SUMMARY
Food deprivation in mammals is typically associated with reduced thyroid hormone (TH) concentrations and deiodinase content and activity to suppress metabolism. However, in prolonged-fasted, metabolically active elephant seal pups, TH levels are maintained, if not elevated. The functional relevance of this apparent paradox is unknown and demonstrates variability in the regulation of TH levels, metabolism and function in food-deprived mammals. To address our hypothesis that cellular TH-mediated activity is upregulated with fasting duration, we quantified the mRNA expression and protein content of adipose and muscle deiodinase type I (DI1) and type II (DI2), and TH receptor beta-1 (THrβ-1) after 1, 3 and 7 weeks of fasting in northern elephant seal pups (N=5–7 per week). Fasting did not decrease the concentrations of plasma thyroid stimulating hormone, total triiodothyronine (tT3), free T3, total thyroxine (tT4) or free T4, suggesting that the hypothalamic–pituitary–thyroid axis is not suppressed, but rather maintained during fasting. Mean mRNA expression of adipose DI1 and DI2 increased threefold and fourfold, respectively, and 20- and 30-fold, respectively, in muscle. With the exception of adipose DI1, protein expression of adipose DI2 and muscle DI1 and DI2 increased twofold to fourfold. Fasting also increased adipose (fivefold) and muscle (fourfold) THrβ-1 mRNA expression, suggesting that the mechanisms mediating cellular TH activity are upregulated with prolonged fasting. The data demonstrate a unique, atypical mechanism of TH activity and regulation in mammals adapted to prolonged food deprivation in which the potential responsiveness of peripheral tissues and cellular TH activity are increased, which may contribute to their lipid-based metabolism.
KEY WORDS: lipid metabolism, reverse T3, seal, thyroxine, triiodothyronine
Introduction
The regulation of thyroid hormone (TH) levels and function during fasting metabolism has long been a subject of research interest, especially because of the diversity of regulatory mechanisms in mammals (Eales, 1988; Reitman et al., 1999; Araujo et al., 2009). THs have been extensively examined in food-deprived humans and rodents (Croxson et al., 1977; Azizi, 1978; Harris et al., 1978; Suda et al., 1978; Azizi et al., 1979; Spencer et al., 1983; Herlihy et al., 1990; Kmiec et al., 1996) as well as metabolically quiescent, hypothermic hibernating mammals such as squirrels, hedgehogs and bears (Demeneix and Henderson, 1978a; Demeneix and Henderson, 1978b; Azizi et al., 1979; Fowler, 1988). However, a comprehensive understanding of the hypothalamic–pituitary–thyroid (HPT) axis and the subsequent cellular activity and function during prolonged fasting in naturally adapted, metabolically active mammals is lacking.
THs regulate metabolism through the regulation of the rate by which several TH-specific genes are transcribed (Gavin et al., 1977; Nguyen et al., 1998; Reitman et al., 1999; Ortiz et al., 2000). The THs 3,5,3′-triiodothyronine (T3) and thyroxine (T4) promote basal metabolism in mammals (van Hardeveld, 1986). Before T3 can promote thyroid-regulated effects, T4 is deiodinated by either deiodinase type I (DI1) or type II (DI2): DI1 can deiodinate either the inner or the outer ring, and DI2 only deiodinates the outer ring of the pro-hormone (Köhrle, 2000). During food deprivation, DI1 is increased to preferentially increase the monodeiodination of the inner ring to promote the production of reverse T3 (rT3), which suppresses cellular metabolism to protect the organism from the energetic burdens imposed during periods of reduced energy intake (LoPresti et al., 1991; St Germain, 1994; Diano et al., 1998). Following monodeiodination of T4, the available T3 can bind to its nuclear receptor, TH receptor beta-1 (THrβ-1), in peripheral tissues (i.e. adipose and skeletal muscle) (McNabb, 1992; Bassett et al., 2003; Oppenheimer et al., 1987). The binding of THrβ-1 activates the transcription of several genes including uncoupling protein 2 (UCP2), which contributes to substrate metabolism, especially lipid (Liang and Ward, 2006).
Because THs regulate basal metabolism in mammals, it is not surprising that their levels and function are altered with prolonged food deprivation (Croxson et al., 1977; Azizi, 1978; Harris et al., 1978; Suda et al., 1978; Spencer et al., 1983; Herlihy et al., 1990; Kmiec et al., 1996; Ortiz et al., 2000). However, alterations in TH alone may not accurately represent TH-mediated changes in cellular metabolism during food deprivation. Thus, changes in deiodination and receptor availability may provide a more meaningful and representative assessment of TH-mediated changes in cellular metabolism (Eales, 1988). For example, the increase in plasma T3 concentration in hibernating ground squirrels results from reduced TH metabolism, accompanied by a reduction in nuclear receptors, suggesting that TH function is suppressed despite the increase in circulating levels (i.e. cryptically hyperthyroid) (Magnus and Henderson, 1988a; Magnus and Henderson, 1988b). Such variability in the regulation of TH levels and function in food-deprived mammals demonstrates the diversity of TH physiology during periods of acute or chronic food deprivation, and highlights the necessity to perform more comprehensive studies into the functional relevance of these differences.
Northern elephant seals, Mirounga angustirostris (Gill 1866), naturally fast from food and water for up to 3 months while on land (Ortiz et al., 1978; Crocker et al., 1998; Le Boeuf and Laws, 1994), and during the post-weaning fast, oxidation of non-esterified fatty acids (NEFAs) accounts for approximately 95% of the pup's metabolic rate (Ortiz et al., 1978; Viscarra et al., 2012). The fasting metabolism of seals is primarily dependent on lipid oxidation (Crocker et al., 1998; Pramfalk et al., 2011; Crocker et al., 2012a; Crocker et al., 2012b; van Heyningen and Glaysher, 2012; Houser et al., 2012; Viscarra et al., 2012). Lipid metabolism in fasting pups is characterized by reduced adipose NEFA uptake and increased triglyceride hydrolysis, which contribute to the maintenance of elevated NEFA during the fast (Viscarra et al., 2012). However, unlike hibernators or other food-deprived mammals, fasting elephant seals remain metabolically active and normothermic (Rea and Costa, 1992; Crocker et al., 1998). Furthermore, unlike other mammals, with the exception of hibernating squirrels (Oppenheimer et al., 1987), prolonged fasting in elephant seals is associated with maintained, if not increased, total TH levels without increasing rT3 (Ortiz et al., 2000; Ortiz et al., 2003), suggesting that the lack of TH suppression may be adaptive to help support the energetic demands imposed during fasting. If so, then this mechanism would be unique among mammalian endocrine systems. However, to properly interpret the significance of the changes in circulating TH levels in prolonged-fasted seals, a comprehensive examination of the cellular TH-mediated responses is necessary. Therefore, to elucidate the mechanisms regulating the cellular function of TH in a mammal naturally adapted to prolonged food deprivation, we quantified the mRNA expression of DI1, DI2, THrβ-1 and the TH target gene, UCP2, as well as their associated proteins in adipose and muscle. We addressed the hypothesis that mRNA expression of DI1, DI2 and THrB-1 increases with fasting duration in northern elephant seal pups.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committees of both the University of California Merced and Sonoma State University. All work was conducted under National Marine Fisheries Service marine mammal permit no. 87-1743.
Sample collection and preparation
We are not allowed to sample pups while nursing (feeding) owing to federal permitting regulations; therefore, instead we examined the changes in circulating and cellular TH factors that occur between 1 and 7 weeks of food deprivation to represent the effects of fasting duration. Eighteen northern elephant seal pups were studied at Año Nuevo State Park, CA, during their natural postweaning fast. Separate cohorts of pups were sampled at three time periods: early (1 week postweaning; N=5), mid (3–4 weeks postweaning; N=7) and late (7–8 weeks postweaning; N=6) fasting. Tagging of pups at birth helped ensure the accuracy of their age to within 1 or 2 days. Sedation procedures, blood sampling and tissue biopsy collections have been detailed previously (Ortiz et al., 2000; Ortiz et al., 2001; Ortiz et al., 2002; Ortiz et al., 2003; Vázquez-Medina et al., 2011; Viscarra et al., 2011a; Viscarra et al., 2011b; Vázquez-Medina et al., 2012; Viscarra et al., 2012). Biopsies were rinsed with cold, sterile saline, placed in cryogenic vials, immediately frozen by immersion in liquid nitrogen, and stored at −80°C until later analyses. Frozen tissue samples were homogenized in 500 μl of hypotonic buffer containing a protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) as previously described (Vázquez-Medina et al., 2011; Viscarra et al., 2011a; Viscarra et al., 2011b; Vázquez-Medina et al., 2012; Viscarra et al., 2012). Nuclear and cytosolic extractions were performed using the Nuclear and Cytoplasmic Extraction Reagents Kit (Thermo Fisher Scientific, Waltham, MA, USA). Total protein content in both cytosolic and nuclear fractions was measured by Bradford assay (Bio-Rad Laboratories, Hercules, CA, USA) in preparation for western blotting.
Quantification of mRNA expression
Total RNA was isolated individually from adipose and muscle samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. RNA integrity was confirmed by measuring the absorbance at 260 nm/280 nm and by 1% agarose gel electrophoresis (Sambrook and Russell, 2006). Contamination of genomic DNA in total RNA was eliminated by digestion with DNase I (Roche, Indianapolis, IN, USA), as specified by the manufacturer. Separate cDNAs from each tissue were synthesized from total DNA-free RNA (1 μg) using oligo-dT and the QuantiTect Reverse Transcription kit (Qiagen, Valencia, CA, USA).
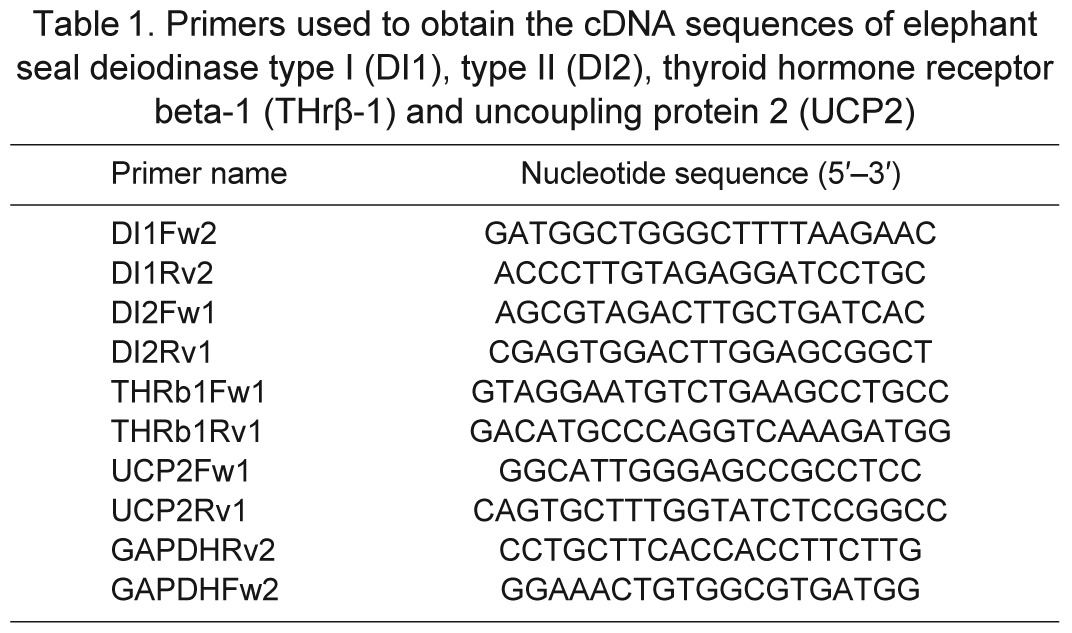
Specific primers for DI1, DI2, THrβ-1 and UCP2 were designed based on homologous mammalian nucleotide sequences (Table 1). The expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard to normalize the expression of each target gene. Gene expression was measured by quantitative RT-PCR using DI1Fw2 + DI1Rv2, DI2Fw1 + DI2Rv2, UCP2Fw1 + UCP2Rv2, THrβ1Fw1 + THrβ1Rv1 and GAPDHFw + GAPDHRv primers. The PCR reactions of each tissue sample were run on a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) in a final volume of 20 μl containing 10 μl of SYBR Green PCR Master Mix (Applied Biosystems), 6 μl of H2O, 0.5 μl of each primer (20 μmol l−1) and 3 μl of cDNA (equivalent to 150 ng of total RNA). After an initial denaturing step at 94°C for 5 min, amplifications were performed for 40 cycles at 94°C for 30 s, 60°C for 30 s and a final step of 30 s at 72°C, with a single fluorescence measurement and a final melting curve program decreasing 0.3°C each 20 s from 95 to 60°C. Positive and negative controls were included. Standard curves for each gene of interest were run to determine the efficiency of amplification using dilutions from 5E–3 to 5E–8 ng μl−1 of PCR fragments. For each measurement, expression levels (ng μl−1) were normalized to the expression of GAPDH. Additional assays were run to confirm that GAPDH expression did not change with fasting duration, confirming its utility for normalizing the other genes.
Table 1.
Primers used to obtain the cDNA sequences of elephant seal deiodinase type I (DI1), type II (DI2), thyroid hormone receptor beta-1 (THrβ-1) and uncoupling protein 2 (UCP2)
Quantification of protein expression by western blotting
The quantity of the biopsy samples limited our ability to quantify the expression of all of the proteins. Because our hypothesis focused on the changes in the deiodinases and the TH receptor, these proteins were given priority. Protein expression was quantified by standard western blot as previously described (Vázquez-Medina et al., 2011; Viscarra et al., 2011a; Viscarra et al., 2011b; Vázquez-Medina et al., 2012; Soñanez-Organis et al., 2012; Viscarra et al., 2012). The primary antibodies for DI1 and DI2, THrβ1, TATA binding protein, histone H3 and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) were diluted 1:500 to 1:5000. The HRP-conjugated secondary antibody (Pierce, Rockford, IL, USA) was diluted 1:10,000, and blots were developed using the Immun-Star Western C kit (Bio-Rad). Blots were visualized and semi-quantified using a Kodak 440 digital science imager (Rochester, NY, USA). In addition to consistently loading the same amount of total protein (20 μg) per well, densitometry values were further normalized by correcting for the densitometry values of β-actin.
Plasma analyses
The plasma concentrations of all TH were measured by radioimmunoassay previously validated for elephant seals (Ortiz et al., 2001; Ortiz et al., 2003). All samples were analyzed in duplicate and run in a single assay with intra-assay percent coefficients of variability of <10% for all assays.
Statistics
Means (±s.d.) were compared by ANOVA adjusted for repeated measures across the fast using a Bonferroni post hoc test. Means were considered statistically different at P<0.05. Statistical analyses were performed using STATISTICA 8 software (StatSoft Inc., Tulsa, OK, USA).
RESULTS
Prolonged fasting does not decrease circulating thyroid hormone concentrations
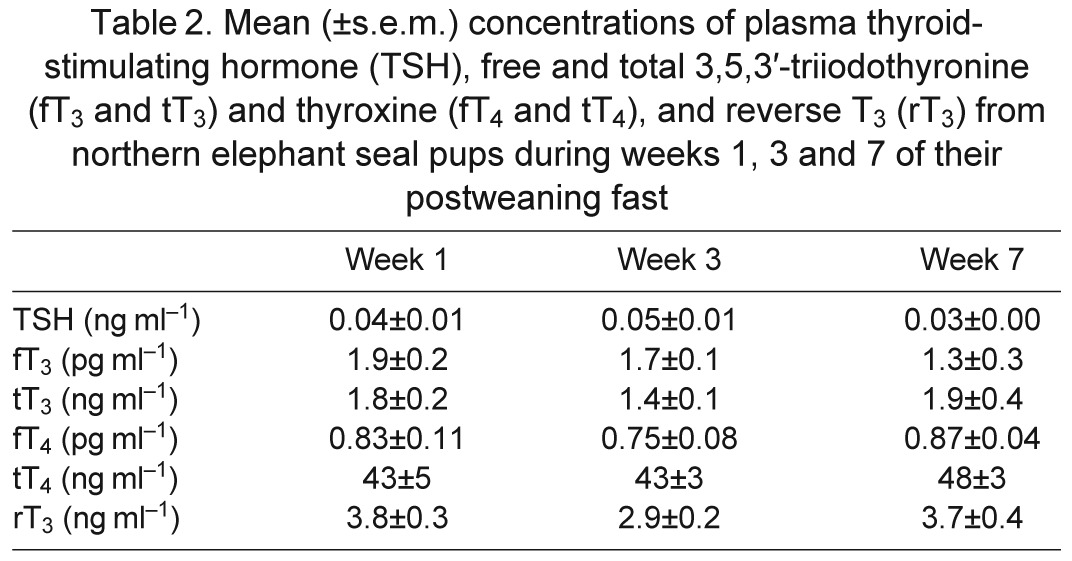
Plasma concentrations of thyroid-stimulating hormone (TSH), total and free T3 and T4, and rT3 were not significantly altered with fasting duration (Table 2).
Table 2.
Mean (±s.e.m.) concentrations of plasma thyroid-stimulating hormone (TSH), free and total 3,5,3′-triiodothyronine (fT3 and tT3) and thyroxine (fT4 and tT4), and reverse T3 (rT3) from northern elephant seal pups during weeks 1, 3 and 7 of their postweaning fast
Prolonged fasting increases adipose and muscle DI1 and DI2 mRNA and protein expression
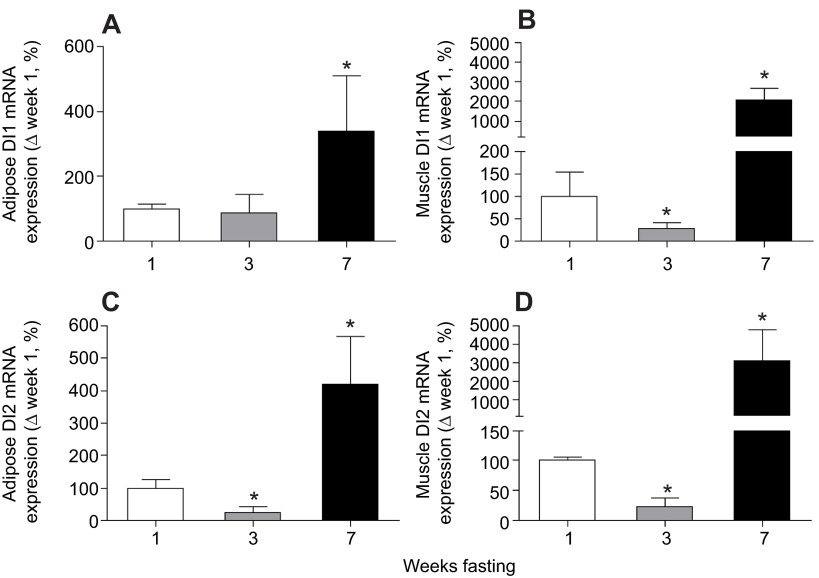
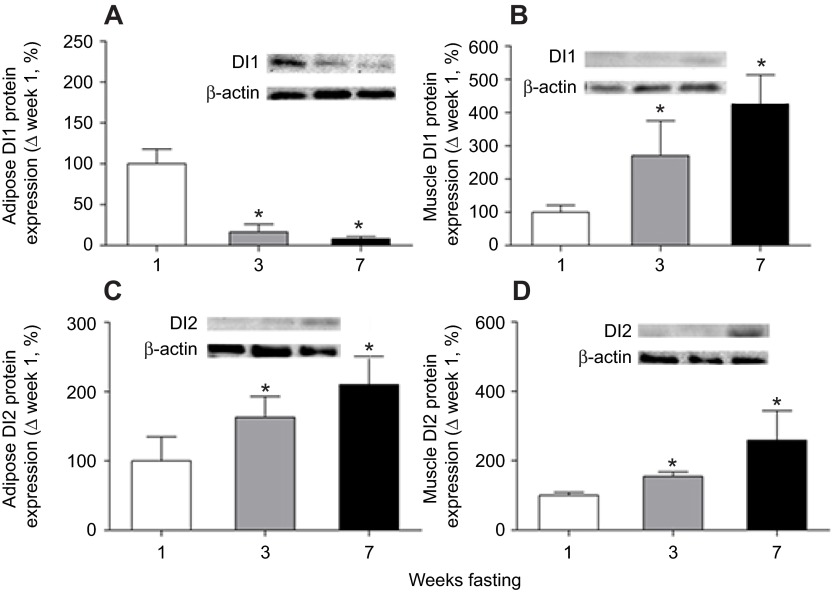
Adipose DI1 mRNA expression was not altered after 3 weeks of fasting, but increased (P<0.05) 3.4-fold by week 7 (Fig. 1A). Mean muscle DI1mRNA expression decreased (P<0.05) by 70% at 3 weeks before increasing (P<0.05) 20-fold by week 7 (Fig. 1B). Similarly, the mean mRNA expression of adipose and muscle DI2 decreased (P<0.05) by 80% in week 3, but increased (P<0.05) fourfold and 30-fold, respectively, after 7 weeks of fasting (Fig. 1C,D). With the exception of adipose DI1 protein expression, the protein expression of muscle DI1 and adipose and muscle DI2 increased (P<0.05) in a step-wise fashion from week 3 to week 7. Mean adipose DI1 protein expression decreased (P<0.05) by 84% by week 3 and by 92% by week 7 compared with week 1 (Fig. 2A). The protein expression of muscle DI1 increased (P<0.05) approximately threefold by week 3 and fourfold by week 7 (Fig. 2B). The protein expression of adipose DI2 increased (P<0.05) by 63% by week 3 and over twofold by week 7 compared with week 1 (Fig. 2C), and muscle DI2 protein expression increased (P<0.05) by 54% by week 3 and over 2.5-fold by week 7 (Fig. 2C,D).
Fig. 1.
Mean (±s.d.) mRNA expression levels of deiodinase type I (DI1) and type II (DI2) in (A,C) adipose and (B,D) muscle from fasting elephant seal pups. Asterisks denote significant (P<0.05) differences from week 1.
Fig. 2.
Mean (±s.d.) protein expression levels of deiodinase type I (DI1) and type II (DI2) in (A,C) adipose and (B,D) muscle from fasting elephant seal pups. Insets: representative western blots for each protein. Asterisks denote significant (P<0.05) differences from week 1.
Prolonged fasting increases adipose and muscle THrβ-1mRNA and protein expression
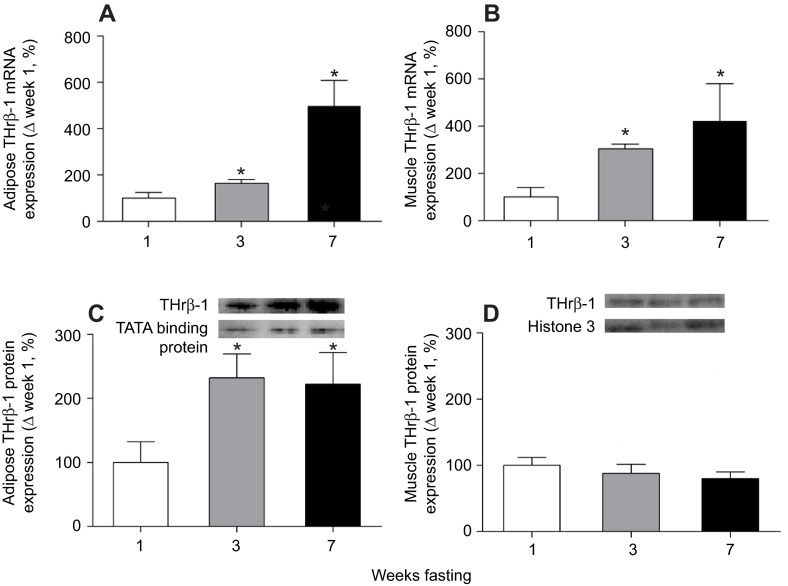
The mRNA expression of adipose THrβ-1 increased (P<0.05) by 63% by week 3 and approximately fivefold by week 7 of fasting compared with week 1 (Fig. 3A). The mRNA expression of muscle THrβ-1 increased (P<0.05) approximately threefold by week 3 and fourfold by week 7 of fasting compared with week 1 (Fig. 3B). The protein expression of adipose THrβ-1 increased (P<0.05) twofold by week 3 and that increase was maintained at week 7 (Fig. 3C). Protein expression of THrβ-1 did not change in muscle (Fig. 3D).
Fig. 3.
Mean (±s.d.) mRNA expression levels of thyroid hormone receptor beta-1 (THrβ-1) in (A) adipose and (B) muscle, and mean (±s.e.m.) protein expression levels of THrβ-1 in (C) adipose and (D) muscle from fasting elephant seal pups. Insets: representative western blots for THrβ-1. Asterisks denote significant (P<0.05) differences from week 1.
Prolonged fasting increases adipose and muscle UCP2 mRNA expression
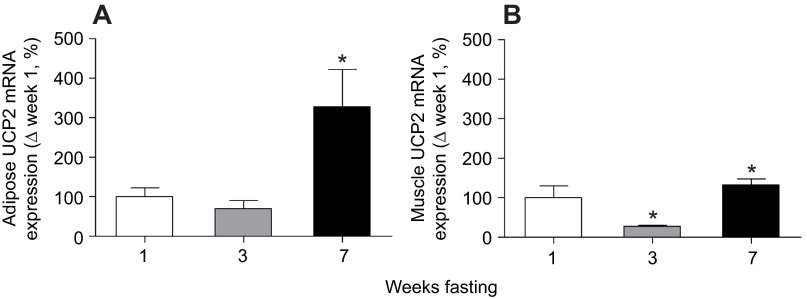
To assess the potential functionality of the increase in THrβ-1, the expression of adipose and muscle UCP2 was also measured. The mRNA expression of adipose UCP2 increased (P<0.05) threefold by week 7 (Fig. 4A). Mean muscle UCP2 mRNA expression decreased (P<0.05) by 74% in week 3, but expression levels more than completely recovered by week 7, increasing (P<0.05) by over 30% from week 1 (Fig. 4B).
Fig. 4.
Mean (±s.d.) mRNA expression levels of uncoupling protein-2 (UCP2) in (A) adipose and (B) muscle from fasted elephant seal pups. Asterisks denote significant (P<0.05) differences from week 1.
DISCUSSION
The typical responses to food deprivation in mammals are: (1) suppression of the HPT axis, (2) a reduction in TH levels and (3) downregulation of deiodinases and TH receptor to minimize the cellular actions of THs (Azizi, 1978; St Germain, 1994; Diano et al., 1998; Köhrle, 2000; Araujo et al., 2008; Vella et al., 2011; Oppenheimer et al., 1987; Araujo et al., 2009). Collectively, these responses curtail the energetic costs associated with TH-mediated cellular functions. Alternatively, metabolically quiescent hibernators such as the ground squirrel respond to hibernation-associated food deprivation differently: reducing TH metabolism, which results in elevated TH levels (Demeneix and Henderson, 1978a; Demeneix and Henderson, 1978b). However, in the presence of reduced receptor number and binding affinity (Magnus and Henderson, 1988a; Magnus and Henderson, 1988b), these elevated levels make these animals cryptically hyperthyroid and render these TH concentrations essentially non-functional. Furthermore, unlike humans and rodents, T3 levels remain constant while T4 levels decrease during fasting in the house musk shrew (Takeuchi et al., 2006; Boelen et al., 2006; Boelen et al., 2008), suggesting that deiodination and T3 utilization are increased, and subsequently cellular metabolism. The present study demonstrates that the TH-mediated response to fasting duration in northern elephant seal pups is atypical. While the TH-associated cellular response to fasting in elephant seals may be atypical, this response may have evolved to facilitate and support the energetic costs imposed by prolonged fasting, which in turn may allow us to characterize their postweaning fast as ‘cryptically fasting’. Prolonged fasting in elephant seals is considered ‘hyper-metabolic’ as they exhibit a metabolic rate that is approximately two times greater than can be estimated by body mass (Kleiber's law) (Rea and Costa, 1992). Furthermore, metabolism in breeding adult elephant seals is associated with increased plasma tT3, which is directly proportional to an increase in energetic expenditure (Crocker et al., 2012a; Crocker et al., 2012b; Kelso et al., 2012), suggesting that THs contribute to the active metabolism of fasting elephant seals regardless of age. Additionally, the present study highlights the diversity of TH-associated cellular response to food deprivation amongst higher vertebrates.
In a previous study on rats, plasma T3 and T4 decreased with acute food deprivation, but the activity and mRNA expression of DI2 increased (Coppola et al., 2005), suggesting that the HPT axis is suppressed. Fasting in humans is typically associated with a decrease in circulating tT3 and fT3 (Gavin et al., 1977; Burman et al., 1979; Spencer et al., 1983) and an increase in rT3 (Palmblad et al., 1977; Gardner et al., 1979; Spencer et al., 1983; Emerson et al., 1988) to diminish the energetic demands that food deprivation places on cellular metabolism (LoPresti et al., 1991). These changes compensate for the metabolic alterations associated with fasting, especially if stored substrates are limited. However, this may not be the case with fasting elephant seal pups as adipose can account for nearly 50% of their body mass during the early phase and is still approximately 45% in the late phase (Ortiz et al., 1978; Crocker et al., 1998; Noren et al., 2003; Crocker et al., 2012a; Crocker et al., 2012b). Thus, given this degree of adiposity throughout the fast and the elephant seals' reliance on lipid oxidation to maintain their metabolism, the lack of a decrease in TH concentrations may indicate that the animals are not substrate limited (Rea and Costa., 1992; Crocker et al., 2012b). Furthermore, the decrease in plasma T3 typically associated with food deprivation in mammals may be attributed to a decrease in DI1 and DI2 activity (Heemstra et al., 2009). In the present study, the mRNA expression of both DI1 and DI2 increased by week 7 in both muscle and adipose, suggesting that fasting duration is associated with an increase in cellular thyroid hormone activation. If not, at least the potential for increased TH-mediated cellular activation exists. With the exception of adipose DI1, the corresponding increases in the tissue content of DI1 and DI2 provide confirmation that the increased expression of the genes translated into increased protein content by week 7, potentially increasing cellular activity. The decrease in adipose DI1 protein despite the increase in mRNA expression suggests that adipose DI1 undergoes post-translational modification over the course of fasting duration. This response may provide an indication of differential metabolism of the enzyme between the two tissues. Furthermore, while some discrepancies exist between mRNA expression and parallel changes in protein expression, especially around week 3, we suspect that transitory changes in substrate metabolism during the early phase of fasting may account for these discrepancies. Thus, TH-related genes and cellular activity may be responding to this transitory phase, resulting in a shift in cellular activity during fasting metabolism. Nonetheless, the changes between weeks 1 and 7 provide a better reflection of the net effects of fasting duration, independent of the transitory changes observed in week 3. Alternatively, because DI1 is primarily responsible for the 5′-monodeiodination of T4 to produce rT3 (Gavin et al., 1977; Suda et al., 1978; Köhrle, 2000), adipose in elephant seals may possess a unique mechanism to suppress the translation of DI1 mRNA to ‘protect’ against the production of rT3 as a means of maintaining an adequate supply of cellular T3 to support TH-mediated activities. Because DI2 can promote the 5′-monodeiodination that is responsible for the formation of T3, the increase in adipose DI2 may compensate for the decrease in DI1 protein content, similar to the changes in hepatic DI1 and DI2 in fasting shrews (Takeuchi et al., 2006). The lack of a significant increase in plasma rT3 suggests that the increase in muscle DI1 protein content was not sufficient to alter circulating rT3 levels or that its increased activity was directed at the 5′ position. Furthermore, DI3, an enzyme that inactivates thyroid hormones, is not likely contributing to the observed levels of tT4, as tT4 levels remained steady in this study (Hernandez et al., 2010). Thus, the increase in DI2 may provide a better metric to assess increased cellular TH activation in peripheral tissues during prolonged food deprivation in fasting-adapted mammals. Nonetheless, the parallel increases in the expression of DI1 and DI2 in both adipose and muscle at week 7 suggest that the net regulation of these genes is similar in peripheral tissues during prolonged fasting.
After 24 h of food deprivation in mice, mRNA expression of THrβ-1 in peripheral tissues decreased (Boelen et al., 2006). In the present study, fasting duration was associated with increased THrβ-1 mRNA and protein expression, providing further indication of increased TH-mediated cellular activation. Similar to DI2, increased mRNA expression of the receptor coincided with increased protein expression, suggesting that, at the very least, adipose and muscle have the potential to increase TH-mediated effects. Additionally, the increase in the THrβ-1 target gene, UCP2, suggests that the increase in THrβ-1 was functional, as UCP2 mRNA expression was measured as a marker to assess the responsiveness of the receptor. While the role UCP2 plays in regulating cellular metabolism is not well defined, decreased hepatic UCP2 is associated with impaired lipid metabolism and antioxidant capacity in insulin-resistant rats (Montez et al., 2012). Because we have demonstrated that insulin resistance develops with fasting duration in elephant seal pups (Viscarra et al., 2011a; Viscarra et al., 2011b; Viscarra et al., 2012), but with enhanced lipid metabolism (Viscarra et al., 2012) and antioxidant capacity (Vázquez-Medina et al., 2011; Vázquez-Medina et al., 2012), the observed increase in UCP2 may contribute to the regulation of both lipid metabolism and oxidative stress in late-fasted pups. Collectively, the increases in the genes measured indicate that fasting duration is associated with upregulation of the TH-mediated signal transduction pathway in adipose and muscle in a select group of mammals uniquely adapted to periods of extensive food deprivation. Because the seal's metabolism during prolonged fasting relies primarily on lipid oxidation (Ortiz et al., 1978; Crocker et al., 2012a; Crocker et al., 2012b; Viscarra et al., 2012), the increase in the THrβ-1 suggests that THs likely contribute to the regulation of lipid metabolism (Liang and Ward, 2006). This is substantiated by the fact that plasma tT3 and daily energy expenditure from the measurement of field metabolic rates are positively correlated in fasting adult elephant seals (Crocker et al., 2012a; Kelso et al., 2012).
In fasted rats, plasma T3 and T4 concentrations fall in response to decreased thyrotropin-releasing hormone and TSH levels (Kmiec et al., 1996), suggesting that food deprivation suppresses the HPT axis. Similarly, circulating levels of TH decreased in wintering bears (Azizi et al., 1979). However, in hibernating ground squirrels, T3 levels increased (Demeneix and Henderson, 1978a; Demeneix and Henderson, 1978b) because of a decrease in metabolic clearance from circulation, and in the presence of reduced TH receptor number and binding affinity (Magnus and Henderson, 1988a; Magnus and Henderson, 1988b). This increase in plasma T3 is characteristic of cryptic hyperthyroidism, reducing the functionality of the elevated circulating levels. Alternatively, increased expression of DI2 facilitated the maintenance of T3 levels in fasting house musk shrews (Takeuchi et al., 2006). Thus, the lack of a decrease in plasma TH levels in the present study and previously reported increases (Ortiz et al., 2000; Ortiz et al., 2003) in the presence of increased TH-mediated signal transduction and cellular activation suggest that the axis must remain active to support the increased cellular metabolism and utilization of circulating levels. The end result is an active and functional HPT axis during prolonged fasting that contributes to TH-mediated cellular functions to support the active, normothermic metabolism of these seals, despite their protracted fast. Otherwise, increased cellular utilization of circulating THs and a suppressed HPT axis would result in significant decreases in circulating total TH concentrations, which was not observed here or previously (Ortiz et al., 2000; Ortiz et al., 2003). Collectively, an examination of the effects of food deprivation on circulating THs suggests that mammals have evolved a dynamic array of mechanisms regulating the HPT axis and TH-mediated cellular activity. This array of mechanisms may be thusly modulated to meet the energy demands of specific animals experiencing varying degrees of altered caloric restriction across a range of respiratory quotients (RQ≈0.73 in this case) (Rea and Costa, 1992).
In summary, despite a prolonged period of absolute food deprivation, we demonstrated that: (1) circulating concentrations of T4 and T3 are maintained, (2) the mRNA expression of DI1, DI2, THrβ-1 and UCP2 are increased, and (3) by week 7 the increases in mRNA expression are translated into increased protein levels. These cellular responses to fasting duration suggest that upregulation of TH-mediated cellular activity contributes, at least in part, to the sustained lipid-based metabolism of these animals (Ortiz et al., 1978; Houser and Costa, 2001; Noren et al., 2003; Houser et al., 2012; Viscarra et al., 2012). This apparent paradox suggests that an increase in TH activity is partially responsible for the maintenance of the relatively high metabolic rates observed in a fasting, but normothermic, mammal such as the northern elephant seal (Rea and Costa, 1992; Crocker et al., 1998). If so, then these animals may be characterized as ‘cryptically fasting’ as the lack of food intake does contribute to substrate depletion during this measurement period. Thus, given the dependence on lipid metabolism in fasting seals, increased cellular TH activity appears to contribute significantly to their lipid metabolism. Additionally, because these animals are weaned and developing, it is very likely that increased cellular TH activity is also contributing to the pups' postweaning development. Clearly, these animals have evolved divergent, yet robust, hormonal mechanisms that have allowed a select group of mammals to tolerate protracted periods of absolute food deprivation.
Perspectives
The RQ in morbidly obese humans is in the range of 0.82–0.85 (Goris and Westerterp, 2000; Marra et al., 2004), indicative of a protein-based metabolism, whereas the RQ in elephant seals is ~0.73 (lipid-based metabolism) throughout their fast (Rea and Costa, 1992); as such, these animals may serve as an intriguing model to better understand the TH-mediated mechanisms that dictate such a reliance on lipid metabolism that is not otherwise present in morbidly obese humans. A better understanding of these cellular metabolic mechanisms may elucidate new therapeutic targets of THs for human obesity. Nonetheless, the present study highlights: (1) a unique, atypical TH-associated response to food deprivation in a mammal reliant on lipid metabolism, and (2) the diversity of evolved endocrine mechanisms in mammals as they relate to prolonged food deprivation.
ACKNOWLEDGEMENTS
We thank M. Tift, S. Tavoni, C. Champagne and J. Cutler for their help sedating the seals. We thank Dr S. Jaques of the Texas Veterinary Medical Diagnostic Laboratory for TSH analysis.
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
J.G.S.-O. was supported by a postdoctoral fellowship from the University of California Institute for Mexico and the United States and Mexico's National Council for Science and Technology (UC MEXUS CONACYT). J.P.V.-M. is supported by fellowships from UC MEXUS, CONACYT and the University of California (Miguel Velez Fellowship, UC Merced GRC). J.A.V. is supported by a National Institutes of Health (NIH) National Heart, Lung and Blood Institute (NHLBI) Supplement to Support Diversity (NIH NHLBI R01HL09176-S). R.M.O. is partially supported by NHLBI (K02HL103787). Research was funded by NIH NHLBI (R01HL09176). Deposited in PMC for release after 12 months.
REFERENCES
- Araujo R. L., de Andrade B. M., de Figueiredo A. S., da Silva M. L., Marassi M. P., Pereira V. S., Bouskela E., Carvalho D. P. (2008). Low replacement doses of thyroxine during food restriction restores type 1 deiodinase activity in rats and promotes body protein loss. J. Endocrinol. 198, 119-125 [DOI] [PubMed] [Google Scholar]
- Araujo R. L., Andrade B. M., da Silva M. L., Ferreira A. C. F., Carvalho D. P. (2009). Tissue-specific deiodinase regulation during food restriction and low replacement dose of leptin in rats. Am. J. Physiol. 296, E1157-E1163 [DOI] [PubMed] [Google Scholar]
- Azizi F. (1978). Effect of dietary composition on fasting-induced changes in serum thyroid hormones and thyrotropin. Metabolism 27, 935-942 [DOI] [PubMed] [Google Scholar]
- Azizi F., Mannix J. E., Howard D., Nelson R. A. (1979). Effect of winter sleep on pituitary-thyroid axis in American black bear. Am. J. Physiol. 237, E227-E230 [DOI] [PubMed] [Google Scholar]
- Bassett J. H. D., Harvey C. B., Williams G. R. (2003). Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol. Cell. Endocrinol. 213, 1-11 [DOI] [PubMed] [Google Scholar]
- Boelen A., Kwakkel J., Vos X. G., Wiersinga W. M., Fliers E. (2006). Differential effects of leptin and refeeding on the fasting-induced decrease of pituitary type 2 deiodinase and thyroid hormone receptor beta2 mRNA expression in mice. J. Endocrinol. 190, 537-544 [DOI] [PubMed] [Google Scholar]
- Boelen A., Wiersinga W. M., Fliers E. (2008). Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid 18, 123-129 [DOI] [PubMed] [Google Scholar]
- Burman K. D., Wartofsky L., Dinterman R. E., Kesler P., Wannemacher R. W., Jr (1979). The effect of T3 and reverse T3 administration on muscle protein catabolism during fasting as measured by 3-methylhistidine excretion. Metabolism 28, 805-813 [DOI] [PubMed] [Google Scholar]
- Coppola A., Meli R., Diano S. (2005). Inverse shift in circulating corticosterone and leptin levels elevates hypothalamic deiodinase type 2 in fasted rats. Endocrinology 146, 2827-2833 [DOI] [PubMed] [Google Scholar]
- Crocker D. E., Webb P. M., Costa D. P., Le Boeuf B. J. (1998). Protein catabolism and renal function in lactating northern elephant seals. Physiol. Zool. 71, 485-491 [DOI] [PubMed] [Google Scholar]
- Crocker D. E., Houser D. S., Webb P. M. (2012a). Impact of body reserves on energy expenditure, water flux, and mating success in breeding male northern elephant seals. Physiol. Biochem. Zool. 85, 11-20 [DOI] [PubMed] [Google Scholar]
- Crocker D. E., Ortiz R. M., Houser D. S., Webb P. M., Costa D. P. (2012b). Hormone and metabolite changes associated with extended breeding fasts in male northern elephant seals (Mirounga angustirostris). Comp. Biochem. Physiol. 161A, 388-394 [DOI] [PubMed] [Google Scholar]
- Croxson M. S., Hall T. D., Kletzky O. A., Jaramillo J. E., Nicoloff J. T. (1977). Decreased serum thyrotropin induced by fasting. J. Clin. Endocrinol. Metab. 45, 560-568 [DOI] [PubMed] [Google Scholar]
- Demeneix B. A., Henderson N. E. (1978a). Thyroxine metabolism in active and torpid ground squirrels, Spermophilus richardsoni. Gen. Comp. Endocrinol. 35, 86-92 [DOI] [PubMed] [Google Scholar]
- Demeneix B. A., Henderson N. E. (1978b). Serum T4 and T3 in active and torpid ground squirrels, Spermophilus richardsoni. Gen. Comp. Endocrinol. 35, 77-85 [DOI] [PubMed] [Google Scholar]
- Diano S., Naftolin F., Goglia F., Horvath T. L. (1998). Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology 139, 2879-2884 [DOI] [PubMed] [Google Scholar]
- Eales J. G. (1988). The influence of nutritional state on thyroid function in various vertebrates. Am. Zool. 28, 351-362 [Google Scholar]
- Emerson C. H., Bambini G., Alex S., Castro M. I., Roti E., Braverman L. E. (1988). The effect of thyroid dysfunction and fasting on placenta inner ring deiodinase activity in the rat. Endocrinology 122, 809-816 [DOI] [PubMed] [Google Scholar]
- Fowler P. A. (1988). Seasonal endocrine cycles in the European hedgehog, Erinaceus europaeus. J. Reprod. Fertil. 84, 259-272 [DOI] [PubMed] [Google Scholar]
- Gardner D. F., Kaplan M. M., Stanley C. A., Utiger R. D. (1979). Effect of triiodothyronine replacement on the metabolic and pituitary responses to starvation. N. Engl. J. Med. 300, 579-584 [DOI] [PubMed] [Google Scholar]
- Gavin L., Castle J., McMahon F., Martin P., Hammond M., Cavalieri R. R. (1977). Extrathyroidal conversion of thyroxine to 3,3′,5′-triiodothyronine (reverse-T3) and to 3,5,3′-triiodothyronine (T3) in humans. J. Clin. Endocrinol. Metab. 44, 733-742 [DOI] [PubMed] [Google Scholar]
- Goris A., Westerterp K. R. (2000). Postabsorptive respiratory quotient and food quotient – an analysis in lean and obese men and women. Eur. J. Clin. Nutr. 54, 546-550 [DOI] [PubMed] [Google Scholar]
- Harris A. R. C., Fang S. L., Azizi F., Lipworth L., Vagenakis A. G., Barverman L. E. (1978). Effect of starvation on hypothalamic-pituitary-thyroid function in the rat. Metabolism 27, 1074-1083 [DOI] [PubMed] [Google Scholar]
- Heemstra K. A., Soeters M. R., Fliers E., Serlie M. J., Burggraaf J., van Doorn M. B., van der Klaauw A. A., Romijn J. A., Smit J. W., Corssmit E. P., et al. (2009). Type 2 iodothyronine deiodinase in skeletal muscle: effects of hypothyroidism and fasting. J. Clin. Endocrinol. Metab. 94, 2144-2150 [DOI] [PubMed] [Google Scholar]
- Herlihy J. T., Stacy C., Bertrand H. A. (1990). Long-term food restriction depresses serum thyroid hormone concentrations in the rat. Mech. Ageing Dev. 53, 9-16 [DOI] [PubMed] [Google Scholar]
- Hernandez A., Quignodon L., Martinez M. E., Flamant F., St Germain D. L. (2010). Type 3 deiodinase deficiency causes spatial and temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology 151, 5550-5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser D. S., Costa D. P. (2001). Protein catabolism in suckling and fasting northern elephant seal pups (Mirounga angustirostris). J. Comp. Physiol. B 171, 635-642 [DOI] [PubMed] [Google Scholar]
- Houser D. S., Crocker D. E., Tift M. S., Champagne C. D. (2012). Glucose oxidation and nonoxidative glucose disposal during prolonged fasts of the northern elephant seal pup (Mirounga angustirostris). Am. J. Physiol. 303, R562-R570 [DOI] [PubMed] [Google Scholar]
- Kelso E. J., Champagne C. D., Tift M. S., Houser D. S., Crocker D. E. (2012). Sex differences in fuel use and metabolism during development in fasting juvenile northern elephant seals. J. Exp. Biol. 215, 2637-2645 [DOI] [PubMed] [Google Scholar]
- Kmiec Z., Kotlarz G., Smiechowska B., Mysliwski A. (1996). Thyroid hormones homeostasis in rats refed after short-term and prolonged fasting. J. Endocrinol. Invest. 19, 304-311 [DOI] [PubMed] [Google Scholar]
- Köhrle J. (2000). The deiodinase family: selenoenzymes regulating thyroid hormone availability and action. Cell. Mol. Life Sci. 57, 1853-1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Boeuf B. J., Laws R. M. (eds) (1994). Elephant seals: an introduction to the genus. In Elephant Seals: Population Ecology, Behavior and Physiology, pp. 1-21 Berkeley, CA: University of California Press; [Google Scholar]
- Liang H., Ward W. F. (2006). PGC-1α: a key regulator of energy metabolism. Adv. Physiol. Educ. 30, 145-151 [DOI] [PubMed] [Google Scholar]
- LoPresti J. S., Gray D., Nicoloff J. T. (1991). Influence of fasting and refeeding on 3,3′,5′-triiodothyronine metabolism in man. J. Clin. Endocrinol. Metab. 72, 130-136 [DOI] [PubMed] [Google Scholar]
- Magnus T. H., Henderson N. E. (1988a). Thyroid hormone resistance in hibernating ground squirrels, Spermophilus richardsoni. I. Increased binding of triiodo-l-thyronine and l-thyroxine by serum proteins. Gen. Comp. Endocrinol. 69, 352-360 [DOI] [PubMed] [Google Scholar]
- Magnus T. H., Henderson N. E. (1988b). Thyroid hormone resistance in hibernating ground squirrels, Spermophilus richardsoni. II. Reduction of hepatic nuclear receptors. Gen. Comp. Endocrinol. 69, 361-371 [DOI] [PubMed] [Google Scholar]
- Marra M., Scalfi L., Contaldo F., Pasanisi F. (2004). Fasting respiratory quotient as a predictor of long-term weight changes in non-obese women. Ann. Nutr. Metab. 48, 189-192 [DOI] [PubMed] [Google Scholar]
- McNabb F. M. A. (1992). Thyroid Hormones. Upper Saddle River, NJ: Prentice Hall PTR; [Google Scholar]
- Montez P., Vázquez-Medina J. P., Rodríguez R., Thorwald M. A., Viscarra J. A., Lam L., Peti-Peterdi J., Nakano D., Nishiyama A., Ortiz R. M. (2012). Angiotensin receptor blockade recovers hepatic UCP2 expression and aconitase and SDH activities and ameliorates hepatic oxidative damage in insulin resistant rats. Endocrinology 153, 5746-5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T., Chapa F., DiStefano J. J., III (1998). Direct measurement of the contributions of type I and type II 5′-deiodinases to whole body steady state 3,5,3′-triiodothyronine production from thyroxine in the rat. Endocrinology 139, 4626-4633 [DOI] [PubMed] [Google Scholar]
- Noren D. P., Crocker D. E., Williams T. M., Costa D. P. (2003). Energy reserve utilization in northern elephant seal (Mirounga angustirostris) pups during the postweaning fast: size does matter. J. Comp. Physiol. B 173, 443-454 [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Mariash C. N., Kinlaw W. B., Wong N. C. W., Freake H. C. (1987). Advances in our understanding of thyroid hormone action at the cellular level. Endocr. Rev. 8, 288-308 [DOI] [PubMed] [Google Scholar]
- Ortiz C. L., Costa D., Le Boeuf B. J. (1978). Water and energy flux in elephant seal pups fasting under natural conditions. Physiol. Zool. 51, 166-178 [Google Scholar]
- Ortiz R. M., MacKenzie D. S., Worthy G. A. (2000). Thyroid hormone concentrations in captive and free-ranging West Indian manatees (Trichechus manatus). J. Exp. Biol. 203, 3631-3637 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Wade C. E., Ortiz C. L. (2001). Effects of prolonged fasting on plasma cortisol and TH in postweaned northern elephant seal pups. Am. J. Physiol. 280, R790-R795 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Wade C. E., Costa D. P., Ortiz C. L. (2002). Renal responses to plasma volume expansion and hyperosmolality in fasting seal pups. Am. J. Physiol. 282, R805-R817 [DOI] [PubMed] [Google Scholar]
- Ortiz R. M., Houser D. S., Wade C. E., Ortiz C. L. (2003). Hormonal changes associated with the transition between nursing and natural fasting in northern elephant seals (Mirounga angustirostris). Gen. Comp. Endocrinol. 130, 78-83 [DOI] [PubMed] [Google Scholar]
- Palmblad J., Levi L., Burger A., Melander A., Westgren U., von Schenck H., Skude G. (1977). Effects of total energy withdrawal (fasting) on thelevels of growth hormone, thyrotropin, cortisol, adrenaline, noradrenaline, T4, T3, and rT3 in healthy males. Acta Med. Scand. 201, 15-22 [DOI] [PubMed] [Google Scholar]
- Pramfalk C., Pedrelli M., Parini P. (2011). Role of thyroid receptor β in lipid metabolism. Biochim. Biophys. Acta 1812, 929-937 [DOI] [PubMed] [Google Scholar]
- Rea L. D., Costa D. P. (1992). Changes in standard metabolism during long-term fasting in northern elephant seal pups (Mirounga angustirostris). Physiol. Zool. 65, 97-111 [Google Scholar]
- Reitman M. L., He Y., Gong D. W. (1999). Thyroid hormone and other regulators of uncoupling proteins. Int. J. Obes. Relat. Metab. Disord. 23 Suppl. 6, S56-S59 [DOI] [PubMed] [Google Scholar]
- Sambrook J., Russell D. W. (2006). SDS-polyacrylamide gel electrophoresis of proteins. Cold Spring Harb. Protoc. 2006, 4540 [Google Scholar]
- Soñanez-Organis J. G., Vázquez-Medina J. P., Zenteno-Savín T., Aguilar A., Crocker D. E., Ortiz R. M. (2012). Prolonged fasting increases purine recycling in post-weaned northern elephant seals. J. Exp. Biol. 215, 1448-1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C. A., Lum S. M. C., Wilber J. F., Kaptein E. M., Nicoloff J. T. (1983). Dynamics of serum thyrotropin and thyroid hormone changes in fasting. J. Clin. Endocrinol. Metab. 56, 883-888 [DOI] [PubMed] [Google Scholar]
- St Germain D. L. (1994). Iodothyronine deiodinase. Trends Endocrinol. Metab. 5, 36-42 [DOI] [PubMed] [Google Scholar]
- Suda A. K., Pittman C. S., Shimizu T., Chambers J. B., Jr (1978). The production and metabolism of 3,5,3′-triiodothyronine and 3,3′,5-triiodothyronine in normal and fasting subjects. J. Clin. Endocrinol. Metab. 47, 1311-1319 [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Suzuki D., Oda S. I., Refetoff S., Seki K., Tsunekawa K., Kasahara T., Murakami M., Murata Y. (2006). Unique regulation of thyroid hormone metabolism during fasting in the house musk shrew (Suncus murinus, Insectivora: Soricidae). Gen. Comp. Endocrinol. 146, 236-241 [DOI] [PubMed] [Google Scholar]
- van Hardeveld C. (1986). Effects of thyroid hormone on oxygen consumption, heat production and energy economy. In Thyroid Hormone Metabolism (ed. Hennemann G.), pp. 579-608 New York, NY: Marcel Dekker; [Google Scholar]
- van Heyningen C., Glaysher J. (2012). Lipid metabolism: thyroid hormone effects on lipid metabolism. Curr. Opin. Lipidol. 23, 584-585 [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Tift M. S., Forman H. J., Crocker D. E., Ortiz R. M. (2011). Apnea stimulates the adaptive response to oxidative stress in elephant seal pups. J. Exp. Biol. 214, 4193-4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Elsner R., Ortiz R. M. (2012). Coping with physiological oxidative stress: a review of antioxidant strategies in seals. J. Comp. Physiol. B 182, 741-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella K. R., Ramadoss P., Lam F. S., Harris J. C., Ye F. D., Same P. D., O'Neill N. F., Maratos-Flier E., Hollenberg A. N. (2011). NPY and MC4R signaling regulate thyroid hormone levels during fasting through both central and peripheral pathways. Cell Metab. 14, 780-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra J. A., Vázquez-Medina J. P., Crocker D. E., Ortiz R. M. (2011a). Glut4 is upregulated despite decreased insulin signaling during prolonged fasting in northern elephant seal pups. Am. J. Physiol. 300, R150-R154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra J. A., Champagne C. D., Crocker D. E., Ortiz R. M. (2011b). 5'AMP-activated protein kinase activity is increased in adipose tissue of northern elephant seal pups during prolonged fasting-induced insulin resistance. J. Endocrinol. 209, 317-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscarra J. A., Vázquez-Medina J. P., Rodriguez R., Champagne C. D., Adams S. H., Crocker D. E., Ortiz R. M. (2012). Decreased expression of adipose CD36 and FATP1 are associated with increased plasma non-esterified fatty acids during prolonged fasting in northern elephant seal pups (Mirounga angustirostris). J. Exp. Biol. 215, 2455-2464 [DOI] [PMC free article] [PubMed] [Google Scholar]