Abstract
Technetium-99 conjugated with methylene diphosphonate (99Tc-MDP) is a novel bisphosphonate derivative without radioactivity and has been successfully used to treat arthritis in China for years. Since bisphosphonate therapy has the potential to induce bisphosphonate-associated osteonecrosis of the jaw (BRONJ), we examine whether 99Tc-MDP represents a new class of bisphosphonate for anti-resorptive therapy to ameliorate estrogen deficiency–induced bone resorption with less risk of causing BRONJ. We showed that 99Tc-MDP-treated ovariectomized (OVX) mice had significantly improved bone mineral density (BMD) and trabecular bone volume in comparison to the untreated OVX group by inhibiting osteoclasts and enhancing osteogenic differentiation of bone marrow mesenchymal stem cells (BMMSCs). To determine the potential of inducing BRONJ, 99Tc-MDP/dexamethasone (Dex) or zoledronate/Dex were administered into C57BL/6J mice via the tail vein, followed by extraction of maxillary first molars. Interestingly, 99Tc-MDP treatment showed less risk to induce osteonecrosis in the maxillary bones compared to zoledronate treatment group, partially because 99Tc-MDP neither suppressed adaptive regulatory T cells (Tregs) nor activated the inflammatory T-helper-producing interleukin 17 cells (Th17). Taken together, our findings demonstrate that 99Tc-MDP therapy may be a promising approach in the treatment of osteoporosis with less risk of causing BRONJ.
Keywords: 99Tc-MDP, bisphosphonate-related osteonecrosis of the jaw, ovariectomized mice, bone mineral density, bone marrow mesenchymal stem cells
Introduction
Postmenopausal osteoporosis is the most common type of osteoporosis, in which estrogen deficiency-induced T cell activation promotes osteoclastogenesis to reduce bone mineral density (BMD) [1–5]. Although bisphosphonate therapy has been widely used in clinics, concerns have been raised by the emergence of side effects such as bisphosphonate-related osteonecrosis of the jaw (BRONJ). Currently, there is no appropriate therapy available in clinics, because we still do not understand the underlying pathophysiological mechanism of BRONJ [1, 6–8]. By establishing a BRONJ mouse model, we recently found that administration of zoledronate causes BRONJ-like disease in mice, in part by suppressing adaptive regulatory T cells, Tregs, while, at the same time, activating the inflammatory T-helper-producing interleukin 17 cells, Th17 [7]. These results suggest that BRONJ is a disease associated with immune dysfunction triggered by administration of bisphosphonate and trauma. Therefore, it is critical to develop appropriate drugs with the capacity of ameliorating osteoporotic phenotype with minimal risk to induce BRONJ.
Technetium-99 conjugated with methylene diphosphonate (99Tc-MDP) is a patented antiinflammatory drug, and it has been used for a safe treatment for rheumatoid arthritis (RA) and ankylosing spondylitis (AS) in China since 1997 [9]. Since 99Tc-MDP is a radioactive-safe decay product of 99mTc-methylene diphosphonate (99mTc-MDP) that has been widely used in bone scintigraphy as a radioactive agent with no significant adverse effects in clinical use. It has been proved that 99Tc-MDP treatment can inhibit macrophage infiltration together with downregulation of proinflammatory cytokines, including TNF-α and IL1-β, ICAM-1, and MMPs [9-10]. Since 99Tc-MDP has both potential antiresorptive effects as a bisphosphonate derivative and anti-inflammatory effects by inhibiting proinflammatory cytokines, it is reasonable to hypothesize that 99Tc-MDP might offer therapeutic effect for osteoporosis. Also, it is important to evaluate whether 99Tc-MDP treatment affects balance between Tregs and Th17, since Treg/Th17 cell imbalance is an important contributing factor for BRONJ [7]. In this study, we demonstrate that administration of 99Tc-MDP results in improvement of BMD in ovariectomy (OVX)-induced osteoporotic mice by inhibiting osteoclasts and enhancing osteogenesis of bone marrow mesenchymal stem cells (BMMSCs). In contrast to zolendronate, we also show that 99Tc-MDP treatment neither increases Th17 levels nor decreases Treg levels, thus avoiding the onset of BRONJ in a mouse model.
Materials and Methods
OVX and BRONJ Mouse Models
Female C3H/HeJ strain mice (8-week-old) were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Generation of OVX mice was performed as described previously [11] and age-matched C3H/HeJ mice receiving sham operation served as control (n=8). In experimental groups (n=11), 99Tc-MDP (Yunke, Chengdu, Sichuan, China. 62.5μg/kg, once per week) or zoledronate (Zometa, Novartis Oncology, East Hanover, NJ, USA. 62.5μg/kg, once per week) were administrated intravenously 2 weeks post-OVX for 2 weeks. Mice were sacrificed 5 weeks after OVX.
To generate the BRONJ mouse model, C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) received intravenous zoledronate (125μg/kg, twice per week) with/without dexamethasone (Dex) (Sigma-Aldrich Co, St. Louis, MO, USA, 5mg/kg, twice per week, n=11 and 10, respectively). Due to the different metabolic rates between mice and human, a body surface area calculation method was recommended by FDA (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf). The dosage used in mice needs to be at least 12 times more than that in humans.
Therefore, we used 12-13 times higher dosage of zoledronate to induce BRONJ mouse model. Mice were injected with 99Tc-MDP (125μg/kg, twice each week) with/without Dex (5mg/kg, twice each week, n=10) via the tail vein. One week after injection, maxillary first molars were extracted under deep anesthesia by i.p. injection of ketamine (Ketaject, 95mg/kg; Phoenix, St. Joseph, MO, USA) and xylazine (Xylaject, 5mg/kg; Phoenix). Two weeks after tooth extraction, the intact maxillas were harvested en bloc. A total of 6 doses of zoledronate/Dex and 99Tc-MDP/Dex were administered for the 2-week follow-up groups, respectively. Untreated mice with tooth extraction were used as controls.
All animal experiments were performed under institutionally approved protocols for the use of animal research at the University of Southern California (USC) (USC#11141, 10941 and 11327).
Antibodies and Reagents
Anti-alkaline phosphatase (ALP) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-β-actin antibody was purchased from Sigma-Aldrich Co. Purified mouse anti-peroxisome proliferator-activated receptor γ (PPARγ) IgG MAb and goat anti-lipoprotein lipase (LPL) IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). APC-conjugated anti-CD4, PE-conjugated anti-FoxP3 and PE-conjugated anti-IL17 antibodies were purchased from eBioscience (San Diego, CA).
Enzyme-linked Immunosorbent (ELISA) Assay
Serum markers of bone turnover, including receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG), were measured using ELISA kits purchased from RD System (Minneapolis, MN, USA) according to the manufacturer's instructions.
MicroCT Analysis
Femurs were harvested and analyzed by Inveon micro-CT system (Siemens AG, Germany). Cross sectional volumetric bone mineral density (BMD) was measured at right femur mid-diaphysis with a density phantom. The specimens were scanned with the following parameters: 14μm resolution, 80 kV voltage, 80 μA current, 2730 ms exposure time. Using 3-dimensional images, a region of interest in secondary spongiosa was manually drawn near the endocortical surface, and trabecular thickness (Tb.Th, mm), bone volume/total volume (BV/TV, %) and bone trabecular number (Tb.n) were assessed as a cancellous bone morphometric parameters.
Bone Histological and Histomorphometric Analysis
For histological and histomorphometric analysis, femurs and tibias were fixed with 4% paraformaldehyde after euthanization. Samples were decalcified with 10% EDTA (pH 8.0), embedded in paraffin and stained with hematoxylin and eosin (H&E) for sections, followed by calculation of trabecular bone volume percentage (Tb percentage, %) using ImageJ software in the area of interest. Trabecular bone only was analyzed by not including sections within two fields from either the growth plate or the cortices. Care was taken to analyze the same, standard site in every animal. At least 5 fields (1 × 1 mm area of bone) per sample were counted. Tartrate-resistant acid phosphate (TRAP) staining was performed according to a previous report [12]. For quantification of bone resorption in the bones, five representative images were analyzed by using ImageJ software. The results were shown as osteoclastic surface per mm bone surface (Oc.S/BS). All the parameters were assessed by a single, experienced technician, blinded to the treatment group of the mice, as previously described [13-14]. Kidney, liver and bladder were also harvested from each mouse, and HE staining was used to detect whether there is any pathological change. Histological analysis in BRONJ model was performed as described previously [7].
Mouse BMMSC and Jaw Bone MSC (JBMSC) Culture
Bone marrow cells were flushed out from bone cavity of femurs and tibias with 2% heat-inactivated fetal bovine serum (FBS; Equitech-Bio, Kerrville, TX, USA) in PBS. Single-cell suspension was obtained by passing through 70 m cell strainers (BD Bioscience, Franklin Lakes, NJ). JBMSCs were isolated from mouse mandibles according to our previous report [28]. Briefly, both attached soft tissues and teeth were removed from the mandibles and all nucleated cells from mandibles were obtained by digestion with 3 mg/ml collagenase type I (Worthington Biochem, Lakewood, NJ, USA) and 4 mg/ml dispase II (Roche Diagnostic, Indianapolis, IN, USA) for 60 min at 37°C. Both BMMSCs and JBMSCs were seeded and cultured with alpha minimum essential medium (α-MEM, Invitrogen Co., Carlsbad, CA, USA) supplemented with 20% FBS, 2 mM L-glutamine (Invitrogen), 55 μM 2-mercaptoethanol (Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Attached cells were cultured for 16 days. To confirm mesenchymal stem cell characteristics, we used flow cytometric analysis to show that these MSCs were positive for CD73, CD90, CD105, CD146, CD166, Sca-1 and SSEA-4, but negative for CD11b, CD31, CD34 and CD45.
CFU-F and BrdU Incorporation Assays
P1 MSCs were seeded in T-25 culture flasks (Nalgene Nunc, Rochester, NY, USA). After 16 days, the flasks were stained with a mixture of 0.1% toluidine blue (Merck, Darmstadt, Germany) and 2% paraformaldehyde (PFA, Merck) solution. Colonies containing > 50 cells were counted as single-colony clusters. Total colony numbers were counted per flask by light microscopy. The CFU-F number was repeated in 5 independent samples per each experimental group. P1 cells (1 × 104 per well) were seeded on 2-well chamber slides (Nunc) for 1–2 days, incubated with BrdU reagent (1:100, Invitrogen) for 24 hrs, and stained with the BrdU staining kit (Invitrogen), following the manufacturer's instructions. Finally, the cells were lightly stained with hematoxylin solution (Invitrogen). To quantify cell proliferation capacity, we used 10 representative images to calculate BrdU-positive nuclei numbers. Cell proliferation was shown as a percentage of BrdU-positive nuclei over total nucleated cells. The BrdU assay was repeated with 5 independent isolated cells for each experimental group.
Osteogenic and Adipogenic Differentiation Assays
For in vitro osteogenesis assay, MSCs were cultured to confluence and changed to an osteoinductive media containing 2mM β-glycerophosphate (Sigma-Aldrich Co.), 100 mM L-ascorbic acid 2-phosphate (Wako Pure Chemical Industries Ltd.) and 10 nM Dex (Sigma-Aldrich Co.). After 4 weeks of osteo-inductive culture, calcium deposits were detected by staining with 1% Alizarin Red (Sigma-Aldrich Co.). The mineralized areas were quantified by using Image J and shown as a percentage of Alizarin Red-positive area over total area. For the adipogenic induction assay, MSCs were cultured to confluence and then induced under an adipogenic medium containing 500 μM isobutyl-methylxanthine, 60 μM indomethacin, 0.5 μM hydrocortisone, and 10 μM insulin for 1 week. Cultures were stained with 0.3% Oil Red-O after adipogenic induction. The number of Oil Red O-positive droplets-containing cells were counted and shown as a percentage of Oil Red O-positive cells over total cells. Three independent experiments were performed for each assay.
Western Blot Analysis
Total protein was extracted using M-PER mammalian protein extraction reagent (Thermo, Rockford, IL). Twenty micrograms of protein were applied and separated on 4–12% NuPAGE gel (Invitrogen Co.), followed by transferring to Immobilon™-P nitrocellulose membranes (Millipore Inc.). Membranes were blocked with 5% non-fat dry milk and 0.1% Tween-20 for 1 hour, followed by incubation with the primary antibodies (1:200–1000 dilution) at 4°C overnight. Horseradish peroxidase-conjugated IgG (Santa Cruz Biotechnology; 1:10,000) was used to treat the membranes for 1 hour, followed by enhancement with a SuperSignal® West Pico Chemiluminescent Substrate (Thermo, Rockford, IL). Bands were detected on BIOMAX MR film (Kodak, Rochester, NY). Each membrane was also stripped using a stripping buffer (Thermo) and reprobed with anti-β-actin antibody to quantify the amount of loaded protein.
Flow Cytometric Analysis of Th17 and Tregs
Cells collected from mouse spleens were treated with ACK lysis buffer (Lonza, Basel, Switzerland) to remove red blood cells. 1−2 × 106 spleen cells were activated with 5 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich Co.), 250 ng/ml ionomycin (Sigma-Aldrich Co.) and 1 μg/ml brefeldin A (GolgiPlug, BD) for 4 hours at 37°C and 5% CO2, followed by incubation with 1 μg of PerCP-conjugated anti-CD4 antibody for 30 min on ice under dark condition. For Treg analysis, additional 1 μg of APC-conjugated anti-CD25 was added during the incubation. After cell fixation and permeabilization using Foxp3 staining buffer kit, cells were stained with 1 μg of PE-conjugated anti-Foxp3 for Tregs and APC-conjugated anti-IFN-γ/PE-conjugated anti-IL17 for Th17. Isotype-matched PE-, APC- or PerCP- conjugated IgGs were used as controls. After washing with PBS/0.4%BSA for 3 times, cells were analyzed by using FACSCalibur flow cytometer (BD Bioscience).
Statistics
SPSS 13.0 was used to perform statistical analysis. Significance was assessed by analysis of variance (ANOVA) followed by Tukey post-hoc test. The P values less than 0.05 were considered significant.
Results
99Tc-MDP Administration Ameliorates OVX-induced BMD Reduction
Since osteoporosis is associated with overactivation of osteoclasts, we hypothesized that 99Tc-MDP might ameliorate osteoporosis phenotypes by inhibiting osteoclast activity. OVX mice, a reliable animal model for estrogen-deficient osteoporosis [2, 12, 15–16], were used to examine the efficacy of 99Tc-MDP treatment (Fig. 1A). In terms of bone turnover markers in serum, ELISA assays showed that RANKL levels were markedly decreased after 99Tc-MDP and zoledronate administration, respectively, whereas OPG levels were markedly increased compared with OVX group (Fig. 1B, 1C). MicroCT analysis showed that 99Tc-MDP administration at 2 weeks post-OVX procedure significantly increased BMD, BV/TV, Tb.Th and Tb.N in comparison to the untreated OVX group, to the extent comparable to zoledronate (Fig. 1D-1H). Histological analysis indicated that trabecular bone structures in 99Tc-MDP-treated OVX mice were significantly improved (Fig. 1I, 1J). Bone resorption was markedly reduced after 99Tc-MDP treatment in OVX mice, as indicated by a reduction in Oc.S/BS (Fig. 1K). Moreover, HE staining showed that 99Tc-MDP treatment failed to impair kidney, liver and bladder (Supplementary Fig. 2), indicating that 99Tc-MDP administration may be not toxic to OVX mice. These data suggest that 99Tc-MDP administration provides an effective treatment for OVX-induced osteoporosis by inhibiting bone resorption.
Figure 1.

99Tc-MDP administration ameliorates OVX-induced BMD reduction.
(A) Experimental design for OVX procedure and 99Tc-MDP (TC) or zoledronate (ZO) treatment. (B, C) RANKL levels in peripheral blood were markedly decreased in both TC- and ZO-treated groups compared with OVX group, whereas OPG levels were markedly increased in both treatment groups. (D) MicroCT analysis showed the reduction of trabecular bone volume in femurs of OVX mice. (E-H) TC administration significantly improved BMD, Tb.Th, BV/TV and Tb.N. compared to untreated OVX mice. (I, J) Histological analysis showed decreased trabecular bone volume (yellow circled area) in femurs of OVX mice when compared to sham mice. TC treatment elevated trabecular bone volume in OVX mice. (K) TRAP staining showed that Oc.S/BS was markedly decreased after TC and ZO treatment compared to the OVX group. The results are presented as means ± SD. Scale bar, 0.2 mm. *p<0.05.
99Tc-MDP Administration Increases Osteoblast Activity in OVX Mice
Since osteoporosis associates with downregulated osteoblast activity, which is caused by the osteogenic deficiency of BMMSCs [12, 17], we examined whether 99Tc-MDP administration could elevate the osteogenic ability of BMMSCs in OVX mice. We harvested BMMSCs and JBMSCs from OVX mice with/without 99Tc-MDP and zoledronate administration, respectively. We found that both drugs reduced CFU-F and proliferation rate in BMMSCs (Fig. 2A, B) and JBMSCs (Appendix Fig. 1A, B), respectively. In osteogenic assay, both drugs increased mineralized nodule formation in BMMSC (Fig. 2C) and JBMSC (Appendix Fig. 1C) groups, as measured by Alizarin Red staining and expression of the osteogenic marker ALP, as shown by Western blot analysis (Fig. 2D, Appendix Fig. 1D). This indicated that 99Tc-MDP treatment elevated the osteogenic capacity of MSCs by sacrificing their proliferation rate in OVX mice. On the other hand, both 99Tc-MDP and zoledronate treatments were able to significantly decrease the adipogenic differentiation capacity of BMMSCs (Fig. 2E) and JBMSCs (Appendix Fig. 1E) derived from OVX mice, as indicated by the decreased number of Oil Red O-positive cells and reduced expression of the adipogenic genes PPARγ and LPL (Fig. 2F and Appendix Fig. 1F, p <0.05). Osteogenic and adipogenic differentiation capacities between sham group and drug-treated groups showed significant difference (p<0.05).
Figure 2.
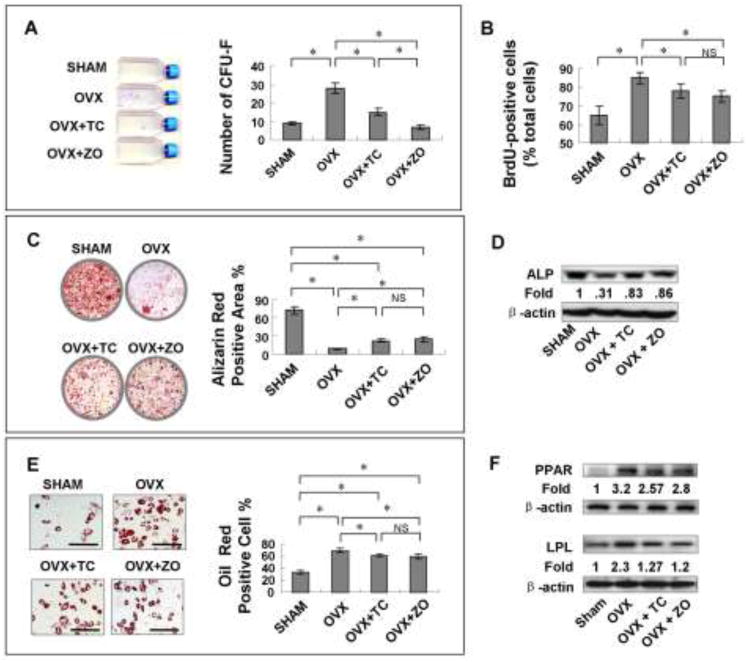
99Tc-MDP administration increases osteoblast activity in OVX mice.
(A) BMMSCs from TC-treated OVX mice generated fewer CFU-Fs compared to OVX BMMSCs. (B) The number of BrdU-positive cells was significantly decreased in the TC-treated OVX group compared to the OVX group. (C) Alizarin Red staining showed that TC treatment increased mineralized nodule formation. (D) Western blot analysis revealed that BMMSCs from TC-treated mice expressed higher levels of ALP after 4 weeks of osteogenic induction. (E, F) TC treatment decreased the adipogenic differentiation of BMMSCs, as indicated by the decreased number of Oil Red O-positive cells and reduced expression of the adipogenic genes PPARγ and LPL (scale bar, 100μm). The results were representative of three independent experiments. *p<0.05.
99Tc-MDP administration diminishes the risk of BRONJ onset by maintaining balance between Tregs and Th17
Since 99Tc-MDP administration shows comparable therapeutic effects on ameliorating osteoporotic phenotypes in OVX mice comparable to those of zoledronate-treated OVX mice, we next asked whether 99Tc-MDP administration could avoid the risk of BRONJ onset. We utilized a BRONJ animal model, as previously described [7], to test this hypothesis. C57BL/6J mice were i.v.-injected with zoledronate or 99Tc-MDP (125 μg/kg) and Dex (5 mg/kg) for 1 week before extraction of the first maxillary molar, and the drug treatment continued twice a week until mice were sacrificed (Fig. 3 A). The maxillary bones were evaluated for clinical signs of BRONJ lesions, following the guidelines established by the American Association of Oral and Maxillofacial Surgeons (AAOMS) and the American Society for Bone and Mineral Research (ASBMR) [18–20]. Clinical examination revealed an incomplete mucosal healing and open sockets with exposed bone in 20% of mice treated with zoledronate and 45% of mice treated with a combination of zoledronate and Dex (Fig. 3B, 3C). The finding of BRONJ in these treated mice was confirmed with histological study revealing a lack of epithelial lining at the alveolar socket (Fig. 3C). Moreover, necrotic bone was found adjacent to the area of intense inflammatory infiltrates, suggesting an association between inflammation and tissue necrosis in BRONJ-like disease (Fig. 3C). In contrast, 100% of the 99Tc-MDP-treated mice, either with or without Dex, showed complete epithelial coverage, which was confirmed by histological study (Fig. 3B, 3C). These data indicated that cumulative dosage of zoledronate, but not 99Tc-MDP, may associate with occurrence of BRONJ in mice undergoing dental extraction.
Figure 3.
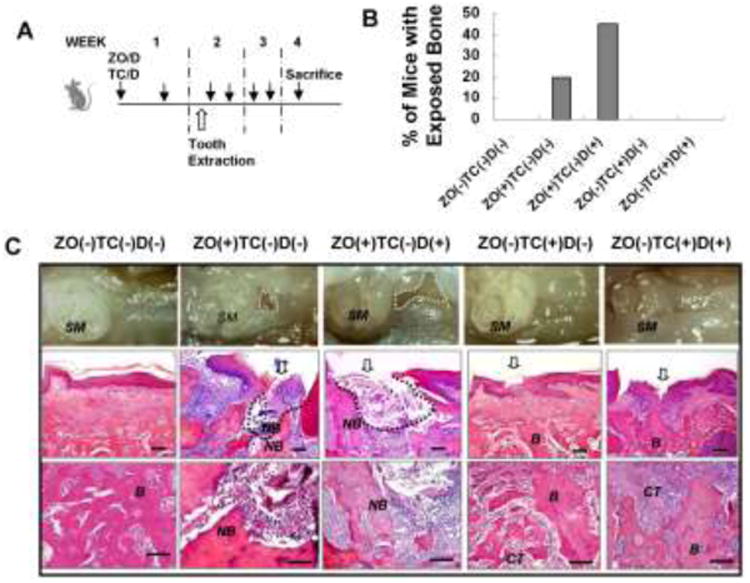
99Tc-MDP administration has less risk of causing BRONJ.
(A) Experimental procedure of inducing BRONJ. C57BL/6J mice were intravenously injected with ZO or TC (125μg/kg) with/without Dex (5mg/kg) twice for 1 week before surgical extraction of the maxillary first molar and continuous injection of ZO/TC and Dex twice weekly for 2 weeks. (B) Incidence of BRONJ, manifested as an unhealed open socket with exposure of bone. (C) Gross observation of gingiva at the extraction sites (open arrow) at 2 weeks after tooth extraction. H&E staining showed the unhealed bone structure in the ZO−treated group, but not the TC group. B, Newly formed bone; CT, connective tissues, NB, necrotic bone. Bottom panel: magnification of the middle panel. Scale bar, 100μm.
It has been proved that nitrogen-containing bisphosphates, e.g. zoledronate, are capable of modulating both innate and adaptive immune responses [21–22]. Importantly, cumulative high dosage of zoledronate can affect the Th17/Treg ratio, thus causing BRONJ-like disease in mice [7]. Therefore, to clarify whether 99Tc-MDP treatment alters Treg/Th17 ratio, we examined the levels of Tregs and Th17 cells in the spleens of mice at 2 weeks after tooth extraction. Zoledronate only and zoledronate/Dex-treated mice showed a significantly decreased level of CD4+CD25+Foxp3+ cells (Tregs) and increased level of CD4+IL17+ cells (Th17) in the splenetic cells compared with untreated control (Fig. 4A). However, 99Tc-MDP-treated groups, with or without Dex, neither significantly decreased the level of Tregs nor increased Th17 levels (Fig. 4A). Consequently, the ratio of Tregs/Th17 was markedly decreased in the zoledronate alone and zoledronate/Dex-treated group compared with control and 99Tc-MDP–treated group (Fig. 4B). These findings suggest that 99Tc-MDP has less risk of causing BRONJ onset in mouse model at the dosage of 125 μg/kg when compared with zoledronate-treated group.
Figure 4.
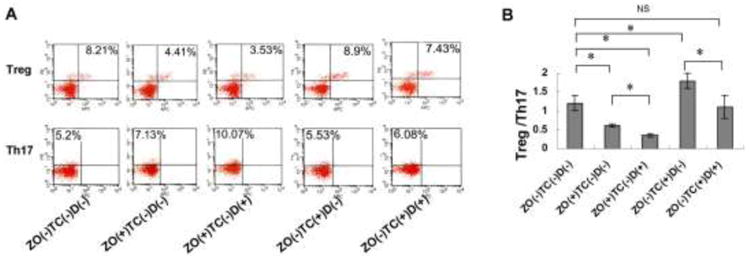
99Tc-MDP, but not zoledronate, keeps the balance between Tregs and Th17 in the BRONJ model.
(A) Flow cytometric analysis showed significantly decreased CD4+CD25+Foxp3+ cells (Tregs) and increased CD4+IL17+ cells (Th17) in the splenetic cells from ZO only and ZO+Dex-treated mice compared with untreated control or TC groups. (B) The Treg/Th17 ratio in spleens of C57BL/6J mice after receiving TC treatment was significantly higher than that with ZO treatment. The results were representative of three independent experiments. *p<0.05.
Discussion
To date, bisphosphonates are the most widely used antiresorptive drugs for osteoporosis. However, BRONJ has become a major concern of bisphosphonate use [1, 6–8]. Although the etiology of BRONJ remains unclear, it is believed that the pathogenesis results, in part, from the suppression of the adaptive Tregs and activation of Th17 cells in newly established BRONJ-like disease models [7, 23]. In order to characterize a novel bisphosphonate derivative, 99Tc-MDP, we treated osteoporotic mice with it and assessed whether it could induce BRONJ. Our data suggest that 99Tc-MDP ameliorates OVX-induced osteoporosis, with effects comparable to zoledronate. Importantly, since 99Tc-MDP does not alter the levels of Th17 cells or Tregs, it does not cause BRONJ-like disease in a mouse model.
OVX mice are a reliable animal model for estrogen deficiency–induced osteoporosis [2, 12, 15– 16]. Since 8-week-old mice show comparable bone turnover markers with older adult mice [24], they have been used as OVX mouse model in previous studies [14, 25–26]. It is well established that osteoclastogenesis is a main cause in estrogen deficiency-induced osteoporosis [3–4, 15], and evidences show that impairment of the osteoblast lineage also contributes to the osteoporotic phenotype in OVX mice [12, 27–28]. In addition to the already established understanding of the inhibition to osteoclast activity by bisphosphonates, recent studies showed that bisphosphonates also stimulated osteoblast function [29]. In this study, we demonstrate that both 99Tc-MDP and zoledronate inhibit bone resorption in OVX mice and affect MSC functions, including inhibition of CFU-F numbers, proliferation rate and adipogenic differentiation, whereas enhancement of osteogenic differentiation, which is in accordance with previous studies [29, 30]. However, the significant differences between sham group and drug-treated OVX groups indicate that the drugs can only partly rescue the deficiency of MSC functions in OVX model by promoting the osteogenic commitment and sacrificing their proliferation and adipogenic differentiation. Therefore, 99Tc-MDP, as a bisphosphonate derivative, may share the same cellular mechanism of inhibiting bone resorption, while stimulating MSC/osteoblast function.
ELISA assay indicated that both 99Tc-MDP and zoledronate administration markedly decreased RANKL levels in serum, whereas increased OPG levels compared with OVX group in short term treatment, which is in accordance with a previously study in rats [31]. However, it has been proved that long term bisphosphonates treatment reduces bone turnover, thereby resulting in increased risk of subtrochanteric femur fractures in osteoporosis patients [32, 33]. These discrepant results may due to, at least in part, the length of bisphosphonates treatment period and intra-specimen variations. Therefore, the long term effects of 99Tc-MDP and zoledronate on the mouse model of OVX still await further studies. Among the many potential risk factors, Treg/Th17 cells are important immune components contributing to BRONJ onset in animal models [7]. Tregs can suppress various immune responses and thus regulate immune homeostasis [34]. Tregs can also suppress IL-17-producing Th17 cells, which play important roles in several autoimmune diseases, including RA, systemic lupus erythematosus (SLE) or multiple sclerosis (MS) [35–37]. Using a newly developed BRONJ mouse model, we confirmed that zoledronate administration could inhibit Tregs and activate Th17 cells, thereby causing BRONJ-like lesions at the site induced by dental extraction. Since most BRONJ patients are also bisphosphonate-treated cancer patients who take immunosuppressant drugs, including Dex, we tested the addition of Dex and found that zoledronate-induced BRONJ-like lesions were enhanced. Since 99Tc-MDP has immunomodulatory properties manifested by downregulation of proinflammatory cytokines, including TNF-α and IL1-β, ICAM-1, and MMPs [9–10], it is reasonable to examine whether 99Tc-MDP regulates Tregs/Th17 cells. Interestingly, in 99Tc-MDP-treated mice, with or without Dex, neither Tregs nor Th17 cells were affected. Moreover, we didn't observe exposed bone in the group treated with 99Tc-MDP + Dex, suggesting that 99Tc-MDP may have less risk of causing BRONJ.
Over the decades, 99mTc-MDP has been used as a radioactive imaging agent for bone scintigraphy. 99Tc-MDP, used in this study, has no radioactivity and is harmless to the human body in long-term clinical treatment of systemic inflammatory diseases such as RA [9]. 99Tc-MDP may be not toxic to mice, as observed no impairment in bladder, kidney and liver. It is important to evaluate long-term effect of multi-dose administration of 99Tc-MDP and elucidate the mechanism by which 99Tc-MDP differs from other bisphosphonates in the context of Treg/Th17 ratio. To the best of our knowledge, this is the first study to show that 99Tc-MDP is equipotent as zoledronate to prevent OVX-induced bone loss, with minimal risks to cause BRONJ. However, whether 99Tc-MDP is effective for treatment of bone loss in other diseases still awaits further study.
In summary, we found that administration of 99Tc-MDP results in increased BMD in OVX-induced osteoporotic mice by inhibiting osteoclasts and enhancing osteogenic differentiation of BMMSCs. Moreover, we showed that 99Tc-MDP, but not zoledronate, can avoid the activation of Th17 cells and the suppression of Tregs in mice, thus preventing the onset of BRONJ. Consequently, 99Tc-MDP therapy may be a promising approach in the treatment of osteoporosis-associated bone resorption with less risk of causing BRONJ.
Supplementary Material
Acknowledgments
This work was supported by grants from the US National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services (R01DE017449, R01DE019932 and R01DE019413 to S.S.) and from the National Basic Research Program (973 Program) of China (2011CB964700).
Footnotes
Competing Interests Statement: The authors have stated that they have no conflict of interest.
References
- 1.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schett G, David JP. The multiple faces of autoimmune-mediated bone loss. Nat Rev Endocrinol. 2010;6:698–706. doi: 10.1038/nrendo.2010.190. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum SL. Postmenopausal osteoporosis, T cells, and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:16711–16712. doi: 10.1073/pnas.0407335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kushner GM, Alpert B. Bisphosphonate-related osteonecrosis of the jaws. Curr Opin Otolaryngol Head Neck Surg. 2011;19(4):302–6. doi: 10.1097/MOO.0b013e328348b257. [DOI] [PubMed] [Google Scholar]
- 7.Kikuiri T, Kim I, Yamaza T, et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res. 2010;25:1668–1679. doi: 10.1002/jbmr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagan J, Scully C, Sabater V, Jimenez Y. Osteonecrosis of the jaws in patients treated with intravenous bisphosphonates (BRONJ): A concise update. Oral Oncol. 2009;45:551–554. doi: 10.1016/j.oraloncology.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Lai K, Xu L, Jin C, Wu K, Tian Z, Huang C, Zhong X, Ye H. Technetium-99 conjugatedwith methylene diphosphonate (99Tc-MDP) inhibits experimental choroidalneovascularization in vivo and VEGF-induced cell migration and tube formation in vitro. Invest Ophthalmol Vis Sci. 2011;52:5702–5712. doi: 10.1167/iovs.10-6370. [DOI] [PubMed] [Google Scholar]
- 10.Yan SX, Wang Y, Peng GJ, Lu XP, Fu Y. Effects of technetium-99 methylenediphosphonate on cytokine-induced activation of retro-ocular fibroblasts from patients with Graves' ophthalmopathy. Nucl Med Commun. 2011;32:142–146. doi: 10.1097/MNM.0b013e32834121cb. [DOI] [PubMed] [Google Scholar]
- 11.Kovacic N, Grcevic D, Katavic V, Lukic IK, Grubisic V, Mihovilovic K, Cvija H, Croucher PI, Marusic A. Fas receptor is required for estrogen deficiency-induced bone loss in mice. Lab Invest. 2010;90:402–413. doi: 10.1038/labinvest.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaza T, Miura Y, Bi Y, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS One. 2008;3:2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daci E, Verstuyf A, Moermans K, Bouillon R, Carmeliet G. Mice lacking the plasminogen activator inhibitor 1 are protected from trabecular bone loss induced by estrogen deficiency. J Bone Miner Res. 2000;15:1510–1516. doi: 10.1359/jbmr.2000.15.8.1510. [DOI] [PubMed] [Google Scholar]
- 14.Okada Y, Morimoto I, Ura K, Nakano Y, Tanaka Y, Nishida S, Nakamura T, Eto S. Short-term treatment of recombinant murine interleukin-4 rapidly inhibits bone formation in normal and ovariectomized mice. Bone. 1998;22:361–365. doi: 10.1016/s8756-3282(97)00296-2. [DOI] [PubMed] [Google Scholar]
- 15.Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Upregulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura M, Chen XD, Allen MR, et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–1713. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen A, Dempster DW, Recker RR, et al. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2011;96(10):3095–105. doi: 10.1210/jc.2011-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 19.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 21.Wolf AM, Rumpold H, Tilg H, Gastl G, Gunsilius E, Wolf D. The effect of zoledronic acid on the function and differentiation of myeloid cells. Haematologica. 2006;91:1165–1171. [PubMed] [Google Scholar]
- 22.Fiore F, Castella B, Nuschak B, Bertieri R, Mariani S, Bruno B, Pantaleoni F, Foglietta M, Boccadoro M, Massaia M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood. 2007;110:921–927. doi: 10.1182/blood-2006-09-044321. [DOI] [PubMed] [Google Scholar]
- 23.Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009;45:164–172. doi: 10.1016/j.oraloncology.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Contie S, Voorzanger-Rousselot N, Litvin J, Bonnet N, Ferrari S, Clezardin P, Garnero P. Development of a new ELISA for serum periostin: evaluation of growth-related changes and bisphosphonate treatment in mice. Calcif Tissue Int. 2010;87:341–350. doi: 10.1007/s00223-010-9391-y. [DOI] [PubMed] [Google Scholar]
- 25.Yamane H, Sakai A, Mori T, Tanaka S, Moridera K, Nakamura T. The anabolic action of intermittent PTH in combination with cathepsin K inhibitor or alendronate differs depending on the remodeling status in bone in ovariectomized mice. Bone. 2009;44:1055–1062. doi: 10.1016/j.bone.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Kemp CJ, Leary CN, Drinkwater NR. Promotion of murine hepatocarcinogenesis by testosterone is androgen receptor-dependent but not cell autonomous. Proc Natl Acad Sci U S A. 1989;86:7505–7509. doi: 10.1073/pnas.86.19.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nat Med. 2009;15:682–689. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaza T, Ren G, Akiyama K, Chen C, Shi Y, Shi S. Mouse mandible contains distinctive mesenchymal stem cells. J Dent Res. 2011;90:317–324. doi: 10.1177/0022034510387796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebert R, Zeck S, Krug R, Meissner-Weigl J, Schneider D, Seefried L, Eulert J, Jakob F. Pulse treatment with zoledronic acid causes sustained commitment of bone marrow derived mesenchymal stem cells for osteogenic differentiation. Bone. 2009;44:858–864. doi: 10.1016/j.bone.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Duque G, Rivas D. Alendronate has an anabolic effect on bone through the differentiation of messenchymal stem cells. J Bone Miner Res. 2007;22:1603–1611. doi: 10.1359/jbmr.070701. [DOI] [PubMed] [Google Scholar]
- 31.Li YF, Zhou CC, Li JH, Luo E, Zhu SS, Feng G, Hu J. The effects of combined human parathyroid hormone (1-34) and zoledronic acid treatment on fracture healing in osteoporotic rats. Osteoporos Int. 2012;23:1463–1474. doi: 10.1007/s00198-011-1751-6. [DOI] [PubMed] [Google Scholar]
- 32.Rizzoli R, Akesson K, Bouxsein M, Kanis JA, Napoli N, Papapoulos S, Reginster JY, Cooper C. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int. 2011;22:373–390. doi: 10.1007/s00198-010-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen MR, Burr DB. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22:1759–1765. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 36.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 37.Awasthi A, Murugaiyan G, Kuchroo VK. Interplay between effector Th17 and regulatory T cells. J Clin Immunol. 2008;28:660–670. doi: 10.1007/s10875-008-9239-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


