Abstract
Hypertension is a major risk factor for intracranial hemorrhage. We therefore investigated the prevalence, treatment and control of hypertension in adult patients with hemophilia (PWH). PWH ≥18 years (n=458) from 3 geographically different cohorts in the United States were evaluated retrospectively for hypertension and risk factors. Results were compared to the nationally representative sample provided by the contemporary National Health and Nutrition Examination Survey (NHANES). PWH had a significantly higher prevalence of hypertension compared to NHANES. Overall, the prevalence of hypertension was 49.1% in PWH compared to 31.7 % in NHANES. At ages 18–44, 45–64, 65–74, and ≥ 75 the prevalence of hypertension for PWH was 31.8%, 72.6%, 89.7%, and 100.0% compared to 12.5%, 41.2%, 64.1%, and 71.7% in NHANES, respectively. Of treated hypertensive PWH, only 27.1% were controlled, compared to 47.7% in NHANES (all p-values <0.05). Age, body mass index, diabetes and renal function were independently associated with hypertension. Among patients with moderate or severe hemophilia there was a trend (~ 1.5-fold) for higher odds of having hypertension compared to patients with mild hemophilia. Based on these results, new care models for adult PWH and further studies for the etiology of hypertension in hemophilia are recommended.
Keywords: Hypertension, Blood Pressure, Hemophilia, cardiovascular disease risk factors, prevalence, NHANES
Introduction
Hemophilia is an X-linked bleeding disorder characterized by deficiencies of Factor VIII or IX. With the advent of safe clotting factor preparations, most of those born with hemophilia survive into adulthood free of human immunodeficiency virus (HIV). As such, almost half of the approximately 20,000 patients with hemophilia (PWH) living in the US are now adults (Center for Disease Control and Prevention/UCSD data report 2011).
Based primarily on previous European studies that reported lower cardiovascular disease (CVD) mortality for PWH compared to age- and sex-matched non-hemophiliacs, CVD prevention in hemophilia has received little attention due to the perception that PWH are protected from CVD due to “hypocoagulability”.1–3 Also previously, life-threatening viral diseases dominated care and PWH died young (median age at death 40.5 to 46 years between 1987–19984). Recently, cardiovascular care for those with hemophilia has gained new attention since, at least in the United States (US), PWH were demonstrated to have twice the prevalence of symptomatic CVD and 3-fold higher mortality rates from CVD than normal age-matched males which included intracranial hemorrhage (ICH).5;6 Since hemophilia is a rare disease the incidence of ICH is mostly provided by event per 105 patient years, whereas ICH incidence in the normal population is mostly provided per 105 subjects during a given time period (often as annual rate). Estimates of the absolute frequency of ICH in PWH are 290–748 per 105 patient years,7;8 and comparatively this is estimated to be 20–50 times more frequent than in the normal population (~13–40 per 105 patient years).7;9;10 ICH is the most severe bleeding event and leading cause of death in PWH,4;11 and carries a high mortality rate. Patients with severe or mild/moderate hemophilia have standard mortality ratios for ICH that are approximately 40-fold and 9-fold higher, respectively, than for normal age-matched males.12 The most significant risk factor for ICH in the normal population is hypertension12–16 and, hypertension was also the most frequent comorbidity in a recent investigation of ICH in Italian PWH.17 Notably, in the general population every 20 point mmHg increase in systolic blood pressure (SBP), or 10 point mmHg increase in diastolic blood pressure (DBP), is associated with a 2-fold increased risk of ICH.13;14 Hence it is concerning that little is currently known about the prevalence and severity of hypertension in PWH. There is emerging evidence from Europe that the prevalence of hypertension in PWH may be increased compared to the normal population or age-matched males, but evidence remains controversial. That is, studies are either small and/or based on patients from ethnically uniform Northern European cohorts.2;18–20
Hence, investigating hypertension in hemophilia is highly relevant. Here, we report the prevalence and control of hypertension, as well as risk factors associated with this condition, from a retrospective analysis of three cohorts with hemophilia located in geographically different areas of the US. Findings were compared to the general population by comparison with contemporary data from the National Health and Nutrition Examination Survey (NHANES).
Materials and Methods
Participants
A retrospective data collection was performed for all male PWH (n=458) aged 18 years and older visiting 3 Hemophilia Treatment Centers (HTCs) in the US: University California San Diego (UCSD), Tulane University (TU) and the Los Angeles Orthopaedic Hospital (LAOH). Data were extracted manually from the electronic medical record and/or paper charts. Record date ranges were 2004–2012 for UCSD, 2008–2011 for TU, and 2005–2012 for LAOH. Patient confidentiality safeguards and data acquisition methods were approved by the Institutional Review Boards of all three institutions.
Health History
Data extracted included demographic information on age, ethnicity, hemophilia type and severity, positive tests for Hepatitis C or HIV by serology or reported history thereof, medication history, prior diagnosis of hypertension and smoking status. Inhibitors (neutralizing antibodies against FVIII or FIX) were documented as present if the patient was positive for inhibitors at any of the recorded blood pressure measurements.
Physical Measurements
Data extracted included laboratory parameters pertaining to diabetes (HbA1c, random blood glucose) and serum creatinine. Laboratory values at all centers were obtained non-fasting during regular health visits. The diagnosis of diabetes was defined according to the 2010 American Diabetes Association Standards of Medical Care in Diabetes as medication use for glycemic control, HbA1c > 6.5 or presence of ≥ 2 random glucose levels above 200 mg/dL.21 Renal function was determined by estimated glomerular filtration rate (eGFR) calculated using the CKD-EPI equation.22 Age, BMI (weight (kg)/height(m2) and creatinine at last recorded blood pressure were used for analysis.
Blood pressure in all clinics was measured in accordance with the current recommendations of the American Heart Association.23 In brief, blood pressures were obtained by licensed staff using calibrated automated manometers with subjects in a chair at rest, arm supported at heart level. All records included at least one recorded blood pressure and no patient was excluded from analysis. The 3 most recent blood pressure measurements were used to evaluate hypertension status (mean number of measurements was 2.6, 2.4, 2.9 for UCSD, TU, and LAOH, respectively). Hypertension was defined as prior physician diagnosis of hypertension and use of antihypertensive medication, or at least two elevated blood pressure measurements (SBP ≥140 mmHg or DBP ≥90 mmHg). Treated hypertension was defined as reported use of antihypertensive medication during the last year of the study period. Controlled hypertension was defined as a treated blood pressure <140 mmHg systolic and <90 mmHg diastolic. For the assessment of controlled hypertension the most recent BP measurements during the last year of the study period were used.
Statistical Analysis
All statistical analyses were performed using SAS Version 9.2 (Cary, NC).
Differences in demographic and health characteristics were evaluated with t-tests or Wilcoxon Mann-Whitney for continuous variables and chi-square tests for categorical variables across treatment centers and hypertension classification.
Overall and age-based prevalence of hypertension in the study cohorts was compared to the prevalence of hypertension for males in published data from NHANES.24 NHANES is a complex, multistage probability sample that represents a statistical model of the entire civilian noninstitutionalized U.S. population conducted by the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS; CDC. National Health and Nutrition Examination Survey Data. Hyattsville, MD: US Department of Health and Human Services, CD, 2010; available at http://www.cdc.gov/nchs/nhanes.htm). NHANES hypertension was defined as elevated blood pressure (average of up to 3 blood pressure measurements, obtained under standard conditions during a single physical examination at the mobile examination center) or report of use of antihypertensive medication (home health interview). One-sample binomial proportion tests were used to test the equality of hypertension prevalence and hypertension control in both cohorts.
Logistic regression was used to evaluate the associations of known risk factors with hypertension. Characteristics with p<0.20 in univariate analysis were included in the multivariate logistic regression analyses for determination of independent predictors of hypertension. An initial model included only hemophilia type and severity. A second model additionally adjusted for age, race, and treatment center, and a final model additionally adjusted for traditional risk factors (smoking history, diabetes, eGFR, and BMI). Effect modification of the association of hemophilia severity with hypertension was evaluated by multiplicative interaction terms within the adjusted models. P-values <0.05 were considered statistically significant for all analyses including interactions. Non-significant interactions were not included in final models.
Results
Basic Cohort Characteristics
Male patients age 18 years and older seen at UCSD (n=114), TU (n=120), and LAOH (n=224) were included in the retrospective analysis. The characteristics of the three cohorts are described in Table 1. The cohorts were not homogeneous and differed by mean age, ethnic composition, inhibitor status, severity of hemophilia, smoking status, hepatitis C, and median creatinine. Conversely, the cohorts were similar regarding proportion of patients with Hemophilia A or B, mean BMI, mean eGFR, HIV status, and prevalence of diabetes.
Table 1.
Baseline Cohort Characteristics
| Cohort | UCSD* | TU* | LAOH* | p-value |
|---|---|---|---|---|
| # of patients | 114 | 120 | 224 | |
| Mean Age | 41.3±14.5 | 37.5 ± 13.5 | 43.2 ± 15.5 | .003 |
| Race | <.0001 | |||
| White | 56(49.1%) | 84(70.0%) | 103(45.8%) | |
| Black | 11(9.7%) | 25(20.8%) | 14(6.2%) | |
| Hispanic | 31(27.2%) | 1(0.8%) | 78(34.7%) | |
| Other | 16(14.0%) | 10(8.3%) | 30(13.3%) | |
| Hemophilia | .44 | |||
| A | 90(79.0%) | 89(74.2%) | 183(81.3%) | |
| B | 24(21.0%) | 31(25.8%) | 41(18.2%) | |
| Unknown | 0 | 0 | 1(0.4%) | |
| Inhibitor | <.0001 | |||
| Positive | 4(3.5%) | 17(14.2%) | 8(3.6%) | |
| Negative | 70(61.4%) | 103(85.8%) | 207(92.0%) | |
| Not Tested | 40(35.1%) | 0 | 10(4.4%) | |
| Severity | <.0001 | |||
| Severe | 50(43.9%) | 69(57.5%) | 134(59.6%) | |
| Moderate | 17(14.9%) | 30(25.0%) | 17(7.8%) | |
| Mild | 46(40.4%) | 21(17.5%) | 66(29.3%) | |
| Unknown | 1(0.9%) | 0 | 8(3.6%) | |
| HIV Status | <.0001 | |||
| Positive | 27(23.7%) | 20(16.7%) | 58(25.8%) | |
| Negative | 62(54.4%) | 93(77.5%) | 167(74.2%) | |
| Not Tested | 25(21.9%) | 7(5.8%) | 0 | |
| Hepatitis | <.0001 | |||
| Positive | 62(54.4%) | 71(59.2%) | 168(74.7%) | |
| Negative | 38(33.3%) | 45(37.5%) | 54(24.0%) | |
| Not Tested | 14(12.3%) | 4(3.3%) | 3(1.3%) | |
| Mean BMI | 27.9 ± 6.6 | 27.1 ± 5.3 | 26.9 ± 5.7 | .31 |
| Smoking | <.0001 | |||
| Ever | 20(16.8%) | 40(33.3%) | 54(34.2%) | |
| Never | 99(83.2%) | 80(66.7%) | 91(57.6%) | |
| Unknown | - | - | 13(8.2%) | |
| Diabetes | 0.17 | |||
| Yes | 7(6.1%) | 5(4.2%) | 24(10.7%) | |
| No | 107 (93.9%) | 115(95.8%) | 200(88.9%) | |
| Unknown | 0 | 0 | 1 (0.4%) | |
| Median Creatinine | 0.84(0.22) | 0.90(0.30) | 0.80(0.30) | .0002 |
| Meane GFR | 106.3 ± 21.7 | 101.9 ± 25.2 | 102.1 ± 24.6 | .28 |
University of California San Diego = UCSD; Tulane University=TU; Los Angeles Orthopedic Hospital (LAOH); BMI = Body Mass Index; eGFR=estimated Glomerular Filtration Rate
Prevalence of hypertension, treatment and control in PWH
There were no statistical differences in the overall or age stratified prevalences of hypertension between the 3 cohorts (45.6% UCSD, 46.7% TU, and 52.2% LAOH, pvalue 0.42) (all p-values > 0.05). Also, there were no differences between percent patients treated for hypertension in each cohort (29.8 in UCSD, 20% in TU, 26.3% LAOH, p-value 0.21). Table 2 shows the details of blood pressure collection (date ranges and mean number of measurements) across the cohorts as well as the prevalence of hypertension overall and according to age group (ages 18–44, 45–64, 65–74 and age ≥ 75) by geographic cohort.
Table 2.
Prevalence of Hypertension and Hypertension Treatment in Patients with Hemophilia
| Blood Pressure |
UCSD* | TU* | LAOH* | |
|---|---|---|---|---|
| Collection Date Range | 2004–2012 | 2008–2011 | 2005–2012 | |
| Mean collected # (± SD) | 2.6 ± 0.7 | 2.4 ± 0.8 | 2.9 ± 0.3 | |
| Prevalence of HTN | p-value | |||
| Overall | 52/114 (45.6%) | 56/120 (46.7%) | 117/224 (52.2%) | .42 |
| 18–44 years | 17/68 (25.0%) | 32/88 (36.4%) | 41/127 (32.3%) | .32 |
| 45–64 years | 23/33 (69.7%) | 19/27 (70.4%) | 56/75 (74.7%) | .83 |
| 65–74 years | 11/12 (91.7%) | 3/3 (100%) | 12/14 (85.7%) | .73 |
| >=75 years | 1/1 (100%) | 2/2 (100%) | 8/8 (100%) | - |
| Treated HTN | 34/114 (29.8%) | 24/120 (20.0%) | 59/224 (26.3%) | .21 |
University of California San Diego = UCSD; Tulane University=TU; Los Angeles Orthopedic Hospital (LAOH). HTN=Hypertension; SD=Standard deviation
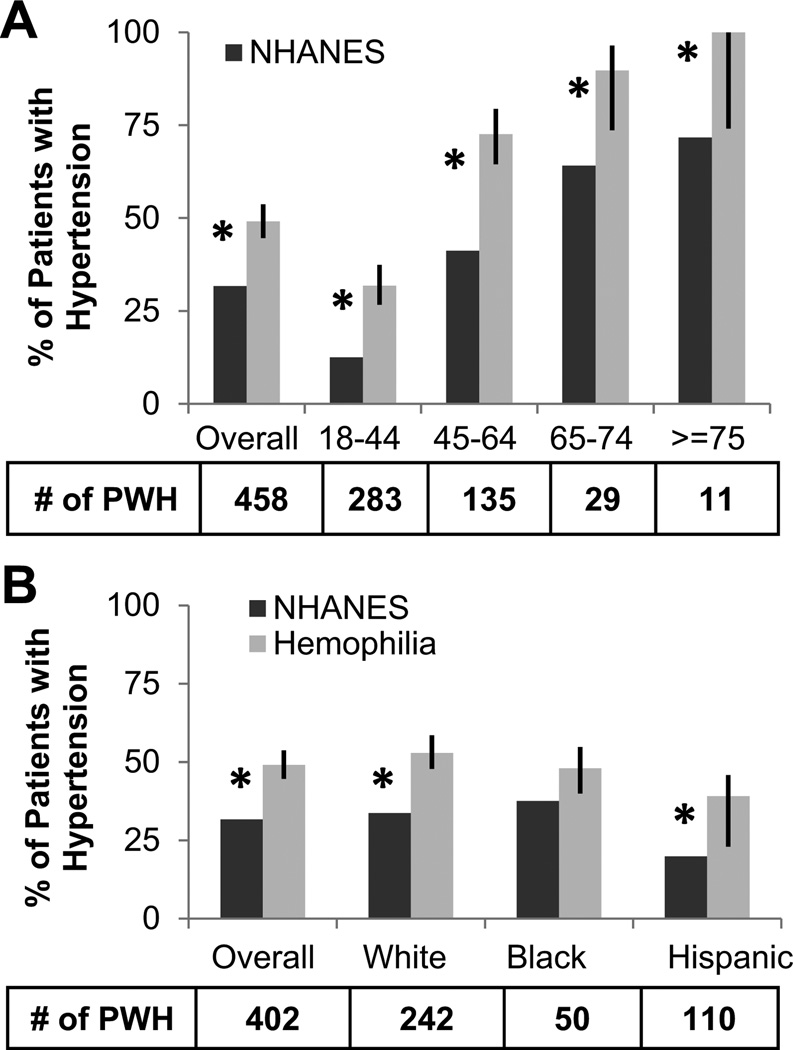
The overall prevalence of hypertension for all cohorts combined (PWH, n=458) and by age group and ethnicities was compared to NHANES (Figures 1a/b). The overall prevalence of hypertension was significantly higher in PWH (49.1%) than NHANES (31.7%) (p-value <0.0001). At ages 18–44, 45–64, 65–74, and ≥ 75 the prevalence of hypertension for PWH was 31.8%, 72.6%, 89.7%, and 100.0% compared to 12.5%, 41.2%, 64.1%, and 71.7% in NHANES, respectively (all p-values ≤ 0.05). PWH hypertension prevalence was significantly higher than NHANES among whites (33.7% vs. 49.1%) and Hispanics (19.9% vs. 39.1%) (p-values ≤ 0.05) and not significantly different among blacks (37.6%, 48.0%, p-value=0.06).
Figure 1. The prevalence of hypertension in patients with hemophilia is significantly higher compared to the general population.
The prevalence of hypertension for patients with hemophilia (PWH) was compared by one sample binomial test to male NHANES data for all age groups combined (overall), and A) divided into different age groups and B) into matching ethnic groups (only white, black, Hispanic included in NHANES). * indicates p-value ≤ 0.5. Error bars indicate 95% Confidence Intervals. NHANES= National Health and Nutrition Examination Survey.
Next, the subject characteristics were analyzed by hypertension status in univariate analysis (Supplemental Table S1). Hemophilia patients with hypertension were significantly older (mean age 48.3 versus 34.4 years, p-value<0.0001), had higher BMI (mean BMI 28.2 versus 26.2, p-value=0.0005), higher creatinine values (median 0.90 versus 0.83, p-value =0.002), and lower eGFR (mean eGFR 94.4 versus 112.0, p-value <0.0001). The prevalence of diabetes (13.8% versus 1.7%, p-value < 0.001) was significantly higher in patients with hypertension. Hepatitis C and Hemophilia severity also differed according to hypertension status. There were no differences in ethnicity, ever smoking, or HIV status.
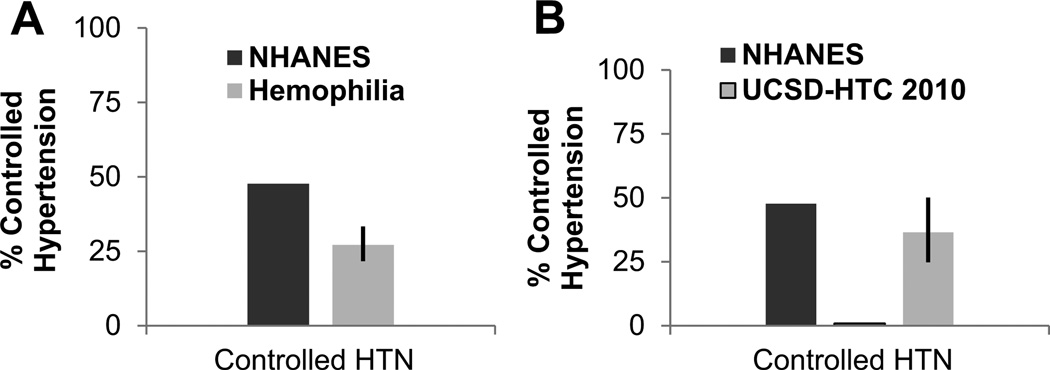
Compared to published results from NHANES 24 control of hypertension was significantly lower among PWH. That is, of treated hypertensive PWH, only 27.1% were controlled, compared to 47.7% in NHANES (p-value <0.0001) (Figure 2a). For PWH seen at UCSD, detailed treatment records were available for data review. Analysis of control during the 2 years preceding data base closure revealed a significant increase of HTN control of PWH between 2010 and 2012 due to health care provider awareness. These records indicated that hypertension control increased from 1% to 36.5% from 2010 to 2012. These percentages are similar to NHANES where 47.7% of treated individuals are controlled (Figure 2b).
Figure 2. The control of hypertension in PWH is significantly lower compared to the general male population.
A. Percent control of treated PWH from all 3 study cohorts combined was compared by one sample binomial test to published male NHANES data. Percent control was significantly lower for PWH than males from NHANES. B. For the HTC at UCSD detailed information regarding control were available for each year of retrospective analysis. Analysis of control during the 2 years preceding data base closure revealed a significant increase of HTN control of PWH between 2010 and 2012 due to health care provider awareness. These data suggest that hypertension of hemophilia can be controlled with usual anti-hypertensives. * indicates p-value ≤ 0.5. Error bars indicate 95% Confidence Intervals. NHANES= National Health and Nutrition Examination Survey; UCSD = University of California San Diego; HTC=Hemophilia Treatment Center. PWH=Patients with Hemophilia.
Independent risk factors of hypertension in hemophilia
Table 3 shows the association of risk factors with hypertension among PWH. Compared to Hemophilia A and in unadjusted analyses, patients with Hemophilia B had a 1.87-fold higher odds of hypertension (p-value=0.009) that was attenuated and no longer significant once adjusted for age, race, treatment center (model 2; OR 1.36; p-value=0.27) and traditional risk factors (model 3; OR 1.63; p-value=0.11). Compared to patients with mild hemophilia and after adjustment for age, race, and treatment center (model 2), patients with moderate/severe hemophilia had a 1.3-fold higher odds of hypertension (p-value=0.32). After further adjustment for traditional risk factors (model 3) this association increased to a 1.51-fold higher odds (p-value 0.15). In the fully adjusted model, higher age (OR 1.36 per 5 years, p-value <0.0001), higher BMI (OR 1.60 per 5 kg/m2, p-value <0.0001), and the presence of diabetes (OR 3.96, p-value=0.05) were associated with higher odds of hypertension, while a higher eGFR (OR 0.83 per 10 mL/min/1.73m2, p-value 0.02) was associated with a decreased odds for hypertension. Model 3 was repeated with log-transformed creatinine in place of eGFR. Higher creatinine (OR 4.43 per log mg/dL, p-value=0.009) was associated with increased odds for hypertension while all other associations were unchanged.
Table 3.
Independent Risk Factors for Hypertension in Hemophilia in a Multi-Stage Model Including Patient Non-Modifiable Risk Factors (Model 2) and Patient Modifiable Risk Factors (Model 3)
| Risk Factors | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Age, per 5 years | - | 1.48(1.35;1.62); <.0001 | 1.36(1.201.53); <.0001 |
| Race | |||
| White | - | Ref | Ref |
| Black | - | 0.86(0.43;1.75); .68 | 0.52(0.241.15); .11 |
| Hispanic | - | 0.91(0.50;1.64); .75 | 0.63(0.32;1.22); 0.17 |
| Other | - | 1.22 (0.61;2.41); .58 | 1.09(0.53;2.23); .82 |
| Center* | |||
| LAOH | - | Ref | Ref |
| UCSD | - | 0.89 (0.52;1.51); .66 | 0.88(0.49;1.57); .66 |
| TU | - | 1.18(0.67;2.09); .56 | 1.26(0.66;2.38); .48 |
| BMI, per 5 kg/m2 | - | - | 1.60(1.28;1.99); <.0001 |
| Ever Smoker | - | - | 1.28(0.76;2.13); .35 |
| Diabetes | - | - | 3.96(1.01;15.56); <.05 |
| eGFR, per 10 mL/min/1.73m2 | - | - | 0.83(0.71;0.96); .02 |
| Hemophilia Type | |||
| A | Ref | Ref | Ref |
| B | 1.87(1.17;2.98); .009 | 1.36(0.79;2.32); .27 | 1.63(0.90;2.94); .11 |
| Severity | |||
| Mild | Ref | Ref | Ref |
| Mod/Severe | 0.87(0.57;1.32); .51 | 1.30(0.78;2.18); .32 | 1.51(0.86;2.67); .15 |
University of California San Diego = UCSD; Tulane University=TU; Los Angeles Orthopedic Hospital (LAOH). BMI = Body Mass Index; eGFR = Estimated Glomerular Filtration Rate;
Discussion
Using a relatively large retrospective analysis, our results indicate that adult PWH who were 18 years and older and from 3 geographically distinct areas in North America have a significantly higher prevalence of hypertension compared to the general male population represented by NHANES. The increased prevalence of hypertension was evident at all ages. Our study populations were ethnically diverse, with approximately 30% PWH of Hispanic ethnicity in California and approximately 20% PWH of African American ethnicity in Louisiana. The high prevalence of hypertension was evident across all ethnicities, but not significant for African Americans likely due to small sample size. Also, hypertension was independently associated with age, presence of diabetes, BMI and renal function by creatinine and eGFR. There was also a trend for hypertension with more severe stages of hemophilia (plasma clotting factor activity ≤5% (severe and moderate hemophilia combined)) compared to mild hemophilia (plasma clotting factor activity >5%). Of concern, control of hypertension was significantly less than that reported for the general male population. These findings are important and clinically relevant since the hypertension of hemophilia may be a serious under-recognized entity, requiring new care models.
An unexplained association between hemophilia and hypertension was described in a Dutch cohort as early as the 1980s2 and more recently in a contemporary Northern European cohort by Fransen van de Putte et al. 20 This cohort, including approximately 700 PWH (mean age 49.8 years), also exhibited a significantly higher prevalence of hypertension in PWH compared to normal males in National Health Registries. Similar to our study, hypertension was higher in patients with severe hemophilia than in those with non-severe hemophilia, similar for Hemophilia A and B, associated with BMI and age, and not associated with HIV. Different from our study results there was no association of hypertension with renal function or hepatitis C. However, renal function in the European study was based on creatinine alone, which may be less sensitive than renal function estimates by eGFR. Results from 2 other contemporary Dutch and Italian case-control studies also support that the prevalence of hypertension may be higher in adult PWH compared to age-matched males. Bierre-Rafi et al. demonstrated that the prevalence of hypertension was 51% in PWH (n=100, mean age 47 years) compared to 37% (p=0.03) in normal males18 and Siboni et al. demonstrated similar findings for 35 elderly PWH (≥65 years).19 Since it was felt that these studies were too small for definite conclusions,20 and since other studies evaluating (cardiovascular) health parameters in PWH did not find an increased prevalence of hypertension25–27 controversy remained.20 Our study is to the best of our knowledge the first in North America to specifically examine the prevalence of hypertension and associated risk factors in PWH, and to report an increased prevalence of hypertension in PWH.
As expected, the prevalence of hypertension increased with age for study participants of NHANES, as well as for PWH. There were relatively fewer numbers of PWH in the older age groups. The attrition may largely be explained by early death either due to lack of clotting factor preparations prior to the 1970s, or to viral infections through contaminated clotting factor preparations provided prior to the mid1980s.1 However, the contribution of premature death due to other events in hemophilia such as catastrophic bleeding is currently unclear. Notably, patients with moderate and severe hemophilia (plasma clotting factor levels <5%) had an approximately 1.5-fold higher odds of hypertension compared to patients with mild hemophilia (plasma clotting factor levels 6–50%) when adjusted for additional risk factors. While this trend was not statistically significant, it may be considered clinically meaningful, especially since similar findings were present in the European cohort.20 The lack of significance may be due to sample size effect, or possibly by survival bias in patients with severe hemophilia and hypertension resulting in their early attrition. When patients were categorized into severe versus non-severe hemophilia no such trend was observed (data not shown), suggesting a threshold effect around moderate hemophilia in association with hypertension. In general, one may speculate that hypertension in patients with more severe bleeding phenotypes (such as moderate and severe hemophilia) may be influenced by their limited ability for vigorous exercise and by forced sedentary lifestyle because of bleeding risk. However, our study is limited by the ability to provide insights regarding diet, exercise, hyperlipidemia (consistent collection of fasting lipid panels was not uniformly performed at all centers) or obstructive sleep apnea. Also, our study lacks information on renal bleeding or intermittent renal (micro)bleeding (which can only be reasonably ascertained by frequent urinalyses), as well as clotting factor usage and history of previously eradicated inhibitors. Inhibitors mostly occur in patients with severe hemophilia and may influence vascular health for example through immune complex formation. Also, our comparator group was not made up of chronically ill patients or patients with regular clinic visits. Hence, the observed higher prevalence of hypertension between PWH and the controls may have been the result of the regular clinic visits of PWH, where hypertension may be more readily diagnosed and more blood pressure measurements are available to make the diagnosis. Conversely, single visits for blood pressure measurements as in NHANES or other National Health Registries may either over- or underestimate the prevalence of hypertension in the general population. However, the highly significant differences between PWH and normal males for all age and ethnic groups in our study are highly suggestive of hypertension of hemophilia. By not having home blood pressure readings we were not able to discern a white-coat hypertension effect in PWH or NHANES. However, one may think that white-coat effects in PWH presenting regularly to a tertiary clinic focused on bleeding complications as opposed to emphasizing blood pressure control in a primary care setting is unlikely. Generally, it should be kept in mind that white-coat hypertension is not a benign condition and has been associated previously with a higher incidence of stroke.28 PWH were not entirely comparable to the NHANES study population with respect to prevalence of traditional risk factors for hypertension such as diabetes, obesity, renal function or smoking.29–33 Specifically, while the mean BMI and prevalence of diabetes were similar between the 2 groups 29;31;32, PWH had a higher prevalence of never smokers (~40% NHANES vs. ~66% PWH)30 and a lower prevalence of renal disease (eGFR<60 ml/min/1.73 m2: ~12% NHANES vs. ~5% PWH (data not shown).33
Our results suggest that traditional risk factors alone are unlikely to explain the higher prevalence of hypertension in PWH, although interactions between these risk factors and hemophilia status cannot be entirely ruled out. Currently, reasons for the high prevalence of hypertension in PWH remain unclear. Potential molecular mechanisms could be both of (epi)genetic or hemostatic origin. Interactions of hemostatic factors with the vessel wall or impact of chronic attenuation of hemostasis on mechanisms regulating vascular tone and endothelial relaxation are conceivable. For instance, decreased thrombin formation influencing activation of thrombin substrates, levels of activated protein C and activated thrombin activatable fibrinolysis inhibitor (TAFIa) may affect blood pressure regulation.34–36 Although less likely because of the more than 1000 different mutations described to date causing hemophilia (hadb.org.uk; factorix.org), genetic predisposition associated with the mutational hemophilia defect on the X-chromosome cannot be excluded to predispose PWH to hypertension.
Perspective
From a pragmatic clinical standpoint our findings may have immediate consequences for hemophilia care. New paradigms to include hypertension control seem necessary. PWH are at extreme risk of mortality from ICH12 and the two most significant risk factors for ICH are age and hypertension.37;38 It is concerning that hypertension went unrecognized, untreated and uncontrolled in many patients at all three HTCs, probably representing general hemophilia care patterns in the US. As evidenced at the UCSD HTC, health care provider awareness and effort can increase hypertension control to what is reported for the general population. And, the hypertension of hemophilia seems treatable with usual anti-hypertensives.
Given that hemophilia is a rare disease, current studies are exploratory and limited by the small number of patients. However, our study results provide evidence that the hypertension of hemophilia is an important comorbidity, present even at young ages, across continents and ethnicities. In addition to informing medical practice, we hope that our findings will stimulate basic research addressing etiology such as the effects of altered hemostasis or genetic associations. In addition, prospective studies of traditional risk factors and variables such as history of inhibitors, clotting factor consumption, diet, exercise, pain level, inflammation, renal bleeding or bleeding phenotypes are required to improve our understanding of this condition.
Supplementary Material
What Is New ?
This study demonstrates for the first time that adult patients with hemophilia living in North America have a higher prevalence of hypertension that is present across all ethnicities and inadequately recognized and controlled compared to the general population in the United States.
What Is Relevant ?
Hypertension is a risk factor for ICH. Current care paradigms at comprehensive hemophilia treatment centers don’t usually include hypertension control and need to be adapted since patients with hemophilia are at high risk for intracranial hemorrhage with a high fatality rate.
Acknowledgments
Funding Sources
This work was supported by a Research Training Award for Fellows from the American Society of Hematology (A.v.D.), unrestricted grant support for “Cardiovascular Health in Hemophilia” from Baxter Biosciences (A.v.D., R.B., J.B) and by National Institutes of Health grants R01HL104165 (L.O.M.).
Footnotes
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
Disclosures
All authors indicate that they have nothing to disclose.
Reference List
- 1.Plug I, Van Der Bom JG, Peters M, Mauser-Bunschoten EP, De Goede-Bolder A, Heijnen L, Smit C, Willemse J, Rosendaal FR. Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study. Journal of Thrombosis and Haemostasis. 2006;4:510–516. doi: 10.1111/j.1538-7836.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosendaal FR, Briët E, Stibbe J, van Herpen G, Leuven JA, Hofman A, Vandenbroucke JP. Haemophilia protects against ischaemic heart disease: a study of risk factors. Br J Haematol. 1990;75:525–530. doi: 10.1111/j.1365-2141.1990.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 3.Sramek A, Kriek M, Rosendaal FR. Decreased mortality of ischaemic heart disease among carriers of haemophilia. Lancet. 2003;362:351–354. doi: 10.1016/s0140-6736(03)14021-4. [DOI] [PubMed] [Google Scholar]
- 4.Chorba TL, Holman RC, Clarke MJ, Evatt BL. Effects of HIV infection on age and cause of death for persons with hemophilia A in the United States. American Journal of Hematology. 2001;66:229–240. doi: 10.1002/ajh.1050. [DOI] [PubMed] [Google Scholar]
- 5.Sharathkumar AA, Soucie JM, Trawinski B, Greist A, Shapiro AD. Prevalence and risk factors of cardiovascular disease (CVD) events among patients with haemophilia: experience of a single haemophilia treatment centre in the United States (US) Haemophilia. 2011;17:597–604. doi: 10.1111/j.1365-2516.2010.02463.x. [DOI] [PubMed] [Google Scholar]
- 6.Soucie JM, Nuss R, Evatt B, Abdelhak A, Cowan L, Hill H, Kolakoski M, Wilber N. Mortality among males with hemophilia: relations with source of medical care. Blood. 2000;96:437–442. [PubMed] [Google Scholar]
- 7.Ljung RC. Intracranial haemorrhage in haemophilia A and B. Br J Haematol. 2008;140:378–384. doi: 10.1111/j.1365-2141.2007.06949.x. [DOI] [PubMed] [Google Scholar]
- 8.Witmer C, Presley R, Kulkarni R, Soucie JM, Manno CS, Raffini L. Associations between intracranial haemorrhage and prescribed prophylaxis in a large cohort of haemophilia patients in the United States. Br J Haematol. 2011;152:211–216. doi: 10.1111/j.1365-2141.2010.08469.x. [DOI] [PubMed] [Google Scholar]
- 9.Giroud M, Milan C, Beuriat P, Gras P, Essayagh E, Arveux P, Dumas R. Incidence and survival rates during a two-year period of intracerebral and subarachnoid haemorrhages, cortical infarcts, lacunes and transient ischaemic attacks. The Stroke Registry of Dijon: 1985–1989. Int J Epidemiol. 1991;20:892–899. doi: 10.1093/ije/20.4.892. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 11.Aronson DL. Cause of Death in Hemophilia-A Patients in the United-States from 1968 to 1979. American Journal of Hematology. 1988;27:7–12. doi: 10.1002/ajh.2830270103. [DOI] [PubMed] [Google Scholar]
- 12.Darby SC, Kan SW, Spooner RJ, Giangrande PL, Hill FG, Hay CR, Lee CA, Ludlam CA, Williams M. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 13.Kim HC, Nam CM, Jee SH, Suh I. Comparison of blood pressure-associated risk of intracerebral hemorrhage and subarachnoid hemorrhage - Korea Medical Insurance Corporation study. Hypertension. 2005;46:393–397. doi: 10.1161/01.HYP.0000177118.46049.e6. [DOI] [PubMed] [Google Scholar]
- 14.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 15.Eastern Stroke and Coronary Heart Disease Collaborative Research Group. Blood pressure, cholesterol, and stroke in eastern Asia. Lancet. 1998;352:1801–1807. (no author names provided) [PubMed] [Google Scholar]
- 16.Song YM, Sung J, Lawlor DA, Davey Smith G, Shin Y, Ebrahim S. Blood pressure, haemorrhagic stroke, and ischaemic stroke: the Korean national prospective occupational cohort study. British Medical Journal. 2004;328:324–325. doi: 10.1136/bmj.328.7435.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanon E, Iorio A, Rocino A, Artoni A, Santoro R, Tagliaferri A, Coppola A, Castaman G, Mannucci PM, Barillari G, Dragani A, Gamba G, Giuffrida A, Lapecorella M, et al. Italian Association of Hemophilia Centers. Intracranial haemorrhage in the Italian population of haemophilia patients with and without inhibitors. Haemophilia. 2012;18:39–45. doi: 10.1111/j.1365-2516.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 18.Biere-Rafi S, Baarslag MA, Peters M, Kruip MJ, Kraaijenhagen RA, Den Heijer M, Büller HR, Kamphuisen PW. Cardiovascular risk assessment in haemophilia patients. Thromb Haemost. 2011;105:274–278. doi: 10.1160/TH10-07-0460. [DOI] [PubMed] [Google Scholar]
- 19.Siboni SM, Mannucci PM, Gringeri A, Franchini M, Tagliaferri A, Ferretti M, Tradati FC, Santagostino E, von Mackensen S Italian Association of Haemophilia Centres (AICE) Health status and quality of life of elderly persons with severe hemophilia born before the advent of modern replacement therapy. Journal of Thrombosis and Haemostasis. 2009;7:780–786. doi: 10.1111/j.1538-7836.2009.03318.x. [DOI] [PubMed] [Google Scholar]
- 20.Fransen van de Putte DE, Fischer K, Makris M, Tait RC, Collins PW, Meijer K, Roosendaal G, Chowdary P, Schutgens RE, Mauser-Bunschoten EP. Increased prevalence of hypertension in haemophilia patients. Thromb Haemost. 2012;108:750–755. doi: 10.1160/TH12-05-0313. [DOI] [PubMed] [Google Scholar]
- 21.Standards of medical care in diabetes--2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics.Health, United States 2011. Hyattsville: MD, Center for Disease Control and Prevention; Hypertension among persons 20 years of age and over, by selected characteristics: United States, selected years 1988–1994 through 2007–2010. Library of Congress Catalog Number 76–641496, Table 70. 2011. Ref Type: Report. [Google Scholar]
- 25.Bilora F, Zanon E, Petrobelli F, Cavraro M, Prandoni P, Pagnan A, Girolami A. Does hemophilia protect against atherosclerosis? A case-control study. Clin Appl Thromb Hemost. 2006;12:193–198. doi: 10.1177/107602960601200207. [DOI] [PubMed] [Google Scholar]
- 26.Foley CJ, Nichols L, Jeong K, Moore CG, Ragni MV. Coronary atherosclerosis and cardiovascular mortality in hemophilia. Journal of Thrombosis and Haemostasis. 2010;8:208–211. doi: 10.1111/j.1538-7836.2009.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh M, Macgregor D, Stuckless S, Barrett B, Kawaja M, Scully MF. Health-related quality of life in a cohort of adult patients with mild hemophilia A. J Thromb Haemost. 2008;6:755–761. doi: 10.1111/j.1538-7836.2008.02929.x. [DOI] [PubMed] [Google Scholar]
- 28.Verdecchia P, Reboldi GP, Angeli F, Schillaci G, Schwartz JE, Pickering TG, Imai Y, Ohkubo T, Kario K. Short- and long-term incidence of stroke in white-coat hypertension. Hypertension. 2005;45:203–208. doi: 10.1161/01.HYP.0000151623.49780.89. [DOI] [PubMed] [Google Scholar]
- 29.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 30.Kahende JW, Adhikari B, Maurice E, Rock V, Malarcher A. Disparities in health care utilization by smoking status--NHANES 1999–2004. Int J Environ Res Public Health. 2009;6:1095–1106. doi: 10.3390/ijerph6031095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Center for Health Statistics Health Data Interactive www.cdc.gov/nchs/hdi.htm. [1-28-2013];Diabetes, ages 20+,US, 1988–2010 (source:NHANES) 2013 Ref Type: Report.
- 32.National Center for Health Statistics Health Data Interactive www.cdc.gov/nchs/hdi.htm. [Accessed on 01/28/2013];Overweight/Obesity, ages 20+:US, 1988–2010 (source:NHANES) 2013 National Center for Health Statistics, Health Data Interactive, www.cdc.gov/nchs/hdi.htm 1-28-2013 Ref Type: Report.
- 33.Saydah SH, Pavkov ME, Zhang C, Lacher DA, Eberhardt MS, Burrows NR, Narva AS, Eggers PW, Williams DE. Albuminuria Prevalence in First Morning Void Compared with Previous Random Urine from Adults in the National Health and Nutrition Examination Survey, 2009–2010. Clin Chem. 2013;59:675–683. doi: 10.1373/clinchem.2012.195644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller SA, Moore CC, Clemens MG, McKillop IH, Huynh T. Activated protein C restores hepatic microcirculation during sepsis by modulating vasoregulator expression. Shock. 2011;36:361–369. doi: 10.1097/SHK.0b013e31822c7380. [DOI] [PubMed] [Google Scholar]
- 35.Koschinsky ML, Boffa MB, Nesheim ME, Zinman B, Hanley AJ, Harris SB, Cao H, Hegele RA. Association of a single nucleotide polymorphism in CPB2 encoding the thrombin-activable fibrinolysis inhibitor (TAF1) with blood pressure. Clin Genet. 2001;60:345–349. doi: 10.1034/j.1399-0004.2001.600504.x. [DOI] [PubMed] [Google Scholar]
- 36.Malyszko J, Tymcio J. Thrombin activatable fibrinolysis inhibitor and other hemostatic parameters in patients with essential arterial hypertension. Pol Arch Med Wewn. 2008;118:36–41. [PubMed] [Google Scholar]
- 37.Chalmers J, Todd A, Chapman N, Beilin L, Davis S, Donnan G, Frommer M, Huxley R, Lenfant C, MacMahon S, Mancia G, Mendis S, Whitworth J, Zanchetti A. International Society of Hypertension (ISH): statement on blood pressure lowering and stroke prevention. J Hypertens. 2003;21:651–663. doi: 10.1097/01.hjh.0000052490.18130.95. [DOI] [PubMed] [Google Scholar]
- 38.Sierra C, Coca A, Schiffrin EL. Vascular mechanisms in the pathogenesis of stroke. Curr Hypertens Rep. 2011;13:200–207. doi: 10.1007/s11906-011-0195-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.