Abstract
Purpose
The overall goal of this project is to enhance ocular delivery of ∆9-Tetrahydrocannabinol (THC) through the topical route.
Methods
Solubility, stability and in vitro transcorneal permeability of the relatively hydrophilic hemiglutarate ester derivative, THC-HG, was studied in the presence of surfactants. The solutions were characterized with respect to micelle size, zeta potential and solution viscosity. In vivo studies were carried out in New Zealand albino rabbits. A previously reported promising THC-HG ion-pair formulation was also studied in vivo.
Results
Aqueous solubility and stability and in vitro transcorneal permeability of THCHG was enhanced significantly in the presence of surfactants. THC levels in the ocular tissues (except cornea) were found to be below detection limits from mineral oil, surfactant or emulsion based formulations containing THC. In contrast, micellar and ion pair based THC-HG formulations produced significantly higher total THC concentrations in the anterior ocular chamber.
Conclusion
In this study, although delivery of THC to the anterior chamber ocular tissues could be significantly increased through the prodrug and formulation approaches tested, further studies are needed to increase penetration to the back-of-the eye.
INTRODUCTION
The visual information in the eye is collected in the retina and transmitted to the brain via the optic nerve. The amacrine cells and the bipolar cells collect the visual information from the rods and the cones in the retina and pass it to the retinal ganglion cells (RGC). The RGC then transmits the visual information from the retina to the brain. Glaucoma is an ocular disease characterized by progressive and irreversible neurodegeneration of the RGC leading to visual field loss(1, 2).
A number of hypotheses have been presented on what triggers RGC cell apoptosis but the exact mechanism is as yet unknown(3). An increase in intraocular pressure (IOP) is a significant risk factor in the development and progression of the disease(4). Thus, achieving a reduction in IOP has been the mainstay for glaucoma therapy(5). The visual field loss, however, continues even after significant IOP reduction(6, 7). Protection of the optic nerve by a mechanism independent of IOP reduction would greatly benefit the treatment and management of glaucoma(8). A number of mechanisms such as excessive glutamate excitotoxicity, oxidative stress, mitochondrial dysfunction, protein misfolding and neurotropin withdrawal have been identified as plausible factors for RGC apoptosis(3). Memantine, a NMDA receptor blocker, the only drug, to our knowledge, that has been studied as a neuroprotectant for glaucoma in phase 3 human clinical trials, failed to meet clinical endpoints(9, 10).
∆9-Tetrahydrocannabinol (THC) is the primary active ingredient of the plant Cannabis Sativa. It has shown promise in the treatment of glaucoma due to its IOP lowering and neuroprotective effects (11, 12). THC may have an advantage over current antiglaucoma agents in that in addition to its IOP lowering activity it could act as a neuroprotectant through independent mechanisms (13, 14). THC is a partial agonist of both CB1 and CB2 receptors present in the eye and these might be involved in its IOP lowering and neuroprotective activity (15, 16). CB1 receptors have been found to be present and functionally active in the iris-ciliary processes and the trabecular meshwork and retina (17, 18) while CB2 receptors are present in the trabecular meshwork and retina(15, 19). Blocking of cannabinoid receptors, however, failed to completely abolish the neuroprotective activity of THC indicating multiple mechanisms might be involved (12, 20, 21). Evidence now suggests that PPARγ could be an additional target for THC(22). PPARγ have been found to be expressed and functionally active in the retina(23). Additionally, THC could also induce vasorelaxation of retinal arteries as well as cause a reduction in ROS activity.
Literature demonstrates that THC has failed to exhibit consistent IOP lowering activity following topical application(24, 25). The observed inconsistency could be due to poor and variable delivery of THC to the target ocular tissues. Formulation of THC as an ophthalmic solution is especially challenging due to its low aqueous solubility (1-2 μg/mL) and high lipophilicity (logP 6.4). Thus, most of the earlier in vivo studies investigating the effect of THC through the topical route used mineral oil or emulsion systems as the vehicle.
In order to improve the physicochemical properties of THC, the relatively water soluble hemiglutarate ester prodrug (THC-HG) was synthesized. The goal of this project was to study the effect of surfactants on the solubility, stability, in vitro permeability and in vivo bioavailability of THC-HG. Effect of including benzalkonium chloride (BAK), a preservative, and ethylenediaminetetraaceticacid (EDTA), a preservative aid, on the in vitro transcorneal permeability was also studied.
In a previous study, we reported the effect of ion pair formation of THC-HG with tromethamine and l-arginine on in vitro transcorneal permeability (13). Both l-arginine and tromethamine significantly improved transcorneal THC delivery. Thus, the ion-pair formulation was also included in this study to compare its delivery in vivo with that of the surfactant formulations. Since l-arginine is a biologically active molecule, tromethamine was selected as the preferred ion pairing agent.
Previous studies exploring the utility of topical THC application in glaucoma, in preclinical and clinical studies, have used light mineral oil as the vehicle (25-27). Some studies have also used a submicron emulsion to deliver THC topically (26). Considering that the positive (27-29) and negative(25, 30, 31) reports in the literature with respect to the IOP lowering characteristics of topical THC could be due to inefficient ocular delivery from such formulations, in the current study we examined the THC levels achieved in the ocular tissues from these formulations also. This investigation thus provides a comparison between the in vivo bioavailability of THC formulated in light mineral oil/emulsion/surfactant formulations and THC-HG formulated in micellar or ion-pair solutions.
METHODS
Materials
Super-refined polysorbate 80 (Croda Inc., Mill Hall, PA). Cremophor RH 40, poloxamer 188 and poloxamer 407 (BASF, Chattanoga, TN) and lipoid E 80 (Lipoid, Ludwigshafen, Germany) were gift samples. Propofol, sigmacote, hydroxypropyl beta cyclodextrin hydroxypropyl methyl cellulose (4000 cps) and tyloxapol were purchased from Sigma (St. Louis, MO). All other chemicals were purchased from Fisher Scientific (St. Louis, MO). Solvents used for analysis were of HPLC grade. Plastic tubes coated with Sigmacote® (St. Louis, MO) or borosilicate glass vials were used for the experiments since THC binds to plastic.
Animal Tissues
Whole eye globes of New Zealand Albino rabbits were purchased from Pel Freez Biologicals (Rogers, AK). Eyes were shipped overnight in Hanks Balanced Salt Solution over wet ice. Corneas were isolated from the whole eye globes and used immediately on receipt. We have previously reported that active and passive transport processes in corneas obtained from ocular globes stored in Hanks Balanced Salt Solution are equivalent to that of freshly excised rabbit corneas(32).
Animals
Male New Zealand White Albino Rabbits were procured from Harlan Labs (Indianapolis, IN). Animal experiments conformed to the tenets of the Association for Research in Vision and Ophthalmology statement on the Use of Animals in Ophthalmic and Vision Research and followed the University of Mississippi Institutional Animal Care and Use committee approved protocols.
Mineral Oil and Emulsion Formulations containing THC
An accurately weighed amount of THC was dissolved in light mineral oil, NF, to prepare the mineral oil based formulation. Emulsion formulations were prepared according to previously published protocols(33, 34). Instead of crude phospholipids, however, Lipoid E 80 was used. The emulsion formulation consisted of super refined soybean oil (14 % w/v), oleic acid (6% w/v), glycerin (2.25% w/v), poloxamer 188 (2% w/v), Δ9-tetrahydrocannabinol (1 % w/v), lipoid E 80 (1 % w/v), α-tocopherol (0.02% w/v) and deionized water to prepare 20 mL. Briefly, THC, α-tocopherol and oleic acid were added to super refined soyabean oil. Poloxamer and glycerin were added to deionized water. Lipoid E 80 was dispersed in the aqueous phase. Both phases were heated to 70 °C. The aqueous phase was added to the oily phase and a coarse emulsion was formed by using a high speed homogenizer, Ultra Turrax T25 (IKA®, Wilmington, NC) at 24000 rpm for 5 minutes. The coarse emulsion was then passed through a high pressure homogenizer Emulsiflex C5 (Avestin®, Ottawa, Canada) at 16000 psi for five cycles. The pH of the final emulsion was adjusted to pH 7.4 using 1% w/v sodium hydroxide and it was filtered through a 0.45 μM membrane filter. The drug loading in the final emulsion was determined by HPLC analysis.
Preparation of THC-HG
THC-HG was synthesized as per previously reported procedures(35).
THC-HG Ion pair formulation
The formulation was prepared as described in our earlier report(13). Briefly, THC-HG (22 mg) was taken from a stock solution (in hexane) in a glass vial and the solvent was evaporated under a stream of nitrogen gas. Ten milliliter of a 8 mM tromethamine solution in isotonic phosphate buffered saline (IPBS) was added to the glass vial and sonicated for 10 minutes. Hydroxy propyl methyl cellulose (HPMC) (0.5% w/v) was added to the formulation and stirred for one hour.
Solubility of THC-HG in Surfactants
Excess THC-HG was taken (stock solution 50 mg/mL of THC-HG in hexane) in a glass vial. Hexane was evaporated under a stream of nitrogen gas. IPBS containing various types and concentrations of surfactants was then added to the glass vials and sonicated for 10 mins. The vials were then placed in a reciprocating water bath (100 shakes per minute) at 25 °C for a period of 24 hours. The solution was subsequently transferred to silicone coated plastic microcentrifuge tubes and centrifuged at 16,000 g for 60 mins (Fisher Scientific acuSpin 17R) at 25 °C. The supernatant was diluted in mobile phase and analyzed for THC-HG by HPLC method described for in vitro samples.
Osmolality Determination
Osmolality of the final solutions was measured using Osmette S (model 4002, Precision Systems Inc., Natick, MA) using the freezing point depression method. The instrument was calibrated using 100 mOsm and 500 mOsm standards.
Determination of Micellar Particle Size, Zeta Potential and Viscosity
Micellar solutions were prepared as described under the solubility studies section. Blank vehicles (no drug) were also evaluated. A dynamic light scattering instrument, Zetasizer Nano ZS (Malvern Instruments Inc., Westbrough, MA) was used for measurements at 25 °C. The Mavern zetasizer is a dynamic light scattering instrument that determines particle size by measuring the rate of intensity fluctuation of scattered light. It also determines the hydrodynamic radius from the Strokes-Einstein equation (Equation 1) by measuring the diffusion co-efficient. A high concentration zeta cell was used to measure micelle size (D90, intensity average) and zeta potential of the micelles.
| (Equation 1) |
Rh – Hydrodynamic radius
D - Translational diffusion co-efficient
K - Boltzmann’s constant
T - Absolute temperature
η - Viscosity
Viscosity measurements were carried out at 25 °C (temperature was maintained using a circulating water bath) using a Brookfield DV II + Pro (Brookfield Engineering, Middleboro, MA) cone and plate viscometer. Rheocalc was used as the data acquisition software. All measurements were carried out in triplicate.
Stability of THC-HG in the Micellar Solutions
THC-HG loaded micellar solutions were prepared as described under the solubility studies section. Stability studies were carried out at 4 °C, 25 °C and 40 °C in glass vials for a period of two months. Aliquots taken at predetermined time points were diluted in mobile phase and THC-HG concentration was determined using HPLC method described for in vitro samples. Apparent degradation rate constants were calculated from the slope of a logarithmic percentage drug remaining vs time plot.
Corneal Permeability Studies
Corneas were extracted from whole ocular globes by making an incision just below the corneal scleral limbus and cutting radially around sclera. It was then mounted between side by side permeation cells (Permgear Inc.). Temperature was maintained at 34 °C (ocular surface temperature) by circulating water through the jacket of the permeation cells. Donor solutions (3 mL), prepared as described under solubility studies, was added to the epithelial side (donor cell) and 3.2 mL of 2.5% hydroxypropyl beta cyclodextrin solution in IPBS was added to the endothelial side (receiver side). The difference in volumes between the donor and the receiver chamber helped maintain the natural curvature of the cornea. Aliquots (0.6 mL) were withdrawn from the receiver side every 30 min and immediately replaced with an equal volume of fresh hydroxypropyl beta cyclodextrin (2.5%) solution in IPBS. Samples were taken for HPLC analysis and analyzed for THC-HG and THC. All of the THC-HG permeated was calculated in terms of THC for permeability calculations.
In Vivo Bioavailability Studies
In vivo bioavailability of THC from the mineral oil/emulsion/micellar formulations, and also total THC from the prodrug (THC-HG) loaded ion pair and micellar formulations, were determined in Male New Zealand albino rabbits weighing between 2-2.5 Kg. Rabbits were anesthetized using a combination of ketamine (35 mg/kg) and xylazine (3.5 mg/kg) injected intramuscularly and maintained under anesthesia throughout the experiment. Topical formulations were prepared as described under the solubility section. Fifty microliters of the formulations were placed in the cul de sac of the right eye of the anesthetized rabbits. At the end of one hour, or three hours in some cases, after topical application, the rabbits were euthanized by an overdose of pentobarbital injected through the marginal ear vein. The eye was washed with ice cold IPBS and immediately enucleated and washed again. The ocular tissues were separated, weighed and placed at -80 °C until further analysis. All formulations were dosed in three animals each.
Analytical Procedure for Solubility and In Vitro Samples
A Waters HPLC system comprising of 600 E pump controller, 717 plus autosampler and 2487 UV detector was used. Data handling was carried out using an Agilent 3395 integrator. THC and THC-HG stock solutions were prepared in hexane and stored at -15 °C. A 85:15 mixture of methanol and 0.84% glacial acetic acid was used as the mobile phase with a Phenomenex Luna PFP(2) 4.6 x 250 mm column at a flow rate of 1.2 mL/min. Detection was carried out at 226 nM. Retention time for THC and THC-HG was 11.3 min and 14.6 min, respectively.
Bioanalytical Method
A HPLC-Fluorescence method was used to determine tissue drug concentration since HPLC-UV method used for analyzing in vitro samples had many protein peaks co -eluting with THC and THC-HG. THC-HG was not observed to be fluorescent but THC is a fluorescent molecule. THC-HG was hydrolyzed to THC under alkaline conditions and total THC was determined. The hydrolysis protocol was validated to ensure complete conversion of the prodrug to THC. Separate studies have established that THC-HG is rapidly converted to THC in the ocular tissue matrix (13). Nevertheless, the additional deconjugation steps were employed to ensure conversion of any intact THC-HG into THC.
Stock solutions of THC, THC-HG and internal standard (propofol) were prepared in acetonitrile. THC/THC-HG was spiked in blank ocular tissues and allowed to stand for 15 minutes before protein precipitation procedure. Standard curves were prepared for THC/THC-HG in aqueous humor (10 ng-200 ng), vitreous humor (20 ng-200 ng), cornea (20ng-200ng), iris ciliary body (10 ng-200 ng), retina choroid (10 ng-200 ng) and sclera (20 ng-200 ng). For THC-HG the standard curve was plotted in terms of total calculated THC. Sample preparation for the various ocular tissues is described as follows.
Aqueous and vitreous humor sample preparation
To 100 μL of aqueous humor or 400 μL of vitreous humor 20 μL of drug (10-200 ng) and 20 μL of internal standard (200 ng) prepared in acetonitrile was added. To the aqueous humor and vitreous humor samples 50 μL and 100 μL respectively of 1N sodium hydroxide was added, and placed at 25 °C. At the end of two hours, 50 and 100 μL of 1N HCl (to neutralize the sodium hydroxide) and 200 μL and 400 μL of ice cold acetonitrile (to precipitate the proteins) was added to the aqueous humor and vitreous humor samples, respectively. All samples were centrifuged at 16,000 g and taken for analysis.
Preparation of other ocular tissue samples
To a weighed amount of the cornea/iris ciliary bodies/RPE choroid or sclera, 20 μL of drug (10-200 ng) and 20 μL of internal standard (200 ng) in acetonitrile was added followed by 600 μL of ice cold acetonitrile and 100 μL of 1 N NaOH. The samples were placed at 25 °C and at end of two hours 1N HCl was added. The samples were centrifuged at 16000 g for 30 min and taken for analysis.
A previously published analytical method using fluorescence detection was modified and used(36). For HPLC analysis, a Phenomenex Luna PFP(2) 4.6 x 250 mm column was used. The mobile phase consisted of 70% Acetonitrile:30% water containing 5.05% v/v of o-phosphoric acid at a flow rate of 1 mL/min. A Waters 2475 detector was set at an excitation wavelength of 220 nm and THC was detected at emission wavelength of 305 nm. EUFS was set at 150 and gain was set at 50. Injection volume was 50 μL. Retention time for propofol and THC were 6.9 min and 11.9 min, respectively.
All standard curves generated had R2 values greater than 0.98. Recovery value of THC from the ocular tissues was determined at three concentrations (low, medium and high). Recovery of THC from the various ocular tissues was calculated using Equation 2.
| (Equation 2) |
Average recovery values were determined in cornea (99.4 %), aqueous humor (93.2 %), vitreous humor (91.2%), iris ciliary body (93.2%), retina (102%) and sclera (91.6%). The detection limit for THC in various ocular tissues is as follows, aqueous humor (5 ng/100 μL), vitreous humor (25 ng/mL), cornea (10 ng/50 mg), iris ciliary body (5 ng/50 mg), retina choroid (5 ng/50 mg) and sclera (10 ng/250 mg).
Data Analysis
All experiments were carried out at least in triplicate. Flux was obtained from a linear regression analysis of the cumulative amount of THC-HG in the receiver chamber versus time. Permeability was calculated by normalizing flux values to donor concentrations.
Unpaired student’s t-test was used to compare between two groups. Statistical analysis between multiple groups was carried out by one way analysis of variance. Levenes test was used to find out variation between the groups. Tukey’s Honestly Significant test was used to differentiate between the groups. Wilcoxon test was used for statistical analysis between groups of unequal variances followed by Dunns method for joint ranking to detect difference between the groups. p < 0.05 was considered to be statistically significant.
RESULTS
Solubility in Surfactant Solutions
Aqueous solubility of THC-HG was significantly improved (Fig. 2). At 0.1% w/v, Polysorbate 80 led to the greatest increase in solubility (36-fold, 678.2 ± 23.4 μg/mL) followed by Poloxamer 407 (29-fold, 507.4 ± 6.6 μg/mL), Tyloxapol (24-fold, 467.6 ± 27.9 μg/mL) and Cremophor RH 40 (22-fold, 424.9 ± 60.5 μg/mL) respectively. Poloxamer 188 led to only a 6-fold improvement in solubility and was thus not studied any further. Solubility of THC-HG was further studied at higher concentrations of Cremophor RH 40 and Poloxamer 407 (maximum FDA approved concentrations for ophthalmic formulations). Solubility of THC-HG in 0.25% w/v and 0.5% w/v of Cremophor RH 40 was found to be 890.7 ± 166.2 μg/mL (47 fold increase) and 2136.2 ± 112.7 μg/mL (113 fold increase), respectively. Solubility of THC-HG at 0.16% Poloxamer 407 was found to be 621.9 ± 77.1 μg/mL (33 fold increase).
Figure 2.
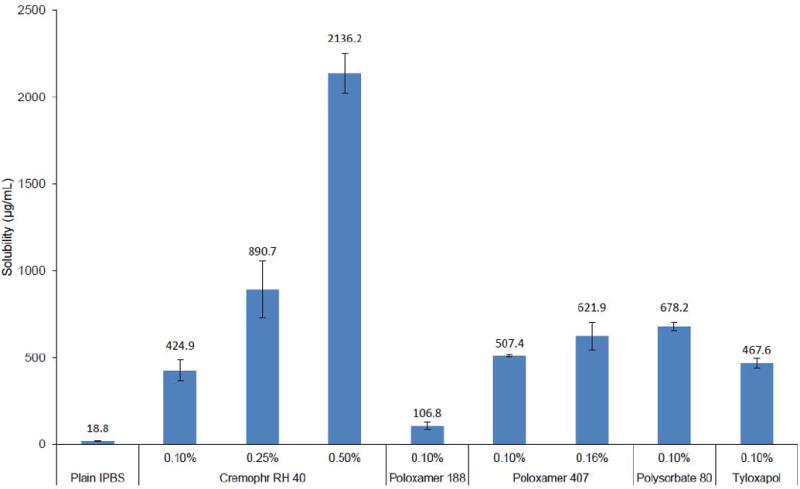
Solubility of THC-HG in Cremophor RH 40, Poloxamer 188, Poloxamer 407, Polysorbate 80 and Tyloxapol in IPBS at 25 °C. Results are depicted as mean ± SD (n=3).
Osmolality of THC-HG Formulations
Inclusion of surfactants and THC / THC-HG in the formulations did not change the osmolality significantly compared to IPBS at the concentrations studied (Table I).
Table I.
Osmolality of THC-HG in Cremophor RH 40, Poloxamer 188, Poloxamer 407, Polysorbate 80 and Tyloxapol in IPBS at 25 °C. Results are depicted as mean ± SD (n=3).
| Excipient | Concentration (% w/v) | Osmolality (mOsm) |
|---|---|---|
| Plain IPBS | - | 281.6 ± 1.7 |
|
| ||
| Cremophor RH 40 | 0.1 | 282.6 ± 0.5 |
|
| ||
| 0.25 | 284.3 ± 0.5 | |
|
| ||
| 0.5 | 292.3 ± 1.2 | |
|
| ||
| Poloxamer 188 | 0.1 | 282 ± 2.2 |
|
| ||
| Poloxamer 407 | 0.1 | 282.9 ± 1.2 |
|
| ||
| 0.16 | 283.6 ± 0.5 | |
|
| ||
| Polysorbate 80 | 0.1 | 282.6 ± 0.5 |
|
| ||
| Tyloxapol | 0.1 | 283 ± 0.8 |
Particle Size and Viscosity Measurements
The hydrodynamic radius, D90 and zeta potential of the blank and THC-HG loaded micellar formulations are presented in Table II.
Table II.
Hydrodynamic radius, D90 and zeta potential of blank and THC-HG loaded (DL) micelles prepared in IPBS at 25 °C. Results are depicted as mean ± SD (n=3).
| Surfactant | Concentration (% w/v) | Hydrodynamic radius (nm) | D90 (nm) | PDI | Zeta potential (mV) | Viscosity (Cp) | |
|---|---|---|---|---|---|---|---|
| Cremophor RH 40 | 0.1 | Blank | 9.4 | 41.8 ± 3.7 | 0.259 | 1 | 1.2 ± 0.01 |
| DL | 16.4 | 68.4 ± 6.9 | 0.220 | -6.4 | 1.26 ± 0.01 | ||
| 0.25 | Blank | 7.9 | 27.5 ± 0.6 | 0.135 | 0.4 | 1.23 ± 0.03 | |
| DL | 11.3 | 41.2 ± 1.6 | 0.164 | -6.4 | 1.25 ± 0.01 | ||
| 0.5 | Blank | 7.6 | 27.2 ± 0.8 | 0.081 | -1.2 | 1.25 ± 0.02 | |
| DL | 14 | 69.3 ± 3.1 | 0.322 | -6.6 | 1.28 ± 0.02 | ||
| Poloxamer 407 | 0.16 | Blank | 8.1 | 30.7 ± 2.9 | 0.162 | -2.3 | 1.22 ± 0.02 |
| DL | 15.9 | 61.7 ± 7.4 | 0.264 | -5.5 | 1.24 ± 0.01 | ||
| Polysorbate 80 | 0.1 | Blank | 11.1 | 36.6 ± 6 | 0.152 | -1.4 | 1.22 ± 0.01 |
| DL | ND* | ND* | ND* | -11 | 1.26 ± 0.01 | ||
| Tyloxapol | 0.1 | Blank | 6.8 | 25.1 ± 1.5 | 0.154 | 0.1 | 1.20 ± 0.01 |
| DL | 14.3 | 65.1 ± 8.3 | 0.237 | -15.8 | 1.24 ± 0.01 | ||
ND - Not determined
Stability in Presence of Surfactants
Hydrolysis of THC-HG followed a first order degradation process. Stability of THC-HG was enhanced significantly in the presence of surfactants and was found to be temperature dependent (Table III). Energy of activation was calculated from the Arrhenius Plot constructed with the data. Formulations containing Poloxamer 407 had the lowest energy of activation (3887 cal/mol). Energy of activation of THC-HG in the presence of Polysorbate 80 (8536 cal/mole), Tyloxapol (9295 cal/mol) and Cremophor RH 40 (9202 cal/mol) was not significantly different. The ion pair formulation demonstrated relatively short half-life of 13.7 ±0.3 days (25 °C) and 4 ± 0.02 days (40 °C). Degradation was however not observed in the ion pair formulation when stored at 4 °C, even after 1 month.
Table III.
Apparent first order rate constants (k, h-1) and half-lives (t1/2, day) of THC-HG in various surfactant solutions in IPBS at 4 °C, 25 °C and 40 °C. Results are depicted as mean ± SD (n=3).
| Formulation | Concentration (% w/v) | 4 °C | 25 °C | 40 °C | Ea (cal/mol) | |
|---|---|---|---|---|---|---|
| IPBS | - | k x 104 | 161 ± 34.5 | 1755.7 ± 84.3 | 2556.7 ± 97.4 | 13722 |
|
| ||||||
| t1/2 | 45.5 ± 11.3 | 3.9 ± 0.2 | 2.7 ± 0.1 | |||
|
| ||||||
| Ion Pair | k x 104 | NDD* | 21.1 ± 0.5 | 72.2 ± 0.3 | ND** | |
|
|
|
|||||
| t1/2 | 13.7 ± 0.3 | 4.0 ± 0.02 | ||||
|
| ||||||
| Cremophor RH 40 | 0.1 | K x 104 | 1.9 ± 0.4 | 5.3 ± 0.1 | 13.6 ± 0.4 | 9202 |
|
| ||||||
| t1/2 | 155.3 ± 34.4 | 54.2 ± 0.5 | 21.1 ± 0.6 | |||
|
| ||||||
| Poloxamer 407 | 0.1 | k x 104 | 4.4 ± 1.8 | 6.8 ± 0.4 | 17.5 ± 0.6 | 3887 |
|
| ||||||
| t1/2 | 77.1 ± 30.1 | 42.2 ± 2.3 | 16.4 ± 0.6 | |||
|
| ||||||
| Polysorbate 80 | 0.1 | k x 104 | 1.7 ± 0.2 | 4.3 ± 0.1 | 10.7 ± 0.5 | 8536 |
|
| ||||||
| t1/2 | 167.7 ± 15.6 | 67.7 ± 2 | 27.1 ± 1.2 | |||
|
| ||||||
| Tyloxapol | 0.1 | k x 104 | 1.8 ± 0.5 | 4.5 ± 0.1 | 13 ± 3.5 | 9295 |
|
| ||||||
| t1/2 | 174.9 ± 52.4 | 64.7 ± 1.8 | 23.7 ± 5.4 | |||
NDD – No degradation detected for one month
ND – Not determined
Permeability of THC-HG in the presence of surfactants
Permeability of THC-HG in the presence of 0.1% w/v Cremophor RH 40 was found to be 14 x 10-6 cm/s. Increasing the concentration of Cremophor RH 40 to 0.25 % w/v (10.5 x 10-6 cm/s) and 0.5 % w/v (10.9 x 10-6 cm/s) did not affect the permeability significantly. Permeability of THC-HG in the presence of 0.16 % w/v Poloxamer 407 was found to be slightly higher (21.4 x 10-6 cm/s) compared to 0.1 % w/v (15 x 10-6 cm/sec) but was not found to be statistically significant. In the presence of 0.1 % Polysorbate 80 and Tyloxapol, permeability was found to be 7.3 x 10-6 cm/s and 12.3 x 10-6 cm/s, respectively. The above data have been illustrated in Fig. 3. At 0.1 % w/v of surfactant concentration permeability of THC-HG in Cremophor RH 40 and Poloxamer 407 were found to be significantly higher compared to Polysorbate 80.
Figure 3.
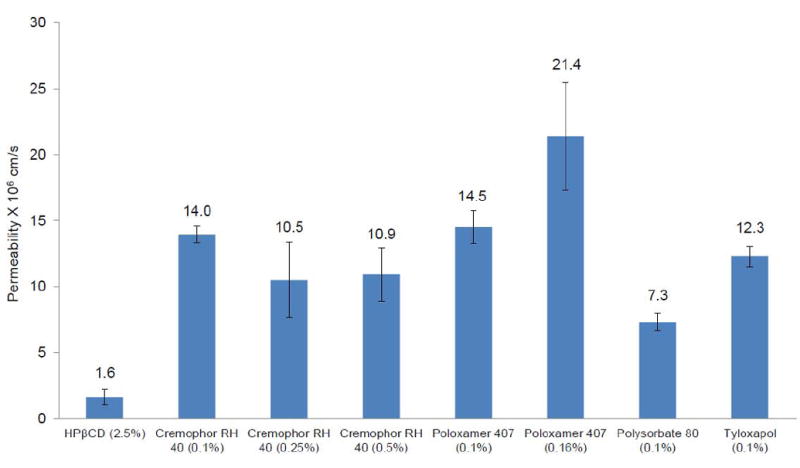
Permeability of THC-HG from various surfactant solutions across isolated rabbit cornea at 34 °C. Receiver solution consisted of IPBS containing 2.5% HPβCD (pH 7.4). Results are depicted as mean ± SD (n=4).
Since transcorneal THC delivery was observed to be the best from Cremophor RH 40 and Poloxamer 407 containing formulations, evaluating the effect of inclusion of BAK and EDTA in these formulations, with respect to permeability, was also considered. Nevertheless, Poloxamer 407 interacted with BAK and led to the formation of a precipitate. Hence the effect of BAK on the permeability of poloxamer 407 based formulations could not be studied. Permeability of THC-HG from formulations containing 0.01% w/v BAK and 0.1% w/v of Cremophor RH-40 was significantly increased to 19.9 x 10-6 cm/s (Fig. 4). Use of 0.02% BAK in 0.1 % w/v Cremophor RH 40 led to a two-fold enhancement in permeability of THC-HG. Addition of 0.1% w/v disodium EDTA to the 0.1% Cremophor RH 40 formulation also led to a significant increase in the permeability of THC-HG (18.6 x 10-6 cm/s). A combination of 0.01% BAK with 0.1% disodium EDTA in 0.1% Cremophor RH 40 yielded a permeability value of 24.2 x 10-6 cm/s. Effect of BAK and EDTA on in vitro permeability of THC-HG at higher Cremophor RH 40 concentrations (0.25% w/v, 0.5% w/v) was not carried out since BAK is known to form mixed micelles or partition into the micelles with other surfactants leading to a loss of permeability enhancing effect(37).
Figure 4.
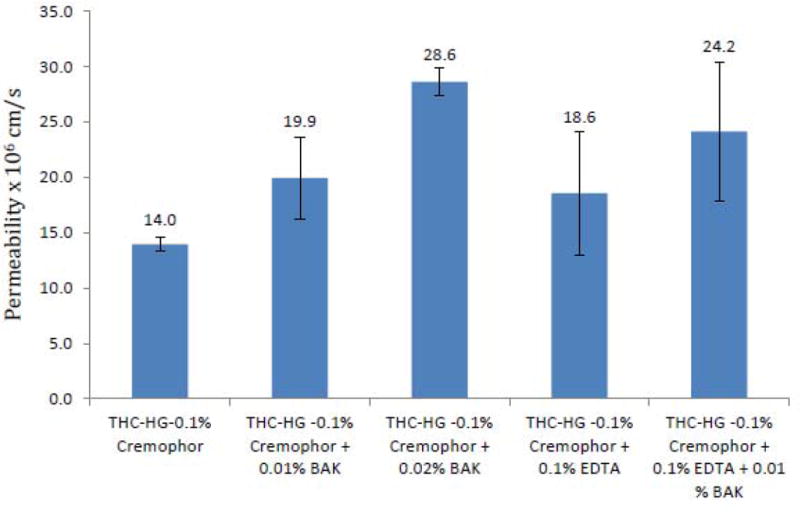
Permeability of THC-HG from donor solutions containing Cremophor RH 40, BAK and/or EDTA across isolated rabbit cornea at 34 °C. Receiver solution used was IPBS containing 2.5% HPβCD (pH 7.4). Results are depicted as mean ± SD (n=4).
In Vivo Bioavailability
The THC or THC equivalent (for the THC-HG formulation) content in the formulations are provided in Tables IV and Table V. THC solubilized in mineral oil (0.1%) or formulated as emulsions (0.37%) did not produce any detectable THC levels in the aqueous humor or the iris ciliary bodies post topical application. On the other hand, THC-HG formulation (0.18% THC equivalent) consisting of 0.5% Cremophor RH 40 + 0.02 % BAK + 0.1 % EDTA + 0.5 % HPMC delivered 32.1 ± 12.6 ng/100 μL to the aqueous humor and 35.6 ± 12.5 ng/50 mg to the iris ciliary bodies. The THC-HG ion pair formulation (0.16% THC equivalent) was able to deliver 52.2 ± 18.7 ng/100 μL to the aqueous humor and 93.1 ± 41.4 ng/50 mg to the iris ciliary bodies (Table V).
Table IV.
Total THC concentrations observed in rabbit ocular tissues 1 h post topical administration of 50 μL of THC in light mineral oil (0.1% w/v), emulsion (0.37% w/v) or micellar solutions (0.125 % w/v THC, 0.5% Cremophor RH 40 + 0.1% EDTA + 0.02% BAK + 0.5% HPMC). Results are depicted as mean ± SD (n=3).
| Tissue | THC
|
|||
|---|---|---|---|---|
| Light mineral oil | Emulsion | 0.5% Cremophor RH 40 + 0.1% EDTA + 0.02% BAK + 0.5% HPMC | ||
| Drug concentration in terms of THC (% w/v) | 0.1 | 0.37 | 0.125 | |
|
| ||||
| pH | NT** | 7.4 | 7.4 | |
|
| ||||
| Osmolality | NT** | NT** | 290 | |
|
| ||||
| Cornea (ng/50 mg Tissue) | 68.8 ± 14.5 | 300.6 ± 79.6 | 553.9 ± 87.4 | |
|
| ||||
| Aqueous Humor (ng/100 μL) | ND* | ND* | ND* | |
|
| ||||
| Iris-Cilliary Body (ng/50 mg Tissue) | ND* | ND* | ND* | |
|
| ||||
| Vitreous Humor (ng/mL) | ND* | ND* | ND* | |
|
| ||||
| Retina-Choroid (ng/50 mg Tissue) | ND* | ND* | ND* | |
|
| ||||
| Sclera (ng/250 mg Tissue) | 104.1 ± 36.1 | 171.1 ± 66.6 | 439.3 ± 280.2 | |
ND - Drug concentration below detection limit
NT - Not Tested
Table V.
Total THC concentrations observed in rabbit ocular tissues post topical administration of 50 μL of THC-HG formulated in, 0.5% Cremophor RH 40 + 0.1% EDTA + 0.02% BAK + 0.5% HPMC (1 h time point) or ion pair formulation (8 mM tromethamine + 0.5% HPMC; 1 and 3 h time points). Results are depicted as mean ± SD (n=3).
| Tissue | THC-HG | ||
|---|---|---|---|
| 0.5% Cremophor RH 40 + 0.1% EDTA+0.02% BAK + 0.5% HPMC | Ion Pair Formulation | ||
| 1 Hour | 1 Hour | 3 Hours | |
| Drug Concentration in terms of THC (% w/v) | 0.18 | 0.16 | 0.16 |
| pH | 7.4 | 7.6 | |
| Osmolality (mOsm) | 289 | 286 | |
| Cornea (ng/50 mg Tissue) | 2451.6 ± 645.6 | 2245.7 ± 240.2 | 1382.7 ± 109.8 |
| Aqueous Humor (ng/100 μL) | 32.1 ± 12.6 | 52.2 ± 18.7 | 27.3 ± 11.3 |
| Iris-Cilliary Body (ng/50 mg Tissue) | 35.6 ± 12.5 | 93.1 ± 41.4 | 67.1 ± 55.6 |
| Vitreous Humor (ng/mL) | ND* | ND* | ND* |
| Retina-Choroid (ng/50 mg Tissue) | ND* | ND* | ND* |
| Sclera (ng/250 mg Tissue) | 354.7 ± 86.4 | 2258.1 ± 1331.1 | 166.1 ± 49.4 |
ND - Drug concentration below detection limit
THC concentrations achieved in the cornea from the THC-HG formulation in Cremophor RH 40 (2451.6 ± 645.6 ng/50 mg) and ion pair formulations (2245.7 ± 240.2 ng/50 mg) were several fold greater than that obtained from the THC loaded mineral oil (68.8 ± 14.5 ng/50 mg) and emulsion (300.6 ± 79.6 ng/50 mg) formulations. THC-HG containing micellar solution (354.7 ± 86.4 ng/250 mg) as well as ion pair formulation (2258.1 ± 1331.8 ng/250 mg) also delivered significantly higher concentrations to the sclera compared to THC formulated in light mineral oil (104.1 ± 36.1 ng/250 mg) or as emulsion (171.1 ± 66.6 ng/250 mg) formulations.
Since significantly higher THC concentrations were detected in the iris ciliary bodies from the ion pair formulation compared to the surfactant formulation, ocular tissue concentrations 3h post instillation of the ion pair formulations was also carried out to determine if any THC reaches the retina with additional time and also evaluate the clearance of THC from the ocular tissues. THC concentrations in the cornea (1382.7 ± 109.8 ng/50 mg), aqueous humor (27.3 ± 11.3 ng/100 μL), iris ciliary (67.1 ± 55.6 ng/50 mg) and sclera (166.1 ± 49.3) were still detectable at the end of three hours (Table V).
When THC, rather than THC-HG, was incorporated into the Cremophor based micellar solution (0.125% w/v THC, 0.5% Cremophor RH 40, 0.02% BAK, 0.1% EDTA, 0.5% HPMC) THC was detected in the cornea (553.9 ± 87.4 ng/50 mg tissue) and sclera (439.3 ± 280.2 ng/250 mg tissue) but not in the aqueous humor, iris ciliary body or the retina choroid.
None of the formulations studied produced THC concentrations in the vitreous humor or the retina choroid.
DISCUSSION
Helper and Frank in 1971 observed that smoking marijuana led to a drop in IOP and subsequently THC was identified as one of the constituents responsible(38). Several preclinical and clinical studies were initiated to determine whether THC could lower IOP when applied topically. The results were, however, ambiguous. One of the reasons behind the observed variability could be lack of an effective ophthalmic THC formulation in these earlier studies. The ocular tissues present significant physiological barriers to the permeation of external moieties (39). Delivery to the posterior segment of the eye is even more challenging (40). On top of these challenges, THC is a highly lipophilic agent and will not efficiently partition into the aqueous precorneal environment from lipophilic formulations to be available for ocular absorption. In the present study, the in vitro transcorneal permeability and in vivo bioavailability of THC has been improved using a combination of hydrophilic prodrug derivatization and formulation approaches. Ocular bioavailability using the prodrug, through the topical route, has also been compared to that of THC from mineral oil and emulsion based formulations, similar to that used in the earlier in vivo studies.
In order to increase the aqueous solubility, stability and permeability of THC-HG, surfactants commonly used in ophthalmic formulations were used. The maximum surfactant concentrations to be used in these studies were determined from the FDA database of inactive excipients. Use of surfactants led to a significant improvement in the aqueous solubility of THC-HG. Moreover, the hydrolysis of the THC-HG ester was significantly reduced in the presence of the surfactants leading to a marked improvement in the stability of THC-HG in aqueous solutions. Surfactant micelles present in dilute solutions usually form spherical/ellipsoid micelles. At higher surfactant concentrations micelles may undergo transformation from spherical/ellipsoid to cylindrical shapes which is usually associated with a significant increase in viscosity(41). Since the viscosity of the drug loaded micellar formulations did not change significantly it probably indicates that the drug loaded micelles were mostly spherical/ellipsoid in shape. Literature reports also suggest that surfactants listed in Table II form spherical/ellipsoid shaped micelles in dilute solutions (42-46).
Amongst the surfactants studied, THC-HG demonstrated highest in vitro transcorneal permeability in the presence of poloxamer 407 and this formulation was thus selected for further evaluation. Although the extent of permeation enhancement achieved with Cremophor RH 40 was less marked than poloxamer 407, Cremophor RH 40 has been used at concentrations up to 0.5% in ophthalmic eyedrops, in contrast to only 0.16% for Poloxamer 407, and can thus provide advantages with respect to higher donor concentrations and flux. Thus, both Poloxamer 407 and Cremophor RH 40 formulations were selected for further investigation. BAK, a cationic surfactant, is commonly used as a preservative in ophthalmic formulations. BAK is known to interact with some surfactants, leading to loss of antimicrobial activity (37). THC-HG being a weak acid is negatively charged at physiological pH and may also interact with positively charged BAK. Furthermore, BAK is also known to act as a permeability enhancing agent (47). Thus, the effect of inclusion of BAK and EDTA in the promising THC-HG surfactant formulations was also investigated.
Poloxamer 407 produced the highest in vitro permeability but addition of 0.02 % BAK to the formulation led to the formation of a precipitate. Formation of a precipitate was however not observed with the blank (no drug added) vehicle. Thus, the observed precipitation could be due to an interaction between BAK and THC-HG. Poloxamer 407 was thus not studied any further. In contrast to the results with poloxamer 407, a clear solution could be prepared with the BAK and Cremophor RH 40 based formulation. This could be due to better shielding of the HG promoiety of THC-HG in the Cremophor RH 40 micelles or packing characteristics of the Cremophor RH 40 micelles.
Based on the in vitro permeability and solubility data, the Cremophor RH 40 based formulation was selected for in vivo evaluation in rabbits. Since, permeability of THCHG in Cremophor RH 40 was found to be independent of the surfactant concentration (within the range studied), 0.5% Cremophor RH 40 concentration, which allowed 2.1 mg/mL of THC-HG loading, was selected for the in vivo studies. BAK (0.02%) and EDTA (0.1%) were also included in the formulation in view of their permeability enhancing effect (Fig. 4) and their common role as preservative/preservative aid in ophthalmic formulations(37). To increase the corneal residence time, 0.5% HPMC was added as a viscosity enhancer.
In an earlier study Green et al reported intraocular tissue concentrations of radiolabelled THC (0.1% w/v) formulated in light mineral oil. Approximate THC concentrations were reported in the cornea (100 ng/50 mg of tissue), iris-ciliary body (15 ng/50 mg) and the aqueous humor (4 ng/100 μL) at the end of one hour(27).
In the current study, topical administration of 0.1% THC in light mineral oil produced similar THC concentrations (68.8 ng ± 14.5 ng/50mg) in the cornea. The, THC levels in the aqueous humor and the iris ciliary bodies, however, were found to be below the detection limits of the analytical method used in this study. The 0.4% w/v THC emulsion formulation was also not able to deliver THC to the intraocular tissues. These results suggest that, in the earlier studies, THC was probably not achieving significant concentrations in the targeted ocular tissues with the mineral oil and emulsion formulations.
Use of the relatively hydrophilic HG ester prodrug of THC and incorporation into a micellar solution markedly improved the delivery of THC to the aqueous humor and the iris ciliary bodies. To delineate the effect of the prodrug from the surfactant system, THC (0.125% w/v) was formulated in the same vehicle and administered topically. In this case, THC levels were not detected in the aqueous humor, iris ciliary body, vitreous humor or the retina choroid. This strongly suggests that, chemical modification of THC into THC-HG led to a significant improvement in the physicochemical properties allowing better partitioning into the aqueous tear fluid and from there into the cornea and deeper ocular tissues. The THC-HG / tromethamine ion pair formulation delivered significantly higher THC concentrations to the iris ciliary bodies, compared to the micellar formulations. Although rapidly cleared from the back-of-the eye tissues, significant THC concentrations were still detectable in the aqueous humor and iris ciliary bodies at the end of three hours after topical instillation.
The overall goal of this research project was to a) improve THC concentrations in the anterior chamber and b) evaluate penetration into the retinal tissue following topical application. While through the approaches evaluated in this study anterior segment THC concentrations could be significantly improved through the topical route, delivery into the back-of-the eye was not successful. Thus, to utilize the neuroprotective potential of THC, through the topical route, further, prodrug modifications and formulation approaches are being evaluated based on the information obtained from this study.
Figure 1.
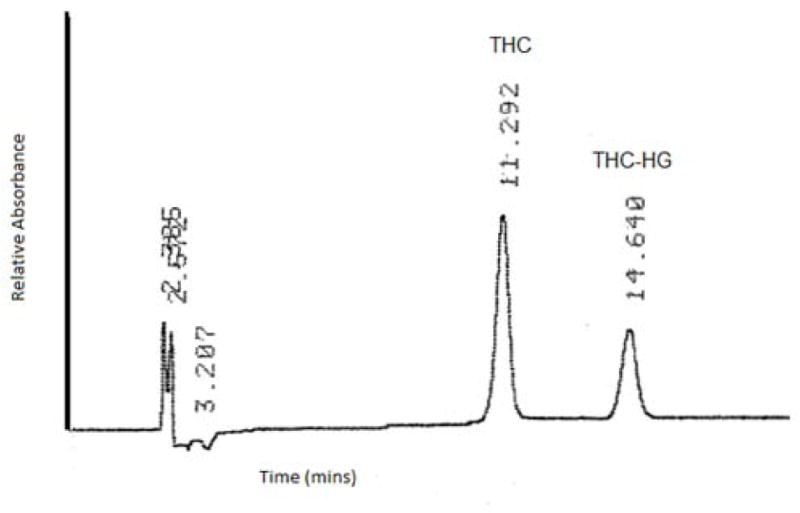
Ultraviolet chromatogram of a mixture of THC (10μg/mL) and THC-HG (10 μg/mL). Column: Phenomenex luna PFP(2) 4.6 x 250 mm, mobile phase: 85:15 mixture of methanol and 0.84% glacial acetic acid, flow rate: 1.2 mL/min. Detection was carried out at 226 nm.
Acknowledgments
This publication was partially supported by grants 1R41EY020042 and 2R42GM067304-02 to ElSohly Laboratories, Incorporated and P20GM104932 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CB1
Cannabinoid Receptor 1
- CB2
Cannabinoid Receptor 2
- RGC
Retinal ganglion cells
- IOP
Intraocular pressure
- NMDA
N-Methyl-D-aspartate
- PPARγ
Peroxisome profiferator-activated receptor gamma
- ROS
Reactive oxygen species
- BAK
Benzalkonium Chloride
- EDTA
Ethylenediaminetetraacetic acid
- THC
∆9-Tetrahydrocannabinol
- THC-HG
Hemiglutarate ester of ∆9-Tetrahydrocannabinol
- NF
National Formulary
References
- 1.Qu J, Wang D, Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 91:48–53. doi: 10.1016/j.exer.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wierzbowska J, Robaszkiewicz J, Figurska M, Stankiewicz A. Future possibilities in glaucoma therapy. Med Sci Monit. 16:RA252–259. [PubMed] [Google Scholar]
- 3.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 31:152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Impagnatiello F, Borghi V, Gale DC, Batugo M, Guzzetta M, Brambilla S, Carreiro ST, Chong WK, Prasanna G, Chiroli V, Ongini E, Krauss AH. A dual acting compound with latanoprost amide and nitric oxide releasing properties, shows ocular hypotensive effects in rabbits and dogs. Exp Eye Res. 93:243–249. doi: 10.1016/j.exer.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Changand EE, Goldberg JL. Glaucoma 2. 0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology. doi: 10.1016/j.ophtha.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JP, Nash MS. Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol. 1999;43(Suppl 1):S102–128. doi: 10.1016/s0039-6257(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 8.Cordeiroand MF, Levin LA. Clinical evidence for neuroprotection in glaucoma. Am J Ophthalmol. 152:715–716. doi: 10.1016/j.ajo.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osborne NN. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol. 2009;87:450–454. doi: 10.1111/j.1755-3768.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- 10.Danesh‐Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol. 22:78–86. doi: 10.1097/ICU.0b013e32834372ec. [DOI] [PubMed] [Google Scholar]
- 11.El‐Remessy AB, Khalil IE, Matragoon S, Abou‐Mohamed G, Tsai NJ, Roon P, Caldwell RB, Caldwell RW, Green K, Liou GI. Neuroprotective effect of (‐)Delta9‐tetrahydrocannabinol and cannabidiol in N‐methyl‐D‐aspartate‐induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol. 2003;163:1997–2008. doi: 10.1016/s0002-9440(10)63558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crandall J, Matragoon S, Khalifa YM, Borlongan C, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and intraocular pressure‐lowering effects of (‐)Delta9‐tetrahydrocannabinol in a rat model of glaucoma. Ophthalmic Res. 2007;39:69–75. doi: 10.1159/000099240. [DOI] [PubMed] [Google Scholar]
- 13.Hingorani T, Gul W, Elsohly M, Repka MA, Majumdar S. Effect of ion pairing on in vitro transcorneal permeability of a Delta(9) ‐tetrahydrocannabinol prodrug: potential in glaucoma therapy. J Pharm Sci. 101:616–626. doi: 10.1002/jps.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (‐)Delta9‐ tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–8273. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heand F, Song ZH. Molecular and cellular changes induced by the activation of CB2 cannabinoid receptors in trabecular meshwork cells. Mol Vis. 2007;13:1348–1356. [PubMed] [Google Scholar]
- 16.Njie YF, Qiao Z, Xiao Z, Wang W, Song ZH. N‐arachidonylethanolamide‐induced increase in aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2008;49:4528–4534. doi: 10.1167/iovs.07-1537. [DOI] [PubMed] [Google Scholar]
- 17.Porcella A, Casellas P, Gessa GL, Pani L. Cannabinoid receptor CB1 mRNA is highly expressed in the rat ciliary body: implications for the antiglaucoma properties of marihuana. Brain Res Mol Brain Res. 1998;58:240–245. doi: 10.1016/s0169-328x(98)00105-3. [DOI] [PubMed] [Google Scholar]
- 18.Stamer WD, Golightly SF, Hosohata Y, Ryan EP, Porter AC, Varga E, Noecker RJ, Felder CC, Yamamura HI. Cannabinoid CB(1) receptor expression, activation and detection of endogenous ligand in trabecular meshwork and ciliary process tissues. Eur J Pharmacol. 2001;431:277–286. doi: 10.1016/s0014-2999(01)01438-8. [DOI] [PubMed] [Google Scholar]
- 19.Lu Q, Straiker A, Maguire G. Expression of CB2 cannabinoid receptor mRNA in adult rat retina. Vis Neurosci. 2000;17:91–95. doi: 10.1017/s0952523800171093. [DOI] [PubMed] [Google Scholar]
- 20.van der Stelt M, Veldhuis WB, Bar PR, Veldink GA, Vliegenthart JF, Nicolay K. Neuroprotection by Delta9‐tetrahydrocannabinol, the main active compound in marijuana, against ouabain‐induced in vivo excitotoxicity. J Neurosci. 2001;21:6475–6479. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabbs JM., Jr Salivary testosterone measurements: reliability across hours, days, and weeks. Physiol Behav. 1990;48:83–86. doi: 10.1016/0031-9384(90)90265-6. [DOI] [PubMed] [Google Scholar]
- 22.O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator‐activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues GA, Maurier‐Mahe F, Shurland DL, McLaughlin A, Luhrs K, Throo E, Delalonde‐ Delaunay L, Pallares D, Schweighoffer F, Donello J. Differential effects of PPARgamma ligands on oxidative stress‐induced death of retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 52:890–903. doi: 10.1167/iovs.10-5715. [DOI] [PubMed] [Google Scholar]
- 24.Merritt JC, Perry DD, Russell DN, Jones BF. Topical delta 9‐tetrahydrocannabinol and aqueous dynamics in glaucoma. J Clin Pharmacol. 1981;21:467S–471S. doi: 10.1002/j.1552-4604.1981.tb02626.x. [DOI] [PubMed] [Google Scholar]
- 25.Greenand K, Roth M. Ocular effects of topical administration of delta 9‐tetrahydrocannabinol in man. Arch Ophthalmol. 1982;100:265–267. doi: 10.1001/archopht.1982.01030030267006. [DOI] [PubMed] [Google Scholar]
- 26.Merritt JC, Olsen JL, Armstrong JR, McKinnon SM. Topical delta 9‐tetrahydrocannabinol in hypertensive glaucomas. J Pharm Pharmacol. 1981;33:40–41. doi: 10.1111/j.2042-7158.1981.tb13699.x. [DOI] [PubMed] [Google Scholar]
- 27.Green K, Bigger JF, Kim K, Bowman K. Cannabinoid penetration and chronic effects in the eye. Exp Eye Res. 1977;24:197–205. doi: 10.1016/0014-4835(77)90260-3. [DOI] [PubMed] [Google Scholar]
- 28.Merritt JC, Perry D, Russell D, Jones B. Topical delta 9‐tetrahydrocannabinol and aqueous dynamics in glaucoma. The Journal of Clinical Pharmacology. 1981;21:467S–471S. doi: 10.1002/j.1552-4604.1981.tb02626.x. [DOI] [PubMed] [Google Scholar]
- 29.Colasanti BK, Powell SR, Craig CR. Intraocular pressure, ocular toxicity and neurotoxicity after administration of delta 9‐tetrahydrocannabinol or cannabichromene. Exp Eye Res. 1984;38:63–71. doi: 10.1016/0014-4835(84)90139-8. [DOI] [PubMed] [Google Scholar]
- 30.Hodges LC, Reggio PH, Green K. Evidence against cannabinoid receptor involvement in intraocular pressure effects of cannabinoids in rabbits. Ophthalmic Res. 1997;29:1–5. doi: 10.1159/000267984. [DOI] [PubMed] [Google Scholar]
- 31.Jayand WM, Green K. Multiple‐drop study of topically applied 1% delta 9‐tetrahydrocannabinol in human eyes. Arch Ophthalmol. 1983;101:591–593. doi: 10.1001/archopht.1983.01040010591012. [DOI] [PubMed] [Google Scholar]
- 32.Majumdar S, Hingorani T, Srirangam R. Evaluation of active and passive transport processes in corneas extracted from preserved rabbit eyes. J Pharm Sci. 99:1921–1930. doi: 10.1002/jps.21979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levyand M, Benita S. Design and characterization of a submicronized o/w emulsion of diazepam for parenteral use. International journal of pharmaceutics. 1989;54:103–112 . [Google Scholar]
- 34.Muchtar S, Almog S, Torracca M, Saettone M, Benita S. A submicron emulsion as ocular vehicle for delta‐8‐tetrahydrocannabinol: effect on intraocular pressure in rabbits. Ophthalmic research. 1992;24:142–149. doi: 10.1159/000267160. [DOI] [PubMed] [Google Scholar]
- 35.Thumma S, Majumdar S, Elsohly MA, Gul W, Repka MA. Preformulation studies of a prodrug of Delta9‐tetrahydrocannabinol. AAPS PharmSciTech. 2008;9:982–990. doi: 10.1208/s12249-008-9136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zoller O, Rhyn P, Zimmerli B. High‐performance liquid chromatographic determination of delta9‐tetrahydrocannabinol and the corresponding acid in hemp containing foods with special regard to the fluorescence properties of delta9‐tetrahydrocannabinol. J Chromatogr A. 2000;872:101–110. doi: 10.1016/s0021-9673(99)01287-x. [DOI] [PubMed] [Google Scholar]
- 37.Jiao J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv Drug Deliv Rev. 2008;60:1663–1673. doi: 10.1016/j.addr.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Heplerand RS, Frank I. Marijuana smoking and intraocular pressure. JAMA. 1971;217:1392. [PubMed] [Google Scholar]
- 39.Barar J, Javadzadeh AR, Omidi Y. Ocular novel drug delivery: impacts of membranes and barriers. Expert Opin Drug Deliv. 2008;5:567–581. doi: 10.1517/17425247.5.5.567. [DOI] [PubMed] [Google Scholar]
- 40.Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG. Drug delivery to the posterior segment of the eye. Drug Discov Today. 16:270–277. doi: 10.1016/j.drudis.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Moitzi C, Freiberger N, Glatter O. Viscoelastic wormlike micellar solutions made from nonionic surfactants: Structural investigations by SANS and DLS. The Journal of Physical Chemistry B. 2005;109:16161–16168. doi: 10.1021/jp0441691. [DOI] [PubMed] [Google Scholar]
- 42.Mermi J, Yajima M, Ebner F. The control of the contraction of myocytes from guinea‐pig heart by the resting membrane potential. Br J Pharmacol. 1991;104:705–713. doi: 10.1111/j.1476-5381.1991.tb12492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitoand Y, Sato T. Micellar formation and micellar structure of poly(oxyethylene)‐ hydrogenated castor oil. Yakugaku Zasshi. 1992;112:763–767. doi: 10.1248/yakushi1947.112.10_763. [DOI] [PubMed] [Google Scholar]
- 44.Amani A, York P, de Waard H, Anwar J. Molecular dynamics simulation of a polysorbate 80 micelle in water. Soft Matter. 2011;7:2900–2908. [Google Scholar]
- 45.Attwood D, Collett J, Tait C. The micellar properties of the poly (oxyethylene)‐poly (oxypropylene) copolymer Pluronic F127 in water and electrolyte solution. International journal of pharmaceutics. 1985;26:25–33. [Google Scholar]
- 46.Zhangand H, Annunziata O. Modulation of drug transport properties by multicomponent diffusion in surfactant aqueous solutions. Langmuir. 2008;24:10680–10687. doi: 10.1021/la801636u. [DOI] [PubMed] [Google Scholar]
- 47.Majumdar S, Hippalgaonkar K, Repka MA. Effect of chitosan, benzalkonium chloride and ethylenediaminetetraacetic acid on permeation of acyclovir across isolated rabbit cornea. Int J Pharm. 2008;348:175–178. doi: 10.1016/j.ijpharm.2007.08.017. [DOI] [PubMed] [Google Scholar]


