Reactions. Steady-state kinetic results for P450 2W1- and 1A1-catalyzed oxidation reactions with GW 610 and 5F 203 are summarized in Table 1, including formation of 4c from GW 610 catalyzed by P450 2W1 and 1A1, formation of 8a and 10 from 5F 203, and formation of 8a and 8b from 5F 203. P450 2W1 was 5-fold more efficient than P450 1A1 in the formation of 4c from GW 610. In contrast, P450 1A1 was ~ 2-fold more efficient than P450 2W1 in terms of oxidation of 5F 203 to form 8a. In addition, while P450 2W1 catalyzed 5F 203 to yield the hydroxylamine 10, P450 1A1 oxidized 5F 203 to 8b much more efficiently.
Table 1.
Steady-state Kinetics of P450 2W1 and 1A1-catalyzed Reactions
| substrate | P450 | product | Km (μM) | kcat (min−1) | kcat / Km (μM−1 min−1) |
|---|---|---|---|---|---|
| GW 610 | 2W1 | 4c | 22± 7 | 3.8 ± 0.4 | 0.18 ± 0.05 |
| 1A1 | 4c | 10± 3 | 0.37 ± 0.03 | 0.036 ± 0.01 | |
|
| |||||
| 5F 203 | 2W1 | 8a | 31 ± 11 | 0.95 ± 0.1 | 0.030 ± 0.01 |
| 10 | 35± 12 | 0.56 ± 0.06 | 0.016 ± 0.005 | ||
|
| |||||
| 1A1 | 8a | 32 ± 9 | 1.7 ± 0.2 | 0.054 ± 0.02 | |
| 8b | 29± 8 | 3.1 ± 0.3 | 0.11 ± 0.03 | ||
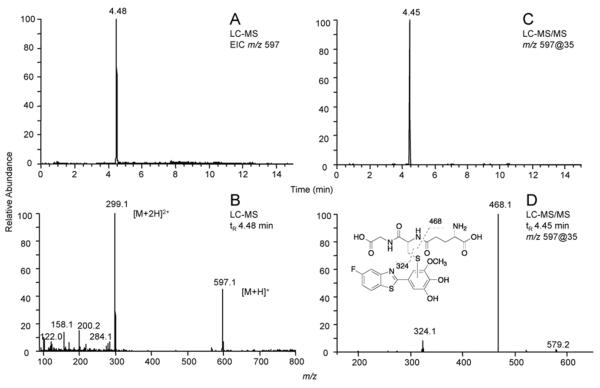
GSH Adduct Formation from GW 610 and 5F 203 Oxidation Products. A peak at tR ~ 4.5 min appeared in total ion chromatograms (ESI+) of all LC-MS analyses for incubation mixtures of GW 610 with P450 2W1 (Figure 3A), P450 1A1, or human liver microsomes (data not shown) in the presence of GSH. The MS of that peak (m/z 597, Figure 3B) was consistent with the structure 4g (the GSH conjugate of 4c'), and another significant signal in the MS spectrum (m/z 299, Figure 3B) was determined to be a doubly charged GSH conjugate ([M+2H]+), in that its fragmentation pattern was found to be identical that of the peak (m/z 597) (data not shown). The LC-MS/MS of the peak at tR ~ 4.5 min (Figure 3C) gave two major fragment ions: m/z 468 (loss of 129) and 324 (loss of 273), commonly seen for GSH conjugates (Figure 3D).
Figure 3.
LC-MS and LC-MS/MS chromatograms and CID spectra showing the presence of a GSH conjugate of GW610 formed with P450 2W1. (A) Extracted ion chromatogram of m/z 597. (B) LC-MS spectrum of the peak at tR 4.48 min. (C) Product ion chromatogram of m/z 597. (B) LC-MS/MS spectrum of the peak at tR 4.45 min.
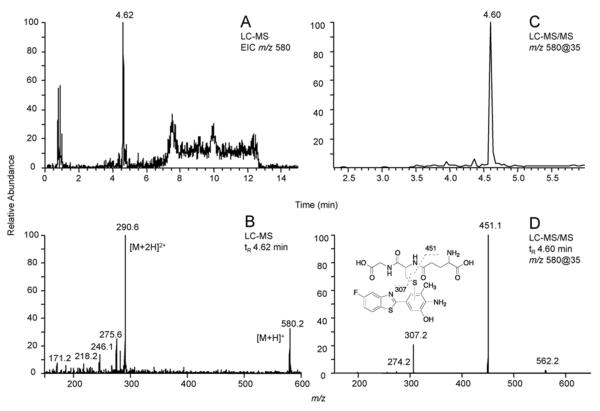
Similarly, the LC-MS total ion chromatograms for reactions of 5F 203 with P450 2W1 and GSH (Figure 4A), as well as P450 1A1 or human liver microsomes (data not shown) in the presence of GSH, all showed a peak at tR ~ 4.6 min under the same conditions as for GW 610. The peak had m/z 580 (Figure 4B), consistent with 8g (the GSH conjugate of 8b) and the predominant signal in the MS spectrum (m/z 290.6, Figure 4B) was also proven to be a doubly charged GSH conjugate ([M+2H]+) upon comparison of its fragmentation pattern with the peak (m/z 580) (data not shown). LC-MS/MS analysis of the peak (m/z 580, Figure 4C) again showed two major fragment ions: m/z 451 (loss of 129) and 307 (loss of 273), providing supportive evidence for the formation of a GSH conjugate (Figure 4D).
Figure 4.
LC-MS and LC-MS/MS chromatograms and CID spectra showing the presence of a GSH conjugate of 5F 203 formed with P450 2W1. (A) Extracted ion chromatogram of m/z 580. (B) LC-MS spectrum of the peak at tR 4.62 min. (C) Product ion chromatogram of m/z 580. (B) LC-MS/MS spectrum of the peak at tR 4.60 min.
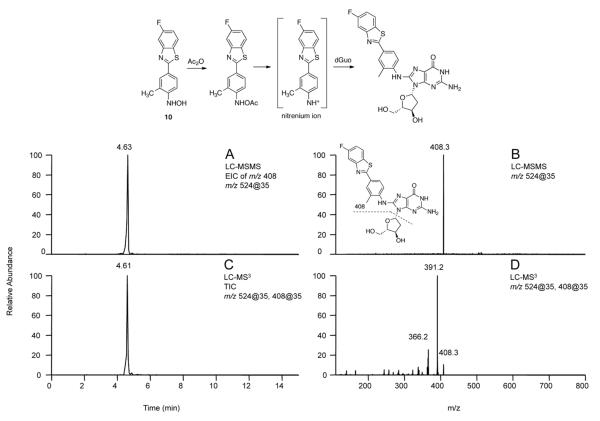
Formation of a dGuo Adduct with Synthetic 10 and a P450 2W1-catalyzed Oxidation of 5F 203. The analyses of samples of dGuo incubated with synthetic 10 and the oxidation product of 5F 203 formed by P450 2W1 (using LC-MS in the positive ion mode) both showed a peak at tR 4.6 min with m/z 524 (MH+), which is consistent with a dGuo adduct of compound 10. From the reaction of 5F 203 P450 2W1 incubation products with dGuo, CID of the peak at tR 4.6 min (Figure 5A, positive ion mode) yielded a characteristic transition of m/z 524 → 408 (Figure 5B), with a loss of 116, very typical of dGuo adducts. LC-MS3 of m/z 408 (Figure 5C) generated a fragment ion with m/z 391 (loss of NH3) as the major peak (Figure 5D), consistent with the proposed structure, providing evidence for the presence of a dGuo adduct. The LC-MS/MS and LC-MS3 analyses of the sample of synthetic 10 with dGuo showed identical fragmentation results (Figure S11) with those found for 5F 203/P450 2W1 incubation products reacting with dGuo.
Figure 5.
LC-MS/MS and LC-MS3 chromatograms and CID spectra showing a dGuo adduct of an oxidation product of 5F 203 formed with P450 2W1. (A) Extracted ion chromatogram of m/z 408. (B) LC-MS/MS spectrum of the peak at tR 4.63 min. (C) Total ion chromatogram of CID of the m/z 408 product ion. (D) LC-MS3 spectrum of the peak at tR 4.61min.
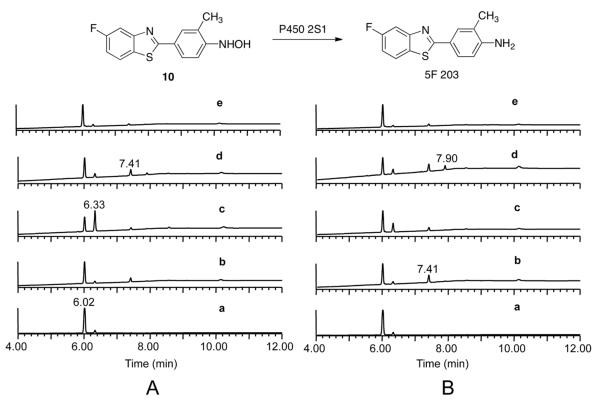
Anaerobic Reduction of 10 Catalyzed by P450 2S1. Freshly prepared 10 was incubated with P450 2S1 under anaerobic conditions (Figure 6A) and normal aerobic conditions (Figure 6B), in parallel. The peaks appearing at tR 6.02, 6.33, and 7.41 min correspond to 10, 5F 203, and 11, respectively. When authentic 10 was incubated in 100 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM EDTA for 45 min, varying amounts of 11 (Figure 6A–b, 6B–b) were formed by oxidation. More 11 was formed under aerobic conditions. In contrast, 5F 203 was produced from the reduction of 10 under both anaerobic and aerobic conditions with P450 2S1 in the presence of NADPH-P450 reductase and an NADPH-generating system (Figure 6A–c, 6B–c, respectively). More 5F 203 was formed under anaerobic conditions. With only the NADPH-generating system deleted, oxidation of 10 to 11 occurred under both anaerobic (Figure 6A–d) and areobic conditions (Figure 6B–d), and unidentified products were also formed, especially under areobic conditions (tR 7.90 min and others, Figure 6B–d). With 10, NADPH-P450 reductase, and the NADPH-generating system in buffer (but in the absence of P450 2S1), oxidation yielded only barely observable amounts of 11 under both anaerobic (Figure 6A–e) and normal aerobic conditions (Figure 6B–e).
Figure 6.
Reduction of 10 by P450 2S1. Freshly prepared 10 (5 μL of a 10 mM solution in DMSO) was incubated with P450 2S1 (total volume 0.5 mL) at 37 °C for 45 min under anaerobic (A) and normal aerobic conditions (B). (a) Standard 10. (b) 10 in 100 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM EDTA, incubated at 37 °C for 45 min. (c) 10 incubated with P450 2S1 in the presence of NADPH-P450 reductase and an NADPH-generating system. (d) 10 incubated with all typical components of the P450 2S1 system with the exception of the NADPH-generating system. (e) 10 incubated with all P450 system components with the exception of P450 2S1.
DISCUSSION
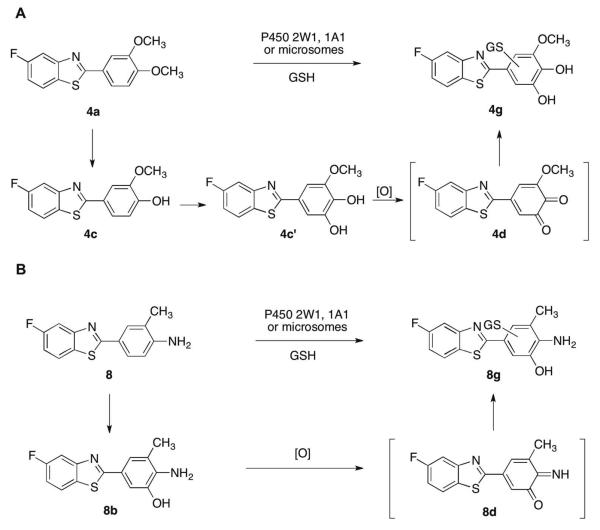
In this study we characterized the P450-mediated bioactivation of GW 610 and 5F 203 to form reactive intermediates, which may lead to their antitumor activity within sensitive tumor cells.13,20 Our results clearly demonstrate that, in addition to P450 1A1, P450 2W1 is also involved in the biotransformation of GW 610. Consistent with a previous hypothesis,11O-demethylation is the first step of the process and it occurred in a regiospecific manner to give 4c as the single initial product. Furthermore, steady-state kinetic studies indicated that P450 2W1 has a 5-fold higher catalytic efficiency towards GW 610 compared to P450 1A1. This result may explain the observation in a previous study that the addition of the P450 1A1 inhibitor resveratrol26 did not affect the potency of GW 610 in sensitive cell lines,19 because P450 2W1 could also contribute to its metabolism. The result may also explain the potent selective antitumor activity of GW 610 in human colon cancer cells, where P450 2W1 was found to be selectively expressed.8, 9 Further oxidation of 4c leads to the formation of a catechol, 4c', which can be readily oxidized to an o-quinone and react with GSH (Scheme 4).
Scheme 4.
GSH conjugates of GW 610 and 5F 203 oxidation products
5F 203 can be oxidized by P450 1A1 as well as 2W1, and the products differ. The two major products of P450 1A1-catalyzed oxidation of 5F 203 were 8a and 8b, both formed by hydroxylations on one aromatic ring. While 8a was also one of the two major products formed by P450 2W1, another product was the hydroxylamine 10, which had been previously proposed as a putative 5F 203 bioactivation product but not characterized.27, 28 A compound analogous to 10 (with no F and methyl groups) was shown to be able to bind to purines and pyrimidines via a nitrenium ion intermediate,28,29 (the mechanism for the formation of C8-substituted dGuo-arylamine adduct was reported previously30). To our knowledge, our in vitro results constitute the first evidence of the formation of 10 in the bioactivation of 5F 203 and, indeed, LC-MS results provide the evidence for the formation of dGuo adduct with activated 10 (Figure 5). The nitroso derivative (11) was also detected in LC-MS (Figure S10).
Among the 2-aryl-benzothiazoles tested in this study, all of the substrates of P450 2W1 contain at least one hydrogen-bonding donor (-NH2, 5F203), acceptor (-OCH3, GW 610), or both (-OCH3, -OH, 4c). In contrast, the 2-phenyl-benzothiazole, without hydrogen-bonding donor or acceptor as substituent on the aromatic ring, is not a substrate for P450 2W1.
The formation of GSH conjugates is usually considered as evidence for chemically reactive intermediates, often associated with drug toxicity and potential adverse drug reactions.31 The GW 610 GSH conjugate is presumably formed via a 1,2-quninone intermediate (Scheme 4A), whereas the 5F 203 GSH conjugate is presumably formed via a quninoneimine (Scheme 4B). The hydroxylamine generated in the biotransformation of 5F 203 could react with dGuo to produce an adduct via a nitrenium ion intermediate, in a similar way as shown in studies using a 5F 203 analogue28, 29 or other carcinogenic arylamines.25
Neither GW 610 nor 5F 203 was a substrate for P450 2S1, i.e. no products were observed from the incubations. However, as shown in the incubation of hydroxylamine 10 with P450 2S1, reduction of 10 to 5F 203 occurred under both anaerobic and aerobic conditions—moreso under anaerobic conditions (Figure 6A–c)—decreasing the production of nitrenium ions and formation of dGuo adducts and thus alleviating the damage caused by 5F 203. These results can explain the role of P450 2S1 in attenuating the antitumor activity of 5F 203, a conclusion reached only on the basis of knock-down experiments.20 It is worth noting that NADPH-P450 reductase appeared to be able to reduce the nitroso derivative 11 (to 10) both anaerobically and aerobically, consistent with similar observations in a previous study of the nitroso derivative of 2-amino-3-methylimidazo[4,5-f]quinoline.32
In conclusion, the biotransformation pathways of the antitumor agents GW 610 and 5F 203 involving human P450s 1A1, 2W1, and 2S1 were studied and the chemical structures of major products were determined. The high catalytic efficiency of P450 2W1 for oxidation of GW 610 may explain the potent and selective antitumor activity to colon cancer cells. The hydroxylamine 10 was identified, for the first time, as a major oxidation product of 5F 203 formed by P450 2W1, which can further react with dGuo to form an adduct (Figure 5). P450 2S1 can catalyze the reduction of 10 back to 5F 203 under anaerobic conditions and (to a lesser extent) aerobic conditions, thus resulting in attenuating the anticancer activity by decreasing the production of nitrenium ions and thus the level of the dGuo adduct and probably other DNA adducts. Oxidation products of GW 610 and 5F 203 can form GSH conjugates, presumably via quinone and quinoneimine intermediates, which may lead to their pharmacological and possible toxicological consequences.
Supplementary Material
Acknowledgments
Funding This work was supported in part by the United States Public Health Service (F.P.G., R37 CA090426, P30 ES000267).
ABBREVIATIONS
- 5F 203
2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole
- COSY
correlated (NMR) spectroscopy
- ESI
electrospray ionization
- GW 610
5-fluoro-2-(3,4-dimethoxyphenyl)-benzothiazole
- LC-MS
liquid chromatography-mass spectrometry
- NOE
nuclear Overhauser effect
- TOCSY
total correlation (NMR) spectroscopy
- UPLC
ultra performance liquid chromatography
Footnotes
Supporting Information
Selective NOE NMR spectra of GW 610, 1H NMR spectrum of 4c', 1H NMR and HH COSY spectra of 8a, 1H NMR spectrum of 8b, UV spectra of GW610, 4b, 4c and 4c', HPLC chromatograms of incubations of GW 610 with P450 2W1 using 4b as internal standard, HPLC chromatograms of incubations of 5F 203 with P450s 2W1 and 1A1, HPLC chromatograms and UV spectra of synthetic 10, 11, and 9, LC-MS chromatograms and spectra of 4c and 4c', LC-MS chromatograms of 5F 203 oxidation products catalyzed by P450s 2W1 and 1A1, and LC-MS/MS and LC-MS3 chromatograms and CID spectra showing the presence of the dGuo adduct formed with synthetic 10. This material is available free of charge via the Internet at http://pubs.acs.org.
Xiao, Y., and Guengerich, F. P. (2012) Metabolomic analysis and identification of a role for the orphan human cytochrome P450 2W1 in selective oxidation of lysophospholipids. J. Lipid Res., in press.
REFERENCES
- (1).Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- (2).Guengerich FP. Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- (3).Guengerich FP, Ortiz de Montellano PR. Cytochrome P450: Structure, Mechanism, and Biochemisty. 3rd ed. Kluwer Academic/Plenum Press; New York: 2005. Human cytochrome P450 enzymes; pp. 377–530. [Google Scholar]
- (4).Guengerich FP, Cheng Q. Orphans in the human cytochrome P450 superfamily: approaches to discovering functions and relevance in pharmacology. Pharmacol. Rev. 2011;63:684–699. doi: 10.1124/pr.110.003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wu ZL, Sohl CD, Shimada T, Guengerich FP. Recombinant enzymes overexpressed in bacteria show broad catalytic specificity of human cytochrome P450 2W1 and limited activity of human cytochrome P450 2S1. Mol. Pharmacol. 2006;69:2007–2014. doi: 10.1124/mol.106.023648. [DOI] [PubMed] [Google Scholar]
- (6).Nishida CR, Lee M, Ortiz de Montellano PR. Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol. Pharmacol. 2010;78:497–502. doi: 10.1124/mol.110.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Xiao Y, Shinkyo R, Guengerich FP. Cytochrome P450 2S1 is reduced by NADPH-cytochrome P450 reductase. Drug Metab. Dispos. 2011;39:944–946. doi: 10.1124/dmd.111.039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gomez A, Karlgren M, Edler D, Bernal ML, Mkrtchian S, Ingelman-Sundberg M. Expression of CYP2W1 in colon tumors: regulation by gene methylation. Pharmacogenomics. 2007;8:1315–1325. doi: 10.2217/14622416.8.10.1315. [DOI] [PubMed] [Google Scholar]
- (9).Gomez A, Nekvindova J, Travica S, Lee MY, Johansson I, Edler D, Mkrtchian S, Ingelman-Sundberg M. Colorectal cancer-specific cytochrome P450 2W1: intracellular localization, glycosylation, and catalytic activity. Mol. Pharmacol. 2010;78:1004–1011. doi: 10.1124/mol.110.067652. [DOI] [PubMed] [Google Scholar]
- (10).Edler D, Stenstedt K, Ohrling K, Hallstrom M, Karlgren M, Ingelman-Sundberg M, Raynhammar P. The expression of the novel CYP2W1 enzyme is an independent prognostic factor in colorectal cancer—A pilot study. Eur. J. Cancer. 2009;45:705–712. doi: 10.1016/j.ejca.2008.11.031. [DOI] [PubMed] [Google Scholar]
- (11).Aiello S, Wells G, Stone EL, Kadri H, Bazzi R, Bell DR, Stevens MF, Matthews CS, Bradshaw TD, Westwell AD. Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648) J. Med. Chem. 2008;51:5135–5139. doi: 10.1021/jm800418z. [DOI] [PubMed] [Google Scholar]
- (12).Hutchinson I, Chua MS, Browne HL, Trapani V, Bradshaw TD, Westwell AD, Stevens MF. Antitumor benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2-(4-aminophenyl)benzothiazoles. J. Med. Chem. 2001;44:1446–1455. doi: 10.1021/jm001104n. [DOI] [PubMed] [Google Scholar]
- (13).Brantley E, Trapani V, Alley MC, Hose CD, Bradshaw TD, Stevens MF, Sausville EA, Stinson SF. Fluorinated 2-(4-amino-3-methylphenyl)benzothiazoles induce CYP1A1 expression, become metabolized, and bind to macromolecules in sensitive human cancer cells. Drug Metab. Dispos. 2004;32:1392–1401. doi: 10.1124/dmd.104.001057. [DOI] [PubMed] [Google Scholar]
- (14).Chua MS, Kashiyama E, Bradshaw TD, Stinson SF, Brantley E, Sausville EA, Stevens MFG. Role of CYP1A1 in modulation of antitumor properties of the novel agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203, NSC 674495) in human breast cancer cells. Cancer Res. 2000;60:5196–5203. [PubMed] [Google Scholar]
- (15).Trapani V, Patel V, Leong CO, Ciolino HP, Yeh GC, Hose C, Trepel JB, Stevens MF, Sausville EA, Loaiza-Perez AI. DNA damage and cell cycle arrest induced by 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203, NSC 703786) is attenuated in aryl hydrocarbon receptor deficient MCF-7 cells. Br. J. Cancer. 2003;88:599–605. doi: 10.1038/sj.bjc.6600722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Omiecinski CJ, Redlich CA, Costa P. Induction and developmental expression of cytochrome-P4501A1 messenger-RNA in rat and human tissues−detection by the polymerase chain-reaction. Cancer Res. 1990;50:4315–4321. [PubMed] [Google Scholar]
- (17).Kitada M, Taneda M, Itahashi K, Kamataki T. 4 forms of cytochrome-P-450 in human fetal liver—Purification and their capacity to activate promutagens. Jpn. J. Cancer Res. 1991;82:426–432. doi: 10.1111/j.1349-7006.1991.tb01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Leong CO, Suggitt M, Swaine DJ, Bibby MC, Stevens MF, Bradshaw TD. In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazoles. Mol. Cancer Ther. 2004;3:1565–1575. [PubMed] [Google Scholar]
- (19).Mortimer CG, Wells G, Crochard JP, Stone EL, Bradshaw TD, Stevens MF, Westwell AD. Antitumor benzothiazoles. 26.1 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J. Med. Chem. 2006;49:179–185. doi: 10.1021/jm050942k. [DOI] [PubMed] [Google Scholar]
- (20).Tan BS, Tiong KH, Muruhadas A, Randhawa N, Choo HL, Bradshaw TD, Stevens MF, Leong CO. CYP2S1 and CYP2W1 mediate 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW-610, NSC 721648) sensitivity in breast and colorectal cancer cells. Mol. Cancer Ther. 2011;10:1982–1992. doi: 10.1158/1535-7163.MCT-11-0391. [DOI] [PubMed] [Google Scholar]
- (21).Turesky RJ, Lang NP, Butler MA, Teitel CH, Kadlubar FF. Metabolic-activation of carcinogenic heterocyclic aromatic-amines by human liver and colon. Carcinogenesis. 1991;12:1839–1845. doi: 10.1093/carcin/12.10.1839. [DOI] [PubMed] [Google Scholar]
- (22).Peng LJ, Turesky RJ. Mass spectrometric characterization of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine N-oxidized metabolites bound at Cys(34) of human serum albumin. Chem. Res. Toxicol. 2011;24:2004–2017. doi: 10.1021/tx2003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Parikh A, Gillam EM, Guengerich FP. Drug metabolism by Escherichia coli expressing human cytochromes P450. Nat. Biotechnol. 1997;15:784–788. doi: 10.1038/nbt0897-784. [DOI] [PubMed] [Google Scholar]
- (24).Guengerich FP, Bartleson CJ. Analysis and characterization of enzymes and nucleic acids. In: Hayes AW, editor. Principles and Methods of Toxicology. 5th Ed. CRC Press; Boca Raton, FL: 2007. pp. 1981–2048. [Google Scholar]
- (25).Kim D, Guengerich FP. Cytochrome P450 activation of arylamines and heterocyclic amines. Annu. Rev. Pharmacol. Toxicol. 2005;45:27–49. doi: 10.1146/annurev.pharmtox.45.120403.100010. [DOI] [PubMed] [Google Scholar]
- (26).Chun YJ, Kim MY, Guengerich FP. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem. Biophys. Res. Commun. 1999;262:20–24. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- (27).Chakraborty M, Jin KJ, Brewer SC, Peng HL, Platz MS, Novak M. Indirect and direct detection of the 4-(benzothiazol-2-yl)phenylnitrenium ion from a putative metabolite of a model anti-tumor drug. Org. Lett. 2009;11:4862–4865. doi: 10.1021/ol901959z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Novak M, Chakraborty M. Reactions of a putative metabolite of the model antitumor drug 2-(4-aminophenyl) benzothiazole with purines and pyrimidines. J. Phys. Org. Chem. 2011;24:960–968. [Google Scholar]
- (29).Chakraborty M, Jin KJ, Glover SA, Novak M. Characterization of the 4-(benzothiazol-2-yl)phenylnitrenium ion from a putative metabolite of a model antitumor drug. J. Org. Chem. 2010;75:5296–5304. doi: 10.1021/jo101275y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Humphreys WG, Kadlubar FF, Guengerich FP. Mechanism of C8 alkylation of guanine residues by activated arylamines: Evidence for initial adduct formation at the N7 position. Proc. Natl. Acad. Sci. USA. 1992;89:8278–8282. doi: 10.1073/pnas.89.17.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Park BK, Boobis A, Clarke S, Goldring CE, Jones D, Kenna JG, Lambert C, Laverty HG, Naisbitt DJ, Nelson S, Nicoll-Griffith DA, Obach RS, Routledge P, Smith DA, Tweedie DJ, Vermeulen N, Williams DP, Wilson ID, Baillie TA. Managing the challenge of chemically reactive metabolites in drug development. Nat. Rev. Drug Discov. 2011;10:292–306. doi: 10.1038/nrd3408. [DOI] [PubMed] [Google Scholar]
- (32).Kim DH, Kadlubar FF, Teitel CH, Guengerich FP. Formation and reduction of aryl and heterocyclic nitroso compounds and significance in the flux of hydroxylamines. Chem. Res. Toxicol. 2004;17:529–536. doi: 10.1021/tx034267y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.