Abstract
Aims
Brugada syndrome is characterized by typical ECG features, ventricular arrhythmias and sudden cardiac death (SCD), more frequent during nighttime. Autonomic cardiovascular control has been implicated in triggering the ventricular arrhythmias. Sleep-disordered breathing (SDB) elicits marked autonomic changes during sleep and it is associated with an increased risk of nighttime SCD. Brugada patients may have a higher likelihood of SDB compared to controls. However, no data are available on cardiac autonomic control in Brugada patients, particularly with regard to the comorbidity of SDB.
Methods
We evaluated autonomic cardiovascular control in Brugada patients with SDB (BRU-SDB, n=9), without SDB (BRU, n= 9), in controls (CON, n=8) and in non-Brugada patients with SDB (n=6), during wakefulness and sleep (N2, N3 and REM). Linear spectral and entropy-derived measures of heart rate variability (HRV) were performed during apnea-free stable breathing epochs.
Results
Total HRV was attenuated in BRU-SDB compared to CON and BRU. During N2 and REM, in BRU-SDB patients sympathetic modulation decreased compared to BRU and CON, while during REM, they showed an increased parasympathetic modulation, compared to the other two groups. BRU-SDB and SDB were similar in terms of spectral components. Entropy-derived indices showed preserved dynamic changes in Brugada patients compared to controls through the different sleep stages.
Conclusion
Brugada syndrome per se does not appear associated with an altered autonomic cardiovascular control during wakefulness and sleep. The comorbidity with SDB may contribute to disrupted autonomic cardiovascular regulation during sleep, possibly predisposing to the increased likelihood of sleep-related ventricular tachyarrhythmias and SCD.
Keywords: Brugada syndrome, sleep disordered breathing, heart rate variability, non linear analysis, sleep, autonomic nervous system
Introduction
Brugada syndrome is a genetic disorder characterized by a typical ECG pattern with ST elevation in right precordial leads and right bundle branch block. The clinical manifestations include syncope, spontaneous ventricular tachyarrhythmias and sudden cardiac death (SCD) [1]. Interestingly, the ventricular tachyarrhythmias and SCD occur predominantly during nighttime than daytime and during sleep compared to wakefulness [2].
It has been hypothesized that the autonomic nervous system (ANS) plays a key role in arrhythmogenesis in Brugada patients, with a predominant vagal modulation of heart rate variability (HRV) during night, associated with altered cardiac sympathetic activity [3]; these phenomena have been linked to the increased risk of cardiac arrhythmias and SCD in these subjects [4]. However, conflicting results have been reported: a presynaptic sympathetic alteration has been described [3], while analyses of HRV using 24-h ECG Holter revealed either altered HRV in Brugada subjects with ventricular fibrillation compared to controls [5,6], or no differences in HRV parameters between healthy subjects and Brugada patients [7].
In keeping with these inconsistencies, two recent papers [8, 9] highlighted the inadequacy of traditional markers of risk stratification to detect Brugada patients at higher risk for SCD, hence calling for evaluation of new risk stratifiers.
Sleep-disordered breathing (SDB) refers to a group of diseases characterized by intermittent episodes of apnea during sleep. These conditions have been strongly associated with marked changes in nocturnal neural circulatory control [10,11] and with increased cardiovascular morbidity and mortality [12-18]. Indeed, like Brugada syndrome, sleep apnea is accompanied by an increased risk of nocturnal SCD [19].
Recently, Macedo and colleagues showed that the prevalence of SDB is higher in Brugada patients compared to controls (45% vs 27% respectively) [20].
Different tools such as spectral analysis and entropy-derived indices have been applied to evaluate HRV during sleep in physiological and pathological conditions [21-25] and they can provide complementary information on neural mechanisms regulating cardiac sinus node function [26, 27].
To our knowledge, no data are available on the modifications of autonomic cardiovascular control in Brugada patients during the different sleep stages nor has the effect of comorbid SDB on sleep-related neural circulatory control been studied.
The aim of the present study was twofold: first, to evaluate cardiac autonomic control in Brugada syndrome patients with and without SDB; second, to assess the dynamic changes of autonomic regulation during the different sleep stages in these patients.
Methods
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [28].
Patients and control subjects are the same as in the study of Macedo et al. [20]. Subject characteristics have been previously reported. In the Brugada group, mean age was 50 ± 15 years, BMI was 24.7 ± 2.7 kg/m2 and 75% of subjects were males.
Patients were diagnosed with Brugada syndrome according to established criteria [1]: 15 subjects had an abnormal baseline ECG and 5 subjects had an abnormal ECG after antiarrhythmic drug challenge with ajmaline or flecainide. Of the 20 patients with Brugada syndrome, 14 had experienced syncope and/or SCD at the time of diagnosis, while 6 patients had a history of SCD in one or more first degree relatives.
A diagnosis of SDB was made in 9 of 20 Brugada patients, while 11 showed a normal sleep study. Two Brugada patients without SDB were excluded due to the excessive supraventricular beats, thus precluding any HRV analysis.
Polysomnographic data
We recruited 20 consecutive patients evaluated at the Hospital Clinic, University of Barcelona. Inclusion criteria were age > 21 years and a definitive diagnosis of Brugada Syndrome. A complete polysomnographic (PSG) study was performed in all subjects. A control group of healthy subjects (CON, n=8) and a group of non-Brugada patients with SDB (SDB, n=6) matched for age, gender and BMI with the Brugada patients, were also studied. The study was approved by the IRB of the University of Barcelona and the Mayo Clinic (Rochester, Minnesota). Informed consent was signed by all participants.
Polysomnographic and autonomic testing laboratory at the University Hospital Barcelona was facilitated using the ANNAlab Portable Research Laboratory Technology (St. Anne’s University Hospital Brno and Institute of Scientific Instruments of Czech Academy of Sciences, Brno, Czech Republic).
PSG studies were performed using a Compumedics Siesta 802 wireless amplifier/recorder (Compumedics, Abbotsford, Victoria, Australia). Electroencephalogram, electro-oculogram, electromyogram, ECG and respiration via oronasal thermal airflow sensor and respiratory impedance plethyismography were recorded continuously. The ECG was sampled at 512 Hz, and the respiratory signal at 32 Hz. Polysomnographic studies were scored by an expert blind sleep technologist. The diagnosis of SDB was made if the apnea/hypopnea index (AHI) was ≥5 events/hour.
Data analysis
From the full polysomnographic recording, we extracted ECG and respiratory traces. These two signals were then divided into wakefulness (W), Non-REM sleep (N2 and N3) and REM sleep by merging the polysomnographic traces with the hypnograms (i.e. text files reporting the sleep scoring). Thus, we obtained segments of ECG and respiratory signals divided into W, N2, N3 and REM.
Analyzing the full sleep study, we considered the first two complete sleep cycles for each subject (a complete sleep cycle is defined by the presence of both NREM and REM sleep).
For Brugada patients diagnosed with SDB and non-Brugada SDB patients, we carefully avoided periods with apneas/hypopneas and we considered only ECG segments associated with stable and regular breathing.
For the autonomic analysis, from the ECG signal we constructed the time series of all the consecutive heart periods, the tachograms. Briefly, after the detection of QRS complexes, the apex of the R wave was located using a parabolic interpolation. QRS detection was checked to avoid missed beats and incorrect detection of R waves. The time series obtained, the tachograms, were linearly detrended. Consecutive samples of short segments of 250-300 beats were selected for spectral and entropy analyses, according to the different sleep stages among the four groups.
Respiratory traces were resampled at 512 Hz and the respiratory rate was assessed from the respiratory signal.
Assessment of cardiac autonomic control
Spectral analysis of HRV
Spectral analysis is a reliable and widely used tool to investigate autonomic oscillations encoded in the heart period time series. This analysis is based on an autoregressive model and is able to evaluate the rhythmic oscillations related to autonomic modulation in sinus node function [21-23; 27]. Three main components can be identified: a very low frequency component (VLF) (below 0.04 Hz), a low frequency component (LF) (bounded between 0.04-0.15 Hz), considered a marker of sympathetic modulation, and a high frequency component (HF), synchronous with respiration and considered a marker of vagal modulation. Each oscillation is described by a specific frequency band and amplitude. The amplitude of the LF and HF oscillations can be expressed in absolute units (ms2) and in normalized units (nu). The nu represent the relative values of each spectral component, LF and HF, in proportion to the total power, calculated as follow: LF nu= [LF absolute units / (total power – VLF power)] and the HF nu = [HF absolute units / (total power-VLF)]. The ratio between LF and HF power was calculated (LF/HF), considered a marker of the sympatho-vagal balance.
Corrected Conditional Entropy (CCE) and Regularity Index
A complete description of the mathematical basis has been published elsewhere [25-26, 29]. Briefly, entropy-derived measures evaluate the complexity of important information on the complexity (or, its opposite, regularity) of the autonomic cardiac control. In fact, several mechanisms work together for the regulation of heart period function (i.e. central oscillators, chemoreflex and baroreflex pathways, sympathetic and parasympathetic modulation of the sinus node, etc). Decreased complexity (and increased regularity) represents a situation characterized by the predominance of one of the regulatory mechanisms involved to the detriment of the others, leading the system less capable of properly responding to external stressor stimuli. Different measures have been used to assess autonomic cardiovascular complexity, and Regularity Index and Corrected Conditional Entropy (CCE) are two of the most common tools.
The definition of CCE is based on the definition of Conditional Entropy, CE, an index that assesses the amount of information carried by the current RR sample (i.e., RR(i)) when L-1 past samples of RR are known (i.e., RRL-1(i-1) =(RR(i-1), …, RR(i-L+1))). This index represents the difficulty in predicting the future values of a time series when the past values are known. Unfortunately the estimate of CE is biased and, thus, CCE was designed. CCE is bounded between 0 and the Shannon entropy, representing the maximum amount of information derived from the RR series.
The CCE decreases to 0 when the new sample is fully predictable, it is of maximum value when the new sample is unpredictable, and it is of minimum value when the knowledge of past values is helpful in reducing the uncertainty associated with future values. From this measure, an index of regularity, Ro, can be derived by dividing the CCE by the Shannon entropy. Ro ranges from 1 (maximum regularity, lowest complexity) to 0 (lowest regularity, maximum complexity).
Statistical analysis
Data are summarized as mean ± SE. SigmaPlot 11 (Systat Software Inc., Chicago, IL, USA) was used for the statistical analysis. A two-way ANOVA was performed to assess the differences between the groups within the sleep stages. The normality test was applied to check whether the distribution was normal. If this was not the case, the Equal Variance Test was performed. A Holm-Sidak method for all pair wise multiple comparison procedures was used. A p<0.05 was considered statistically significant.
Results
HRV analysis was performed in 9 Brugada patients without SDB (BRU), 9 Brugada patients with SDB (BRU-SDB), 8 controls (CON) and 6 non-Brugada SDB patients (SDB). Data are summarized in Tables 1.
Table 1.
HRV during wakefulness and sleep in Brugada patients and control subjects
| Wake | N2 | N3 | REM | |
|---|---|---|---|---|
| Heart Rate (bpm) | ||||
| Bru (n=9) | 61 ± 2 | 54 ± 2‡ | 54 ± 3 | 55 ± 2 |
| Bru-SDB (n=9) | 63 ± 1 | 60 ± 1‡ | 64 ± 1*,† | 59 ± 2 |
| Con (n=8) | 60 ±2 | 54 ± 1‡ | 54 ± 2 | 57 ± 2 |
| SDB (n=6) | 63±7 | 60±7 | 61±7*,† | 61±6 |
| Total power(ms2) | ||||
| Bru (n=9) | 8077±3853 | 4627± 1191 | 2650±590 | 9157±4182 |
| Bru-SDB (n=9) | 1529 ± 254*,† | 935 ± 142 | 798 ±177 | 662± 133*,† |
| Con (n=8) | 7994 ±1254 | 6362 ± 740 | 3565±565 | 6152±268 |
| SDB(n=6) | 1838±768*,† | 3824±1397 | 1731±506 | 3000±1552*,† |
| LF power (ms2) | ||||
| Bru (n=9) | 3054±1500 | 2324 ± 509 | 1291±261 | 3220± 1441 |
| Bru-SDB (n=9) | 414±171 | 305 ± 90* | 337 ± 82 | 1247± 63† |
| Con (n=8) | 1608±241 | 3765± 596 | 1905±301 | 2834± 345 |
| SDB(n=6) | 463±266 | 2190±1038 | 818±209 | 1980±1188 |
| HF power (ms2) | ||||
| Bru (n=9) | 1219±674 | 809 ± 209 | 702 ± 234 | 1096±349 |
| Bru-SDB (n=9) | 137 ± 29 | 192 ± 27 | 217 ± 41 | 130 ± 19 |
| Con (n=8) | 1392± 825 | 1327±390 | 1094±307 | 814±249 |
| SDB (n=6) | 302±164 | 613±194 | 363±107 | 413±184 |
| LF nu | ||||
| Bru (n=9) | 63 ± 6 | 67 ± 6 | 64 ± 7 | 62 ± 7 |
| Bru-SDB (n=9) | 49 ± 5 | 47 ± 6*,† | 51 ± 10 | 32 ± 7*,† |
| Con (n=8) | 58 ± 6 | 69 ± 5 | 60 ± 5 | 73 ± 4 |
| SDB(n=6) | 33±8 | 52±9* | 44±6 | 57±13 |
| HF nu | ||||
| Bru (n=9) | 32 ± 5 | 32 ± 6 | 34 ± 7 | 36 ± 7 |
| Bru-SDB (n=9) | 43 ± 6 | 46 ±6 | 44 ±10 | 59 ± 7*,† |
| Con (n=8) | 37 ± 6 | 30 ± 5 | 39 ± 5 | 25 ± 4 |
| SDB(n=6) | 62±7 | 44±7 | 51±4 | 36±5* |
| LF/HF | ||||
| Bru (n=9) | 4.2 ± 1.0 | 6.4 ± 2.1 | 4.7 ± 1.4 | 5.3 ± 1.7 |
| Bru-SDB (n=9) | 3.6 ± 1.1 | 2.2 ± 0.6 | 2.1 ± 0.8 | 1.5 ± 0.5 |
| Con (n=8) | 2.6 ± 0.5 | 4.7 ± 1.2 | 2.5 ± 0.5§ | 7.2 ± 1.7‡ |
| SDB(n=6) | 3.4±2.5 | 4±2.7 | 4±3.1 | 6±3.4 |
| HF Hz Resp | ||||
| Bru (n=9) | 0.27 ± 0.02 | 0.27 ± 0.03 | 0.27 ± 0.03 | 0.27 ± 0.04 |
| Bru-SDB (n=9) | 0.26 ± 0.01 | 0.24 ± 0.02 | 0.23 ± 0.02 | 0.24 ± 0.02 |
| Con (n=8) | 0.25 ± 0.03 | 0.23 ± 0.03 | 0.24 ± 0.03 | 0.24 ± 0.03 |
| SDB(n=6) | 0.27 ±0.01 | 0.25 ± 0.004 | 0.26 ±0.01 | 0.27± 0.01 |
Data are presented as mean ± SEM.
p< 0.05 vs CON
p<0.05 vs BRU
p<0.05 vs SDB
p< 0.05 vs Wake
p< 0.05vs REM.
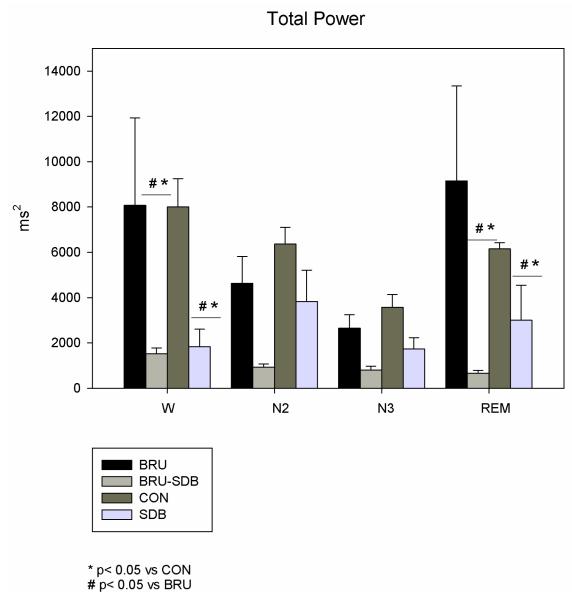
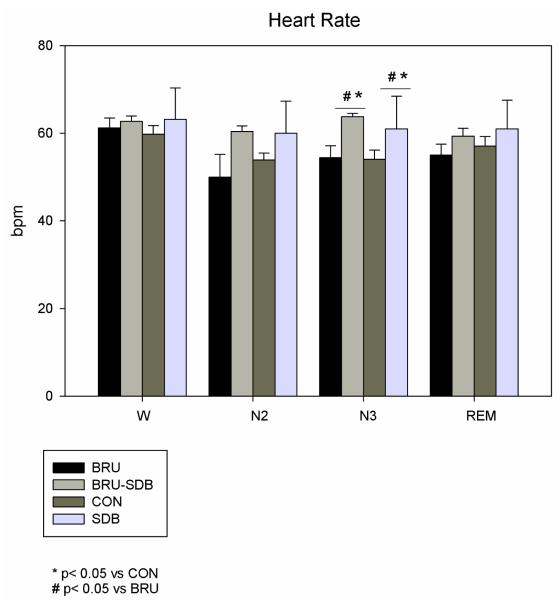
Mean heart rate (HR) was similar among the four groups during W, N2 and REM, and higher during N3 in BRU-SDB and SDB compared to CON (p=0.017) and BRU (p=0.019). Considering the within-sleep stage analysis, HR during W was higher than during N2 (see Table. 1). Total variance decreased during Wake and REM in BRU-SDB and SDB patients compared to CON (p=0.02) and BRU (p=0.01) (see Fig. 2).
Figure 2.
Total power (TP) in BRU-SDB compared to BRU, CON and SDB during W and sleep. TP is reduced in BRU-SDB and SDB compared to BRU and CON in all the sleep stages.
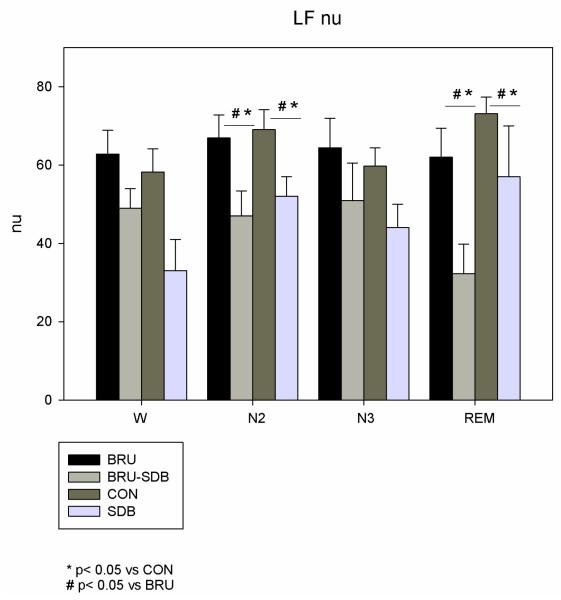
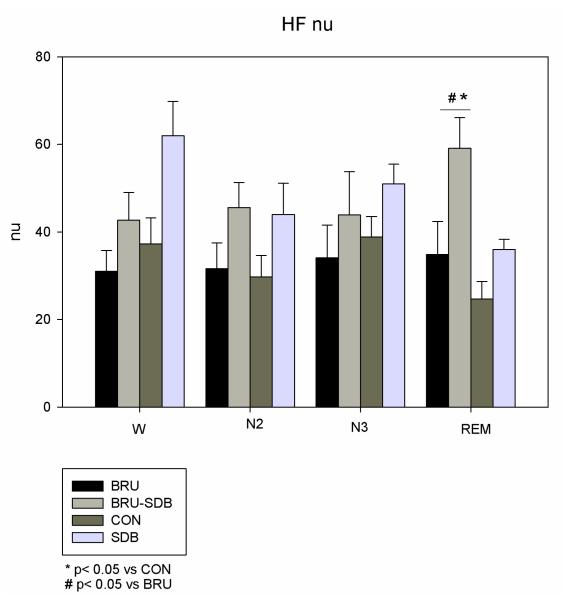
LFnu was lower during N2 and REM in BRU-SDB and SDB compared to BRU (p=0.02) and CON (p=0.01) (see Fig. 3). During REM sleep, HF nu was higher in BRU-SDB compared to BRU (p=0.012) and CON (p=0.001) (see Fig. 4). The non-Brugada SDB group did not significantly differ in terms of power spectral components from the BRU-SDB group.
Figure 3.
LFnu in BRU-SDB compared to BRU, CON and SDB during W and sleep. LFnu decreased during N2 and REM in BRU-SDB and SDB compared to BRU and CON.
Figure 4.
HFnu in BRU-SDB compared to BRU, CON and SDB during W and sleep. HFnu increased in BRU-SDB and SDB compared to BRU and CON during REM sleep.
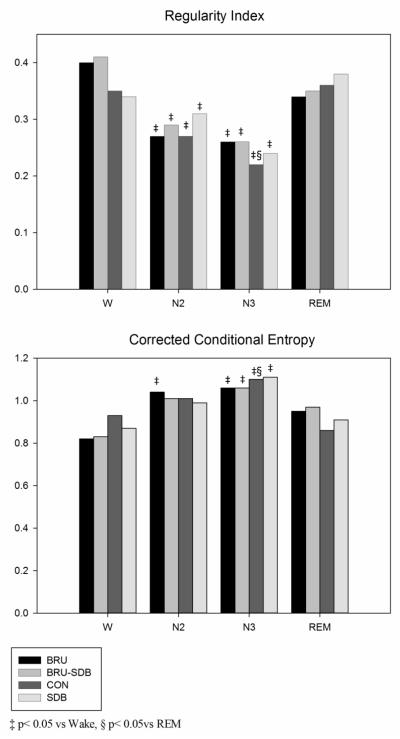
Ro and CCE entropy measures showed no differences between the four groups during sleep stages. However, Ro was significantly different during N2 and N3 compared to W in BRU-SDB and BRU. In the CON group, Ro significantly changed in N3 compared to W and REM. Similarly, CCE changed during N3 compared to W in BRU-SDB and BRU and also compared to REM in CON (see Figure 5).
Figure 5.
Regularity Index and Corrected Conditional Entropy (CCE) during W and sleep in BRU, BRU-SDB, CON and SDB. Regularity Index is lower in N2 and N3 compared to Wake and also compared to REM in Controls. CCE is higher during N3 compared to Wake and also to REM in Controls.
No changes in respiratory frequency were observed between the three groups and within the different sleep stages.
There were no differences in terms of clinical manifestations and number of patients who received an implantable cardioverter-defibrillator (ICD) in the groups of Brugada patients. However, the electrophysiological study (EPS) was positive, i.e. able to induce VF, in 1 BRU patient without SDB and in 4 out of 9 BRU-SDB patients.
Discussion
The major findings of our study are first, that Brugada patients without co-morbid SDB have preserved neural control of HRV during wakefulness and sleep, while the presence of SDB is accompanied by altered neural cardiac regulation, namely an increase of vagal modulation more evident during REM sleep. Second, the dynamic changes in the complexity of neural control are similar in Brugada patients with and without SDB, in comparison with controls. These observations are of clinical significance, particularly with regard to stratifying Brugada patients at higher risk.
Brugada syndrome is associated with specific ECG characteristics and predisposes to VF, more frequent during night and sleep than wakefulness [2], while patients with the majority of heart diseases have the highest incidence of SCD during the early morning [30, 31].
The dominant vagal modulation that characterizes deep sleep could be an arrhythmogenic trigger in Brugada patients [4]. The key role played by the ANS has been shown in previous studies [3, 32-34].
The available data on HRV in Brugada patients are contradictory. Pierre et al [35] reported that 24-h ECG Holter of symptomatic Brugada patients were characterized by lower LF and HF components [6, 36], while other studies showed no differences in HRV between Brugada and healthy subjects, either during day or night [7]. When interpreting the contrasting results, several factors must be considered carefully: first, 24-h ECG Holter recordings do not differentiate between wake and sleep; second, comorbid (and often undiagnosed) SDB could potentially contribute to autonomic alterations; third, respiratory irregularities and apneas due to SDB would confound HRV measures; fourth, cardiovascular autonomic control is not stable during sleep, so it is essential to consider the different sleep stages. Moreover, the high prevalence of SDB in Brugada patients [20] may also contribute to the inconsistency in data. We therefore suggest a novel approach to these patients, taking into consideration SDB.
We showed that during sleep, total variability was lower in BRU-SDB patients compared to CON and BRU, was further reduced during REM sleep, accompanied by a decreased sympathetic modulation. A predominant vagal modulation was present in BRU-SDB during REM sleep, consistent with previous observations [4, 6].
We observed reduced total power associated with vagal predominance in BRU-SDB during REM sleep. One possible explanation is that REM sleep may be characterized by coactivation of the sympathetic and parasympathetic branches. While there is agreement in considering cardiac vagal modulation protective and sympathetic activation detrimental for cardiovascular control [36, 38], a coactivation of the two branches of the ANS may elicit a highly unstable electrophysiologic milieu, triggering life-threatening arrhythmias [39, 40]. This phenomenon may occur during apneas in patients with SDB [41, 42], when chemoreflex excitation is associated with simultaneous vagal and sympathetic activation. Therefore, our data suggest that in normal conditions, Brugada patients have preserved HRV control and a predominant vagal modulation, which could become a pro-arrhythmic substrate in situations characterized by surges of sympathetic bursts such as apnea, more evident during REM sleep, a condition of potential risk factor for the cardiovascular system [26], when both vagal modulation in Brugada and sympathetic surges associated with apneas are greater.
In addition, it is conceivable that during REM, autonomic cardiac control is characterized by an increased cardiac vagal outflow from arterial baroreflex, which overpowers the concomitant peripheral (and cardiac) sympathetic activation, typical of REM sleep. This mechanism could also help explain the lack of an increase in HR despite sympathetic activation as previously reported in healthy subjects [43, 44].
An additional finding supporting this hypothesis relates to HRV total variance, a powerful tool to stratify the arrhythmic risk after cardiovascular events [45, 46]. In our study, Brugada patients had an overall variance similar to controls, while those with SDB showed significantly reduced variance both during wakefulness and sleep, as has been reported for patients with SDB [47]. SDB is associated with altered breathing patterns in sleep, with repetitive hypoxemic episodes causing frequent arousals during sleep. SDB is strongly related to an increased cardiovascular morbidity and mortality, and an impairment of autonomic cardiovascular control is thought to play a key role in this phenomenon [18]. We did not observe alterations of autonomic profile in Brugada syndrome, in contrast with previous studies [4-7]. However, this is the first study in which Brugada patients have been divided into two groups, with or without SDB, providing support for the hypothesis that comorbidity with SDB is the main contribution to altered autonomic control. Brugada patients without SDB and Controls showed a similar autonomic profile, supporting the concept that Brugada with and without SDB should be considered as two different populations in terms of autonomic regulation and thus, risk profile.
We further investigated the dynamic changes of autonomic control using complexity indices of HRV. A decreased complexity, or its opposite, an increased regularity, represent a situation characterized by the predominance of one of the regulatory mechanisms that collaborate to regulate sinus node function, leading the system to become less capable of properly responding to external stressor stimuli. It has been shown that different sleep stages are characterized by changes in the complexity of cardiac control, namely an increased CCE in slow wave sleep (N3) compared to Wake and REM, more evident in older subjects [26]. The present results confirm that complexity changes during different sleep stages, with a decreased regularity during slow wave sleep (N2 and N3) compared to Wake in all the groups and also compared to REM in Controls, and an increased CCE during N3 compared to Wake and also to REM in Controls. These observations suggest first, that the dynamic changes of complexity are similar in the four groups, with slow wave sleep characterized by lower regularity and higher complexity compared to Wake. Second, in Controls these changes are evident also compared to REM sleep, according to previous data, suggesting an autonomic control more capable of responding to external stressor stimuli during this stage.
These results might in part explain why traditional electrocardiographic markers of risk stratification failed to identify Brugada patients at higher risk for sudden cardiac death [8,9].
Interestingly, although BRU and BRU-SDB patients were similar in terms of clinical manifestations, we observed a difference in terms of induction of VF during EPS, being positive in 1 BRU and in 4 BRU-SDB respectively. Although this is a small population, we speculate that this observation may be of clinical relevance, considering that recent data suggest that EP testing may predict events in Brugada syndrome patients [48].
We therefore propose that screening for SDB in Brugada patients may provide important information for risk stratification. As our study was not designed to evaluate new markers, prospective studies are required to properly assess the validity and reliability of the proposed measures in the Brugada population.
Study limitations
First, the small number of subjects in each experimental group could be responsible for the lack of significant changes of HRV parameters between the sleep stages despite clear trends.
However, it is worth noting that Brugada syndrome is a relatively uncommon disease among general the population, with a prevalence estimated at 5/10000. Nevertheless, we propose that Brugada patients need to be screened for SDB since this comorbidity may contribute to cardiovascular deregulation.
Second, a direct or indirect measure of peripheral sympathetic control such as MSNA or arterial blood pressure is lacking, since we limited our study to data acquired during sleep; in addition, for reasons of subject safety, we did not performed any pharmacological autonomic blockade in our subjects during sleep.
Finally, we consider the inducibility of VF at PVS as a surrogate index of “high risk patients”, despite the fact that there is not yet consensus on this issue. However, screening for SDB in Brugada patients would be important, because this comorbidity, through impaired autonomic control, may possibly contribute to ventricular arrhythmias.
Conclusions
In conclusion, our data suggest that the presence of Brugada syndrome per se is not associated with an altered autonomic cardiovascular control during wakefulness and sleep, meaning that the abnormalities observed in Brugada patients cannot be fully explained by autonomic dysregulation. Both sleep apnea and Brugada syndrome are accompanied by an increased risk of nocturnal SCD. Therefore the strong comorbidity of Brugada syndrome with SDB may result in altered cardiac regulation, and possibly contribute to the pathogenesis of ventricular tachyarrhythmias and SCD. Although the present study does not aim to draw conclusive results on the mechanisms responsible for major cardiac events in Brugada syndrome, we believe that these data may help in better stratifying these patients in terms of risk profile.
Figure 1.
Mean HR in BRU-SDB compared to BRU, CON and SDB during W and sleep. HR is higher in BRU-SDB and SDB compared to BRU and CON during N3.
Acknowledgments
This work was supported by an unrestricted grant of the University of Milan, Italy to ET; a National Institute of Health [2R01 HL65176-05] to VKS; Czech Ministry of Health [No. NS 10098-4/2008] and by European Regional Development Fund - Project FNUSA-ICRC [No. CZ.1.05/1.1.00/02.0123] to VKS, TK and PL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest Dr. Somers has served as a Consultant for ResMed, Respicardia, Apnex Medical, Deshum, NeuPro, Johnson and Johnson, and Medtronic Corporation and has been a co-investigator on research grants funded by the ResMed Foundation. Mayo Foundation has received a gift from the Phillips-Respironics Foundation for the study of sleep apnea and cardiovascular disease. For all the other authors, none conflict of interest is declared.
All the authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo K, Kurita T, Inagaki M, et al. The circadian pattern of the development of ventricular fibrillation in patients with Brugada Syndrome. Eur Heart J. 1999;20:465–470. doi: 10.1053/euhj.1998.1332. [DOI] [PubMed] [Google Scholar]
- 3.Wichter T, Matheja P, Eckardt L, et al. Cardiac Autonomic Dysfunction in Brugada Syndrome. Circulation. 2002;105:702–706. doi: 10.1161/hc0602.103677. [DOI] [PubMed] [Google Scholar]
- 4.Wichter T. What role for autonomic dysfunction in Brugada Syndrome? Pathological and prognostic implication. Europace. 2008;10:782–783. doi: 10.1093/europace/eun117. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa K, Sakurai T, Takagi A, et al. Autonomic imbalance as a property of symptomatic Brugada syndrome. Circ J. 2003;67(6):511–4. doi: 10.1253/circj.67.511. [DOI] [PubMed] [Google Scholar]
- 6.Krittayaphong R, Veerakul G, Nademanee K, Kangkagate C. Heart rate variability in patients with Brugada syndrome in Thailand. Eur Heart J. 2003;24(19):1771–8. doi: 10.1016/j.ehj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Kostopoulou A, Koutelou M, Theodorakis G, et al. Disorders of the Autonomic Nervous System in Patients With Brugada Syndrome: A Pilot Study. J Cardiovasc Electrophysiol. 2010;21(7):773–9. doi: 10.1111/j.1540-8167.2009.01702.x. [DOI] [PubMed] [Google Scholar]
- 8.Raju H, Papadakis M, Govindan M, et al. Low prevalence of risk markers in cases of sudden death due to Brugada syndrome relevance to risk stratification in Brugada syndrome. J Am Coll Cardiol. 2011;57(23):2340–5. doi: 10.1016/j.jacc.2010.11.067. [DOI] [PubMed] [Google Scholar]
- 9.Priori SG, Gasparini M, Napolitano C. Risk Stratification in Brugada Syndrome Results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) Registry. J Am Coll Cardiol. 2012;59(1):37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 10.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328(5):303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 11.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kales A, Bixler EO, Cadieux RJ. Sleep apnoea in a hypertensive population. Lancet. 1984;2(8410):1005–8. doi: 10.1016/s0140-6736(84)91107-3. [DOI] [PubMed] [Google Scholar]
- 13.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336(8710):261–4. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 14.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27(3):401–7. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- 15.Bliwise DL, Bliwise NG, Partinen M, Pursley AM, Dement WC. Sleep apnea and mortality in an aged cohort. Am J Public Health. 1988;78(5):544–7. doi: 10.2105/ajph.78.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNicholas WT, Bonsigore MR, Management Committee of EU COST ACTION B26 Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 17.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79(8):1036–46. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 18.Somers VK, White DP, Amin R, et al. American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation. Sleep apnea and cardiovascular disease. Circulation. 2008;118:1080–1011. [Google Scholar]; J Am Coll Cardiol. 19(52):686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 20.Macedo PG, Brugada J, Leinveber P, et al. Sleep-disordered breathing in patients with the Brugada syndrome. Am J Cardiol. 2011;107(5):709–13. doi: 10.1016/j.amjcard.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malliani A, Pagani M, Montano N, Mela GS. Sympathovagal balance: a reappraisal. Circulation. 1998;98(23):2640–3. [PubMed] [Google Scholar]
- 22.Malliani A, Montano N. Heart rate variability as a clinical tool. Ital Heart J. 2002;3(8):439–45. [PubMed] [Google Scholar]
- 23.Montano N, Porta A, Cogliati C, et al. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev. 2009;33:71–80. doi: 10.1016/j.neubiorev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Viola AU, Brandenberger G, Buchheit M, et al. Sleep as a tool for evaluating autonomic drive to the heart in cardiac transplant patients. Sleep. 2004;27(4):641–7. doi: 10.1093/sleep/27.4.641. [DOI] [PubMed] [Google Scholar]
- 25.Porta A, Guzzetti S, Montano N, et al. Entropy, entropy rate and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans Biomed Eng. 2001;48:1282–1291. doi: 10.1109/10.959324. [DOI] [PubMed] [Google Scholar]
- 26.Viola AU, Tobaldini E, Chellappa SL, Casali KR, Porta A, Montano N. Short-term complexity of cardiac autonomic control during sleep: REM as a potential risk factor for cardiovascular system in aging. PLoS One. 2011;6(4):e19002. doi: 10.1371/journal.pone.0019002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 28.Coats AJS, Shewan LG. Statement on Authorship and Publishing Ethics in the International Journal of Cardiology. Int J Cardiol. 2011;153:239–40. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 29.Porta A, Gnecchi-Ruscone T, Tobaldini E, Guzzetti S, Furlan R, Montano N. Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J Appl Physiol. 2007;103(4):1143–9. doi: 10.1152/japplphysiol.00293.2007. [DOI] [PubMed] [Google Scholar]
- 30.Muller JE, Ludmer PL, Willich SN, et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75(1):131–8. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 31.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987;60(10):801–6. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol. 1996;27(5):1061–70. doi: 10.1016/0735-1097(95)00613-3. [DOI] [PubMed] [Google Scholar]
- 33.Kies P, Wichter T, Schäfers M, et al. Abnormal myocardial presynaptic norepinephrine recycling in patients with Brugada syndrome. Circulation. 2004;110(19):3017–22. doi: 10.1161/01.CIR.0000146920.35020.44. [DOI] [PubMed] [Google Scholar]
- 34.Paul M, Meyborg M, Boknik P, et al. Autonomic Dysfunction in Patients with Brugada Syndrome: Further Biochemical Evidence of Altered Signaling Pathways. Pacing Clin Electrophysiol. 2011;34(9):1147–53. doi: 10.1111/j.1540-8159.2011.03127.x. [DOI] [PubMed] [Google Scholar]
- 35.Pierre B, Babuty D, Poret P, et al. Abnormal nocturnal heart rate variability and QT dynamics in patients with Brugada syndrome. Pacing Clin Electrophysiol. 2007;30(Suppl 1):S188–91. doi: 10.1111/j.1540-8159.2007.00635.x. [DOI] [PubMed] [Google Scholar]
- 36.Mizumaki K, Fujiki A, Tsuneda T, et al. Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2004;15(6):667–73. doi: 10.1046/j.1540-8167.2004.03601.x. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz PJ. Vagal stimulation for heart diseases: from animals to men. - An example of translational cardiology. Circ J. 2010;75(1):20–7. doi: 10.1253/circj.cj-10-1019. [DOI] [PubMed] [Google Scholar]
- 38.Verrier RL, Lau TR, Wallooppillai U, et al. Primary vagally mediated decelerations in heart rate during tonic rapid eye movement sleep in cats. Am J Physiol. 1998;274:R1136–41. doi: 10.1152/ajpregu.1998.274.4.R1136. [DOI] [PubMed] [Google Scholar]
- 39.Guzzetti S, Borroni E, Garbelli PE, et al. Symbolic dynamics of heart rate variability: a probe to investigate cardiac autonomic modulation. Circulation. 2005;112(4):465–70. doi: 10.1161/CIRCULATIONAHA.104.518449. [DOI] [PubMed] [Google Scholar]
- 40.Paton JF, Boscan P, Pickering AE, Nalivaiko E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res Rev. 2005;49(3):555–65. doi: 10.1016/j.brainresrev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Calvin AD, Somers VK. Obstructive sleep apnea and cardiovascular disease. Curr Opin Cardiol. 2009;24(6):516–20. doi: 10.1097/HCO.0b013e328330c2ed. [DOI] [PubMed] [Google Scholar]
- 42.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Legramante JM, Marciani MG, Placidi F, et al. Sleep-related changes in baroreflex sensitivity and cardiovascular autonomic modulation. J Hypertens. 2003;21(8):1555–61. doi: 10.1097/00004872-200308000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Iellamo F, Placidi F, Marciani MG, et al. Baroreflex buffering of sympathetic activation during sleep: evidence from autonomic assessment of sleep macroarchitecture and microarchitecture. Hypertension. 2004;43(4):814–9. doi: 10.1161/01.HYP.0000121364.74439.6a. [DOI] [PubMed] [Google Scholar]
- 45.La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107(4):565–70. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 46.Guzzetti S, La Rovere MT, Pinna GD, et al. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J. 2005;26(4):357–62. doi: 10.1093/eurheartj/ehi067. [DOI] [PubMed] [Google Scholar]
- 47.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98(11):1071–7. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 48.Brugada J, Brugada R, Brugada P. Electrophysiologic testing predicts events in Brugada syndrome patients. Heart Rhythm. 2011;8(10):1595–7. doi: 10.1016/j.hrthm.2011.07.011. [DOI] [PubMed] [Google Scholar]