Abstract
Women who take folic acid periconceptionally reduce their risk of having a child with a neural tube defect (NTD) by >50%. A variant form of methylenetetrahydrofolate reductase (MTHFR) (677C→T) is a known risk factor for NTDs, but the prevalence of the risk genotype explains only a small portion of the protective effect of folic acid. This has prompted the search for additional NTD-associated variants in folate-metabolism enzymes. We have analyzed five potential single-nucleotide polymorphisms (SNPs) in the cytoplasmic, nicotinamide adenine dinucleotide phosphate–dependent, trifunctional enzyme methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase (MTHFD1) for an association with NTDs in the Irish population. One SNP, R653Q, in this gene appears to be associated with NTD risk. We observed an excess of the MTHFD1 “Q” allele in the mothers of children with NTD, compared with control individuals. This excess was driven by the overrepresentation of QQ homozygotes in the mothers of children with NTD compared with control individuals (odds ratio 1.52 [95% confidence interval 1.16–1.99], P=.003). We conclude that genetic variation in the MTHFD1 gene is associated with an increase in the genetically determined risk that a woman will bear a child with NTD and that the gene may be associated with decreased embryo survival.
Neural tube defects (NTDs) are common congenital malformations that present mainly as anencephaly, encephalocele, and spina bifida. Genetic and environmental factors that produce alterations in folate metabolism are likely to play a major role in the development of NTDs. Use of folic acid to supplement periconceptional maternal diet is known to prevent the majority of NTDs (MRC Vitamin Study Research Group 1991; Czeizel and Dudas 1992). In addition, decreased levels of folate in red blood cells and plasma (Kirke et al. 1993; Daly et al. 1995) and elevated levels of homocysteine in plasma are associated with an increased risk of an NTD-affected pregnancy (Mills et al. 1995). A genetic component is evidenced by the increased risk to sibs of a child with NTD, compared with the risk in the general population (λs>10) and by the observation that NTD rates vary between different ethnic groups. For example, rates in people of Celtic origin are relatively high, compared with levels in African Americans (Elwood et al. 1992). The identification and importance of these genetic factors has not been completely elucidated. The genes that code for the enzymes of the folate pathway are obvious candidates to screen for variation associated with NTD risk. A polymorphism (A222V) dbSNP rs1801133 (historically referred to as “C677T”) in the enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR [MIM 236250]) (Frosst et al. 1995) was subsequently associated with lower folate status and increased risk of NTDs in some populations (van der Put et al. 1995; Whitehead et al. 1995; Ou et al. 1996; de Franchis et al. 1998; Christensen et al. 1999). However, the association of the MTHFR 677C→T polymorphism with NTDs remains controversial, with several studies finding no association (Papapetrou et al. 1996; Mornet et al. 1997; Speer et al. 1997; Koch et al. 1998; Shaw et al. 1998; Weitkamp et al. 1998; Boduroglu et al. 1999). Several additional folate-related genes have also been examined in relation to NTDs; however, no convincing associations have been found, to date. Some of these genes include methionine synthase, methionine synthase reductase, several folate receptors, and cystathionine β-synthase (reviewed in Lucock 2000; Melvin et al. 2000; Gelineau-van Waes and Finnell 2001).
MTHFR plays an important role in folate metabolism by catalyzing the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which acts as a methyl group donor (Scott and Weir 1994) (fig. 1). The MTHFR 677C→T polymorphism results in conversion of an alanine to a valine, resulting in a “thermolabile” variant of the enzyme (Kang et al. 1988). Individuals who are homozygous for the thermolabile variant of MTHFR (TT) have an increased risk of hyperhomocysteinemia and lower levels of folate in plasma and red blood cells (Molloy et al. 1997). This polymorphism, in combination with low folate status, was the first genetic variant to show an association with NTDs in the Irish population (Whitehead et al. 1995). The predominant MTHFR-related genetic effect is observed in developing embryos homozygous for the valine-containing allele (TT). This genotype is estimated to account for 11.4% of the population-attributable fraction (Shields et al. 1999). A meta-analysis of NTD risk associated with the MTHFR 677TT case genotype results in a pooled odds ratio (OR) of 1.8 (95% CI 1.4–2.2) (Botto and Yang 2000). Intervention trials and case-control studies indicate that >50% of NTDs are preventable by periconceptional supplementation with folic acid (Scott et al. 1994). Therefore, the MTHFR 677C→T polymorphism does not account for the majority of NTDs in the Irish population, and other genetic and/or environmental factors may also be involved.
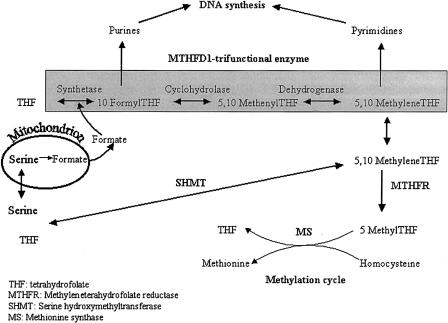
Figure 1.
Role of MTHFD1 in DNA synthesis. The main role for MTHFD1 (shaded) is in providing 10-formyl THF and 5,10-methylene THF for purine and pyrimidine synthesis. 10-Formyl THF can be synthesized directly from formate and THF, via the synthetase activity, or from 5,10-methenyl THF, via the cyclohydrolase activity, which is channeled from 5,10-methylene THF via the dehydrogenase activity. MTHFD1 plays an indirect role in homocysteine metabolism by providing some of the 5,10-methylene THF pool, but the major source of this is SHMT. The enzymes MTHFR and MS are directly involved in homocysteine metabolism.
We have analyzed several potential polymorphisms in another folate-dependent enzyme, methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase/formyltetrahydrofolate synthetase (MTHFD1 [MIM 172460]), for an association with NTDs. MTHFD1 is a trifunctional nicotinamide adenine dinucleotide phosphate (NADP)–dependent cytoplasmic enzyme (often referred to as “C1-THF synthase”), which catalyzes the conversion of tetrahydrofolate to the corresponding 10-formyl, 5,10-methenyl, and 5,10-methylene derivatives (fig. 1). 10-Formyltetrahydrofolate and 5,10-methylenetetrahydrofolate are the donor cofactors for de novo purine and pyrimidine biosynthesis and, thus, the biosynthesis of DNA. In eukaryotes the trifunctional polypeptide exists as a homodimer with two functionally distinct domains (Hum et al. 1988). The NADP-dependent dehydrogenase and cyclohydrolase activities share an overlapping active site on the NH2-terminal domain (Barlowe et al. 1989). The COOH-terminal domain possesses the formyltetrahydrofolate synthetase activity and provides the source of 10-formyltetrahydrofolate for purine biosynthesis. The synthetase is also thought to play a noncatalytic role in purine biosynthesis by forming part of a purine-synthesizing multienzyme complex (Barlow and Appling 1990).
Our study population consisted of children with NTDs, plus their parents (triads), whom we recruited throughout Ireland from 1993 to the present with the assistance of various branches of the Irish Association for Spina Bifida and Hydrocephalus. The population consisted of 319 complete NTD triads and a small number of incomplete triads, in which DNA was not available from all three family members (22 additional children with NTD, 13 mothers, and 2 fathers). An additional 83 mothers of children with NTD were drawn from a bank of previously collected samples (described below and by Kirke et al. [1993]). Individuals from incomplete triads and the bank sample were used in the case-control comparisons only. Information about the type of defect was available for 320 of the children with NTD, and the group included 303 (95%) children with spina bifida and 17 (5%) children with encephalocele. Blood samples were collected from two populations in addition to the NTD study population. Samples were obtained between 1986 and 1990 from 56,049 pregnant women attending the three main maternity hospitals in the Dublin area. Details of this collection have been described elsewhere (Kirke et al. 1993; Daly et al. 1995; Mills et al. 1995). A set (83 individuals) of the 56,049 pregnant women was selected for genotyping and biochemical analyses, because they subsequently gave birth to an NTD-affected child. Information on the type of defect was available for 80 of the NTD-affected pregnancies, which led to the birth of 40 (50%) children with spina bifida, 33 (41%) children with anencephaly or anencephaly plus spina bifida, and 7 (9%) children with encephalocele or iniencephaly. This group of case mothers was included with the case mothers from the NTD study population in the final genotype comparisons. Control population I comprised a randomly selected sample of these pregnant women (699 individuals) who did not give birth to a child with NTD and who had no previous history of an NTD-affected pregnancy. Genotyping was performed on all of control population I, and biochemical analyses were performed on a subset (200 individuals) of this group. A further control population (control population II) consisted of 318 nonpregnant women of childbearing age who were selected from the students and staff of one of the Dublin maternity hospitals. Genotyping and biochemical analyses were performed on samples from all members of this control group. Details of control population II have been described elsewhere (Molloy et al. 1997). Informed consent and ethical approval were obtained for all samples collected.
Several potential MTHFD1 SNPs were identified from the public databases and the literature. A total of five potential SNPs were chosen for analysis, because they result in an amino acid change and therefore may alter enzyme function. Of these, three were obtained from the Single Nucleotide Polymorphism (dbSNP) Database and were described as “tentative” SNPs (rs1950902, 401 G→A [R134K]; rs1803950, 2777 C→T [P926L]; and rs1803951, 2380 G→T [G794C] [dbSNP]). The fourth SNP was identified from the CGAP-GAI database (SNP 616138, 2282 C→T [T761M] [Cancer Genome Anatomy Project–Genetic Annotation Initiative]). Hol et al. (1998) previously reported an additional SNP within the coding region of MTHFD1, which also results in an amino acid change (1958 G→A [R653Q]).
PCR assays for the MTHFD1 polymorphisms R134K, P926L, G794C, and T761M were designed using the genomic sequence of MTHFD1 (NT-025892 [Entrez] and Primer Express (PE Applied Biosystems). To avoid nonspecific amplification of the MTHFD-processed pseudogene on the X chromosome (Italiano et al. 1991), PCR primer sets were designed so that at least one of the primers annealed to an intronic region of the MTHFD1 sequence. Genotyping was performed using either allele-specific–oligonucleotide (ASO) hybridization or PCR-RFLP analysis. MTHFD1 SNPs P926L, G794C, and T761M were not found to be variable in 230–300 Irish individuals. The MTHFD1 R134K is polymorphic in the Irish population; it was PCR amplified using forward primer 5′-TTCCTTCTTATTTCCATCACTT-3′ and reverse primer 5′-TTAGGCGTACAAGGAATGA-3′, and it was genotyped by ASO. The MTHFD1 R653Q polymorphism was analyzed, essentially, as described elsewhere (Hol et al. 1998), except that the restriction enzyme MspI was used for the RFLP analysis. Although the PCR primers for the MTHFD1 R653Q polymorphism were designed within the coding region, amplification of the MTHFD pseudogene on the X chromosome (Italiano et al. 1991) would be unlikely because of two internal mismatches in the reverse primer when aligned with the pseudogene sequence (data not shown). In addition, the primers flank a 98-bp intron, which allows detection of nonspecific pseudogene amplification on the basis of size difference (i.e., 330 bp for the functional MTHFD1 gene and 232 bp for the pseudogene). Nonspecific amplification of the pseudogene was never observed in our sample set. Moreover, the PCR products of several randomly selected samples were sequenced, and all contained the expected 98-bp intron. A final quality control check included repeat MTHFD1 R653Q genotyping of ∼10% of our samples with an independent PCR assay using a different set of intronic primers and the restriction enzyme AlwI.
The SNPs MTHFD1 R653Q and R134K were polymorphic in our sample set, and we were able to assign unambiguous R653Q genotypes to 98% (2,053/2,094) of our samples and to assign R134K genotypes to 97.5% (2,042/2,094). The rate of genotype failure did not differ between the groups. The allele/genotype frequencies and comparisons for each group are summarized in tables 1 and 2. There was no evidence of linkage disequilibrium between the two SNPs (data not shown). Allele and genotype frequencies did not differ significantly between the two control groups. These groups were pooled for subsequent comparisons. Analysis of the R653Q allele frequencies show a significant excess of the Q-containing allele in the mothers of children with NTD, compared with control individuals (OR 1.20 [95% CI 1.02–1.42], P=.025). The increased frequency of the “Q” allele is due to the increased frequency of maternal QQ homozygotes, because comparisons based on genotype (QQ vs. RQ/RR) show a highly significant difference in the frequency of QQ homozygotes in case mothers, compared with controls (OR 1.52 [95% CI 1.16–1.99], P=.003) (table 1). Similar results are obtained when case mothers are compared with control populations separately (control population I OR 1.54 [95% CI 1.15–2.06, P=.004; control population II OR 1.48 [95% CI 1.03–2.12], P=.033). Moreover, when type of NTD is taken into account, maternal QQ homozygosity remains a risk factor in each NTD subgroup, compared with controls (spina bifida [n=337] OR 1.43 [95% CI 1.07–1.92], P=.017; anencephaly or anencephaly plus spina bifida [n=33] OR 2.46 [95% CI 1.19–5.09], P=.015; encephalocele [n=17] OR 3.01 [95% CI 1.13–8.02], P=.027). Despite small numbers, the maternal effects are strong enough to reach statistical significance in each subgroup. The frequency of MTHFD1 R653Q heterozygotes did not differ between the study and control groups. Therefore, heterozygotes and RR homozygotes were combined to calculate the population-attributable risk (the proportion of cases in the total population that can be attributed to the risk fact) associated with homozygosity for the Q allele. Under the assumption that it is an independent risk factor, 8.9% (95% CI 2.8%–14.6%) of NTDs can be attributed to this allele. Analysis of the R134K data shows some enrichment of the “K” allele in children with NTD, compared with controls (KK/RK vs. RR), but this is not statistically significant (OR 1.25 [95% CI 0.96–1.62], P=.098) (table 2).
Table 1.
Allele and Genotype Frequencies/Comparisons of the MTHFD1 Polymorphism R653Q in NTD Study Groups and Control Individuals[Note]
|
Frequencies among Membersof Families with NTD |
Frequencies amongControl Groups |
|||||
| Fathers(n = 310) | Children(n = 336) | Mothers(n = 410) | Ia(n = 691) | IIb(n = 306) | I and II(n = 997) | |
| Genotype: | ||||||
| RR | 106 (.34) | 101 (.30) | 108 (.26) | 185 (.27) | 98 (.32) | 283 (.28) |
| RQ | 144 (.46) | 178 (.53) | 195 (.48) | 377 (.55) | 149 (.49) | 526 (.53) |
| 60 (.19) | 57 (.17) | 107 (.26) | 129 (.19) | 59 (.19) | 188 (.19) | |
| Allele: | ||||||
| R | .57 | .57 | .50 | .54 | .56 | .55 |
| Q | .43 |
.43 |
.50 |
.46 |
.44 |
.45 |
| Comparisons of family members and control groups (QQ vs. RQ/RR): | OR |
OR (LL)c |
OR (UL)d |
Pe |
||
| Mothers/control groups | 1.52 | 1.16 | 1.99 | .003 | ||
| Children/control groups | .88 | .63 | 1.22 | .439 | ||
| Fathers/control groups | 1.03 | .75 | 1.43 | .845 | ||
Note.— Data in parentheses are genotype frequencies. Because of rounding, some columns of genotype frequencies may not sum to 1.
Control group I consists of pregnant women, as described in the text.
Control group II consists of nonpregnant women of childbearing age, as described in the text.
LL = lower limit of 95% CI.
UL = upper limit of 95% CI.
Assessed with use of χ2 analysis.
Table 2.
Allele and Genotype Frequencies/Comparisons of the MTHFD1 Polymorphism R134K in NTD Study Groups and Control Individuals[Note]
|
Frequencies among Membersof Families with NTD |
Frequencies amongControl Groups |
|||||
| Fathers(n = 314) | Children(n = 335) | Mothers(n = 404) | I(n = 676) | II(n = 313) | I and II(n = 989) | |
| Genotype: | ||||||
| RR | 205 (.65)D | 216 (.64) | 267 (.66) | 474 (.70) | 212 (.68) | 686 (.69) |
| RK | 101 (.32) | 112 (.33) | 122 (.30) | 187 (.28) | 90 (.29) | 277 (.28) |
| KK | 8 (.03) | 7 (.02) | 15 (.04) | 15 (.02) | 11 (.04) | 26 (.03) |
| Allele: | ||||||
| R | .81 | .81 | .81 | .84 | .82 | .83 |
| K | .19 |
.19 |
.19 |
.16 |
.18 |
.17 |
| Comparisons of family members and control groups (KK/RK vs. RR): | OR |
OR (LL) |
OR (UL) |
P |
||
| Mothers/control groups | 1.16 | .91 | 1.49 | .233 | ||
| Children/control groups | 1.25 | .96 | 1.62 | .098 | ||
| Fathers/control groups | 1.20 | .92 | 1.58 | .176 | ||
Note.— Because of rounding, some columns of genotype frequencies may not sum to 1. Control groups and abbreviations are as defined in table 1.
The transmission/disequilibrium test (TDT) (Spielman et al. 1993) was performed on informative MTHFD1 R653Q and R134K heterozygotes from our NTD triad sample set. Comparisons of allele transmission from informative heterozygous parents to children with NTD was performed by use of the McNemar test. Allele transmission from informative R653Q heterozygotes (n=215) (triads in which both parents and cases are heterozygous or homozygous are not informative) showed favorable transmission of the wild-type “R” allele (R: 58%, n=126; Q: 41%, n=89; P=.014, McNemar χ2 6.03). In addition to the standard TDT, the five conditional models described by Schaid (1999) were also fitted to the triad data. The best-fitting model was the recessive model, which includes only the relative risk for the QQ allele in the cases. This was estimated to be 0.61 (SE 0.12) (χ2=6.20, P=.013). Transmission of the R allele was even more apparent when the TDT was performed on heterozygotes (n=125) only where a QQ homozygote case is possible (i.e., exclusion of triads in which one parent is RR [R: 63%, n=79; Q: 37%, n=46; P=.004, McNemar χ2 8.19]). TDT analysis of informative R134K heterozygotes (n=150) showed approximately equal transmission of each allele (R: 49%, n=74; K: 51%, n=76; P=.935, McNemar χ2 0.007). However, when the TDT was performed only on heterozygotes (n=33) where a KK homozygote case is possible, again preferential transmission of the wild-type R allele is observed (R: 83%, n=24; K: 17%, n=9; P=.015, McNemar χ2 5.94).
We analyzed our R653Q triad data further by applying log-linear models, which provide a powerful test of allele transmission, assuming Hardy-Weinberg equilibrium (Weinberg et al. 1998) (table 3). An important feature of the log-linear model is that it can include both case and maternal effects, both individually and jointly in the same model. Models for both recessive (Case QQ and Mother QQ effects in table 3) and dominant (Case QQ or RQ and Mother QQ or RQ) effects were separately fitted. Like the results in table 1, this analysis shows an increased QQ maternal risk (relative risk 1.42 [SE 0.24], P=.034). Moreover, it demonstrates a significantly lower number of QQ cases than expected (relative risk 0.62 [SE 0.11], P=.007). The relative risk is very similar to the estimate obtained using the recessive model of Schaid, which does not permit the inclusion of maternal effects. A joint effect of the maternal and case genotypes is also evidenced (P=.003) (table 3). Consistent with the results shown in table 1, this analysis, table 3, shows an increased QQ maternal risk (relative risk 1.42, P=.034). Moreover, it also demonstrates a significantly lower number of QQ cases than expected (relative risk 0.62, P=.007). We also performed an additional log-linear–model analysis by including the controls to provide a more accurate estimate of Hardy-Weinberg equilibrium (table 3). This analysis gives similar results. The log-linear models could not be applied to the R134K data because of limited genotype numbers.
Table 3.
MTHFD1 R653Q Log-Linear Models of Case and Maternal Effects within Parent-Case Triads Alone and Combined with Control Individuals[Note]
| Genotype | CaseG2 | RelativeRisk | P | Case and ControlG2 | RelativeRisk | P |
| No effect | 17.37 | 21.77 | ||||
| Case QQ | 10.09 | .62 | .0070 | 16.98 | .70 | .029 |
| Case QQ or RQ | 17.14 | .93 | .63 | 21.74 | .98 | .85 |
| Mother QQ | 12.89 | 1.42 | .034 | 17.47 | 1.35 | .038 |
| Mother QQ or RQ | 16.14 | 1.19 | .27 | 20.37 | 1.18 | .24 |
| Combined QQ: | 5.60 | .0027 | 10.13 | .0030 | ||
| Case QQ | .62 | .0070 | .64 | .0067 | ||
| Mother QQ | 1.42 | .034 | 1.48 | .0088 | ||
| Combined QQ/RQ: | 15.91 | .48 | 19.28 | .28 | ||
| Case QQ or RQ | .93 | .63 | .93 | .30 | ||
| Mother QQ or RQ | 1.19 | .27 | 1.20 | .12 |
Note.— G2 is the likelihood ratio goodness-of-fit statistic for the model containing the particular effect(s). Differences of these values are χ2 statistics for the effect(s). G2 = 0.21 (likelihood-ratio χ2 statistic with 1 df) for equality of Hardy-Weinberg equilibrium (frequency of Q allele) between cases and controls. 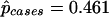 ;
; 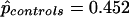 .
.
The MTHFD1 R653Q and R134K polymorphisms may result in disturbance of the folate-mediated homocysteine pathway and therefore may alter folate or homocysteine levels. Assays that measure levels of folate in plasma and red blood cell, as well as homocysteine levels in plasma, had been performed previously on a subset of our study and control populations (Kirke et al. 1993; Daly et al. 1995; Mills et al. 1995; Molloy et al. 1997, 1998). Results of each assay were grouped on the basis of each genotype and were compared. No biochemical differences were observed between genotypes among control nonpregnant women, case pregnant mothers, and control pregnant mothers (data not shown).
In summary, our analysis of five SNPs in the MTHFD1 gene showed that both MTHFD1 R653Q and R134K SNPs are polymorphic in the Irish population. The MTHFD1 R653Q allele frequencies reported here are similar to those previously found in the Dutch (Hol et al. 1998) and Turkish (Akar and Akar 1999) populations (i.e., ∼0.55 for the R allele and 0.45 for the Q allele). As in the Dutch study (Hol et al. 1998), we did not observe a significant difference in allele or genotype frequencies between the case and control groups. However, our study also included the parents of children with NTD, and a comparison between groups shows an overrepresentation of QQ homozygotes among the case mothers, compared with controls. Our results predict that mothers with the MTHFD1 “QQ” genotype have an increased risk (∼1.5- to 2.0-fold) of having an NTD-affected pregnancy. The absence of any difference between children with NTD and controls (table 1) suggests that this is a maternal effect only, and we would therefore expect approximately equal transmission of either allele, by the TDT. However, we observe preferential transmission of the wild-type R allele to children with NTD (P=.014), and this transmission is enhanced even further when the TDT is performed only when a QQ homozygote case is possible (P=.004). In addition to these data, the log-linear approach shows similar results (table 3), in that QQ homozygosity is overrepresented in mothers of children with NTD and underrepresented in children with NTD. This is in contrast to what is expected for a maternal risk factor (i.e., approximately equal transmission of either allele, by the TDT, and a possible enrichment of the risk allele in the cases because of their mothers’ genotype [Labuda et al. 2002]). This suggests that the QQ genotype may be having a different effect in the embryo. One possibility is that the QQ genotype in the embryo results in decreased viability. The QQ genotype may produce more severely affected NTD embryos that do not survive to birth or may exert a viability effect unrelated to NTDs. This effect may be influenced by the maternal genotype as the log-linear analysis does indicate a combined effect of the mother and case genotypes (table 3). A second possibility is that, in QQ mothers, the QQ genotype in the embryo may be protective against NTDs, and therefore we observe fewer QQ homozygote NTD cases than were expected. Thus, there appears to be both a clear maternal NTD risk with the QQ genotype and a suggestion that QQ homozygosity in the embryo is associated with decreased viability or is protective against NTDs. The MTHFD1 R134K polymorphism has an allele frequency of 0.17 and therefore is less abundant than the R653Q polymorphism. This SNP may have a small risk for NTD cases, but a sample size larger than that described here will be required to establish the significance of this finding.
The MTHFD1 R653Q polymorphism lies within the 10-formyltetrahydrofolate synthetase domain of MTHFD1. This residue is also an arginine in the rat, mouse, and some fungal orthologs of MTHFD1. MTHFD1 in other species (including numerous prokaryotes, insects, plants, and fungi) contain a nearly equivalent lysine at this residue. This cross-kingdom conservation suggests that the replacement of this amino acid with a glutamine may have direct functional consequences. Alternatively, this SNP may be in linkage disequilibrium with another, as yet undescribed, variant that alters function. The MTHFD1 R134K lies within the dehydrogenase/cyclohydrolase domain of the enzyme, but this arginine residue is not conserved in other species and is a lysine in the rat and a glutamic acid in yeast. Therefore, the evidence for an association with NTDs is more convincing for the R653Q variant than for the R134K variant.
The MTHFD1 R653Q data show a clear excess of QQ homozygotes in mothers of children with NTD, compared with controls, and suggest that this variant (or a variant in linkage disequilibrium) plays a role in abnormal neural tube closure. Analysis by NTD type shows that the maternal QQ genotype remains a risk factor in spina bifida, anencephaly or anencephaly plus spina bifida, and encephalocele groups. We did not observe an obvious correlation between MTHFD1 R653Q genotype and levels of folate in plasma or red blood cells or between genotype and levels of homocysteine among mothers of patients with NTD or control individuals. However, this is not unexpected, because MTHFD1 is an important provider of carbon-1 units for de novo purine and pyrimidine synthesis (West et al. 1996). Although it indirectly provides carbon-1 units via 5,10-methylenetetrahydrofolate for methylation reactions, its role in this is less important than that of cytoplasmic serine hydroxymethyltransferase (which also provides 5,10-methylenetetrahydrofolate) (fig. 1). Consequently, its function in supplying methyl groups for methylation reactions may be unimportant, and thus an alteration of its activity caused by a polymorphic variant, such as MTHFD1 R653Q, might not be expected to result in elevation of plasma homocysteine levels. Moreover, the synthetase domain is also thought to play a noncatalytic role in purine synthesis (Barlowe and Appling 1990; West et al. 1996), and thus a polymorphism that has no effect on its enzyme activity may have an effect on DNA synthesis because of an alteration of a necessary multiprotein complex. Thus, the MTHFD1 enzyme may play a key role in cell division and therefore may have an important influence on the maternal environment. At the time of neural tube closure (days 21–27), the embryo is entirely dependent upon maternally derived cells that protect and nurture its development. The synthetase activity provides the source of 10-formyltetrahydrofolate, which is essential for rapidly dividing cells, which require 2 mol of 10-formyltetrahydrofolate per mole of purine ring. MTHFD1 QQ homozygote mothers may have less efficient purine or pyrimidine synthesis, which is critical during early development. The preferential transmission of the wild-type R allele and the less-than-expected frequency of QQ cases also suggests that this variant (or a variant in linkage disequilibrium with R653Q and R134K) may be linked to embryo loss or may be protective against NTDs, but further analysis is required.
The identification of the maternal MTHFD1 R653Q genotype as a genetic risk factor for NTDs emphasizes both the importance of folate-dependent pathways and the maternal influence on neural tube development. MTHFD1 is only the second folate-related gene to be identified as a risk factor for NTDs, and identification of the gene helps to explain the protective effect of folic acid against NTDs. Further analyses will aid in elucidating the exact mechanism by which genetic variants, such as MTHFR 677C→T and MTHFD1 R653Q, affect neural tube closure.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development, National Institutes of Health, and the Health Research Board of Ireland (in particular by their provision of the ABI 377 automated DNA sequencer). We thank the Irish Association for Spina Bifida and Hydrocephalus for their assistance with subject recruitment. We wish to thank Helen Burke, Miriam Lynch, Sharon Murray, Deborah Watson, and Marie Sutton for subject recruitment and data collection. We thank the three Dublin maternity hospitals (National Maternity Hospital Holles Street, the Coombe Womens’ Hospital, and the Rotunda Hospital) and, particularly, Dr. Sean Daly, Master of the Coombe Womens’ Hospital, for control recruitment. We thank Mary Cuneen, Regina Dempsey, Tracey Claxton, and Rebecca Seltzer for their technical assistance.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Cancer Genome Anatomy Project–Genetic Annotation Initiative (CGAP-GAI), http://cgap.nci.nih.gov/ (for MTHFD1 2282 C→T [SNP 616138])
- Entrez, http://www.ncbi.nlm.nih.gov/Entrez/ (for MTHFD1 sequence [NT-025892])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MTHFR [MIM 236250] and MTHFD1 [MIM 172460])
- Single Nucleotide Polymorphism Database, http://www.ncbi.nlm.nih.gov/SNP/ (for MTHFD1 401 G→A [rs1950902], MTHFD1 2777 C→T [rs1803950], MTHFD1 2380 G→T [rs1803951], and MTHFR 677C→T [rs1801133])
References
- Akar N, Akar E (1999) Methylenetetrahydrofolate-dehydrogenase 1958 G→A (R653Q) polymorphism in Turkish patients with venous thromboembolism. Acta Haematol 102:199–200 [DOI] [PubMed] [Google Scholar]
- Barlowe CK, Appling DR (1990) Molecular genetic analysis of Saccharomyces cerevisiae C1-tetrahydrofolate synthase mutants reveals a noncatalytic function of the ADE3 gene product and an additional folate-dependent enzyme. Mol Cell Biol 10:5679–5687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe CK, Williams ME, Rabinowitz JC, Appling DR (1989) Site-directed mutagenesis of yeast C1-tetrahydrofolate synthase: analysis of an overlapping active site in a multifunctional enzyme. Biochemistry 28:2099–2106 [DOI] [PubMed] [Google Scholar]
- Boduroglu K, Alikasifoglu M, Anar B, Tuncbilek E (1999) Association of the 677C→T mutation on the methylenetetrahydrofolate reductase gene in Turkish patients with neural tube defects. J Child Neurol 14:159–161 [DOI] [PubMed] [Google Scholar]
- Botto LD, Yang Q (2000) 5,10-Methyleneterahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 151:862–877 [DOI] [PubMed] [Google Scholar]
- Christensen B, Arbour L, Tran P, Leclerc D, Sabbaghian N, Platt R, Gilfix BM, Rosenblatt DS, Gravel RA, Forbes P, Rozen R (1999) Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet 84:151–157 [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I (1992) Prevention of the first occurrence of neural tube defects by periconceptual vitamin supplementation. N Engl J Med 327:1832–1835 [DOI] [PubMed] [Google Scholar]
- Daly LE, Kirke PN, Molloy AM, Weir DG, Scott JM (1995) Folate levels and neural tube defects: implications for prevention. JAMA 274:1698–1702 [DOI] [PubMed] [Google Scholar]
- de Franchis R, Buoninconti A, Mandato C, Pepe A, Speerandeo MP, Del Gado R, Capra V, Salvaggio E, Andria G, Mastroiacovo P (1998) The C677T mutation of the 5,10-methylenetetrahydrofolate reductase gene is a moderate risk factor for spina bifida in Italy. J Med Genet 35:1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood JM, Little J, Elwood JH (1992) Epidemiology and control of neural tube defects. Oxford University Press, Oxford [Google Scholar]
- Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJH, den Heijer M, Kluijtmans LAJ, van den Heuvel LP, Rozen R (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113 [DOI] [PubMed] [Google Scholar]
- Gelineau-Van Waes J, Finnell RH (2001) Genetics of neural tube defects. Semin Pediatr Neurol 8:160–164 [DOI] [PubMed] [Google Scholar]
- Hol FA, van der Put NMJ, Geurds MPA, Heil SG, Trijbels FJM, Hamel BCJ, Mariman ECM, Blom HJ (1998) Molecular genetic analysis of the gene encoding the trifunctional enzyme MTHFD (methylenetetrahydrofolate-dehydrogenase, methenyltetrahydrofolate-cyclohydrolase, formyltetrahydrofolate synthetase) in patients with neural tube defects. Clin Genet 53:119–125 [DOI] [PubMed] [Google Scholar]
- Hum DW, Bell AW, Rozen R, MacKenzie RE (1988) Primary structure of a human trifunctional enzyme: isolation of a cDNA encoding methylenetetrahydrofolate-dehydrogenase, methenyltetrahydrofolate-cyclohydrolase, formyltetrahydrofolate synthetase. J Biol Chem 263:15946–15950 [PubMed] [Google Scholar]
- Italiano C, John SW, Hum DW, MacKenzie RE, Rozen R (1991) A pseudogene on the X chromosome for the human trifunctional enzyme MTHFD (methylenetetrahydrofolate-dehydrogenase, methenyltetrahydrofolate cyclohydrolase, formyltetrahydrofolate synthetase). Genomics 10:1073–1074 [DOI] [PubMed] [Google Scholar]
- Kang S-S, Wong PWK, Zhou J, Sora J, Lessick M, Ruggie N, Grcevich G (1988) Thermolabile methylenetetrahydrofolate reductase in patients with coronary artery disease. Metabolism 37:611–613 [DOI] [PubMed] [Google Scholar]
- Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM (1993) Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 86:703–708 [PubMed] [Google Scholar]
- Koch MC, Stegmann K, Ziegler A, Schroter B, Ermert A (1998) Evaluation of the MTHFR C677T allele and the MTHFR gene locus in a German spina bifida population. Eur J Pediatr 157:487–492 [DOI] [PubMed] [Google Scholar]
- Labuda D, Krajinovic M, Sabbagh A, Infante-Rivard C, Sinnett D (2002) Parental genotypes in the risk of a complex disease. Am J Hum Genet 71:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucock M (2000) Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab 71:121–138 [DOI] [PubMed] [Google Scholar]
- Melvin EC, George TM, Worley G, Franklin A, Mackey J, Viles K, Shah N, Drake CR, Enterline DS, McLone D, Nye J, Oakes WJ, McLaughlin C, Walker ML, Peterson P, Brei T, Buran C, Aben J, Ohm B, Bermans I, Qumsiyeh M, Vance J, Pericak-Vance MA, Speer MC (2000) Genetic studies in neural tube defects—NTD Collaborative Group. Pediatr Neurosurg 32:1–9 [DOI] [PubMed] [Google Scholar]
- Mills JL, McPartlin JM, Kirke PN, Lee YJ, Conley MR, Weir DG, Scott JM (1995) Homocysteine metabolism in pregnancies complicated by neural tube defects. Lancet 345:149–151 [DOI] [PubMed] [Google Scholar]
- Molloy AM, Daly S, Mills JL, Kirke PN, Whitehead AS, Ramsbottom D, Conley MR, Weir DG, Scott JM (1997) Thermolabile variant of 5,10-methylenetetrahydrofolate reductase associated with low red-cell folates: implications for folate intake recommendations. Lancet 349:1591–1593 [DOI] [PubMed] [Google Scholar]
- Molloy AM, Mills JL, Kirke PN, Ramsbottom D, McPartlin JM, Burke H, Conley M, Whitehead AS, Weir DG, Scott JM (1998) Low blood folates in NTD pregnancies are only partly explained by thermolabile 5,10-methylenetetrahydrofolate reductase: low folate status alone may be the critical factor. Am J Med Genet 78:155–159 [PubMed] [Google Scholar]
- Mornet E, Muller F, Lenvoise-Furet A, Delezoide A-L, Col J-Y, Simon-Bouy B, Serre J-L (1997) Screening of the C677T mutation on the methylenetetrahydrofolate reductase gene in French patients with neural tube defects. Hum Genet 100:512–514 [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group (1991) Prevention of neural tube defects: results of the Medical Research Council vitamin study. Lancet 338:131–137 [PubMed] [Google Scholar]
- Ou CY, Stevenson RE, Brown VK, Schwartz CE, Allen WP, Khoury MJ, Rozen R, Oakley GP Jr, Adams MJ Jr (1996) 5,10-Methylenetetrahydrofolate reductase genetic polymorphism as a risk factor for neural tube defects. Am J Med Genet 63:610–614 [DOI] [PubMed] [Google Scholar]
- Papapetrou C, Linch SA, Burn J, Edwards YH (1996) Methylenetetrahydrofolate reductase and neural tube defects. Lancet 348:58 [DOI] [PubMed] [Google Scholar]
- Schaid DJ (1999) Likelihoods and TDT for the case-parent design. Genet Epidemiol 16:250–260 [DOI] [PubMed] [Google Scholar]
- Scott JM, Weir DG (1994) Folate/vitamin B12 inter-relationships. Essays Biochem 28:63–72 [PubMed] [Google Scholar]
- Scott JM, Weir DG, Molloy A, McPartlin J, Daly L, Kirke P (1994) Folic acid metabolism and mechanisms of neural tube defects. In: Bock G, Marsh J (eds) Ciba Foundation Symposium 181: Neural tube defects. John Wiley & Sons, Chichester, United Kingdom, pp 180–191 [DOI] [PubMed] [Google Scholar]
- Shaw GM, Rozen R, Finnell RH, Wasserman CR, Lammer EJ (1998) Maternal vitamin use, genetic variation of infant methylenetetrahydrofolate reductase, and risk for spina bifida. Am J Epidemiol 148:30–37 [DOI] [PubMed] [Google Scholar]
- Shields DC, Kirke PN, Mills JL, Ramsbottom D, Molloy AM, Burke H, Weir DG, Scott JM, Whitehead AS (1999) The “thermolabile” variant of methylenetetrahydrofolate reductase and neural tube defects: an evaluation of genetic risk and the relative importance of the genotypes of the embryo and the mother. Am J Hum Genet 64:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer MC, Worley G, Mackey JF, Melvin E, Oakes WJ, George TM, Group NC (1997) The thermolabile variant of methylenetetrahydrofolate reductase (MTHFR) is not a major risk factor for neural tube defect in American Caucasians. Neurogenetics 1:149–150 [DOI] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ (1993) Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 52:506–511 [PMC free article] [PubMed] [Google Scholar]
- van der Put NMJ, Steegers-Theunissen RPM, Frosst P, Trijbels FJM, Eskes TKAB, van den Heuvel LP, Mariman ECM, den Heyer M, Rozen R, Blom HJ (1995) Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet 346:1070–1071 [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, Lie RT (1998) A log-linear approach to case-parent–triad data: assessing effects of disease genes that act either directly or through maternal effects and that may be subject to parent imprinting. Am J Hum Genet 62:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitkamp LR, Tackels DC, Hunter AGW, Holmes LB, Schwartz CE (1998) Heterozygote advantage of the MTHFR gene in patients with neural-tube defect and their relatives. Lancet 351:1554–1555 [DOI] [PubMed] [Google Scholar]
- West MG, Horne DW, Appling DR (1996) Metabolic role of cytoplasmic isozymes of 5,10-methylenetetrahydrofolate dehydrogenase in Saccharomyces cerevisiae. Biochemistry 35: 3122–3132 [DOI] [PubMed] [Google Scholar]
- Whitehead AS, Gallagher P, Mills JL, Kirke PN, Burke H, Molloy AM, Weir DG, Shields DC, Scott JM (1995) A genetic defect in 5,10-methylenetetrahydrofolate reductase in neural tube defects. Q J Med 88:763–766 [PubMed] [Google Scholar]