Abstract
Objective
The primary aim of this study was to design prediction models based on a functional marker (preoperative gait-speed) to predict readiness for home discharge time of ≤ 90 minutes, and to identify those at risk for unplanned admissions, after elective ambulatory surgery.
Design
This prospective observational cohort study evaluated all patients scheduled for elective ambulatory surgery. Home discharge readiness and unplanned admissions were the primary outcomes. Independent variables included preoperative gait speed, heart rate, and total anesthesia time. The relationship between all predictors and each primary outcome was determined in separate multivariable logistic regression models.
Results
After adjustment for covariates, gait speed with adjusted odds ratio = 3.71 (95% CI: 1.21-11.26), p=0.02; was independently associated with early home discharge readiness ≤90 minutes. Importantly, gait speed dichotomized as greater or less than 1 m/s predicted unplanned admissions with odds ratio = 0.35 (95% CI: 0.16 to 0.76, p=0.008) for those with speeds ≥ 1 m/s in comparison to those with speed < 1 m/s. In a separate model, prior history of cardiac surgery with adjusted odds ratio =7.5 (95% CI: 2.34-24.41)(p=0.001) was independently associated with unplanned admissions after elective ambulatory surgery, when other covariates were held constant.
Conclusions
This study demonstrates use of novel prediction models based on gait speed testing to predict early home discharge and to identify those patients at risk for unplanned admissions, after elective ambulatory surgery.
Keywords: Gait Speed, Ambulatory Surgery, Discharge Planning, Quality Outcomes
Gait speed is a powerful predictor of long-term mortality in the elderly.1 Recent studies suggest an association between habitual gait speed (HGS) and clinical outcomes in surgical inpatients older than 65-years.2 In particular, slow gait speed was shown to correlate with mortality from strokes and cardiovascular disease.3 It is generally thought that older patients (> 65 years old) are likely to have worse surgical outcomes.4 Yet, old age remains a crude and unreliable predictor of post-surgical outcomes. 4 Due to the heterogeneity and variance among patients of physiological and chronological age, measurements of frailty or functional status may be superior to age as markers of perioperative risk. 5 It has been suggested that gait speed may provide a quick, simple, objective, inexpensive, and reproducible test that will allow effective stratification of patients’ functional status and has shown promise in some investigations. 6,7 As a singular measure of frailty, gait speed also predicts costs of hospitalizations, length of hospital stay and emergency department visits after major surgery. 8 Given that total time to postoperative discharge in the post-anesthesia care unit (PACU) is an important determinant of the overall costs of care, 9 understanding how gait speed in the ambulatory surgical population relates to discharge time may provide an important preoperative tool for stratifying anticipated healthcare resources. 10 Prior studies of gait speed have used retrospective data, and relatively small sample sizes in an inpatient setting, making it difficult to generalize results and implement findings within ambulatory surgical practice. 11 Thus, there is the need for larger prospective studies of gait speed within the ambulatory setting.
With the rise in demand for early discharge after ambulatory surgery and anesthesia, several criteria have been proposed to determine home readiness. 12-14 Yet, there are no standardized discharge readiness cut-off times to guide clinical decision-making. 15-17 Standard discharge criteria and discharge readiness times will allow for better comparative analysis of patient outcomes in the ambulatory setting. Safe and early home discharge (60-90 mins after surgery) has been shown to be key for patient satisfaction, an indicator of quality outcomes.18-20 A related measure of quality care is the incidence of unplanned admissions after surgery. Traditionally, there has been strong association between high levels of co-morbidity and the risk of unplanned admissions, with the likelihood of an unplanned admission highest in the immediate post-discharge period. 21 Current evidence shows that clinicians cannot reliably predict which patients will be re-admitted.22 This may be due to the lack of focus on measures of preoperative physical function. Therefore predictive tools inclusive of performance measures, such as gait speed, could provide an anticipatory window of time for clinicians and care managers/coordinators to coordinate and align resources for those at risk of converted discharges (unplanned admissions). Reports in the literature suggests that early rehabilitative training months in advance of surgery and immediately after acute illness reduces the risk of postoperative readmissions, morbidity, and mortality. 23,24 Thus, renewed emphasis on physical function as targets of rehabilitative interventions holds promise to help reduce unplanned admissions and to improve quality of patient care.
This prospective study therefore sought to design prediction models based on gait-speed to determine early home discharge (defined here as readiness for home discharge in ≤ 90 minutes), as well as to identify those at risk for unplanned admissions. It is anticipated that identifying those specific preoperative predictors of early discharge and unplanned admissions after ambulatory surgery, will better assist clinicians to provide cost-effective care and to meet the postoperative needs of patients.
In spite of the apparent validity of the gait speed-test in the inpatient setting,2,3,8 the role of gait speed among patients undergoing elective ambulatory surgery remains unexplored.
METHODS
Study Design
With Institutional Review Board approval, including a waiver of written informed consent, oral consent was obtained and patients were evaluated prior to elective ambulatory surgery. Patients fulfilling the inclusion criteria were all adult patients ( ≥ 18 years) undergoing elective ambulatory surgical procedures at a major academic hospital center. Exclusion criteria included: patients demonstrating a history — or with obvious findings — of back pain and movement disorders, as well as those with anticipated lower limb surgery or the inability to complete the gait speed test.
While in street clothes and shoes, consenting patients completed a 6.10 m (20-ft) walk test on a level, non-carpeted floor in a well-lit area. The gait test was demonstrated to study participants prior to performance to ensure they understood how to perform the test. Participants were then asked to walk at a self-selected usual pace from a standstill position. All gait measurements were performed by the same examiner (CAO). Time to walk the 6.10 m track was defined as the time between the first footfall after the 0-mark and the first footfall after the 6.10 m mark.25 The following clinical and demographic variables were also collected and assessed: age, gender, race, surgery and anesthesia technique (general anesthesia [endotracheal tube, laryngeal mask airway], monitored anesthesia care, regional anesthesia), American Society of Anesthesiologist’s Physical Status [ASAPS], Body Mass Index [BMI] in kg/m2, preoperative blood pressure (mmHg) and respiratory rate, self-reported health, prior surgery and hospitalization, history of atrial fibrillation, hypercholesterolemia, stroke, heart disease, diabetes, hypertension, chronic obstructive pulmonary disease, asthma, emphysema, smoking and exercise history, total surgery time [time between surgical incision to placement of surgical dressing], total anesthesia time [time between preoperative evaluation in holding area to arrival time in PACU], total operating room time [time between arrival and departure from operating room], postoperative and PACU blood pressure, respiratory rate, oxygen saturation and pain (as measured via the validated visual analog scale). The primary outcomes were: 1) home discharge readiness time ≤ 90 minutes; and 2) unplanned admissions from the PACU (i.e discharges that were converted to admissions). Secondary outcomes were the occurrence of 24-hr postoperative complications of nausea/vomiting, minor bleeding and pain. Determination of home discharge readiness was made by PACU nursing and anesthesia staff at regular 15-minute intervals using the validated and well established modified Aldrete scoring system discharge criteria (Appendix 1). 26,27 A patient was only considered to be discharge-ready when they met all discharge criteria or had a minimum score of ≥ 8 using the modified Aldrete scoring system .26,27 The final time at which a patient met discharge criteria was recorded in the patient chart. All personnel performing discharge evaluations were blinded to results of gait speed test. To assess postoperative complications, all subjects were contacted 24-hours postoperatively by PACU nursing staff to ascertain the occurrence of nausea/vomiting, bleeding, post-operative pain and other self-reported complications.
Statistical Analysis
To determine the optimal number of patients required to observe a statistically significant association between gait speed and discharge readiness in the ambulatory surgical setting, we performed a priori power analysis using the Power Analysis and Sample Size software (PASS, 2008, Kaysville, Utah). The analysis showed that assuming a 0.2 association between the predictor and outcome, a sample size of 600 gave us at least 80% power to reject the null hypothesis of zero association between gait speed and time to discharge readiness, using a two-sided hypothesis test with p<0.05 considered significant.
All remaining statistical analyses were conducted using SAS software, version 9.2® (Cary, NC). The Shapiro-Wilk test was used to test for normality of continuous outcomes. Descriptive statistics were calculated: continuous and normally distributed were presented as mean ± standard deviation (SD) and were compared with the Student t test; non-normally distributed data were presented as median (inter-quartile range, IQR) and were compared with the Wilcoxon-rank sum test for unpaired data. Seven outliers (defined statistically as discharge readiness times greater than 3-standard deviations from the mean value), were removed prior to analysis.
The association between each variable and each primary outcome was quantified by bivariate analysis. All candidate variables with p ≤ 0.2 in the bivariate analysis were retained for entry in the multivariable logistic regression models to identify explanatory predictors that were independently associated with the primary outcomes. Separate models were created for each primary outcome. Interaction effects were also considered in the analysis. Gait speed was treated as a continuous variable in all models. A complementary-log log-function was used to analyze the outcome of unanticipated admissions due to the highly uneven distribution. The model predicting discharge readiness time ≤ 90 minutes was internally validated using Monte-Carlo Cross validation and bootstrapping methods with 2×104 iterations. 28,29 The model predicting unanticipated admissions could not be cross-validated by the same methods due to the skewed distribution of the outcome. The association of gait speed with secondary outcomes (nausea, vomiting, and bleeding) by logistic regression analysis, with occurrence of one or more complications given a value of 1, and no complications, given a value of 0. All statistical analysis was done with SAS, version 9.2® (Cary, NC), and p<0.05 was considered statistically significant.
RESULTS
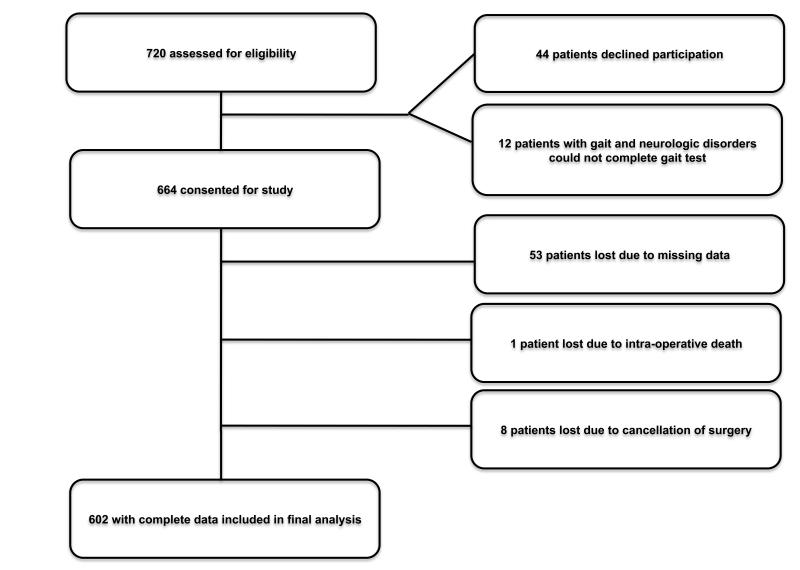
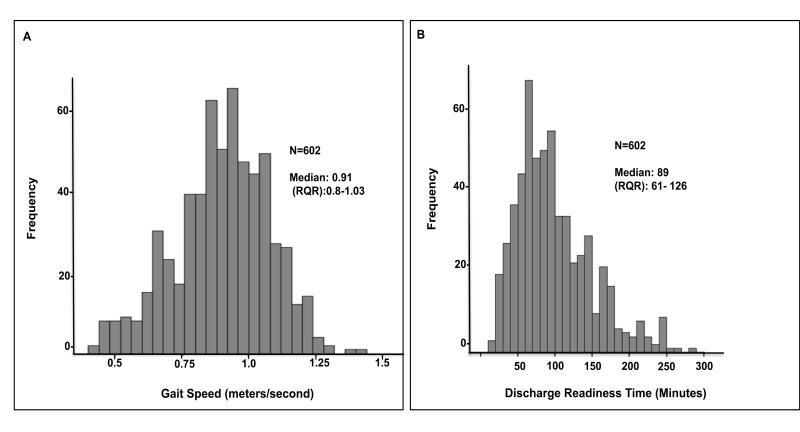
Of 720 patients approached: 664 consented for inclusion in the study, 44 declined participation and 12 did not meet inclusion criteria. Of those consented and completing the study: 53 patients were lost due to missing data, 1 patient died intra-operatively from major vessel rupture and organ perforation, 8 were lost due to surgery cancellation. The data of 602 patients was used in the final statistical analysis. (Figure 1) Demographics and clinical characteristics of study population are listed in Table 1. The median gait speed was 0.91 (m/s) with an interquartile range of 0.80-1.03 m/s (Figure 2 A) and the median home discharge-readiness time was 89 minutes (interquartile range 61-126) (Figure 2 B). The rate of unplanned admissions was 9.5% (N=57) and the rate of early discharge was 51% (N=307). Bivariate analysis identified the following explanatory variables for both primary outcomes (p ≤ 0.2) which were entered into the multivariable logistic regression models: age, female gender, BMI (overweight), gait speed, anesthesia technique, surgical risk, preoperative mean arterial pressure and heart-rate, number of chronic conditions, and postoperative pain.
Figure 1.
Flow diagram of patient enrollment.
Table 1.
Demographic and clinical characteristics of study participants
| Variable | Summary |
|---|---|
| Age in years, median (range) | 55 (18 - 91) |
| Male, n, % | 275 (46) |
| Female, n, % | 327 (54) |
| Race, n, % | |
| Caucasian/White | 445 (74) |
| Black | 75 (12) |
| Asian | 14 (2) |
| Hispanic | 68 (11) |
| BMI, median (range) kg/m2 | 28.1 (24.3-32.4) |
| BMI Groups, n, % | |
| Underweight (<18.5) | 4 (0.7) |
| Normal weight (18.5-24.9) | 167 (27.3) |
| Overweight (24.9-29.5) | 197 (32) |
| Obese (30-40) | 193 (32) |
| Morbid Obesity (>40) | 46 (8) |
| Time in operating room, median (range) minutes | 101 (65-153) |
| Total Anesthesia Time, median (range), minutes | 112 (77-169 |
| Self Reported Health, n, % | |
| Excellent | 71 (11.8) |
| Very good | 174 (28.9) |
| Good | 249 (41.4) |
| Fair | 97 (16.1) |
| Poor | 11 (1.8) |
| Surgical Procedures, n, % | |
| Arthroscopy | 105 (17) |
| Bladder and Kidney | 60 (10) |
| Upper GI (endoscopy, hernia & others) | 122 (20) |
| Lower GI (colonoscopy & polyp biopsy) | 80 (13) |
| Lens and Cataract | 107 (18) |
| Skin/wound debridement | 81 (14) |
| Septoplasty and tympanoplasty | 47 (8) |
Figure 2.
A: Histogram distribution of gait speed (meters/sec = m/s). B: Histogram distribution of Home Discharge-Readiness (minutes)
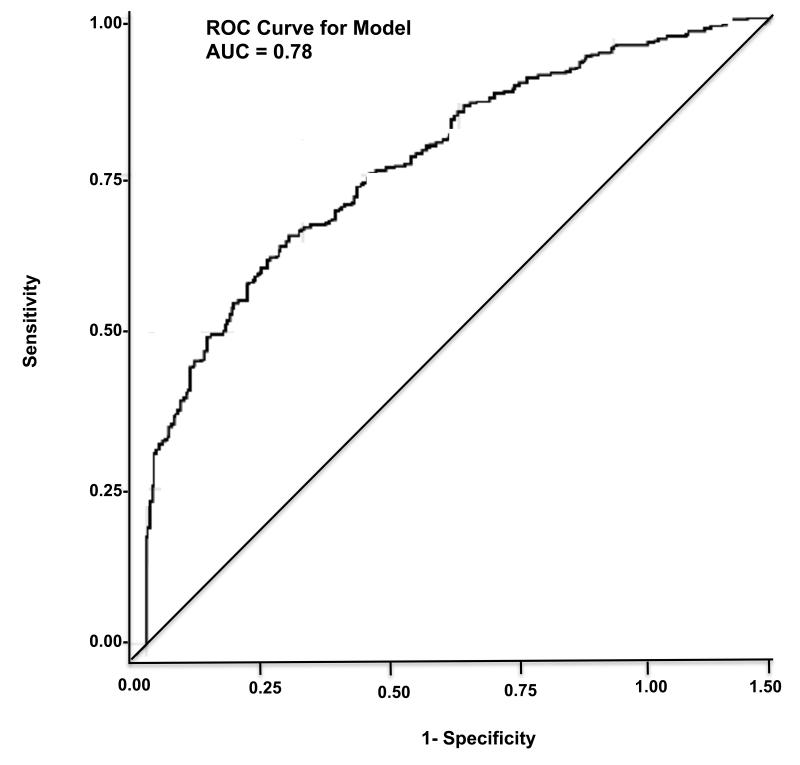
In the multivariable logistic regression models, the following independent predictors were associated with early home discharge readiness ≤90 minutes: gait speed (continuous variable) with adjusted odds ratio = 3.71 (95% CI: 1.21-11.26), p=0.02; and preoperative heart rate with adjusted odds ratio = 1.02 (95% CI: 1.008-1.04), p=0.004 (Table 2). The covariates of pre-operative mean arterial pressure (p=0.001), and co-morbidity status (p=0.02) were also significant predictors. The area under the curve for this model was=0.78 (95% CI: 0.74 to 0.82) (Figure 3). In a separate logistic regression model (Table 3), the following predictors were found to be independently associated with unplanned admissionse after elective ambulatory surgery, when other covariates were held constant: prior history of cardiac surgery or cardiac disease with adjusted odds ratio =7.5 (95% CI: 2.34-24.41)(p=0.001) and the history of prior hospitalizations (p=0.0001). The area under the curve for this model was 0.72 (95% CI: 0.67-0.79) (Table 3).
Table 2.
Predicting the likelihood of early home discharge readiness (≤ 90 minutes). Area under the curve for model=0.78 (95% CI: 0.74 to 0.82)
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|
| Age | 1.007 (0.99 to 1.02) | 1.01 (0.99 to 1.03) |
| Preoperative Heart Rate | 1.007 (0.99 to 1.02) | 1.02 (1.008 to 1.04) |
| Preoperative Gait Speed | 1.92 (0.80 to 4.72) | 3.70 (1.21 to 11.26) |
| Postoperative Pain | 1.02 (0.97 to 1.07) | 1.01 (0.96 to 1.08) |
OR: Odds ratio
Model was adjusted for gender (p=0.07), anesthesia technique (p=0.14), surgical risk (p=0.8), pre-operative mean arterial pressure (p=0.001), and co-morbidity status (p=0.02).
For a 0.1 unit change in gait speed, the OR of early discharge was 1.14 (1.02 to 1.27).
Surgical risk was categorized as major intervention vs. minor intervention.
Minor intervention was defined as all of the following: surgery duration <1h, expected blood loss <500 ml, and no opening of visceral cavity (except in case of diagnostic laparoscopic procedures).
Major intervention was defined as any of the following: duration of procedure ≥1h, expected blood loss ≥500 ml, opening of visceral cavity, potential massive respiratory or hemodynamic effects due to surgery.
Figure 3.
Area-under the curve (AUC) for model predicting early home discharge readiness (≤ 90 minutes)
Table 3.
Predicting the likelihood of unplanned admissions. Area under the curve/cstatistic for model=0.72 (95% CI: 0.67 to 0.79)
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|
| Age | 1.004 (0.99 to 1.02) | 0.995 (0.975 to 1.02) |
| Prior cardiac surgeries | 3.94 (1.35 to 11.47) | 7.5 (2.34 to 24.41) |
| Preoperative gait speed | 0.40 (0.09 to 1.81) | 0.30 (0.06 to 1.57) |
| Postoperative pain | 1.008 (0.92 to 1.10) | 1.02 (0.93 to 1.12) |
OR: Odds ratio
Model was adjusted for gender (p=0.05), anesthesia technique (p=0.71), surgical risk (p=0.08) and history of prior hospitalizations (p=0.0001).
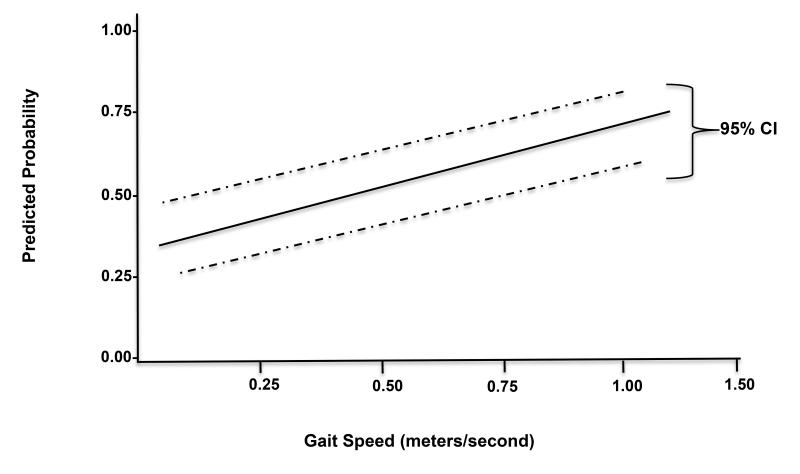
Gait speed as a continuous variable was not significantly associated with unplanned admissions (Table 3). However, when stratified by groups (< 1 m/s vs. ≥ 1 m/s) based on well-established and validated 1 m/s cut-off, 30 the odds ratio of unplanned admissions for those with gait speed ≥ 1 m/s was 0.35 (95% CI: 0.16 to 0.76, p=0.008) in comparison to those with gait speed < 1 m/s (Table 4). The positive and negative predictive values were 85.9 (95% CI: 74.2 to 93.7) and 31.6 (95% CI: 27.7 – 35.7), respectively (Table 4). Overall, the predicted probability of early discharge increased proportionally with increasing gait speed (Figure 4). There were no significant interaction effects identified among the predictors for either models of early discharge or unplanned admissions. For secondary outcomes, one patient died intraoperatively (laparoscopic surgery) from major vessel rupture and organ perforation (0.17%); 126 patients (20.93%) experienced significant post-operative pain as measured by the visual-analog scale score >5/10 in the PACU. Less than 2% of patients (10/602) experienced postoperative nausea and vomiting in the PACU. At the twenty-four hour follow-up phone call, fifty one percent of patients (306/602) responded. Of these, 27.7% (85/306 patients) reported nausea and vomiting, while 10.1% (31/306) reported minor bleeding (Table 5). Gait speed did not predict the incidence or number of 24-hour postoperative complications (data not shown).
Table 4.
Likelihood of unplanned admissions stratified by gait speed
| Gait Speed (m/s) | N, % | OR (95% CI) |
|---|---|---|
| < 1 | 422 (70) | Reference |
| ≥ 1 | 180 (30) | 0.35 (0.16 to 0.76)** |
OR: Odds ratio
p=0.008 compared to reference group with gait speed < 1 m/s (chi square analysis)
Positive Predictive Value = 85.9 (95% CI: 74.2 to 93.7)
Negative Predictive Value = 31.6 (95% CI: 27.7 – 35.7)
Figure 4.
Predicted probability of early home discharge increases proportionally with increasing gait speed.
Table 5.
Incidence of 24-hour postoperative complications among respondents (N=306) after elective ambulatory surgery
| Variable | Summary n, % |
|---|---|
| 24-hour postoperative complications | |
| Nausea and vomiting | 85 (27.7) |
| Minor Bleeding | 31 (10.1) |
| Not voiding | 48 (15.7) |
| Poor activities of daily living | 9 (2.9) |
| Did not return to normal diet | 80 (26.1) |
DISCUSSION
This prospective observational study is first to show that preoperative gait speed serves as a clinically important predictive marker for early home discharge readiness (≤ 90 minutes) in the ambulatory surgical setting. Stratified by a validated 1 m/s cut-off, 30 there was a 65% decrease in the odds of unplanned admissions for those with gait speed greater than 1 m/s as compared to those with gait speed less than 1 m/s, with good predictive reliability (Table 4). These findings corroborate the results of other studies which demonstrate that gait speed predicts length of hospital stay in older surgical and stroke patients.30 The study results underscore a potential novel use of gait speed in the preoperative screening and evulation of patients within the clinical setting, in anticipation of elective surgery. Importantly, the significant association with early discharge suggests a plausible use of gait speed to identify those patients at the highest levels of function who may not require post-anesthesia care services and thus may be eligible for a fast-track discharge pathway. Presumably, these patients with higher physical functional status could be sent home directly after surgery. Therefore gait screening holds the potential for an efficient means to reduce overall costs of care. Given the reported association between patient satisfaction and early discharge, this may help improve overall health quality outcomes.18-20
While physiatrists recognize and emphasize function in the clinical care of patients, there is a general underappreciation of the role of physical functional markers in surgical care. 31 The predictive association of gait speed with early discharge as well as unplanned admissions therefore marks an important opportunity to re-emphasize the importance of an interdisciplinary team-based approach to preoperative screening and evaluation of patients. Predictive tools inclusive of mobility measures, such as gait speed, could provide an anticipatory window for referral of those at the lowest levels of function to physiatrists and other care providers to help improve functional performance and overall health outcomes. This allows for a more comprehensive and collaborative approach to addressing patients’ needs. As demonstrated by previous reports, early rehabilitative training months in advance of surgery, and immediately after acute illness, reduces the risk of postoperative readmissions and complications. 23,24 Therefore, an interdisciplinary team-based approach to care in the clinical setting with a focus on physical function could help improve quality outcomes within the context of elective ambulatory surgery.
This study’s findings of the independent association of patients’ prior history of cardiac surgery and prior hospitalizations with unplanned admissions, corroborates recent reports by others which indicates that preoperative factors such as age, type of surgery, and medical history, may be better predictors of perioperative complications and adverse outcomes, than are expensive routine preoperative laboratory tests.4 This suggests that in the current climate of increasing healthcare costs, it is clinically more expedient to shift focus to less expensive but reliable and validated preoperative measurements that allow for better risk stratification and prognostication of outcomes. By showing the association of preoperative gait speed, the history of cardiac surgeries and prior hospitalizations with unanticipated admissions in the ambulatory surgical setting, the results of this study further emphasize the importance of a simple test, such as gait speed, and the need for a good medical history, in the preoperative screening and assessment of patients.
The association of gait speed with ambulatory surgical outcomes begs the question of why a simple functional marker such as gait speed appears to be a potent predictor of outpatient surgical outcomes. As demonstrated in prior studies, gait parameters are influenced by both central and peripheral neurologic factors.32,33 Seminal work by Strotmeyer et al., shows that a decline in peripheral nerve function secondary to peripheral vascular disease is an important source of mobility decline.34,35 Gait speed has also been proven to have good relationship with lower limb power and strength.32,36-38 In view of this, gait serves as a seemingly good proxy of health attributes that are important to recovery from physiologic perturbation due to surgery. Although a simple walking test, gait speed captures very essential cognitive, central and peripheral neurological components of an individual’s health, which belies its complexity.33 That all these aspects of health could be accurately reflected in this simple test with predictive association with important postsurgical outcomes, further underscores the potential utility of gait testing in the ambulatory surgical setting.
Importantly, the identification of reliable preoperative predictors offers an interesting area for future research to consider how functional measures such as gait speed could be incorporated into a composite preoperative risk score that takes into account other factors such as age, gender, surgical risk, preoperative heart rate and postoperative pain. This composite score may provide a more robust clinically relevant prediction of extended and delayed PACU stay, and allow for a rapid reliable way to identify high-risk ambulatory surgical patients, who may require more postoperative care.
Given that various clinical scenarios (such as surgical complications, postoperative nausea and vomiting, pain, hemodynamic disturbances and cognitive problems) may increase the length of stay in the PACU, home discharge readiness was chosen as the surrogate marker due to the feasibility and simplicity of measuring this indicator of quality outcomes in the busy setting of the ambulatory surgical center. This study thus sets the stage for future research to investigate the predictive association of preoperatitve markers such as gait speed, with specific postoperative outcomes, such as pain, nausea and vomiting, which may have a potential influence on discharge outcomes. Overall, the rather low incidence of nausea and vomiting (< 2%) in the PACU suggest that this factor may not play a significant role in the determination of discharge outcomes. Of note, adjusting for all other covariates, postoperative pain did not associate with discharge readiness. This may be due to the attenuating effects of postoperative analgesics in patients’ experience of pain, leading to a mixed effect on overall recovery time. Future studies focusing on predictors of reported postoperative pain severity in relation to preoperative functional status and preoperative pain levels, may add to our understanding of this important outcome.
The strength of the present study lies in the fact that it prospectively tested and evaluated readiness to discharge rather actual discharge time from the PACU. This made it possible to bypass some of the logistical issues of lack of escorts as well as other systemic delays that could have biased the findings and prolonged home discharge time disproportionately. Secondly, in contrast to previous gait studies that used retrospective data, this study is the first prospective observational study of gait speed among a large cohort of patients in an ambulatory surgical setting. The study demonstrates the feasibility of novel use of gait-speed for preoperative evaluation of patients within the constraints of the ambulatory surgical suite. In additon, determination of time to discharge readiness was performed by nursing and medical staff who were blinded to subjects’ comordity status, or anesthetic management, hence limiting the potential for observer bias. Lastly, the study models suggest potential utility of preoperative gait speed in the ambulatory surgical context as well as other settings where delayed pass through may have economic implications both on healthy and unhealthy patients, and potentially help delineate those who may be eligible for fast-track recovery.
Study Limitations
While the broad population sample may lend generalizability to the study results, it may also have been a limitation, since the heterogeneity of patients from multiple surgical subpopulations may have made it challenging to compare gait speed among groups of surgical patients. Future studies may address this issue by focusing on intra-group analysis and using a more homogenous group of patients (for example all patients undergoing only cataract surgery).
Second, this study was performed at a single academic medical center. Hence, determination of the primary end point of discharge readiness, while following the standardized Aldrete scoring protocols, may have adaptations unique to this center that may have influenced the actual discharge readiness time. Thus, the relationship between the primary predictor, gait speed, and the meaured primary outcomes of discharge readiness and unplanned admissions, may have to be externally validated at other academic centers and in different ambulatory surgical settings. Nonetheless, the prediction models used in this study add to the body of literature on the association between preoperative functional markers (such as gait speed) and discharge readiness outcomes.
In contrast to studies of gait speed within the in-patient surgical population, failure to find a predictive association between gait speed and secondary outcomes of nausea/vomiting, and minor bleeding at 24-hr postoperative surgery, underscores the relatively infrequent occurrence of these events within the context of elective ambulatory surgery. Longer term follow-up (1-week and 1-month) in the future may allow more accurate assessments of any possibly delayed complications. However, the twenty-four follow-up for complications is the standard of practice. 39
The exclusion of patients with movement disorders or those with findings of mobility limiting back pain, while necessary to limit the effects of confounders, make it difficult to extend the findings of this study to all patients receiving elective surgery in the ambulatory surgical setting. One may surmise that patients with movement disorders and mobility limitations may have slower gait speeds, thus would have more prolonged home-discharge readiness times. It is also plausible that the 1m/s cut-off used in this study may be too stringent of a threshold for this subgroup of patients. 11 Thus, one may have to recalibrate a different gait-speed cut-off when performing gait-testing among a cohort of patients who are mobility-limited. The wide range of gait-variability among different older adult populations makes establishing a standard cut-off a daunting task. 40 A way forward may be population-specific determinations that account for mobility-related disorders that potentially influence performance on gait testing. Future studies may consider conducting such gait speed testing among those with mobility-limitations within the preoperative clinical setting. Overall, however, gait-speed appears to hold some promise as a preoperative screening tool in the context of ambulatory surgery. Nonetheless, definitive commendations for clinical use and interpretation of gait-speed testing within the ambulatory surgical setting should be tempered by the limitations of this study.
CONCLUSIONS
This study adds to the growing body of literature, which suggests that gait speed is predictive of clinical outcomes in selected populations. In support of studies of general and cardiac surgical in-patients, where gait speed predicted morbidity and mortality,2 the study demonstrates new use of gait speed testing in predicting early discharge and also identifying those patients at risk for unplanned admissions, in the ambulatory surgical setting. Future studies focused on the design of a risk score based on the predictive associations of preoperative gait speed with other risk classification measures, may provide a cost-effective tool to screen high risk patients and help guide perioperative resource allocation.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the nursing and medical staff of the Yale New Haven Hospital Express Admission Surgery and Ambulatory Surgery Unit, without whose cooperation and kind assistance, this study would have been impossible.
This work was funded by: Yale University School of Medicine, Office of Student Research Grant (Charles Odonkor);National Institute of Health (NIH) Research Training Grant T32 GM086287, P.I. Laura Niklason, and CTSA Grant Number UL1 RR024139 from the NIH National Center for Advancing Translational Sciences (NCATS) (Robert Schonberger).
Footnotes
Disclosures: Part of the study results were presented in oral session poster format at the Poster Grand Rounds at the 2013 annual meeting of the Association of Academic Physiatrists (AAP), New Oleans, LA. Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
REFERENCES
- 1.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. Journal of the American Geriatrics Society. 2007 Nov;55(11):1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 2.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait Speed as an Incremental Predictor of Mortality and Major Morbidity in Elderly Patients Undergoing Cardiac Surgery. Journal of the American College of Cardiology. 2010 Nov 9;56(20):1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 3.Cleveland JC., Jr Frailty, aging, and cardiac surgery outcomes: the stopwatch tells the story. Journal of the American College of Cardiology. 2010 Nov 9;56(20):1677–1678. doi: 10.1016/j.jacc.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Fritsch G, Flamm M, Hepner DL, Panisch S, Seer J, Soennichsen A. Abnormal pre-operative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta anaesthesiologica Scandinavica. 2012 Mar;56(3):339–350. doi: 10.1111/j.1399-6576.2011.02593.x. [DOI] [PubMed] [Google Scholar]
- 5.Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age and ageing. 2009 Mar;38(2):156–162. doi: 10.1093/ageing/afn293. [DOI] [PubMed] [Google Scholar]
- 6.Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Critical reviews in oncology/hematology. 2011 May;78(2):138–149. doi: 10.1016/j.critrevonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Toumpoulis IK, Anagnostopoulos CE. Can EuroSCORE accurately predict long-term outcome after cardiac surgery? Nature clinical practice. Cardiovascular medicine. 2005 Dec;2(12):620–621. doi: 10.1038/ncpcardio0375. [DOI] [PubMed] [Google Scholar]
- 8.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA : the journal of the American Medical Association. 2011 Jan 5;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toumpoulis IK, Anagnostopoulos CE, Swistel DG, DeRose JJ., Jr Does EuroSCORE predict length of stay and specific postoperative complications after cardiac surgery? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2005 Jan;27(1):128–133. doi: 10.1016/j.ejcts.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Twersky RS, Sapozhnikova S, Toure B. Risk factors associated with fast-track ineligibility after monitored anesthesia care in ambulatory surgery patients. Anesthesia and analgesia. 2008 May;106(5):1421–1426. doi: 10.1213/ane.0b013e31816a6600. table of contents. [DOI] [PubMed] [Google Scholar]
- 11.Verghese J, Wang C, Holtzer R. Relationship of clinic-based gait speed measurement to limitations in community-based activities in older adults. Archives of physical medicine and rehabilitation. 2011 May;92(5):844–846. doi: 10.1016/j.apmr.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall SI, Chung F. Discharge criteria and complications after ambulatory surgery. Anesthesia and analgesia. 1999 Mar;88(3):508–517. doi: 10.1097/00000539-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Patel RI. Discharge criteria and postanesthetic complications following pediatric ambulatory surgery. Journal of post anesthesia nursing. 1988 Apr;3(2):114–117. [PubMed] [Google Scholar]
- 14.Pittoni G, Toffoletto F, Davia G, Zanette G, Della Puppa A. [Discharge criteria and postoperative complications] Minerva anestesiologica. 1999 Jun;65(6):397–400. [PubMed] [Google Scholar]
- 15.Partridge BL, Stabile BE. The effects of incisional bupivacaine on postoperative narcotic requirements, oxygen saturation and length of stay in the post-anesthesia care unit. Acta anaesthesiologica Scandinavica. 1990 Aug;34(6):486–491. doi: 10.1111/j.1399-6576.1990.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 16.Waddle JP, Evers AS, Piccirillo JF. Postanesthesia care unit length of stay: quantifying and assessing dependent factors. Anesthesia and analgesia. 1998 Sep;87(3):628–633. doi: 10.1097/00000539-199809000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Ajis A, Tan KJ, Myerson MS. Ankle Arthrodesis vs TTC Arthrodesis: Patient Outcomes, Satisfaction, and Return to Activity. Foot & ankle international. / American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society. 2013 Mar 6; doi: 10.1177/1071100713478929. [DOI] [PubMed] [Google Scholar]
- 19.Otani K, Herrmann PA, Kurz RS. Improving patient satisfaction in hospital care settings. Health services management research : an official journal of the Association of University Programs in Health Administration / HSMC, AUPHA. 2011 Nov;24(4):163–169. doi: 10.1258/hsmr.2011.011008. [DOI] [PubMed] [Google Scholar]
- 20.Conry MC, Humphries N, Morgan K, et al. A 10 year (2000-2010) systematic review of interventions to improve quality of care in hospitals. BMC health services research. 2012;12:275. doi: 10.1186/1472-6963-12-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Bruijne MC, van Rosse F, Uiters E, et al. Ethnic variations in unplanned readmissions and excess length of hospital stay: a nationwide record-linked cohort study. European journal of public health. 2013 Feb 6; doi: 10.1093/eurpub/ckt005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allaudeen N, Schnipper JL, Orav EJ, Wachter RM, Vidyarthi AR. Inability of providers to predict unplanned readmissions. J Gen Intern Med. 2011 Jul;26(7):771–776. doi: 10.1007/s11606-011-1663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puhan MA, Scharplatz M, Troosters T, Steurer J. Respiratory rehabilitation after acute exacerbation of COPD may reduce risk for readmission and mortality -- a systematic review. Respiratory research. 2005;6:54. doi: 10.1186/1465-9921-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takagi H, Kawai N, Umemoto T. Preoperative inspiratory muscle training and postoperative complications. JAMA : the journal of the American Medical Association. 2007 Feb 21;297(7):698. doi: 10.1001/jama.297.7.698-a. author reply 698-699. [DOI] [PubMed] [Google Scholar]
- 25.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005 Oct;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 26.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesthesia and analgesia. 1970 Nov-Dec;49(6):924–934. [PubMed] [Google Scholar]
- 27.Brown I, Jellish WS, Kleinman B, et al. Use of postanesthesia discharge criteria to reduce discharge delays for inpatients in the postanesthesia care unit. Journal of clinical anesthesia. 2008 May;20(3):175–179. doi: 10.1016/j.jclinane.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Bian X, Cai W, Shao X, Chen D, Grant ER. Detecting influential observations by cluster analysis and Monte Carlo cross-validation. The Analyst. 2010 Nov;135(11):2841–2847. doi: 10.1039/c0an00345j. [DOI] [PubMed] [Google Scholar]
- 29.Patterson ES, Doebbeling BN, Fung CH, Militello L, Anders S, Asch SM. Identifying barriers to the effective use of clinical reminders: bootstrapping multiple methods. Journal of biomedical informatics. 2005 Jun;38(3):189–199. doi: 10.1016/j.jbi.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Cesari M. Role of gait speed in the assessment of older patients. JAMA : the journal of the American Medical Association. 2011 Jan 5;305(1):93–94. doi: 10.1001/jama.2010.1970. [DOI] [PubMed] [Google Scholar]
- 31.Culp WC, Jr., Beyer EA. Preoperative inspiratory muscle training and postoperative complications. JAMA : the journal of the American Medical Association. 2007 Feb 21;297(7):697–698. doi: 10.1001/jama.297.7.697-b. author reply 698-699. [DOI] [PubMed] [Google Scholar]
- 32.Odonkor CA, Thomas JC, Holt N, et al. A Comparison of Straight- and Curved-Path Walking Tests Among Mobility-Limited Older Adults. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013 May 8; doi: 10.1093/gerona/glt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. Journal of the American Geriatrics Society. 2012 Nov;60(11):2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Archives of physical medicine and rehabilitation. 2001 Aug;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 35.Leishear K, Boudreau RM, Studenski SA, et al. Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. Journal of the American Geriatrics Society. 2012 Jun;60(6):1057–1063. doi: 10.1111/j.1532-5415.2012.03998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. The journals of gerontology. 2004 Series;59(11):1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 37.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? The journals of gerontology. Series A, Biological sciences and medical sciences. 2003 Aug;58(8):728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 38.Bean JF, Kiely DK, LaRose S, Goldstein R, Frontera WR, Leveille SG. Are changes in leg power responsible for clinically meaningful improvements in mobility in older adults? Journal of the American Geriatrics Society. 2010 Dec;58(12):2363–2368. doi: 10.1111/j.1532-5415.2010.03155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hitchens JT. Ambulatory ophthalmic surgery and the Health Care Financing Administration’s Outpatient Surgery Generic Quality Screen Guidelines. CRNA : the clinical forum for nurse anesthetists. 1992 Feb;3(1):2–6. [PubMed] [Google Scholar]
- 40.Bryant MS, Rintala DH, Hou JG, et al. Gait variability in Parkinson’s disease: influence of walking speed and dopaminergic treatment. Neurological research. 2011 Nov;33(9):959–964. doi: 10.1179/1743132811Y.0000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.