Summary
Background
Reolysin® is reovirus serotype 3-Dearing strain, a double-stranded replication-competent RNA non-enveloped icosahedral virus. It induces cytopathic and anti-cancer effects in cells with an activated ras pathway due to inhibition of the dsRNA-activated protein kinase.
Methods
This was a single center dose escalation trial of Reolysin administered intravenously every 4 weeks in doses ranging from 1×108 to 3×1010 tissue culture infective dose (TCID)50. Serum for neutralizing antibody, and serum, stool, saliva, and urine for viral shedding were collected. Tumor samples were analyzed for activating mutations in the ras and braf oncogenes.
Results
Eighteen patients received 27 doses of Reolysin in 6 dose cohorts accomplishing a 300 fold dose escalation without a protocol-defined dose limiting toxicity. Drug related grade 2 toxicities included fatigue and fever (1 patient each). All patients developed neutralizing antibody during the course of the study. Viral shedding was observed in 6 patients. One patient with anthracycline and taxane refractory breast cancer experienced a partial response (PR) and her tumor had a ras G12A mutation. Biopsy from her chest wall mass showed evidence of necrosis and viral replication by electron microscopy. Overall clinical benefit (1 PR + 7 stable disease) rate was 45%, and appeared higher in patients with viral shedding (67%) than those without (33%).
Conclusion
Reolysin administered monthly as a one-hour infusion is safe and well-tolerated even in multiple doses. Reolysin has anti-tumor activity as a single agent warranting further evaluation, including in combination with chemotherapy. Viral shedding may suggest intrapatient replication yielding a benefit and should be studied carefully in future studies.
Keywords: Phase I, Clinical trials, Reolysin®, Reovirus, Neutralizing antibody
Introduction
Reolysin® (Oncolytics Biotech Inc., Calgary, Alberta, Canada) is a purified live replication-competent form of the reovirus serotype 3 Dearing strain. REOvirus (Respiratory Enteric Orphan), originally named by Sabin [1], is a 70 nm, naturally occurring, ubiquitous, non-enveloped, icosahedral shaped virus with a genome of 10 segments of double stranded RNA. It belongs to the genus Orthoreovirus, family Reoviridae that consists of six genera of which only three infect humans – rotavirus, orbivirus, and reovirus [2]. Reovirus is the least pathogenic and has 3 distinct serotypes based on the neutralization and hemagglutination inhibition tests [3].
Serology studies estimate that between 50% and 100% of human adults have antibodies to the reovirus suggesting exposure at some point during their life time [4, 5]. Community acquired reovirus infection in humans is usually mild and asymptomatic, and restricted to the upper respiratory and gastrointestinal tracts [4, 6, 7]. The application of reovirus as an anti-cancer drug stems from its predilection for cells with an activated ras signaling pathway. In wild-type ras cells, the presence of viral transcripts leads to autophosphorylation and activation of double-stranded RNA (dsRNA) activated protein kinase (PKR) that in turn phosphorylates eIF2 (eukaryotic translation initiation factor 2) alpha, resulting in a block in viral translation and a suspension of its protein synthesis, subsequently inhibiting viral spread [8, 9]. Ras activated cells inhibit this autophosphorylation of PKR, maintaining it in an inactive state, thereby allowing viral infection and translation to continue unabated, eventually resulting in oncolysis [9]. Reovirus is selectively cytopathic to many human cancer cells in vitro and in metastatic models of human adenocarcinomas in immune competent mice [10]. Similarly, encouraging anti-cancer activity has been observed using xenografts from lymphomas, breast, prostrate, pancreas, brain, ovarian and colon cancers [11–15].
The ras signaling pathway can be activated as a result of a mutated ras or braf proto-oncogene, or by activation of growth factor receptor signaling pathways such as the epidermal growth factor receptor [16]. Ras mutations are present in multiple cancers - 90% of pancreatic cancers, 50% of thyroid cancers, 40% of colon cancers, 30% of non-small cell lung cancer, and 30% of myeloid leukemias thereby making Reolysin an appealing therapeutic agent in these cancers [17–22]. Reolysin has been tested in humans using a direct intratumoral, and intrathecal mode of administration, and has shown evidence for direct and systemic effect. Based on this encouraging pre-clinical and clinical data, we performed a phase I trial to evaluate the effects of increasing doses of systemic intravenous administration of Reolysin in patients with advanced solid tumors delivered once every 4 weeks.
Patients and methods
Patient selection
Eligible patients were adults (age ≥18 years) with an histologically confirmed advanced or metastatic solid malignancy, refractory to standard treatment; with measurable or evaluable disease; Karnofsky’s performance status ≥70%; life expectancy greater than 6 months; and adequate organ function as defined by absolute neutrophil count (ANC) ≥1000/μl, platelet count ≥75,000/μl, hemoglobin ≥9 gm/dl; serum creatinine and bilirubin within 1.5 times the institutional normal limits (INL); ALT and AST≤5 times INL; and adequate cardiac function, defined as ejection fraction >50% by echocardiogram or MUGA scan. Pregnant or nursing women were excluded and patients of childbearing age were required to practice contraception. Patients were required to have at least a 4-week interval from all previous surgery, chemotherapy, and radiotherapy, prior to study initiation. Patients were excluded if they had brain metastases or were on immunosuppressive therapy or with known HIV infection or hepatitis B or C. All patients gave written informed consent approved by the Institutional Review Board at Montefiore Medical Center.
Study design, endpoints and definition of toxicities
This was a single center, open label, single arm standard phase I dose escalation trial without intra-patient dose escalation [23]. The primary endpoint was determination of safety and feasibility of intravenous administration of Reolysin and determination of the maximum tolerated dose (MTD). A minimum of three patients was to receive at least one dose of therapy at each level. Accrual to the next higher dose level was begun after the last patient at the previous level had been observed for 4 weeks after the first dose. For purposes of defining MTD, only cycle 1 toxicities were considered. DLT was defined as any grade 3 or 4 toxicity except grade 3 neutropenia or fever or nausea less than 72 h duration. The initial approval for the study from the United States Food and Drug Administration (USFDA) was for a single dose administration, with requirement for single patient approval for repeat administration. After the first four dose cohorts, the study was amended to include a USFDA approval for multiple doses for the fifth and sixth dose cohorts.
Study drugs and treatment plan
Reolysin was supplied by Oncolytics Biotech Inc. as a translucent to clear, colorless to light blue liquid in vials containing 7.2×1011 tissue culture infective dose (TCID)50 per ml of reovirus in a phosphate-buffered solution and stored at minus 60°C. No prophylactic medications were administered, however, an emergency cart with epinephrine (0.5–1 mL subcutaneous injection 1:1000) and diphenhydramine HCl 50 mg (or equivalent) for intravenous injection was available at the bedside for use in case of an immune-mediated reaction to this agent. The starting dose of Reolysin® was 1×108 TCID50 (cohort 1) and subsequent doses were in half log increments with doses of 3×108 (cohort 2), 1×109 (cohort 3), 3×109 (cohort 4) 1×1010 (cohort 5), and 3×1010 TCID50 (cohort 6). The drug was administered as a 60-minute intravenous infusion every 28 days.
As Reolysin is a live virus, strict precautions were observed. Patients were advised to adhere to the following recommendations after their Reolysin treatment: for the first five days: - stay at home as much as possible; and for 2 weeks: - wear a mask that was provided when around others including family members; avoid close contact (such as kissing), and sexual activity; sleep in a room separate from others; avoid sharing towels and eating utensils; wash hands frequently, especially after using the washroom, blowing the nose or coughing; if a surface, such as a toilet seat, comes in contact with urine or stool, wipe that area clean using chlorine bleach; avoid contact with anyone who may have lowered immunity (children 6 years or younger, elderly, recipient of an organ transplant; patients with AIDS or HIV infection or cancer).
Patient evaluation and follow-up
All patients underwent a complete medical history, physical examination, and performance status evaluation within 2 weeks of entry into the study, and prior to the start of each cycle. Complete blood count with differential, chemistry profile, serum tumor markers, cardiac enzymes, coagulation tests, and urinalysis was performed both pre-and post-dose of Reolysin of each cycle. Vital signs were performed at 30, 60, 90, and 120 min after the end of infusion. EKG was performed at baseline and within two hours after the end of infusion. All tests were repeated on days 3, 7, 10, 14, 21, and 28.
Imaging studies of the chest, abdomen and pelvis for response evaluation (using the RECIST criteria) [24] and cardiac ejection fraction measurements were performed at baseline, and after 1, 3, 5, and 7 cycles. Toxicity assessments were performed on days 1, 3, 7, 10, 14, 21, 28, and graded as per the NCI Common Toxicity Criteria version 2.0 [25]. The CT scans from all responding patients underwent an independent review and a confirmation of response by an independent radiologist prior to this final report of the study.
Virologic studies
Samples of plasma, urine, stool and saliva were screened for extent of viral shedding by evaluation of viral RNA by RT-PCR at baseline, days 1, 7, 14, 21, 28, and at 2 months, at CIRION Biopharma Research (Quebec, Canada). Viral RNA was isolated from serum by using the QIAGEN QIAamp Ultrasens virus kit and from urine, stool and saliva by the QIAGEN viral RNA mini-kit. The integrity of the RNA isolates was confirmed by electrophoresis on a denatured agarose gel for quality of extraction. The RT-PCR was performed with the QIAGEN one step RT-PCR kit. The primers used for this reaction were F: 5′-AATTCGATTTAGGTGACACTATAGCTATTGGTCGGGATG-3′ and R: 5′-CCCTTTTGACAGTGATGCTCCGTTATCACTCG-3′. The assay sensitivity was tested by isolation of viral RNA from the pure viral stock.
Neutralizing antibodies
An antibody-neutralizing assay was used to detect antibody titers at baseline and weekly during the first month and at the 2-month time-point. Reovirus neutralizing antibodies were detected in all the pre-treatment (naïve) serum samples and differed considerably between samples. Therefore, a sample was considered positive (having an in vitro neutralizing potential) if the inhibitory percentage (% viability) of post treatment sample was at least double that of pretreatment (naïve) serum sample at any given dilution. The assay was developed in L929 cells which were cultured and infected with 1500 fold diluted reovirus stock to produce cytopathic effect in 90% of cells and a standard curve was prepared using goat polyclonal serum [26]. Two-fold dilutions of heat inactivated patient serum from baseline and all the time points were mixed with reovirus to determine the NA titer. This validated cell-based assay was developed by CIRION BioPharma Research [27].
Determination of tumor genotype for Ras and Braf mutational status using Pyrosequencing
DNA was extracted from formalin fixed paraffin embedded tissue using the QIAamp DNA FFPE Tissue Kit. Briefly, tissue was scraped off the slides into a 1.5 micro-centrifuge tube, to which 1 ml xylene was added, followed by ethanol extraction. After ensuring complete ethanol evaporation, the pellet was resuspended in buffer ATL followed by addition of proteinase K. This was incubated at 56°C until complete lysis. In addition, the sample was incubated at 90°C in buffer ATL to reverse formaldehyde modification of nucleic acids. This was followed by addition of buffer AL and alcohol. The sample was then washed using buffer AW1, AW2 and finally eluted in buffer ATE. The DNA yield was confirmed and quantified using the Nanodrop N-1000 spectrophotometer.
The DNA was PCR amplified using primers and PCR set up as detailed in Table 1. The pyrosequencing assay was designed to evaluate for mutations in ras codon 12, (nucleotide bases 34 and 35), ras codon 13 (bases 37 and 38), ras codon 61 (bases 182 and 183) and braf codon 600 (base 1799) [17, 28, 29]. Since the DNA under analyses was a mix of tumor and stromal tissue, the presence of a non wild-type base in 5% or greater fraction was considered to represent a mutation in the tumor. This threshold presumes that the mutation is heterozygous and at least 10% of the amplified DNA is tumoral while the rest is stromal tissue.
Table 1.
Primers and PCR set up for pyrosequencing
| Gene | Exon | Codon | Primer | Tm for PCR |
|---|---|---|---|---|
| Ras | Exon 2 | 12 and 13 | F: 5′-GGCCTGCTGAAAATGACTG-3′ R: 5′-(BioTEG) GCTGTATCGTCAAGGCACTCT-3′ S: 5′-CTTGTGGTAGTTGGAGC-3′ |
58 |
| Ras | Exon 3 | 61 | F: 5′-AATTGATGGAGAAACCTGTCTCTT-3′ R: 5′-(BioTEG) TACTGGTCCCTCATTGCACTGTA-3′ S: 5′-ATATTCTCGACACAGCAG-3′. |
65 |
| Braf | Exon 15 | 600 | F: 5′-TGAAGACCTCACAGTAAAAATAGG-3′ R: 5′-(BioTEG) TCCAGACAACTGTTCAAACTGAT-3′ S: 5′-TGATTTTGGTCTAGCTACA-3′ |
60 |
Abbreviations: F forward primer, R reverse primer, S sequencing primer, The PCR reactions were set as follows: 95°C for 5 min; 95°C for 15 s, Tm (° C) for 30 s, 72°C for 15 s, repeat steps 2 through 4 (44 times), 72°C for 5 min, and 4°C forever
Results
Patient characteristics
Eighteen patients with a median age of 57 (range 40–72) years and performance status of 1–2 were enrolled (Table 2). Fourteen (77%) patients were women; 6 (33%) patients had a diagnosis of ovarian and 4 (22%) of colorectal cancer; all patients had received prior therapy (including both biologic and chemotherapy) with a median of 5 (range 2–16) regimens. All patients are evaluable for toxicity and response.
Table 2.
Patient characteristics
| Age | Median (Range) | 57 (40–72) |
| Sex | Female | 14 |
| Male | 4 | |
| Performance Status | 1 | 17 |
| 2 | 1 | |
| Diagnosis | Ovary | 6 |
| Colon | 4 | |
| Breast | 2 | |
| Cervical | 2 | |
| Others* | 4 | |
| # prior chemotherapy regimens | 1–3 | 6 |
| 4–8 | 8 | |
| ≥9 | 4 |
Others: leiomyosarcoma (1), carcinoid (1), prostate (1), non small cell lung cancer (1)
Toxicities
No protocol-defined dose-limiting toxicities were observed. Overall, toxicities were minor, with only 2 patients experiencing grade 2 events (Table 3). One patient at dose level 5 received 7 cycles of Reolysin developed grade 2 fever and grade 1 chills that progressively worsened with repeated administration. She experienced a quicker onset of higher fever (in °C, but not in CTC grade). A second patient at dose level 2 experienced grade 2 fatigue as her worst toxicity. In all, the commonest grade 1 toxicies included fever and chills, myalgias, cold like symptoms, gastrointestinal upset and fatigue. Most were observed within the first week of administration and did not extend into the second or later weeks. None of the toxicities required any dose reductions or delays. They did not require any management as they resolved prompty with time. Dose escalation progressed without any delays or modifications as specified in the protocol. No hematologic toxicities were observed.
Table 3.
Toxicities across all patients across all doses
| Dose Level | 1
|
2
|
3
|
4
|
5
|
6
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCID50 | 1×108
|
3×108
|
1×109
|
3×109
|
1×1010
|
3×1010
|
||||||
| G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | |
| Fever | 2 | 1 | 3 | |||||||||
| Chills | 1 | 1 | 1 | 1 | ||||||||
| Myalgia | 1 | 2 | 3 | 2 | ||||||||
| Headache | 2 | 1 | 1 | 1 | ||||||||
| Sore throat/Nasal fullness | 2 | 1 | 2 | |||||||||
| Fatigue | 2 | 1 | 1 | 2 | 1 | |||||||
| Dehydration | 1 | |||||||||||
| Nausea | 1 | 1 | 1 | 2 | ||||||||
| Vomiting | 1 | 1 | 1 | |||||||||
| Diarrhea | 1 | 3 | 1 | |||||||||
| Constipation | 1 | 1 | ||||||||||
| Bloating | 1 | |||||||||||
| Anorexia | 1 | 1 | 1 | 1 | ||||||||
| Dysgeusia | 1 | |||||||||||
| Skin rash | 1 | |||||||||||
Abbreviations: TCID50 tissue culture infective dose, G1 grade 1, G2 grade 2
Antibody response
All patients developed anti-Reovirus neutralizing antibody during the course of the study (Table 4). The neutralizing antibody titer increased dramatically from baseline in all 18 patients (Fig. 1); however, the time to development varied considerably between the patients (16 patients by day 7, 2 patients by day 28). There was no relationship between neutralizing antibody formation and clinical benefit.
Table 4.
Dosing, mutation status and virology
| Seq # | Dose Level | Diagnosis | # Rx | BR | Mutation Status
|
NA | Viral shedding
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ras (codon)
|
Braf (codon) | Serum | Saliva | Urine | Feces | ||||||||
| 12 | 13 | 61 | 600 | ||||||||||
| 101 | 1 | Ovarian | 1 | SD | WT | WT | WT | WT | D8 | N | N | N | N |
| 102 | 1 | Colon | 1 | PD | WT | WT | WT | WT | D8 | N | N | N | N |
| 103 | 1 | Leio* | 1 | SD | WT | WT | WT | WT | D8 | N | N | N | N |
| 201 | 2 | Colon | 1 | PD | G>V | WT | WT | WT | D8 | N | N | N | N |
| 202 | 2 | Ovarian | 3 | SD | WT | WT | WT | WT | D8 | N | N | N | N |
| 203 | 2 | Carcinoid | 1 | SD | ND | ND | ND | ND | D8 | D1 | D3 | D3 | N |
| 301 | 3 | Cervical | 1 | PD | WT | WT | WT | WT | D5 | N | N | N | N |
| 302 | 3 | Ovarian | 1 | SD | WT | WT | WT | WT | D5 | D3 | N | D3 | N |
| 303 | 3 | Prostate | 1 | PD | G>A | WT | WT | WT | D28 | N | N | N | N |
| 401 | 4 | Ovarian | 1 | PD | WT | WT | WT | WT | D5 | N | N | N | N |
| 402 | 4 | Colon | 1 | PD | WT | WT | WT | WT | D5 | N | N | N | N |
| 403 | 4 | Cervical | 1 | PD | WT | WT | WT | WT | D8 | N | N | N | N |
| 501 | 5 | Breast | 7 | PR | G>A | WT | WT | WT | D14 | N | D149 | N | D64 |
| 502 | 5 | Breast | 1 | PD | G>A | WT | WT | V>E | D8 | N | N | N | N |
| 503 | 5 | Lung | 1 | PD | WT | WT | WT | WT | D8 | D21 | N | D8 | N |
| 601 | 6 | Colon | 1 | PD | WT | WT | WT | WT | D8 | N | N | D14 | N |
| 602 | 6 | Ovarian | 1 | SD | WT | WT | WT | WT | D8 | N | N | N | N |
| 603 | 6 | Ovarian | 2 | SD | G>V | WT | WT | WT | D1 | D1 | D1 | D1 | D8 |
Abbreviations: Leio* leiomyosarcoma, # Rx number of doses, BR best response, SD stable disease, PD progressive disease, PR partial response, NA neutralizing antibody, N not detected, D earliest day of detection, ND not done, WT wild type, G>V glycine>valine (mutation), G>A glycine >alanine (mutation), V>E valine>glutamic acid (mutation)
Fig. 1.
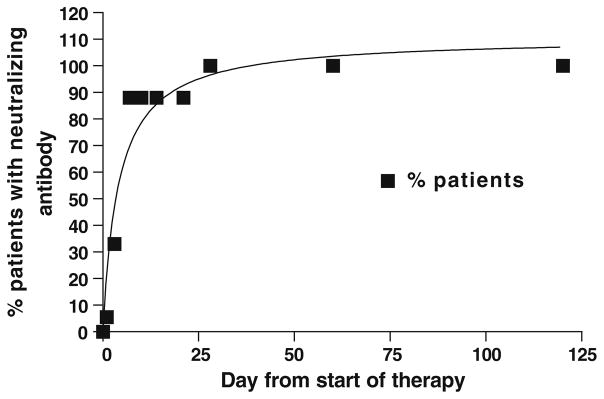
Systemic Immune Reaction: Graph depicts percentage of patients developing neutralizing antibodies as a function of time. While 88% of the patients demonstrated an antibody response within the first week, 2 patients only did so after 4 weeks
Virology and virokinetics
Viral shedding was observed in 6 patients [urine (5), serum (4), saliva (3), and stool (2)] (Table 4). The electron microscopy (EM) images from a patient’s biopsy of a chest wall mass revealed evidence of viral replication and capsids/ghosts in the patient’s tumor (Fig. 2b), reflecting the prolonged interval between the first dose of Reolysin and the biopsy (93 days). Prior animal work has shown that in tumor samples collected after a prolonged dosing interval, the virus lacked intracellular organelles and appeared to have only old remnant capsids (“ghosts”).
Fig. 2.
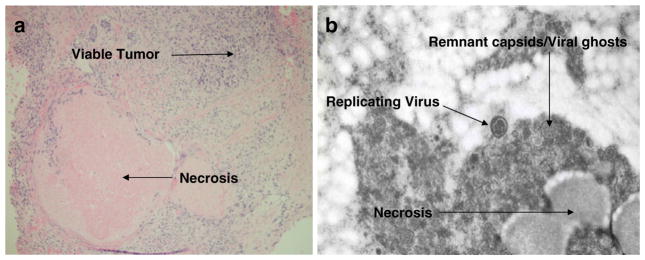
Biopsy taken from a chest wall mass of a 60 year old woman with anthracycline and taxane pre treated breast cancer. The biopsy was taken 93 days after the first dose of Reolysin, (48 h after the third dose). Panel 2a: Pharmacodynamic Effect: Hematoxylin and Eosin stain of the biopsy showing extensive necrosis (also seen in 2b) of the tumor suggestive of anti-tumor activity. Panel 2b: Virokinetics: Electron Microscopy of the same biopsy specimen showing viral replication and remnant capsids/ghosts, typical of findings after prolonged interval between viral exposure and tissue collection, and with evidence of tissue necrosis
Antitumor response
Although response evaluation was not the primary end point of this study, 16 patients were evaluable for objective response by imaging studies. The two patients who were unable to have repeat imaging were deemed to have clinical progression. One patient, a 60 year old woman with anthracycline and taxane refractory breast cancer experienced a partial response with a 34% decrease in tumor burden by RECIST criteria. She demonstrated successive tumor shrinkage at each imaging time interval and achieved a PR after 5 cycles. The response lasted for 9 weeks. Additionally, 7 patients (ovarian-5, carcinoid-1, leiomyosarcoma-1) demonstrated stable disease as the best response. Interestingly, of the 6 patients with ovarian cancer that entered the trial, 5 had stable disease. There did not appear to be a relationship between a higher dose level and higher incidence of clinical benefit.
Pharmacodynamics
Detailed examination of the post treatment biopsy revealed extensive necrosis in the dermis, well demonstrated on the standard hematoxylin and eosin staining (Fig. 2a), and in the EM images (Fig. 2b), consistent with a clinical response in this patient. The necrosis is clearly suggestive of anti-cancer activity.
Mutation screening
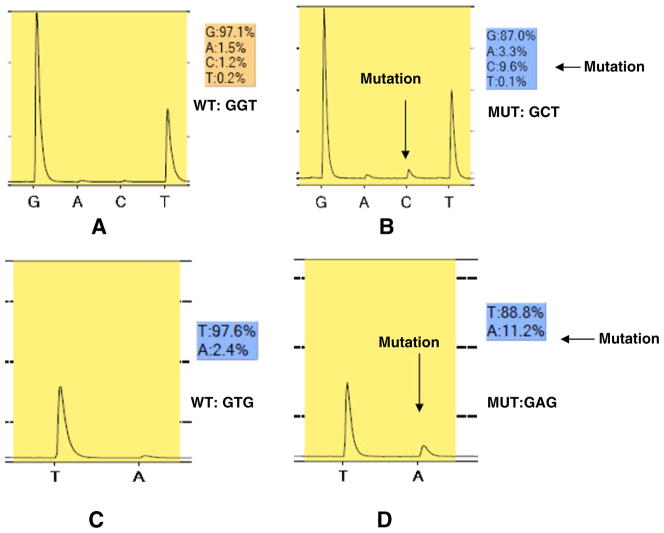
Tumor samples sufficient for DNA extraction were available for 17 of the 18 patients. Of these, 5 were found to have mutation in the ras oncogene, and 1 in the braf oncogene (Table 4). Most interestingly, the only patient who experienced an objective response was found to have a mutation in ras codon 12 [glycine (GGT) to alanine (GCT)] in her tumor. As shown in Fig. 3b, she had a C in codon 12 at 9.6% while the expected level is <5%, confirming the presence of a mutation.
Fig. 3.
Results of mutational analysis of tumors for ras codon 12 and 13 and braf. DNA was extracted from formalin fixed paraffin embedded tissue, PCR amplified, and analyzed by pyrosequencing. Panel a: Sample from patient in first dose cohort, showing the wild type ras sequence, GGT. Panel b: Sample from the patient with breast cancer who experienced a partial response (# 501-Table 4), showing the mutated ras sequence, GCT. Panel c: Sample from patient in first dose cohort, showing the wild type braf sequence, GTG. Panel D: Sample from the patient with breast cancer (# 502-Table 4), showing the mutated braf sequence GAG
Discussion
We report the first human trial of systemic administration of Reolysin in the U.S. as a one-hour intravenous infusion and observed that it is safe and well tolerated at a dose of 3×1010 TCID50 and can be delivered as multiple cycles every 28 days. We were able to achieve a 300-fold increase in the dose through 6 dose levels without much toxicity. Grade 2 toxicities were only experienced by 2 patients, and there was no cumulative or protocol defined dose-limiting toxicity. Once the protocol-specified highest dose of 3×1010 TCID50 was reached, the protocol was terminated. We did not proceed to expand this highest cohort, as there was emerging data from the UK that on a daily times five schedule, a similar dose was safe [30]. It was encouraging to observe one partial response in a heavily pre-treated woman with metastatic breast cancer. She received seven doses of Reolysin and only experienced grade 2 fever as her worst toxicity.
Viral approaches to cancer have been attempted for over half a century with little success [31, 32]. One of the most extensively studied approaches has been with ONYX-015, an attenuated chimeric human group C adenovirus, which preferentially replicates within and lyses tumor cells that are p53 negative [33] and has obtained commercial approval in China (H101) in the treatment of advanced head and neck cancer [34]. Other approaches using virus-mediated oncolysis have included the use of the oncolytic adenovirus ICOVIR-5 as a treatment for malignant gliomas, and PV 701, an attenuated form of the Newcastle virus [35, 36]. Other studies with Reolysin have been performed using both local (intra-prostatic injection) and systemic approaches in Canada and the UK [30, 37].
This study highlights important issues in virus-mediated oncolysis. First, it establishes the safety of systemically administering a live replication-competent purified virus. Previous trials have either demonstrated the safety of systemic administration of replication deficient or attenuated viruses, or the intralesional administration of live replication-competent viruses. Second, it demonstrates that virus administration is safe and well tolerated. The toxicities observed with intravenous injection of Reolysin have been minimal and resemble symptoms of a natural infection with the reovirus, typically a mild flu-like syndrome. Third, it reconfirms that virus therapy can be effective, as demonstrated by the partial response in a heavily pre-treated woman with metastatic breast cancer and clinical benefit observed in seven others on this trial.
The virus was found to be extremely well tolerated with grade 2 toxicities in only 2 patients. All the patients experienced at least one toxicity event; however, none were significant enough to warrant dose delay or reduction or interruption. As expected, a majority of the toxicities resembled the symptoms of a natural infection by the reovirus; mild flu-like symptoms that resolved within the first week after virus administration. The toxicities did not increase with increasing dose of the virus.
Further, mutational analysis of the tumor in the woman with a partial response revealed a ras mutation in codon 12, in further support of the hypothesis that ras transformed cells are susceptible to reovirus mediated oncolysis. While ras mutations are rare in breast cancer [38], in vitro studies have demonstrated activity when reovirus has been used to treat breast cancer cell lines [11, 15]. Consistent with the ras mutation, this patient’s tumor was negative for her-2 gene amplification. Since her-2 overexpression drives the EGFR pathway and thereby serves the same function as a constitutively active ras (i.e., activating mutation); patients with this category of breast cancer are potential beneficiaries of Reolysin-based anti-cancer therapy. Further investigation of Reolysin in her-2 overexpressing, trastuzumab (anti her-2 monoclonal antibody) resistant breast cancer is warranted.
It was encouraging to observe a partial response and stable disease in 7 patients. As is common in phase I trials of systemic administration of investigational drugs, all patients had been heavily pre-treated (80% had ≥3 prior regimens). In contrast to the recently reported trial of multiday dosing of Reolysin that reported that only 4 of the 30 (13%) tested patients had viral shedding [30], we found that 6 of the 18 (33%) patients were shedding virus, possibly related to the use of a more sensitive assay. Moreover, patients with viral shedding had a higher clinical benefit (66% versus 33%) than the patients who did not, and is deserving of further study. This may suggest viral shedding to be a marker of viral replication, and patients whose internal milieu supports replication are more likely to have a higher viral load leading to a better oncolytic effect. However, similar to the other study, we found an almost universal development of anti reovirus NA. While development of the NA may raise concern of development of resistance; the presence of viral capsids in the tumor biopsy 13 weeks after initial dose and the documentation of PR in this patient and SD in 7 patients suggest that clinical resistance is unlikely.
We observed mutations in 5 of the 17 analyzed tumor samples with a breast cancer being mutant in ras and braf. With only one patient who experienced a PR and another 7 with SD, we were not able to clearly demonstrate an association between ras/braf mutations, and susceptibility of the cancer to the oncolytic effects of Reolysin (Fisher exact test, p=0.4). We would however suggest that this association be tested in larger cohorts and less pre-treated patients. Other possible reason for this lack of correlation between kras/braf mutations and clinical benefit is that all the patients were heavily pre-treated and are not the best candidates to test efficacy. While PKR/ras/braf system with viral replication permissiveness plays a key role in the anti-cancer activity of Reolysin, it is likely that it is far more complex and the exact mechanism is currently being actively investigated. While active as a single agent, Reolysin is probably best combined with systemic chemotherapy. Synergistic activity has been reported with gemcitabine in both in vitro and in vivo models using HCT 116 colon cancer cells [39].
In summary, these results clearly demonstrate that Reolysin is safe and effective in patients with chemotherapy refractory malignancies and the systemically administered mode of administration of this live replication-competent virus is safe over a period of multiple doses. The clinical safety data as a single agent and the synergy data from the pre-clinical models has led to further studies of Reolysin as a single agent (melanoma, ovarian cancer, and bone and soft tissue sarcoma) and in combination with cytotoxic chemotherapy (non small cell lung cancer, and advanced head and neck cancer) and the results are eagerly awaited.
Acknowledgments
This study was supported by a grant from Oncolytics Biotech Inc., Calgary, Alberta, Canada.
Footnotes
Presented in part at the 36th Annual Meeting of the American Society of Clinical Oncology, Chicago, 2007.
This study was subject to a US FDA audit in Sep 2006 without issue of form 483.
Contributor Information
Radharani Gollamudi, Department of Oncology, Montefiore Medical Center, Bronx, NY, USA.
Mohammad H. Ghalib, Department of Oncology, Montefiore Medical Center, Bronx, NY, USA
Kavita K. Desai, Department of Oncology, Montefiore Medical Center, Bronx, NY, USA
Imran Chaudhary, Department of Oncology, Montefiore Medical Center, Bronx, NY, USA.
Benny Wong, Department of Oncology, Montefiore Medical Center, Bronx, NY, USA.
Mark Einstein, Albert Einstein College of Medicine and Cancer Center, 1825 Eastchester Road, Bronx, NY 10461, USA. Division of Gynecologic Oncology, Department of Obstetrics & Gynecology and Women’s Health, Montefiore Medical Center, Bronx, NY, USA.
Matthew Coffey, Oncolytics Biotech Inc., Calgary, Alberta, Canada.
George M. Gill, Oncolytics Biotech Inc., Calgary, Alberta, Canada
Karl Mettinger, Oncolytics Biotech Inc., Calgary, Alberta, Canada.
John M. Mariadason, Department of Oncology, Montefiore Medical Center, Bronx, NY, USA. Albert Einstein College of Medicine and Cancer Center, 1825 Eastchester Road, Bronx, NY 10461, USA
Sridhar Mani, Department of Oncology, Montefiore Medical Center, Bronx, NY, USA. Albert Einstein College of Medicine and Cancer Center, 1825 Eastchester Road, Bronx, NY 10461, USA.
Sanjay Goel, Email: sgoel@montefiore.org, Department of Oncology, Montefiore Medical Center, Bronx, NY, USA. Albert Einstein College of Medicine and Cancer Center, 1825 Eastchester Road, Bronx, NY 10461, USA.
References
- 1.Sabin AB. Reovirus: a new group of respiratory and enteric viruses formerly classified as ECHO type 10 is described. Science. 1959;130:1387–1389. doi: 10.1126/science.130.3386.1387. [DOI] [PubMed] [Google Scholar]
- 2.Tyler KL, Fields BN. Reovirus. In: Fields BN, Knipe DM, Chanock RM, editors. Virology. Raven; New York: 1990. pp. 1307–1328. [Google Scholar]
- 3.Rosen L. Serologic grouping of reoviruses by hemagglutination-inhibition. Am J Hyg. 1960;71:242–249. doi: 10.1093/oxfordjournals.aje.a120107. [DOI] [PubMed] [Google Scholar]
- 4.Jackson GG, Muldoon RL. Viruses causing common respiratory infection in man. IV. Reoviruses and adenoviruses. J Infect Dis. 1973;128:811–866. doi: 10.1093/infdis/128.6.811. [DOI] [PubMed] [Google Scholar]
- 5.Selb B, Weber B. A study of human reovirus IgG and IgA antibodies by ELISA and western blot. J Virol Methods. 1994;47:15–25. doi: 10.1016/0166-0934(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 6.Rosen L, Evans HE, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- 7.Jackson GG, Muldoon RL, Cooper GS. Reovirus type 1 as an etiologic agent of the common cold. J Clin Invest. 1961;40:1051. meeting abstract. [Google Scholar]
- 8.Coffey MC, Strong JC, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 9.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. Embo J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirasawa K, Nishakawa SG, Normal KL, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–353. [PubMed] [Google Scholar]
- 11.Norman KL, Coffey MC, Hirasawa K, et al. Reovirus oncolysis of human breast cancer. Human gene therapy. 2002;13:641–652. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–1701. [PubMed] [Google Scholar]
- 13.Alain T, Hirasawa K, Pon KJ, et al. Reovirus therapy of lymphoid malignancies. Blood. 2002;100:4146–4153. doi: 10.1182/blood-2002-02-0503. [DOI] [PubMed] [Google Scholar]
- 14.Etoh T, Himeno Y, Matsumoto T, et al. Oncolytic viral therapy for human pancreatic cancer cells by reovirus. Clin Cancer Res. 2003;9:1218–1223. [PubMed] [Google Scholar]
- 15.Yang WQ, Senger DL, Lun XQ, et al. Reovirus as an experimental therapeutic for brain and leptomeningeal metastases from breast cancer. Gene therapy. 2004;11:1579–1589. doi: 10.1038/sj.gt.3302319. [DOI] [PubMed] [Google Scholar]
- 16.Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW. Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Scie USA. 2004;101:11099–11104. doi: 10.1073/pnas.0404310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 18.Bos JL, Fearon ER, Hamilton SR, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 19.Lemoine NR, Mayall ES, Wyllie FS, et al. Activated ras oncogenes in human thyroid cancers. Cancer Res. 1988;48:4459–4463. [PubMed] [Google Scholar]
- 20.Needelman SW, Kraus MH, Srivastava SK, Levine PH, Aaronson SA. High frequency of N-ras activation in acute myelogenous leukemia. Blood. 1986;67:753–757. [PubMed] [Google Scholar]
- 21.Rodenhuis S, Slebos RJ, Boot AJ, et al. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48:5738–5741. [PubMed] [Google Scholar]
- 22.Grunewald K, Lyons J, Frohlich A, et al. High frequency of Ki-ras codon 1w mutations in pancreatic adenocarcinomas. Int J Cancer. 1989;43:1037–1041. doi: 10.1002/ijc.2910430614. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase I clinical trial design in cancer drug development. J Clin Oncol. 2000;18:684–692. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25. [accessed Jun 3, 2009]; ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.
- 26.White CL, Twiggen KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–920. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 27. [Accessed Jun 3, 2009]; http://www.cirion.com/
- 28. [accessed Jun 3, 2009]; www.pyrosequencing.com.
- 29.Ogino S, Kawasaki T, Brahmandam M, et al. Sensitive sequencing method for KRAS mutation detection by pyrosequencing. J Mol Diagn. 2005;7:413–421. doi: 10.1016/S1525-1578(10)60571-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 dearing in patients with advanced cancer. Clin Can Res. 2008;14:7127–7137. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 31.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 32.Csatary LK. Viruses in the treatment of cancer. Lancet. 1971;2:825. doi: 10.1016/s0140-6736(71)92788-7. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 34.Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 35.Alonso MM, Gomez-Manzano C, et al. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 2007;14:756–761. doi: 10.1038/sj.cgt.7701067. [DOI] [PubMed] [Google Scholar]
- 36.Pecora AL, Rizvi N, Cohen GI, et al. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid tumors. J Clin Oncol. 2002;20:2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Reolysin Investigator Brochure. Oncolytics Biotech Inc; Data on File. Release Date January 31, 2009. [Google Scholar]
- 38.Rochlitz CF, Scott GK, Dodson JM, et al. Incidence of activating ras oncogene mutations associated with primary and metastatic human breast cancer. Cancer Res. 1989;49:357–360. [PubMed] [Google Scholar]
- 39.Lane ME, Fahey JM, Besanceney C, et al. In vivo synergy between oncolytic reovirus and gemcitabine in ras-mutated human HCT116 xenografts. AACR Meeting Abstracts; Apr 2007; 2007. p. 4812. [Google Scholar]