SUMMARY
Deep hibernators such as golden-mantled ground squirrels (Callospermophilus lateralis) have multiple challenges to cardiac function during low temperature torpor and subsequent arousals. As heart rates fall from over 300 beats min−1 to less than 10, chamber dilation and reduced cardiac output could lead to congestive myopathy. We performed echocardiography on a cohort of individuals prior to and after several months of hibernation. The left ventricular chamber exhibited eccentric and concentric hypertrophy during hibernation and thus calculated ventricular mass was ~30% greater. Ventricular ejection fraction was mildly reduced during hibernation but stroke volumes were greater due to the eccentric hypertrophy and dramatically increased diastolic filling volumes. Globally, the systolic phase in hibernation was ~9.5 times longer, and the diastolic phase was 28× longer. Left atrial ejection generally was not observed during hibernation. Atrial ejection returned weakly during early arousal. Strain echocardiography assessed the velocity and total movement distance of contraction and relaxation for regional ventricular segments in active and early arousal states. Myocardial systolic strain during early arousal was significantly greater than the active state, indicating greater total contractile movement. This mirrored the increased ventricular ejection fraction noted with early arousal. However, strain rates were slower during early arousal than during the active period, particularly systolic strain, which was 33% of active, compared with the rate of diastolic strain, which was 67% of active. As heart rate rose during the arousal period, myocardial velocities and strain rates also increased; this was matched closely by cardiac output. Curiously, though heart rates were only 26% of active heart rates during early arousal, the cardiac output was nearly 40% of the active state, suggesting an efficient pumping system. We further analyzed proportions of cardiac myosin heavy-chain (MyHC) isoforms in a separate cohort of squirrels over 5 months, including time points before hibernation, during hibernation and just prior to emergence. Hibernating individuals were maintained in both a 4°C cold room and a 20°C warm room. Measured by SDS-PAGE, relative percentages of cardiac MyHC alpha were increased during hibernation, at both hibernacula temperatures. A potential increase in contractile speed, and power, from more abundant MyHC alpha may aid force generation at low temperature and at low heart rates. Unlike many models of cardiomyopathies where the alpha isoform is replaced by the beta isoform in order to reduce oxygen consumption, ground squirrels demonstrate a potential cardioprotective mechanism to maintain cardiac output during torpor.
KEY WORDS: hibernatiaon, MyHC, echocardiogram, atrial contraction, cardiac function
INTRODUCTION
The heart of mammals is a wondrously adaptable organ, responding to changing hemodynamic conditions on a minute-to-minute basis. The cardiac phenotype of any species is complex and can be altered by neural input, metabolic conditions, hormonal or chemical alterations and aging. Hibernating mammals provide powerful opportunities to study unusual cardiovascular biology and muscle adaptations in animals undergoing dramatic ranges of hemodynamic conditions. How cardiac muscle senses and responds to varying stimuli, including the hibernation state, captures biological as well as clinical interest. Hibernators provide an unusually altered perspective on mechanisms that control cardiac chamber activity, functional capacity, muscle mass and protein expression.
The golden-mantled ground squirrel (Callospermophilus lateralis) is a prototypical deep hibernator, manifesting dramatic seasonal and within-season metabolic variation. Individuals transition from an active, euthermic state (37°C) to a hibernating state where torpid body temperature falls to 3–5°C but is punctuated repeatedly by arousals to euthermy. During bouts of torpor over a 6 month hibernation, these animals may be completely inactive for 2 weeks at a time at near-freezing temperatures, while arousals may last 24 h. Cardiovascular adaptations must occur for the myocardium to remain healthy and efficient during a period of extremely low temperatures, and low heart rate and cardiac output (Folk et al., 1970; Caprette and Senturia, 1984; Milsom et al., 1993; Milsom et al., 1999; Burlington and Darvish, 1998; Brauch et al., 2005). Non-hibernators that suffer from low heart rate conditions alone will develop cardiac chamber remodeling and congestive heart failure over time (Kertesz et al., 1997; Verduyn et al., 2001; Schoenmakers et al., 2003). The mechanisms that circumvent cardiac dysfunction during hibernation are not well understood, but they may partly involve alterations in sarcomeric proteins (Nelson et al., 2008; Barrows et al., 2011).
The myosin heavy-chain isoforms (MyHC) are crucial determinants of contractile performance in cardiac muscle tissue. These motor proteins differ in their parameters of velocity and force produced (Alpert et al., 2002; Galler et al., 2002; Stelzer et al., 2007); cardiac alpha (α) and beta (β) MyHC isoforms can be differentially expressed by heart chamber, as well as by mass and age of the individual (Dool et al., 1995; Miyata et al., 2000; Barrows et al., 2011). The myocardium is plastic, similar to skeletal muscle, and mechanical loading and other factors can shift the relative isoform abundance of the cardiac motor proteins. Exercise training or, conversely, relative sedentary states can radically alter cardiac muscle mass as well as the expression of MyHC isoforms (Schreiber et al., 1981; Pagani and Solaro, 1983; McDermott et al., 1985; Schaible and Scheuer, 1985; Izumo et al., 1987; Morris et al., 1990; Morgan and Baker, 1991; Klein et al., 1992; Tanamura et al., 1993; Geenen et al., 1994; Wade et al., 1999).
Ground squirrels have several challenges to cardiac function during the months of hibernation. First, cardiac pumping function must be maintained at low temperatures, far beyond the onset of human myocardial contraction failure. Some of the adaptations to permit this are related to calcium channel function (Wang et al., 2002; Hauton et al., 2011). However, the force from motor proteins at low temperatures is reduced (Rall and Woledge, 1990; Piazzesi et al., 2003; Woledge et al., 2009), and yet ground squirrel hearts function at near-freezing temperatures, while simultaneously supporting cardiac performance when body temperature returns to euthermy, a temperature differential of over 30°C. Second, the activity of the heart, as quantified by heart rate, ranges from over 350–400 beats min−1 prior to hibernation, to a low of ~4–5 beats min−1 during torpor. Interbout arousals necessitate a resumption of relatively high heart rates, with unknown consequences of compromised chamber function following 2 weeks of inactivity, and therefore minimal cardiac pumping. Early observations of this phenomenon suggest a marvelous paradox that the heart may undergo hypertrophy during hibernation (Wickler et al., 1991). Would this be influenced by requirements of pumping at low temperature and low heart rates, or by repeatedly supporting highly aerobic arousals from torpor?
Echocardiography is a powerful diagnostic tool that can provide in vivo measurements of cardiac pumping function and blood flow dynamics, and assessment of heart chamber wall hypertrophy. Because echocardiography is non-invasive, it may facilitate evaluation of more natural physiologic responses. Echocardiography has been applied previously to a single hibernator, the grizzly bear (Nelson et al., 2008; Nelson and Robbins, 2010). Comparison of the deep hibernation and low temperature of squirrels with the shallow hibernation and warmer body temperature of bears is an additional aim, and is aided by application of nearly identical methodologies (Barrows et al., 2011). We predicted that hibernating ground squirrels would demonstrate more profound changes in cardiac remodeling and functional parameters because of the added challenges of near-freezing body temperature and the far greater change in heart rate.
We evaluated cardiac function and myosin isoform expression in the myocardium of ground squirrels during deep hibernation and arousal bouts, compared with the seasonally active period just prior to hibernation. For echocardiography, we utilized unanesthetized subjects to avoid the confounding effect of anesthesia on cardiac function. Based on indications of cardiac plasticity during hibernation in bears and ground squirrels (Wickler et al., 1991; Wang et al., 2002; Nelson et al., 2008; Barrows et al., 2011; Hauton et al., 2011), we hypothesized that MyHC isoforms also would shift expression in response to low heart rate and subsequent hemodynamic changes. These changes in myosin isoform expression would allow cardiac adaptation to low temperatures and slower contractile indices during hibernation. Given a previous observation of hypertrophy in hearts of hibernating squirrels (Wickler et al., 1991), and the well-described responses to hypertrophy in non-hibernating rodents (Pagani and Solaro, 1983; Schaible and Scheuer, 1985; Izumo et al., 1987; Dool et al., 1995; Wade et al., 1999), we predicted an increase in MyHC-β. We more fully characterized a hypertrophy of the heart chambers with hibernation, but found instead an increase in MyHC-α. This is similar to hamsters (Morano et al., 1992), but ground squirrels differ in pre-emptively altering MyHC isoform profiles, which change even without low temperature torpor. The unusual MyHC plasticity in squirrels may represent an important mechanism preventing the negative consequences of chronic hypothermia and reduced cardiac output on heart function, while also serving the dramatic metabolic and pumping requirements of repeated arousals from torpor.
MATERIALS AND METHODS
Experimental animals and groups
Two cohorts of adult golden-mantled ground squirrels, C. lateralis (Say 1823), were field-captured near Redding, CA, USA, in August 2007, and in September 2009, under a California Department of Fish and Game Scientific Collecting Permit (BCR). They were transported to California State University, Long Beach, in group cages by automobile following a 1 week field quarantine. Individuals were sorted by sex into large (1 m3) group cages of 4–6 animals, and quarantined for an additional week. The 2007 cohort was used for the tissue sampling, and was composed of individuals (N=60; 39 females, 21 males) used in a related and simultaneous study on hindlimb musculature (see Nowell et al., 2011). Molecular protocols were only carried out on animals from the 2007 cohort, sorted into seven seasonal groups. Echocardiogram measurements were predominantly from the 2009 cohort (20 animals), but with some 2007 animals (4, including one lab-born juvenile).
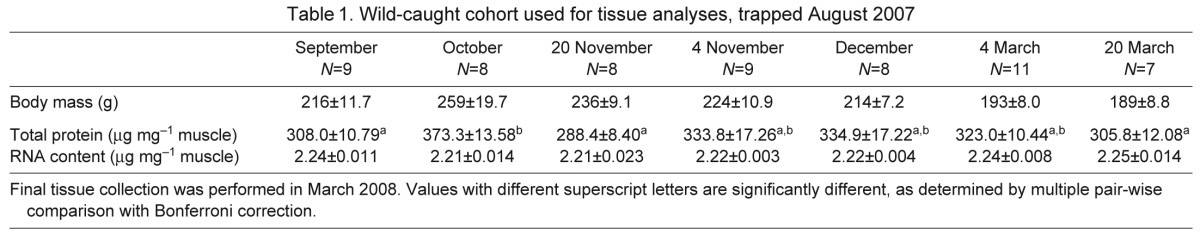
The colony room was held between 18 and 20°C, and lighting was changed daily to coincide with local sunrise and sunset. Animals were given cotton bedding over woodchips. Squirrels were provided with water, rodent blocks and sunflower seeds ad libitum, supplemented with fresh fruit and vegetables. Following quarantine, the 2007 cohort of squirrels was matched for mass, placed singly into small cages and sorted among groups. These were killed progressively as the hibernation season continued over the next 6 months from September 2007 until March 2008 (N for each group, see Table 1). One group was killed in September and one in October, and the remaining groups were designated as hibernators. In October, three hibernating groups were placed into a darkened cold room hibernaculum at 4°C, while two remained in the colony room at 18–20°C. This gave seven groups: autumn (September), pre-hibernators (October), early hibernators (November, at 20 and 4°C), mid-hibernators (December, at 4°C only), and late hibernators (March, at 20 and 4°C). At each time point, the group was killed within 48 h from the start.
Table 1.
Wild-caught cohort used for tissue analyses, trapped August 2007
Hibernators were provided food and water, but squirrels at 4°C typically did not eat, while those maintained at 20°C did so sporadically. Both warm and cold room hibernators entered bouts of torpor lasting several days up to a maximum of 12 days. We placed temperature dataloggers (iButton, Maxim Integrated Products Inc., Sunnyvale, CA, USA; sample rate 10 min) directly under the cotton bedding of the nested squirrels at 4°C. These were used to help confirm our daily observations of activity and torpor status. Arousals were clearly observable as increases in the probe temperature, but primary determination was performed via daily observation of the animals to record posture and breathing rate. Cold room animals exhibited prototypical bouts of deep torpor and periodic arousal to euthermy. Warm room animals showed pronounced torpor behavior of curled posture and unresponsiveness to handling, but were unable to decrease body temperature below the room temperature of 18–20°C. This shallow torpor was spontaneous and not induced by a decrease in room temperature or withdrawal of food.
All animals from which cardiac tissues were collected were killed by intracardiac injection of sodium pentobarbital, following ketamine anesthesia. Cold room hibernators were killed while in deep torpor at 4°C, and warm room animals were killed during shallow torpor. All animal care protocols were approved by the California State University, Long Beach, Institutional Animal Care and Use Committee, and carried out in accordance with NIH guidelines.
Twenty-four (12 female, 12 male) squirrels were used for gathering echocardiogram data for this study. Prehibernation data were collected from the 24 squirrels in September 2009, but only 16 were subsequently sampled in January 2010 during hibernation because of investigator travel constraints (8 females, 8 males). These squirrels were used unanesthetized to avoid the potential confounding effects of anesthesia on in vivo assessment of cardiac function by echocardiography (Roth et al., 2002; Stein et al., 2007). Cardiac function thus was measured during the active phase of the year (September) and the hibernation period (January).
Echocardiography
Squirrels were manually restrained for a complete transthoracic echocardiographic examination that included 2-dimensional, M-mode, spectral and color-flow Doppler evaluations. Echocardiography was performed by the same person (O.L.N.) using commercially available equipment (Biosound Esaote, MyLab 30, Indianapolis, IN, USA). Standard imaging planes and function calculations have been previously described for rodents (Collins et al., 2001; Stypmann et al., 2006). Left ventricular mass calculations were performed on M-mode exam taken from the right parasternal short-axis view at the level of the papillary muscles (Fig. 1). All measures were performed in accordance with the American Society of Echocardiography for cardiac volume and functional calculations (Schiller, 1991; Kuecherer et al., 1991; Ommen et al., 2000; Garcia et al., 2001). Video images were captured using a commercially available digital echocardiography software program and data were collected off-line using a workstation (Biosound Esaote, MyLab Desk, Indianapolis, IN, USA). The mean of four consecutive measurements was considered average for each parameter during each data collection period.
Fig. 1.
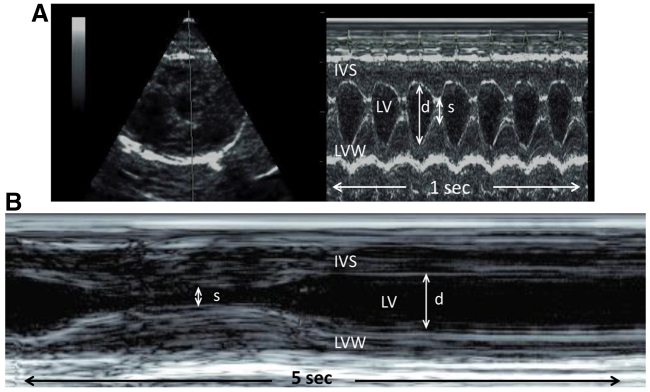
M-mode echocardiography in an active (A) and hibernating (B) ground squirrel. Left ventricular free wall (LVW) and interventricular septum (IVS) in diastole used to calculate cardiac mass. The left ventricular (LV) internal chamber dimensions are depicted by the arrowed lines in diastole (d) and in systole (s).
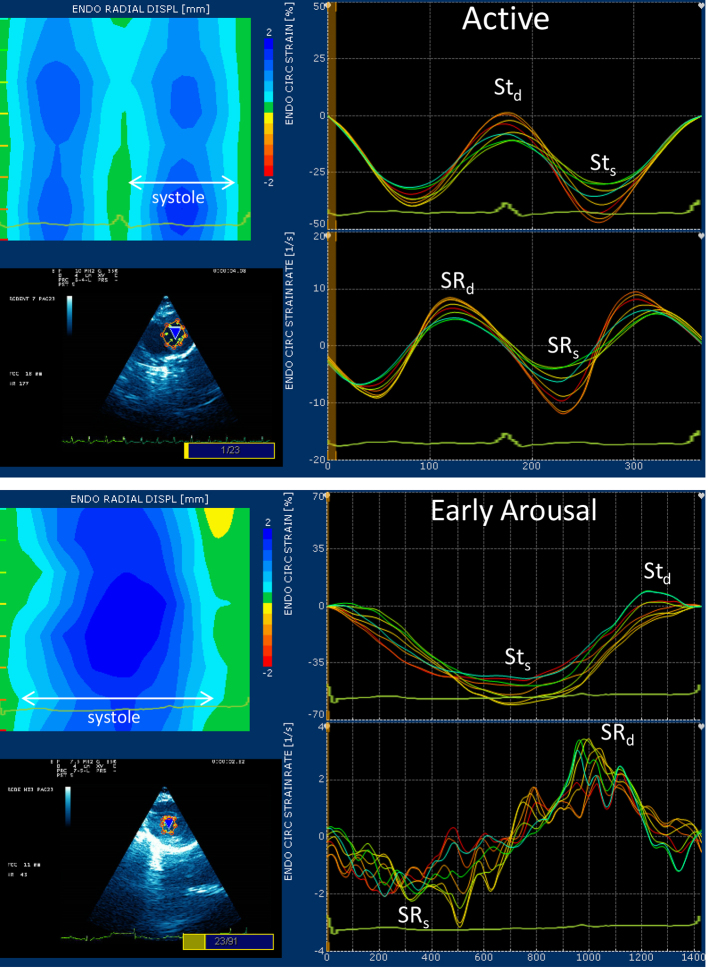
Myocardial strain and strain rate evaluation is a 2-dimensional ultrasound technique that uses Doppler tissue imaging to assess segmental myocardial function non-invasively (Fig. 2). This technique defines myocardial timing, contractility and deformation and, unlike conventional Doppler, is not dependent on the angle of incidence (Langeland et al., 2005; Notomi et al., 2005). Strain (St) imaging examines myocardial deformation (percentage change in distance traveled by the regional segments) and strain rate (SR) measures the rate of change of segments. Strain imaging was performed from the right parasternal short axis view at the level of the papillary muscles, and the variables recorded were radial myocardial velocity (systole, diastole), and strain and strain rate (systole, diastole). Eight points of interest were tagged along the circumferential endocardial border. Each point depicted movement of that specific myocardial point, and is traced over time. A best-fit line was generated and considered the average value of the eight regions. The strain software package had a lower detection limit of 40 beats min−1 based on an instantaneous heart rate calculation determined by measuring the time interval between two consecutive beats. Thus, the first strain analysis obtained in January was during the early arousal period when heart rates reached 40 beats min−1 or greater.
Fig. 2.
Strain echocardiography graphs in a representative Callospermophilus lateralis during active and hibernating/early arousal states. The lower left-hand corner of each panel depicts the left ventricular chamber with eight points tagged to the circumferential endocardial region. Movement of each of these regions is displayed as a colored line in the graphs to the right (endocardial circumferential strain and strain rate). Higher values of systolic strain (Sts) were found in the hibernation–early arousal period; diastolic strain (Std) was not significantly different. Strain rates were lower during early arousal, particularly the rate of systolic strain (SRs), which was a fraction of the active SRs, in comparison with the rate of diastolic strain (SRd), which tended to be closer to active SRd. Note the overall undulation of the SR values. This finding was common in early arousal and suggests heterogeneity in the rates of myocardial deformation across regions and throughout progression of the cardiac cycle. The upper left-hand corner of each panel depicts radial displacement of endocardial wall motion. Increasing intensities of blue color indicate increasing distances of myocardial movement as systole progresses. Note the greater gradation of movement and the undulating pattern of the early arousal period that correlates to the heterogeneity of the strain rate curve. In this individual, systolic contraction was 155 ms in the active period and 1090 ms during early arousal.
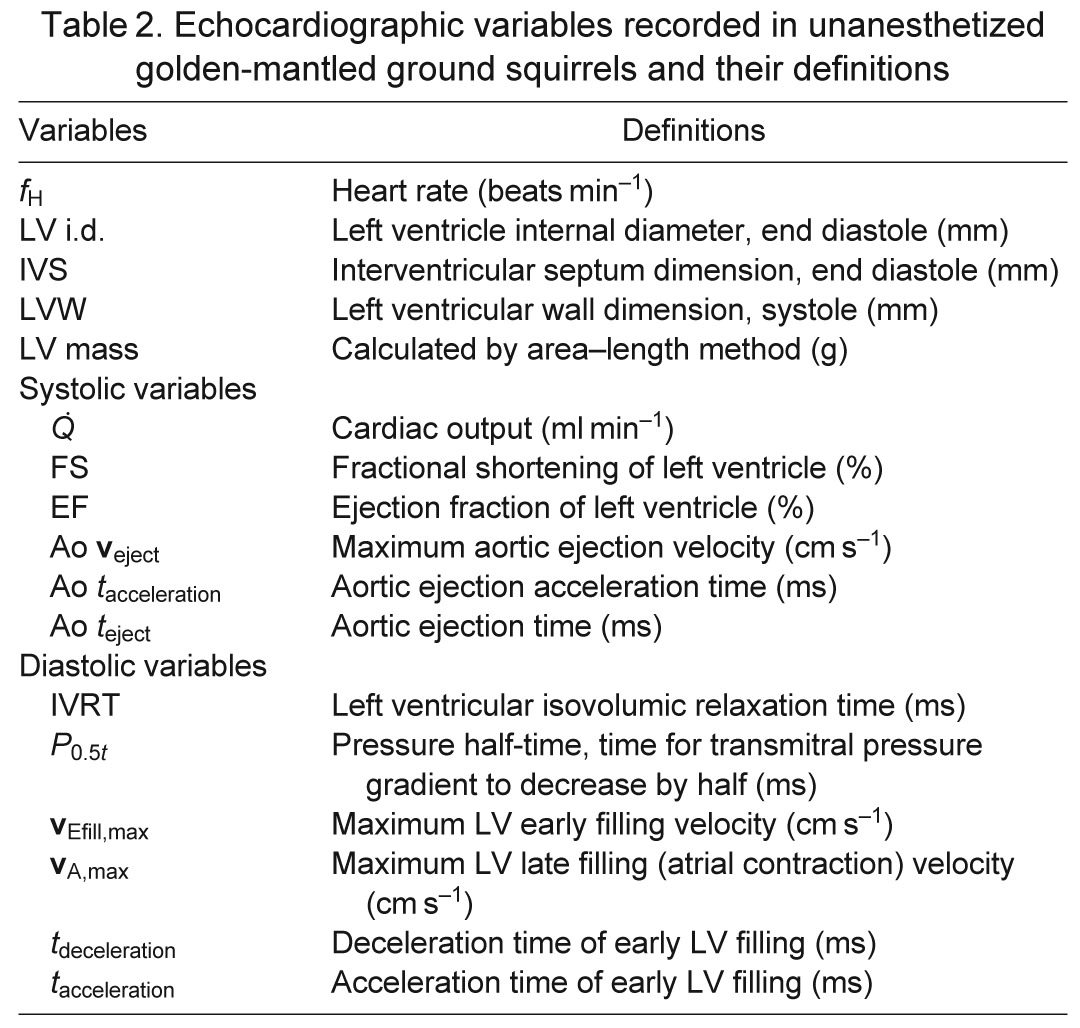
Studies were performed at a room temperature of 20°C during the active period (September 2009), and at 4 and 20°C during hibernation (January 2010). During hibernation, equipment and personnel remained in the cold room with the animals, and a complete echocardiographic study was performed. The animals then were allowed to arouse for comparative study at different heart rates. During this time the animals and equipment were removed from the cold room and monitored until heart rates reached 40 and 100 beats min−1, at a room temperature of 20°C. Body temperature was unknown during this portion of echocardiography. The average time from initial handling to a heart rate of 40 beats min−1 was ~8 min, whereas the average time from initial handling to a heart rate of 100 beats min−1 was ~22 min. The four groups used for echocardiography were categorized as active (A), torpid state while in cold room (H), and aroused from torpor to a heart rate of ~40 beats min−1 (fH40) and subsequently to a heart rate of ~100 beats min−1 (fH100) at a room temperature of 20°C. Table 2 lists the standard echocardiography variables and definition for each.
Table 2.
Echocardiographic variables recorded in unanesthetized golden-mantled ground squirrels and their definitions
Tissue preparation, and protein and nucleic acid content
Immediately after the animals of the 2007–2008 cohort were killed, the heart was rapidly frozen on dry ice, and then transferred to −80°C until further processing. Hearts could not be weighed reliably because of the requirement for an intracardiac injection of pentobarbital to ensure lethality while the animals remained in torpor. A 20–30 mg sample of ventricles (mixed right and left) was homogenized with a glass pestle for 15 s in 19 vol. of ice-cold protein buffer (250 mmol l−1 sucrose, 100 mmol l−1 KCl, 5 mmol l−1 EDTA) for total protein determination and electrophoresis. Total protein concentration was assayed in triplicate on a microplate spectrophotometer (BioTek, Winooski, VT, USA) using a commercially available kit (Bio-Rad, Hercules, CA, USA) and IgG as standard.
A second, small (15–20 mg) sample of ventricular muscle was homogenized at high speed with a 5 mm metal grinder in TriReagent (Molecular Research Center, Cincinnati, OH, USA) for extraction of total RNA. Phase separation was initiated by addition of BCP reagent (Molecular Research Center), and a fixed volume of the aqueous portion was withdrawn. Pellets were precipitated with isopropanol, and then rinsed twice with 75% ethanol. The RNA pellets were dried in an evaporator, and solubilized in 20 ml of DNase/RNase-free water. Absorbance was read on a UV microplate (BioTek) at 260 and 280 nm.
MyHC isoform analyses
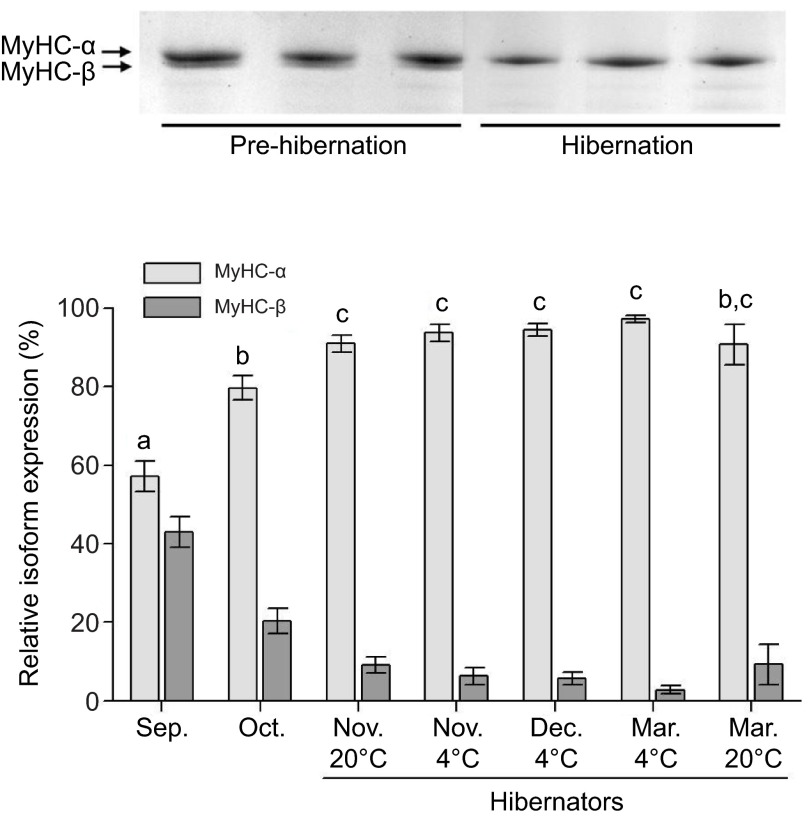
MyHC protein isoforms were separated by SDS-PAGE analysis, as described previously for this species (Rourke et al., 2004a) (modified from Talmadge and Roy, 1993). Although cardiac isoforms in rodent models are more typically assayed by native-conformation protein gels, in C. lateralis we were fortunate to resolve MyHC-α and MyHC-β easily by SDS-PAGE, using exactly the same conditions as for skeletal muscle. Electrophoresis of individual muscle samples utilized total protein fractions from each muscle, and was performed in duplicate (see Fig. 3). Gels were visualized with silverstain (Bio-Rad) and relative isoform percentages were analyzed by densitometry (ImageQuant, GE Healthcare, Piscataway, NJ, USA). Skeletal muscle samples containing type I/beta protein were used as a molecular marker in SDS gels.
Fig. 3.
(Top) SDS-PAGE of cardiac myosin heavy-chain (MyHC)-α and MyHC-β protein isoforms in C. lateralis. (Bottom) MyHC isoform protein profiles of ventricles from ground squirrels, hibernating during the months of November through to March. Hibernators include animals that displayed torpor in 4 and 20°C hibernacula. October individuals show a seasonal increase in MyHC-α prior to hibernation; 20°C March individuals show a return to pre-hibernating isoform profiles.
The cardiac MyHC-β gene is equivalent to slow type 1 MyHC when expressed in skeletal muscle. Therefore, corroboration of the isoform types was aided by our earlier identification of skeletal slow type 1 MyHC, through antibody typing and partial sequencing of cDNA (Rourke et al., 2004a; Rourke et al., 2004b). Here, we partially sequenced the MyHC-α gene using identical methodology, starting with total RNA isolated from ventricular tissue of adult squirrels and 3′ RACE.
MyHC mRNA expression
Total RNA was reverse-transcribed (1 μg) to cDNA using SuperScript III (Invitrogen, Carlsbad, CA, USA), and was used for determination of mRNA expression of cardiac MyHC-α and MyHC-β genes. Primers for PCR reactions of cardiac MyHC were developed for this study (Table 3) as implemented in rats (see Wright et al., 1997), and now numerously in hibernators [C. lateralis skeletal muscle (Rourke et al., 2004b; Choi et al., 2009), prairie dog and black bear (Rourke et al., 2006), grizzly bear heart (Barrows et al., 2011)]. All reactions were carried out on a thermocycler (Eppendorf, Hamburg, Germany), at an annealing temperature of 54°C, with an initial 3 min, 94°C start, 28 cycles of annealing and extension (94°C 45 s, 54°C 45 s, 72°C 30 s; see below for 68°C permutation) and a final 72°C step. PCR products were separated in horizontal 2% agarose gels, stained with SybrGreen (Invitrogen), and photographed with a digital camera and a SybrGreen filter. Bands were analyzed by densitometry in ImageQuant. In the primary implementation of this protocol, two multiplex reactions were performed for each muscle sample, composed of a separate reaction for MyHC-α and for MyHC-β. Each reaction amplified two substrates as well: one a cDNA from the cardiac muscle tissue, and one a synthetic fragment constructed with annealing sites for the alpha and beta primers. The synthetic fragment allows correction for reaction efficiency differences between primer sets for each MyHC isoform.
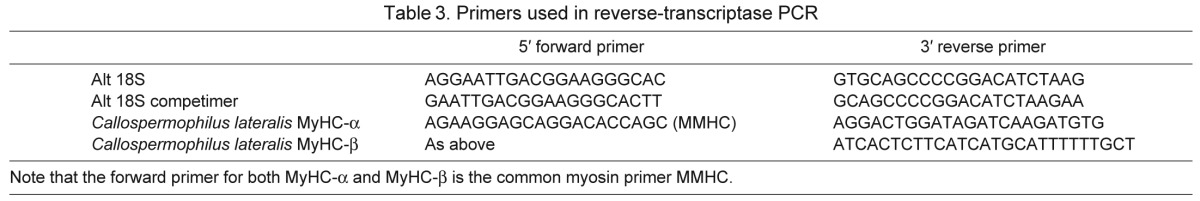
Table 3.
Primers used in reverse-transcriptase PCR
Interpretation of the mRNA signal of the cardiac myosin genes was complicated by several factors previously described in rat hearts (Haddad et al., 2003; Haddad et al., 2006b; Haddad et al., 2007; Haddad et al., 2008), and which we believe also act in these squirrels. We subsequently varied our protocols for both reverse-transcription and the PCR reactions attempting to account for antisense mRNA expression in the squirrel heart. We ultimately carried out PCR reactions for cardiac myosin genes under conditions where the temperature and commercial brand of enzyme were varied, as suggested by Haddad et al., utilizing Taq (Qiagen, Valencia, CA, USA) and Platinum Taq DNA polymerase (Invitrogen). Initially we used a constructed DNA control as called for by our earlier methodology (see Wright et al., 1997; Rourke et al., 2004b; Rourke et al., 2006; Barrows et al., 2011), but later performed reactions with no synthetic control fragment, or utilizing a ribosomal 18S subunit gene for control expression (as modified by our lab from AMBION/Applied Biosystems, Austin, TX, USA). Lastly, we ran all reactions on cDNA reverse-transcribed from RNA in the following ways: (1) with oligo dT and random primers; (2) with no random primers; and (3) with MMHC as the initial primer for reverse-transcription.
Statistical analysis
For echocardiograms, analysis was performed using commercial statistical software (Minitab statistical software, version 15). Repeated measures ANOVA was used to compare echocardiography parameters among the groups, and active, hibernating and arousal states. Additionally, cohort and sex were tested as the data were pooled. Assumptions of ANOVA were satisfied; a P-value of 0.05 was considered significant. All echocardiogram data are presented as means ± s.d.
For myosin, each group was assessed for significance from all other groups by one-way ANOVA with multiple pair-wise comparisons, using a Bonferroni post hoc correction. The main focus of the study was the progression of myosin changes from the autumn-active state, through deep hibernation at 4°C, and persisting until near-emergence 6 months later. The September group was treated as the active, wild-caught state for comparisons, and 20°C torpid groups were included specifically to test whether low temperature was required to elicit cardiac remodeling during hibernation. Increasing the pair-wise comparisons by including 20°C torpid groups did not alter the conclusions regarding the progression of MyHC changes in the 4°C deep hibernation groups. A value of P<0.05 (following Bonferroni) was accepted as significant. All statistical tests were performed using the Systat 10.2 statistical package (SPSS, Chicago, IL, USA), and data are means ± s.e.m.
RESULTS
Echocardiography
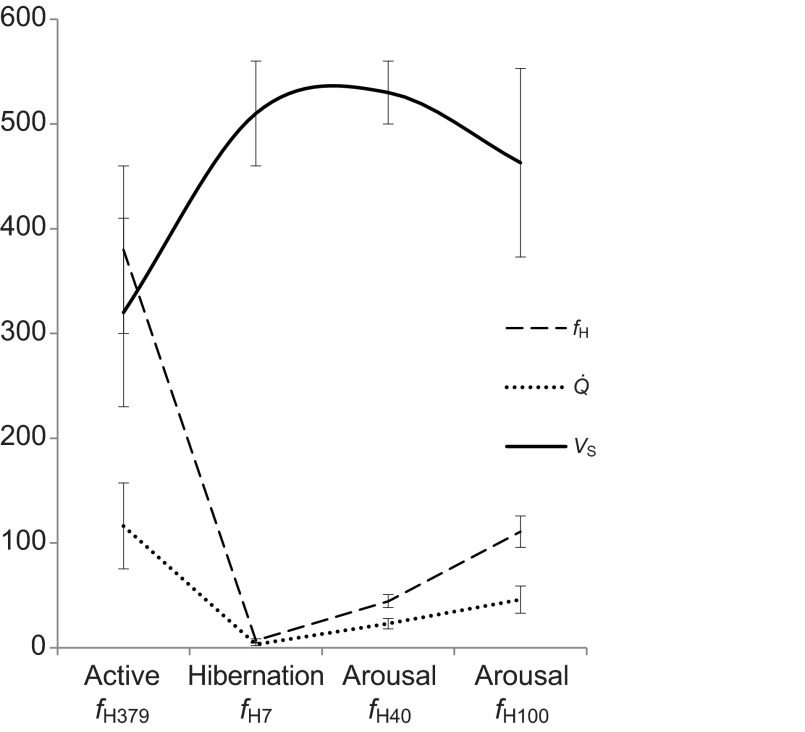
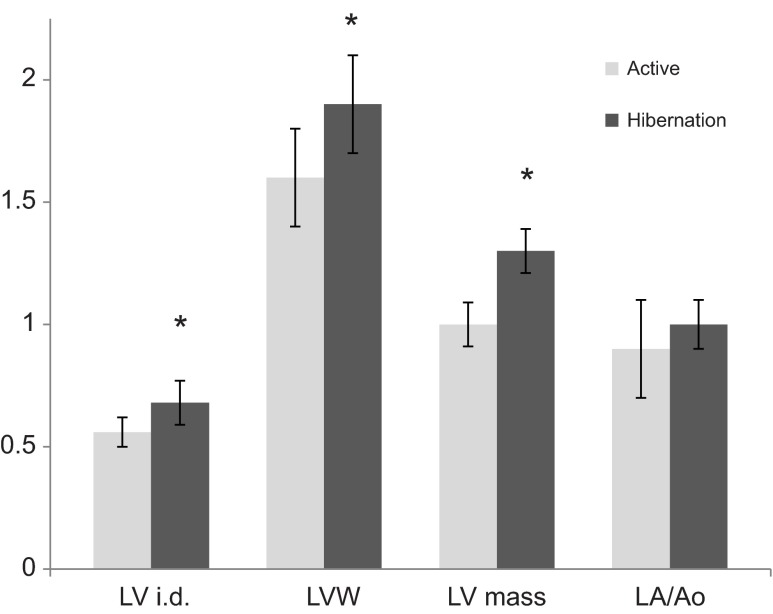
Large differences in heart rate and cardiac output were noted from active to hibernating states, not unexpectedly (Fig. 4, Table 4). Left ventricular (LV) chamber size demonstrated significant eccentric and concentric hypertrophy during hibernation and thus calculated LV mass was also ~30% greater (Fig. 5). The left atrial chamber dimension was not notably different between the active and hibernating state. Global LV systolic function as measured by ejection fraction (EF) was mildly reduced in hibernation while stroke volume (VS) was increased in hibernation. Even though LV systolic function was mildly reduced, the LV chamber had a substantially larger filling dimension in diastole and thus ejected a greater stroke volume during systolic contraction (Fig. 4, Table 4). Atrial contraction waveforms (vA,max) were absent in 14 of the 16 hibernating animals. Most animals manifest only early ventricular filling waveforms (vEfill,max). Of the two animals that manifested vA,max, the velocities were markedly reduced compared with the active state (no statistical comparison; Fig. 6A,B). Rates of LV contraction and relaxation are significantly slower during hibernation. This is supported by reduced aortic flow velocities (Aoveject), and slower aortic ejection acceleration times (Ao tacceleration) and aortic ejection times (Ao teject) for the systolic contraction phase. Longer isovolumic relaxation times (IVRT), LV pressure decline (pressure half-time, P0.5t), LV inflow acceleration and deceleration (tacceleration, tdeceleration), and reduced early LV inflow velocities (vEfill,max) support a slower diastolic filling phase. Globally, the systolic contraction phase was ~9.5 times longer in hibernation and the diastolic phase was 28 times longer.
Fig. 4.
Heart rate (fH), stroke volume (VS) and cardiac output (Q) recorded in golden-mantled ground squirrels (C. lateralis) evaluated during the active, hibernating and arousal period with corresponding mean heart rates (379, 7, 40 and 100 beats min−1). Hibernating ground squirrels manifest higher stroke volume than during the active period. As heart rate increased during arousal, mean stroke volume declined.
Table 4.
Cohort used for imaging analyses, trapped August 2007 and 2009
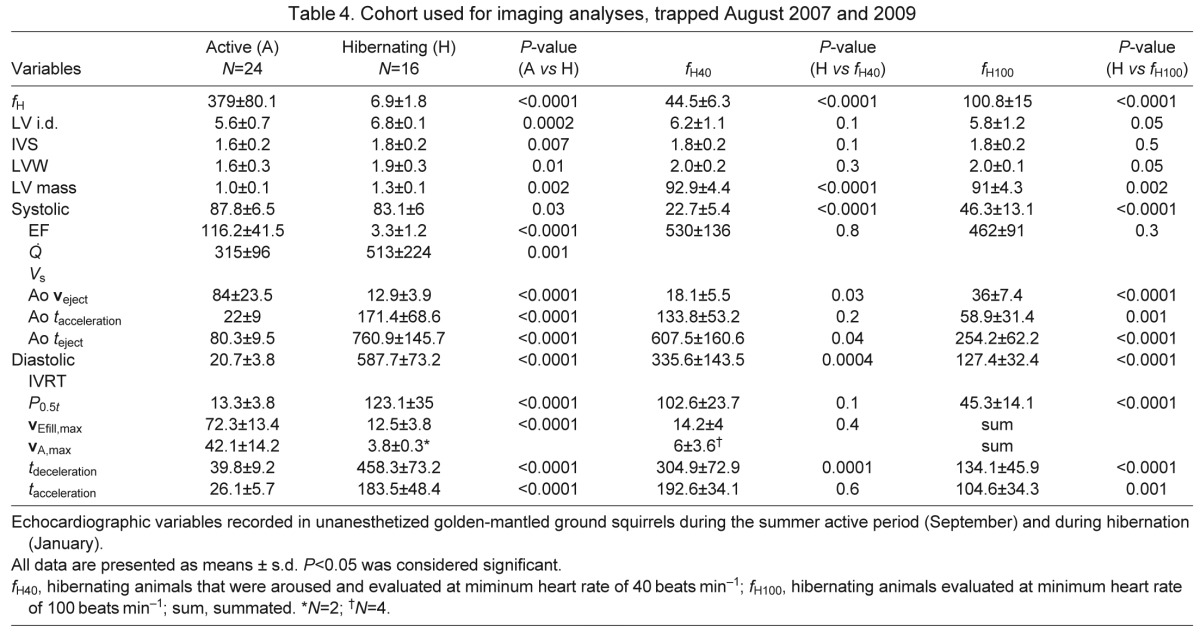
Fig. 5.
Left ventricular (LV) and atrial dimensions in active and hibernating ground squirrels: LV i.d., left ventricular internal diameter dimension (cm); LVW, left ventricular wall dimension (mm); LV mass, left ventricular mass (g); LA/Ao, left atrial size expressed as a ratio of the left atrium (LA) to aortic (Ao) root dimension. Values are expressed as means ± s.d. *P<0.05.
Fig. 6.
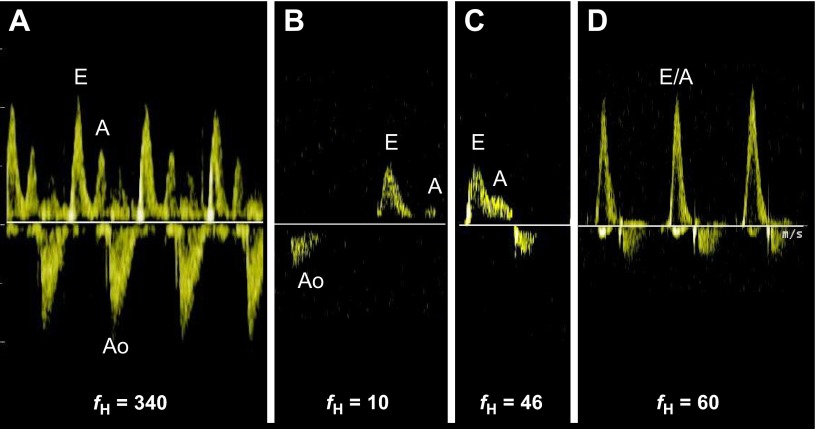
Mitral valve (left ventricular) inflow profiles during active and hibernation periods, and two points of arousal in ground squirrels. (A) During the active period, early ventricular filling (E) and atrial contraction (A) flow profiles are identified in a typical relationship to each other. The aortic flow profile (Ao) is also seen. (B) Only two animals exhibited atrial contraction flow profiles during hibernation, of very low velocity. (C) During early arousal, atrial contraction profiles were frequently identified. (D) With increasing heart rate, atrial contraction flow became summated with the early filling flow, suggesting that early filling was not yet complete at the time of atrial contraction. Note: Ao flow profiles are not optimized in all images. Heart rate (fH) is in beats min−1.
During the early arousal period when heart rate increased from a mean of 6.9±1.8 to 44.5±6.3 beats min−1, four of the six systolic variables were significantly affected. EF,  and Ao veject increased while Ao teject decreased in the systolic phase. EF was greater than active measures. IVRT and tdeceleration decreased in the diastolic phase during this early arousal period as the heart rate increased. Four of the animals resumed atrial contraction waves (vA,max) at a very low velocity. Once aroused to a heart rate of 100.8±15 beats min−1, all variables tended towards active values, demonstrating progressively increasing ejection and contraction speeds with rising heart rate; however, there still remained a marked difference between the active
and arousal states at this time (Table 4). One exception was VS, which remained elevated during both arousal state measurements compared with the active state even; mean VS did decrease slightly at a heart rate of 100 beats min−1. Atrial contraction flow profiles occurred more regularly at a heart rate of 40 beats min−1, but were summated with the early filling flow profiles at higher heart rates, suggesting incompletely recovered diastolic filling function (Fig. 6C,D).
and Ao veject increased while Ao teject decreased in the systolic phase. EF was greater than active measures. IVRT and tdeceleration decreased in the diastolic phase during this early arousal period as the heart rate increased. Four of the animals resumed atrial contraction waves (vA,max) at a very low velocity. Once aroused to a heart rate of 100.8±15 beats min−1, all variables tended towards active values, demonstrating progressively increasing ejection and contraction speeds with rising heart rate; however, there still remained a marked difference between the active
and arousal states at this time (Table 4). One exception was VS, which remained elevated during both arousal state measurements compared with the active state even; mean VS did decrease slightly at a heart rate of 100 beats min−1. Atrial contraction flow profiles occurred more regularly at a heart rate of 40 beats min−1, but were summated with the early filling flow profiles at higher heart rates, suggesting incompletely recovered diastolic filling function (Fig. 6C,D).
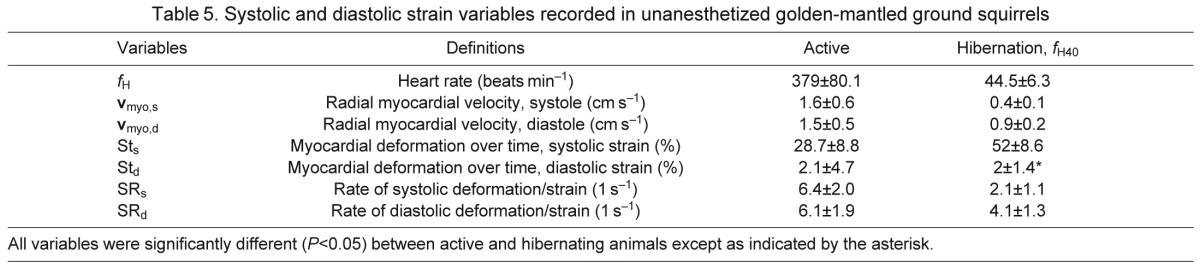
Strain imaging performed during early arousal (Table5) showed that total movement distance, systolic strain (STS), was significantly greater than in the active state, which correlated with enhanced ventricular EF. Even though total contraction was enhanced, strain echocardiography confirmed slower myocardial segment velocities and rates of change during contraction and relaxation compared with the active period. The velocity of myocardial contraction (vmyo,s) was ~25% of active and the velocity of myocardial relaxation (vmyo,d) was ~60% of the active state. The rate of myocardial contraction (systolic phase, SRs) was 33% of active rates while the rate of relaxation (early diastolic phase, SRd) was closer to active rates (67% of active) at a heart rate of 40 beats min−1 (Fig. 2). The entire diastolic filling phase was 28 times longer during hibernation than during the active period. The lengthening of this phase is primarily due to lengthening of passive filling and diastasis as strain imaging identified that the rate of actual myocardial movement during arousal is more similar to that during the active period in diastole than in systole. Strain rates revealed greater oscillations of the baseline, and some heterogeneity in contraction movement within myocardial segments during the early arousal period (Fig. 2). Even though heart rates were only 26% of active heart rates during arousal, the cardiac output was nearly 40% that of the active state, suggesting pumping is maintained by increased VS and ventricular EF even though these processes take a longer period of time to complete.
Table 5.
Systolic and diastolic strain variables recorded in unanesthetized golden-mantled ground squirrels
MyHC protein isoforms
Ground squirrels show a dramatic, progressive increase in MyHC-α expression in the ventricles from September to March (Fig. 3). The initial increase is large and rapid, from 57% MyHC-α in September to 80% just one month later in October, and this occurs prior to the onset of hibernation. In the first month of hibernation, MyHC-α continues to accumulate to over 90%. The next 4 months of hibernation see little further change in MyHC-α, with values all remaining above 90%. The March 20°C group shows the first decline in MyHC-α levels, and no longer differs significantly from levels just prior to hibernation in October. One male individual from this group had a sharply decreased MyHC-α expression to ~40% (not included in Fig. 3), while remaining individuals are at 90–100%. After removing this one individual, 20 and 4°C groups do not differ from one another for November (early hibernation) or March (pre-emergence). Captivity effects have been explicity tested for previously (by B.C.R.), and are not apparent in isoform profiles of skeletal or cardiac muscle.
MyHC isoform mRNA expression
In multiple attempts incorporating several different methodologies, we obtained paradoxical results regarding mRNA expression of cardiac MyHC-α and MyHC-β. Our difficulties included an inability to optimize the concentration of the control fragment to be sensitive simultaneously to the primer reactions for MyHC-α and MyHC-β. This resulted in one of the isoform products outcompeting the other in the reaction, which therefore biased the results dramatically. However, when the reactions were carried out with no control fragment, comparing the expression of mRNA for MyHC-α or MyHC-β in separate reactions, no significant differences were observed for the groups. We do not present these data as they are not rigorously quantitative without the controls for efficiency of reverse-transcription and primer annealing, or for the routine discrepancy in pipetting volume. Further attempts using the 18S subunit as a correction for expression also were unsatisfactory.
DISCUSSION
Despite prolonged periods of extremely low heart rates and cardiac output, ground squirrel myocardium remains apparently healthy and maintains efficiency over broad physiological states. Our goal was to provide a greater mechanistic understanding of cardiovascular adaptations of this prototypical deep hibernator, by describing contractile properties influenced by motor protein expression, and combining in vivo imaging of cardiac pumping. At a point approximately 3 months into hibernation, two hibernating states, deep torpor and early arousal, were compared with a pre-hibernating active period. Our study used unanesthetized ground squirrels to avoid the well-known confounding effects of drugs on cardiac function (Hellyer et al., 1988; Roth et al., 2002; Stein et al., 2007); this provided observations of more natural cardiac physiologic responses, and we feel any response to handling stress was minor.
The deep torpor that ground squirrels utilize during hibernation presents considerable challenges to cardiovascular function. Cardiac cycle alterations in hibernators bear many similarities to human disease states. Low heart rates and cardiac output generally lead to chamber dilation due to volume overload (Verduyn et al., 2001; Schoenmakers et al., 2003). These conditions in hibernators may have led to a noted variety of cardiac adaptations (Folk et al., 1970; Caprette and Senturia, 1984; Milsom et al., 1993; Milsom et al., 1999; Burlington and Darvish, 1998; Brauch et al., 2005). In humans, cardiovascular function ceases with a cessation of pacemaker conduction at about 20°C, and this acute failure masks more chronic failure of the heart where pumping function can be maintained at a lower temperature in rats and mice. A number of electrical and conduction-related adaptations have been suggested (Johansson, 1996), as well as calcium-handling mechanisms which may adjust the duration of contraction and relaxation times (Wang et al., 2002; Yatani et al., 2004; Li et al., 2011). The challenges for deeply torpid squirrels would seem to be the most severe among mammals – prolonged periods of infrequent contractions at extraordinarily low heart rates (4 beats min−1) at near-freezing temperatures, punctuated by highly aerobic periods of arousal and resumption of relatively high heart rates.
Another hibernator studied with identical methodology, the grizzly bear (Ursus arctos horribilis), shows no cardiac dilation, but also a variety of contractile protein shifts for myosin and titin isoforms (Nelson et al., 2008; Nelson and Robbins, 2010; Barrows et al., 2011). Both bears and squirrels show an increase in MyHC-α, which is localized to the left atrium of bears (Barrows et al., 2011), and a larger increase of MyHC-α in combined ventricle tissues of squirrels (current study). The larger ursid hibernators differ in torpor physiology quite significantly from squirrels (Wickler et al., 1991; Nelson et al., 2008), particularly by being less hypothermic (33°C) than hibernating rodents (5–7°C) (Hissa et al., 1994). The overall hibernation period is also considerably more homogeneous in an ursid as wide fluctuations in metabolism and heart rate with arousals are absent. Ground squirrels are almost completely inactive during bouts of torpor, but awaken slowly and periodically during arousals. In contrast, bears are easily awakened, appearing quite alert but a cyclic, spontaneous arousal period is not present. Temperatures below 30°C are required to create significant changes in calcium handling and contraction dynamics (Narahashi et al., 1987; Walsh et al., 1989; Sitsapesan et al., 1991), and thus calcium handling dynamics and blood viscosity also may be less problematic for bears.
Echocardiography in ground squirrels
Unsurprisingly, the heart rate and cardiac output of the ground squirrels was significantly decreased during hibernation (Fig. 4). We observed increased left ventricular mass and internal dimension (LV i.d.) during hibernation (Fig. 5), consistent with previous reports in deep hibernators (Popovic, 1964; Kirkebö, 1968a; Wickler et al., 1991). Eccentric left ventricular hypertrophy, or chamber dilation, occurs as a result of passive mechanisms lengthening diastole, and increasing the chamber filling phase due to increased venous return (Popovic, 1964; Kirkebö, 1968a). This pattern of eccentric hypertrophy is compensatory, such that the heart sustains cardiac output at low heart rates by sustaining a high stroke volume (Lorell and Carabello, 2000). In general, increased diastolic filling volume equates to increased stroke volume, as follows from Starling's Law. In addition to increased left ventricular dilation, increased ventricular wall thickness (concentric hypertrophy) also contributed to the calculation of greater cardiac mass during hibernation. Because systolic stress is a major determinant of ejection performance, the normalization of systolic wall stress by myocardial hypertrophy helps to maintain a more optimal ejection fraction even when needing to generate high levels of systolic performance (Gunther and Grossman, 1979). That is, contractile force and pressure generation are maintained against afterload issues of increased resistance – either due to increased absolute pressure or increased duration of force generation. Concentric hypertrophy has not been previously evaluated in hibernating rodents but this observation is congruent with hemodynamics of deep hibernators of slow contractile indices (this study) and increased peripheral vascular resistance (Johansen, 1967; Kirkebö, 1968a; Kirkebö, 1968b; Caprette and Senturia, 1984).
Total peripheral vascular resistance rises during hibernation in ground squirrels and hedgehogs, in part due to modest peripheral vasoconstriction, but primarily due to the large increase in blood viscosity at low body temperatures (Johansen, 1967; Kirkebö, 1968a; Kirkebö, 1968b; Maclean, 1981). Even though systemic arterial blood pressure overall decreases during hibernation compared with the active state (Chatfield and Lyman, 1950; Johansen, 1967; Kirkebö, 1968a), the slower rate of cardiac contraction combined with increased peripheral vascular resistance results in a slower diastolic flow movement down the arterial system. The windkessel effect promotes blood flow along vessels but with the prolonged diastole of slow heart rates, diastolic flow movement down the system does not proceed rapidly. Therefore, despite the fall in systolic pressure and prolongation of diastolic phase, the diastolic pressure, and thus mean pressure, remains relatively high (Lyman and O'Brien, 1963; Burlington and Darvish, 1998). Prolonged duration of systolic strain during hibernation as identified by strain echocardiography (discussed below) could also play a role in the hypertrophic response (Ritter and Neyses, 2003). These factors would be expected to generate a mechanical signal that initiates a cascade of biological events leading to coordinated cardiac growth according to the law of LaPlace. These structural changes in chambers may be required to maintain an optimal level of cardiac output, even markedly reduced, that matches oxygen consumption and tissue needs during hibernation.
Some cardiovascular adaptations in ground squirrels seem quite opposite to those of bears. Grizzly bears evaluated over four hibernation seasons did not demonstrate any left ventricular chamber dilation or increase in stroke volume (Nelson and Robbins, 2010). This was attributed to increased ventricular stiffness and shifts in myocardial titin isoform expression which may inhibit chamber distension (Nelson et al., 2008). In addition, left ventricular mass was reduced during hibernation as compared with the active season (Nelson et al., 2008; Nelson and Robbins, 2010) and ventricular insulin-like growth factor-I mRNA expression was reduced by ~50%, consistent with the ventricular atrophy observed in these bears (Barrows et al., 2011). Some explanation for these structural and functional differences may lie in the depth of hibernation between the two species. While bears reduce their cardiac output in hibernation to roughly 25% that of the active season (Nelson and Robbins, 2010), their sizable body mass confers far greater ability to store large volumes of fat for over-wintering. Ground squirrels do not have this luxury and must reduce their metabolic needs to an even greater extent; indeed, cardiac output is reduced to ~3% of active values. The dramatic differences in heart and metabolic rate, diastolic pauses, body temperature and peripheral vascular resistance are likely to account for the alternative cardiac phenotypes manifested by the two species.
To evaluate the plasticity of cardiovascular responses during the arousal period, the squirrels were allowed to warm progressively to two target heart rates. After the initial echocardiography in the cold room, animals were moved to a room temperature of ~25°C and monitored until heart rate reached ~40 and 100 beats min−1. Heart rate steadily climbed during arousal, closely matched by increasing contractile speed and cardiac output. Stroke volume remained fairly consistent from 7 to 40 beats min−1, but stroke volume fell with further increases in heart rate (Fig. 4). Declining stroke volume accompanied by rebounding heart rate has also been described during arousal bouts in hedgehogs (Kirkebö, 1968a). Stroke volume in these squirrels paralleled left ventricular internal dimension changes during the arousal period, which became smaller with progressive increases in heart rate (Table 4). With less time for ventricular filling in diastole at higher rates, ejection volumes declined. Although cardiac output was still increasing up to a heart rate of 100 beats min−1, the increase in output was tempered by these progressively smaller stroke volumes.
Cardiac contractile indices were significantly increased during arousal compared with the baseline active state. Rising heart rate and cardiac contractility are likely associated with increases in sympathetic tone and the fall of peripheral vascular resistance known to occur during arousal (Lyman and O'Brien, 1963; Milsom et al., 1993). Myocardial systolic strain during early arousal was significantly greater than in the active state, indicating greater total contractile movement of individual muscle segments. Increased systolic strain mirrored increased ejection fraction, as initially measured by standard echocardiography. Enhanced contractile strain maintained for prolonged periods, even episodically during arousal, may provide evidence of greater mechanical stress, which could contribute to the hypertrophic response (Sadoshima and Izumo, 1997; Berenji et al., 2005). During late arousal, cardiac output was disproportionately greater at lower heart rates than during the active state. Increased VS and EF contribute despite heart rate being only 26% of active. Thus, structural and functional changes may be required to maintain a highly flexible but optimal level of cardiac output as rapid changes in oxygen demand occur during arousal.
Atrial contraction flow profiles occurred rarely in the hibernating squirrels and the velocities were markedly reduced compared with those during the active period when they were noted (Fig. 6A,B). This observation suggests that either the atria are not contracting or they are contracting too weakly to produce detectable flow signals in most animals. A similar finding has been observed in hibernating brown bears (Nelson and Robbins, 2010; Barrows et al., 2011). Atrial contraction profiles occurred more regularly at increasing heart rates (~40 beats min−1) during arousal (Fig. 6C); however, at faster heart rates, profiles were summated with early ventricular filling (Fig. 6D). Atrial contraction flow profiles were more likely to be summated during the active period at the higher heart rates (>400 beats min−1) but this finding was variable at the high rates. The observation of summated flow profiles at a lower heart rate during arousal suggests incompletely recovered diastolic filling function at this time, i.e. early filling is not yet complete before atrial contraction occurs. Our hemodynamic assessment suggests a dramatic downregulation of atrial chamber function during the hibernation period but that rapid ‘recovery’ of contractile activity occurs with arousal. It is reasonable to postulate that a programmed reduction in atrial chamber function in ground squirrels during hibernation is protective against atrial fatigue, which could occur with atrial contraction against an optimally filled, larger ventricle. The functional significance of this adaptation may be to allow the bradycardic hibernator to further reduce energy consumption in an energetically costly organ, the heart, and to avoid atrial chamber dilation and congestion during this period.
Cardiac myosin isoform expression in ground squirrels
The cardiac MyHC-α and MyHC-β isoforms differ in contraction speed, duration of force and maximum force produced. MyHC-α isoforms may contract 2–3 times faster, with generally lower force, but species-specific differences are apparent; average force can be similar between MyHC-α and MyHC-β (Sugiura et al., 1998; Alpert et al., 2002; Galler et al., 2002; Malmqvist et al., 2004; Narolska et al., 2005a; Narolska et al., 2005b). The expression of the isoforms varies by heart chamber, and by sex, age and mass of the individual (Cummins and Lambert, 1986; O'Neill et al., 1991; Reiser and Kline, 1998; Barrows et al., 2011). Larger animals typically have greater amounts of the slower, but perhaps more forceful, MyHC-β isoform [>90% in humans (Miyata et al., 2000), 100% in grizzly bears (Barrows et al., 2011)]. Greater MyHC-α expression is more commonly observed in the atria and in smaller rodents.
Numerous factors control the regulation of these contractile proteins in mammals (Schreiber et al., 1981; Pagani and Solaro, 1983; McDermott et al., 1985; Schaible and Scheuer, 1985; Izumo et al., 1987; Morris et al., 1990; Morgan and Baker, 1991; Klein et al., 1992; Tanamura et al., 1993; Baldwin and Haddad, 2001), and a number of pathologies in the heart are noted (Gupta, 2007). Shifts in the relative expression of isoforms are common, and confer critical changes in the contractile strength and speed of the chamber contraction (Narolska et al., 2005a; Narolska et al., 2005b). Atrophy typically induces isoform transitions (Klein et al., 1992; Geenen et al., 1994; Tang et al., 2005), and cardiomyopathy generally leads to a loss of MyHC-α (Buttrick et al., 1991; Takahashi et al., 1992; Miyata et al., 2000; Reiser et al., 2001), at least in non-hibernators.
The increase in MyHC-α during hibernation in ground squirrels is remarkable because it mimics many instances of cardioprotective expression of MyHC-α in other paradigms. Many models of cardiomyopathy, including pressure-overload and dilation, show an isoform switch to MyHC-β under failing or stressed heart function. This may represent an energy- and oxygen-saving mechanism at the expense of force production and peak power (Litten et al., 1982; Litten et al., 1985; Hasenfuss et al., 1991; Herron and McDonald, 2002; Korte et al., 2005). The lower power in turn contributes to depressed systolic function in end-stage heart failure (Stelzer et al., 2007). While MyHC-α decreases in failing hearts, it increases with recovery (Haworth, 2007), and may be cardioprotective in stressed myocardium (Marian, 2005). In dilated rabbit hearts with tachycardia-induced cardiomyopathy, transgenic expression of MyHC-α was cardioprotective (James et al., 2005). In transgenic mice, MyHC-α to MyHC-β switching was detrimental; the energy saving reduced function nonetheless (Krenz and Robbins, 2004). In humans, only a small amount of MyHC-α (7%) is found in healthy hearts, but is notably absent in ventricles of failing hearts (Nakao et al., 1997; Miyata et al., 2000). Perhaps related to the unusual MyHC mRNA expression of our ground squirrels, humans have proportions of MyHC-α mRNA approaching 35%, even with no incorporation into myocardium (Lowes et al., 1997; Nakao et al., 1997; Miyata et al., 2000; Reiser et al., 2001).
In the other study demonstrating an increase in MyHC-α in a hibernating rodent, Morano et al. (Morano et al., 1992) reveal species-specific mechanisms of cardiac plasticity. Hamsters alter their cardiac isoforms, but it was not apparent when, as July summer-active animals were compared with hibernators in December and January, after 1–2 months of torpor. This allows the possibility that hamsters might shift expression before hibernation, as in C. lateralis (our October, pre-hibernation data). Usefully, the Morano study also compared winter-active animals; these hamsters did not experience low temperatures and crucially fail to show the increase in MyHC-α. Morano et al. argue that this demonstrates a requirement for torpor and low body temperature as a stimulus for their observed cardiac plasticity. We find precisely the opposite in C. lateralis, as for cardiac muscle (this study) and skeletal muscle (Nowell et al., 2011), similar changes in muscle phenotype occurred seasonally prior to torpor, and in warm-room animals during hibernation.
It is tempting to invoke fluctuating thyroid hormone concentration as a stimulus to seasonal changes in cardiac MyHC isoforms. Thyroid hormone is extremely important to MyHC expression, and hyperthyroidism will increase the expression of MyHC-α (Morkin, 2000; Baldwin and Haddad, 2001; Haddad et al., 2010). There is resistance to the action of the hormone during hibernation, as increases in binding affinity reduce free hormone level, but thyroid hormone secretion may be elevated during arousals in related squirrels (Magnus and Henderson, 1988). In rats, cold acclimation can promote increased MyHC-α, but without any ventricular hypertrophy (Adler et al., 1991). Here, thermogenic requirements of cold adaptation and subsequent hyperthyroid state may be a strong stimulus, as it conflicts with other hypertrophic stimuli that would promote MyHC-β. We argue that a role for thyroid hormone, and the inception of a stimulus to myocardial cells during the first few bouts of torpor, is instead refuted by our data. Firstly, in C. lateralis the increase in MyHC-α happens before the first bout of torpor is observed, and therefore prior to any elevated thyroid state during hibernation. Secondly, while thyroid hormone levels may increase in some species during hibernation, they do not clearly do so in our wild-caught C. lateralis (B.C.R., unpublished data), and they decline in bears (Tomasi et al., 1998). Lastly, warm room squirrels not experiencing either low temperature torpor or significant arousals still show identical increases in MyHC-α.
Greater MyHC-α expression in hibernating ground squirrels may represent a mechanism to combat dilated myopathy, as well as to deal with increased viscosity of blood at near-freezing temperatures. Combined with a greater ventricular wall thickness, stroke volume is enhanced, perhaps to counteract further losses in contractile protein function at low temperatures. Periodic arousals benefit from, and may have necessitated, the increase in MyHC-α and the hypertrophy of the ventricle. Prevention of chamber dilation at low heart rate may be the primary stimulus, however, as much larger bears increase MyHC-α with decreases in body temperature of only a few degrees during hibernation (Barrows et al., 2011). Similarly, the comparison of squirrels at 20 and 4°C in November or March demonstrates that the lower temperature of deep hibernation is not a requirement in the cardiac plasticity of MyHC isoforms, and that like skeletal muscle (Nowell et al., 2011), seasonal cues are more important than absolute body temperature. A difference in Q10 for the isoforms favoring preservation of function for MyHC-α is also conceivable. In skinned rat fibers, some crossbridge parameters, but not tension, can vary thusly (Rossmanith et al., 1986). We hesitate to draw parallels to C. lateralis without direct measurements, given the suite of adaptations to torpor absent in rats, and the lack of low temperature as a required stimulus in both bears and ground squirrels.
A final assessment of the rather uncommon timing of cardiac muscle changes, occurring prior to changes in actual loading or torpor, reveals the untested possibility that hypertrophy of the ventricles also precedes hibernation. The heart presumably encounters greater work and increased metabolic demands in the first arousal from torpor, and given that MyHC changes pre-empt torpor, could not hypertrophy as well? The minimal time presented by hibernation to remodel the heart, as many crucial turnover rates are reduced dramatically during torpor, might favor changes to muscle proteins prior to alterations in actual loading. Unfortunately, this was not addressed because our echocardiogram measurements were confined to only two sessions, and we did not sample during the month that this cardiac remodeling could have been observed. With other seasonal regulation clearly a strategy in this species, future work should pursue the mechanisms of regulation, and the potential anticipatory preparation of the heart for the pumping demands of hibernation.
Conclusions
Hibernating ground squirrels face challenges to cardiac function wrought by low temperature, and low cardiac output, with widely varying ranges of temperature and pumping demands due to periodic torpor and arousals. Echocardiography measured a compensatory increase in left ventricular mass and dilation during hibernation, which resulted in a dramatically increased stroke volume. Atrial ejection was minimal during torpor and early arousal. Myocardial contractile indices were reduced during hibernation from the active state, but contraction increased to a significantly greater level during early arousal than in the torpid or active state. This functional change occurs rapidly, contributing to the ability to maintain efficient cardiac output at low heart rates with minimal atrial ejection. An accompanying increase in MyHC-α isoform expression may aid cardiac output by increasing total chamber contraction as seen in early arousal, as well as affecting the velocity at low temperature where viscosity and wall stiffness might otherwise compromise function. Ground squirrels show cardiac plasticity akin to that of grizzly bears, but less similar to that of hamsters with regard to stimuli and mechanisms. The increase in MyHC-α expression in squirrels is contrary to the typical loss of that isoform during cardiomyopathy and heart failure in humans and rodent models. Ground squirrels thus show protective mechanisms against cardiac dilation and reduced cardiac output at body temperatures and heart rates far below what non-hibernators can tolerate.
ACKNOWLEDGEMENTS
Expert technical assistance (B.C.R.) was provided by Nicole Choi, Akino Higa, Megan Nowell, Lauren Sims and Karine Vu. We thank one annonymous reviewer in particular for helpful and thoughtful remarks which greatly improved the manuscript.
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
Funding was provided by the National Insititutes of Health (NIH) Minority Biomedical Research Support of Competitive Research [2 S06 GM063119 to B.C.R.]. Deposited in PMC for release after 12 months.
REFERENCES
- Adler K., Boels P., Ganten U., Ganten D., Morano I. (1991). The influence of cold stress on the myosin heavy chain expression of cardiac and smooth muscle in normotensive and spontaneously hypertensive female rats. Circ. Res. 69, 1640-1644 [DOI] [PubMed] [Google Scholar]
- Alpert N. R., Brosseau C., Federico A., Krenz M., Robbins J., Warshaw D. M. (2002). Molecular mechanics of mouse cardiac myosin isoforms. Am. J. Physiol. 283, H1446-H1454 [DOI] [PubMed] [Google Scholar]
- Baldwin K. M., Haddad F. (2001). Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J. Appl. Physiol. 90, 345-357 [DOI] [PubMed] [Google Scholar]
- Barrows N. D., Nelson O. L., Robbins C. T., Rourke B. C. (2011). Increased cardiac alpha-myosin heavy chain in left atria and decreased myocardial insulin-like growth factor (Igf-I) expression accompany low heart rate in hibernating grizzly bears. Physiol. Biochem. Zool. 84, 1-17 [DOI] [PubMed] [Google Scholar]
- Berenji K., Drazner M. H., Rothermel B. A., Hill J. A. (2005). Does load-induced ventricular hypertrophy progress to systolic heart failure? Am. J. Physiol. 289, H8-H16 [DOI] [PubMed] [Google Scholar]
- Brauch K. M., Dhruv N. D., Hanse E. A., Andrews M. T. (2005). Digital transcriptome analysis indicates adaptive mechanisms in the heart of a hibernating mammal. Physiol. Genomics 23, 227-234 [DOI] [PubMed] [Google Scholar]
- Burlington R., Darvish A. (1998). Low-temperature performance of isolated working hearts from a hibernator and a nonhibernator. Physiol. Zool. 61, 387-395 [Google Scholar]
- Buttrick P., Perla C., Malhotra A., Geenen D., Lahorra M., Scheuer J. (1991). Effects of chronic dobutamine on cardiac mechanics and biochemistry after myocardial infarction in rats. Am. J. Physiol. 260, H473-H479 [DOI] [PubMed] [Google Scholar]
- Caprette D. R., Senturia J. B. (1984). Isovolumetric performance of isolated ground squirrel and rat hearts at low temperature. Am. J. Physiol. 247, R722-R727 [DOI] [PubMed] [Google Scholar]
- Chatfield P. O., Lyman C. P. (1950). Circulatory changes during process of arousal in the hibernating hamster. Am. J. Physiol. 163, 566-574 [DOI] [PubMed] [Google Scholar]
- Choi H., Selpides P. J. I., Nowell M. M., Rourke B. C. (2009). Functional overload in ground squirrel plantaris muscle fails to induce myosin isoform shifts. Am. J. Physiol. 297, R578-R586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K. A., Korcarz C. E., Shroff S. G., Bednarz J. E., Fentzke R. C., Lin H., Leiden J. M., Lang R. M. (2001). Accuracy of echocardiographic estimates of left ventricular mass in mice. Am. J. Physiol. 280, H1954-H1962 [DOI] [PubMed] [Google Scholar]
- Cummins P., Lambert S. J. (1986). Myosin transitions in the bovine and human heart. A developmental and anatomical study of heavy and light chain subunits in the atrium and ventricle. Circ. Res. 58, 846-858 [DOI] [PubMed] [Google Scholar]
- Dool J. S., Mak A. S., Friberg P., Wahlander H., Hawrylechko A., Adams M. A. (1995). Regional myosin heavy chain expression in volume and pressure overload induced cardiac hypertrophy. Acta Physiol. Scand. 155, 396-404 [DOI] [PubMed] [Google Scholar]
- Folk G. E., Brewer M. C., Sanders D. (1970). Cardiac physiology of polar bears in winter dens. Arctic 23, 130-131 [Google Scholar]
- Galler S., Puchert E., Gohlsch B., Schmid D., Pette D. (2002). Kinetic properties of cardiac myosin heavy chain isoforms in rat. Pflugers Arch. 445, 218-223 [DOI] [PubMed] [Google Scholar]
- Garcia M. J., Firstenberg M. S., Greenberg N. L., Smedira N., Rodriguez L., Prior D., Thomas J. D. (2001). Estimation of left ventricular operating stiffness from Doppler early filling deceleration time in humans. Am. J. Physiol. 280, H554-H561 [DOI] [PubMed] [Google Scholar]
- Geenen D. L., Malhotra A., Buttrick P. M., Scheuer J. (1994). Ventricular pacing attenuates but does not reverse cardiac atrophy and an isomyosin shift in the rat heart. Am. J. Physiol. 267, H2149-H2154 [DOI] [PubMed] [Google Scholar]
- Gunther S., Grossman W. (1979). Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation 59, 679-688 [DOI] [PubMed] [Google Scholar]
- Gupta M. P. (2007). Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J. Mol. Cell. Cardiol. 43, 388-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F., Bodell P. W., Qin A. X., Giger J. M., Baldwin K. M. (2003). Role of antisense RNA in coordinating cardiac myosin heavy chain gene switching. J. Biol. Chem. 278, 37132-37138 [DOI] [PubMed] [Google Scholar]
- Haddad F., Adams G. R., Bodell P. W., Baldwin K. M. (2006a). Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J. Appl. Physiol. 100, 433-441 [DOI] [PubMed] [Google Scholar]
- Haddad F., Qin A. X., Bodell P. W., Zhang L. Y., Guo H., Giger J. M., Baldwin K. M. (2006b). Regulation of antisense RNA expression during cardiac MHC gene switching in response to pressure overload. Am. J. Physiol. 290, H2351-H2361 [DOI] [PubMed] [Google Scholar]
- Haddad F., Qin A. X., Giger J. M., Guo H., Baldwin K. M. (2007). Potential pitfalls in the accuracy of analysis of natural sense-antisense RNA pairs by reverse transcription-PCR. BMC Biotechnol. 7, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F., Qin A. X., Bodell P. W., Jiang W., Giger J. M., Baldwin K. M. (2008). Intergenic transcription and developmental regulation of cardiac myosin heavy chain genes. Am. J. Physiol. 294, H29-H40 [DOI] [PubMed] [Google Scholar]
- Haddad F., Jiang W., Bodell P. W., Qin A. X., Baldwin K. M. (2010). Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am. J. Physiol. 299, H1968-H1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss G., Mulieri L. A., Blanchard E. M., Holubarsch C., Leavitt B. J., Ittleman F., Alpert N. R. (1991). Energetics of isometric force development in control and volume-overload human myocardium. Comparison with animal species. Circ. Res. 68, 836-846 [DOI] [PubMed] [Google Scholar]
- Hauton D., May S., Sabharwal R., Deveci D., Egginton S. (2011). Cold-impaired cardiac performance in rats is only partially overcome by cold acclimation. J. Exp. Biol. 214, 3021-3031 [DOI] [PubMed] [Google Scholar]
- Haworth S. (2007). The cell and molecular biology of right ventricular dysfunction in pulmonary hypertension. Eur. Heart J. Suppl. 9, H10-H16 [Google Scholar]
- Hellyer P., Muir W. W., 3rd, Hubbell J. A., Sally J. (1988). Cardiorespiratory effects of the intravenous administration of tiletamine-zolazepam to cats. Vet. Surg. 17, 105-110 [DOI] [PubMed] [Google Scholar]
- Herron T. J., McDonald K. S. (2002). Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ. Res. 90, 1150-1152 [DOI] [PubMed] [Google Scholar]
- Hissa R., Siekkinen J., Hohtola E., Saarela S., Hakala A., Pudas J. (1994). Seasonal patterns in the physiology of the European brown bear (Ursus arctos arctos) in Finland. Comp. Biochem. Physiol. 109A, 781-791 [DOI] [PubMed] [Google Scholar]
- Izumo S., Lompré A. M., Matsuoka R., Koren G., Schwartz K., Nadal-Ginard B., Mahdavi V. (1987). Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J. Clin. Invest. 79, 970-977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J., Martin L., Krenz M., Quatman C., Jones F., Klevitsky R., Gulick J., Robbins J. (2005). Forced expression of alpha-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation 111, 2339-2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen B. J. (1967). Heart and circulation in hibernators. In Mammalian Hibernation III (ed. Fischer K. C., Dawe A. R., Lyman C. P., Schonbaum E., South F. E., Jr) pp. 200-218 New York, NY: American Elsevier; [Google Scholar]
- Johansson B. W. (1996). The hibernator heart – nature's model of resistance to ventricular fibrillation. Cardiovasc. Res. 31, 826-832 [DOI] [PubMed] [Google Scholar]
- Kertesz N. J., Friedman R. A., Colan S. D., Walsh E. P., Gajarski R. J., Gray P. S., Shirley R., Geva T. (1997). Left ventricular mechanics and geometry in patients with congenital complete atrioventricular block. Circulation 96, 3430-3435 [DOI] [PubMed] [Google Scholar]
- Kirkebö A. (1968a). Temperature effects on the viscosity of blood and the aorta distension from a hibernator, Erinaceus europaeus L. Acta Physiol. Scand. 73, 385-393 [DOI] [PubMed] [Google Scholar]
- Kirkebö A. (1968b). Cardiovascular investigations on hedgehogs during arousal from the hibernating state. Acta Physiol. Scand. 73, 394-406 [DOI] [PubMed] [Google Scholar]
- Klein I., Ojamaa K., Samarel A. M., Welikson R., Hong C. (1992). Hemodynamic regulation of myosin heavy chain gene expression. Studies in the transplanted rat heart. J. Clin. Invest. 89, 68-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte F. S., Herron T. J., Rovetto M. J., McDonald K. S. (2005). Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am. J. Physiol. 289, H801-H812 [DOI] [PubMed] [Google Scholar]
- Krenz M., Robbins J. (2004). Impact of beta-myosin heavy chain expression on cardiac function during stress. J. Am. Coll. Cardiol. 44, 2390-2397 [DOI] [PubMed] [Google Scholar]
- Kuecherer H. F., Kusumoto F., Muhiudeen I. A., Cahalan M. K., Schiller N. B. (1991). Pulmonary venous flow patterns by transesophageal pulsed Doppler echocardiography: relation to parameters of left ventricular systolic and diastolic function. Am. Heart J. 122, 1683-1693 [DOI] [PubMed] [Google Scholar]
- Langeland S., D'hooge J., Wouters P. F., Leather H. A., Claus P., Bijnens B., Sutherland G. R. (2005). Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation 112, 2157-2162 [DOI] [PubMed] [Google Scholar]
- Li X. C., Wei L., Zhang G. Q., Bai Z. L., Hu Y. Y., Zhou P., Bai S. H., Chai Z., Lakatta E. G., Hao X. M., et al. (2011). Ca2+ cycling in heart cells from ground squirrels: adaptive strategies for intracellular Ca2+ homeostasis. PLoS ONE 6, e24787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten R. Z., 3rd, Martin B. J., Low R. B., Alpert N. R. (1982). Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ. Res. 50, 856-864 [DOI] [PubMed] [Google Scholar]
- Litten R. Z., Martin B. J., Buchthal R. H., Nagai R., Low R. B., Alpert N. R. (1985). Heterogeneity of myosin isozyme content of rabbit heart. Circ. Res. 57, 406-414 [DOI] [PubMed] [Google Scholar]
- Lorell B. H., Carabello B. A. (2000). Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 102, 470-479 [DOI] [PubMed] [Google Scholar]
- Lowes B. D., Minobe W., Abraham W. T., Rizeq M. N., Bohlmeyer T. J., Quaife R. A., Roden R. L., Dutcher D. L., Robertson A. D., Voelkel N. F., et al. (1997). Changes in gene expression in the intact human heart. Downregulation of alpha-myosin heavy chain in hypertrophied, failing ventricular myocardium. J. Clin. Invest. 100, 2315-2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman C. P., O'Brien R. C. (1963). Autonomic control of circulation during the hibernating cycle in ground squirrels. J. Physiol. 168, 477-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean G. S. (1981). Blood viscosity of two mammalian hibernators: Spermophilus tridecemlineatus and Tamias striatus. Physiol. Zool. 54, 122-131 [Google Scholar]
- Magnus T. H., Henderson N. E. (1988). Thyroid hormone resistance in hibernating ground squirrels, Spermophilus richardsoni. I. Increased binding of triiodo-l-thyronine and l-thyroxine by serum proteins. Gen. Comp. Endocrinol. 69, 352-360 [DOI] [PubMed] [Google Scholar]
- Malmqvist U. P., Aronshtam A., Lowey S. (2004). Cardiac myosin isoforms from different species have unique enzymatic and mechanical properties. Biochemistry 43, 15058-15065 [DOI] [PubMed] [Google Scholar]
- Marian A. J. (2005). On mice, rabbits, and human heart failure. Circulation 111, 2276-2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott P., Daood M., Klein I. (1985). Contraction regulates myosin synthesis and myosin content of cultured heart cells. Am. J. Physiol. 249, H763-H769 [DOI] [PubMed] [Google Scholar]
- Milsom W. K., Burlington R. F., Burleson M. I. (1993). Vagal influence of heart rate in hibernating ground squirrels. J. Exp. Biol. 185, 25-32 [Google Scholar]
- Milsom W. K., Zimmer M. B., Harris M. B. (1999). Regulation of cardiac rhythm in hibernating mammals. Comp. Biochem. Physiol. 124A, 383-391 [DOI] [PubMed] [Google Scholar]
- Miyata S., Minobe W., Bristow M. R., Leinwand L. A. (2000). Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ. Res. 86, 386-390 [DOI] [PubMed] [Google Scholar]
- Morano I., Adler K., Agostini B., Hasselbach W. (1992). Expression of myosin heavy and light chains and phosphorylation of the phosphorylatable myosin light chain in the heart ventricle of the European hamster during hibernation and in summer. J. Muscle Res. Cell Motil. 13, 64-70 [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Baker K. M. (1991). Cardiac hypertrophy. Mechanical, neural, and endocrine dependence. Circulation 83, 13-25 [DOI] [PubMed] [Google Scholar]
- Morkin E. (2000). Control of cardiac myosin heavy chain gene expression. Microsc. Res. Tech. 50, 522-531 [DOI] [PubMed] [Google Scholar]
- Morris G. S., Baldwin K. M., Lash J. M., Hamlin R. L., Sherman W. M. (1990). Exercise alters cardiac myosin isozyme distribution in obese Zucker and Wistar rats. J. Appl. Physiol. 69, 380-383 [DOI] [PubMed] [Google Scholar]
- Nakao K., Minobe W., Roden R., Bristow M. R., Leinwand L. A. (1997). Myosin heavy chain gene expression in human heart failure. J. Clin. Invest. 100, 2362-2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. (1987). Characterization of two types of calcium channels in mouse neuroblastoma cells. J. Physiol. 383, 231-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narolska N. A., Eiras S., van Loon R. B., Boontje N. M., Zaremba R., Spiegelen Berg S. R., Stooker W., Huybregts M. A., Visser F. C., van der Velden J., et al. (2005a). Myosin heavy chain composition and the economy of contraction in healthy and diseased human myocardium. J. Muscle Res. Cell Motil. 26, 39-48 [DOI] [PubMed] [Google Scholar]
- Narolska N. A., van Loon R. B., Boontje N. M., Zaremba R., Penas S. E., Russell J., Spiegelenberg S. R., Huybregts M. A., Visser F. C., de Jong J. W., et al. (2005b). Myocardial contraction is 5-fold more economical in ventricular than in atrial human tissue. Cardiovasc. Res. 65, 221-229 [DOI] [PubMed] [Google Scholar]
- Nelson O. L., Robbins C. T. (2010). Cardiac function adaptations in hibernating grizzly bears (Ursus arctos horribilis). J. Comp. Physiol. B 180, 465-473 [DOI] [PubMed] [Google Scholar]
- Nelson O. L., Robbins C. T., Wu Y., Granzier H. (2008). Titin isoform switching is a major cardiac adaptive response in hibernating grizzly bears. Am. J. Physiol. 295, H366-H371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi Y., Lysyansky P., Setser R. M., Shiota T., Popović Z. B., Martin-Miklovic M. G., Weaver J. A., Oryszak S. J., Greenberg N. L., White R. D., et al. (2005). Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J. Am. Coll. Cardiol. 45, 2034-2041 [DOI] [PubMed] [Google Scholar]
- Nowell M. M., Choi H., Rourke B. C. (2011). Muscle plasticity in hibernating ground squirrels (Spermophilus lateralis) is induced by seasonal, but not low-temperature, mechanisms. J. Comp. Physiol. B 181, 147-164 [DOI] [PubMed] [Google Scholar]
- O'Neill L., Holbrook N. J., Fargnoli J., Lakatta E. G. (1991). Progressive changes from young adult age to senescence in mRNA for rat cardiac myosin heavy chain genes. Cardioscience 2, 1-5 [PubMed] [Google Scholar]
- Ommen S. R., Nishimura R. A., Appleton C. P., Miller F. A., Oh J. K., Redfield M. M., Tajik A. J. (2000). Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102, 1788-1794 [DOI] [PubMed] [Google Scholar]
- Pagani E. D., Solaro R. J. (1983). Swimming exercise, thyroid state, and the distribution of myosin isoenzymes in rat heart. Am. J. Physiol. 245, H713-H720 [DOI] [PubMed] [Google Scholar]
- Piazzesi G., Reconditi M., Koubassova N., Decostre V., Linari M., Lucii L., Lombardi V. (2003). Temperature dependence of the force-generating process in single fibres from frog skeletal muscle. J. Physiol. 549, 93-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic V. (1964). Cardiac output in hibernating ground squirrels. Am. J. Physiol. 207, 1345-1348 [DOI] [PubMed] [Google Scholar]
- Rall J. A., Woledge R. C. (1990). Influence of temperature on mechanics and energetics of muscle contraction. Am. J. Physiol. 259, R197-R203 [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Kline W. O. (1998). Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am. J. Physiol. 274, H1048-H1053 [DOI] [PubMed] [Google Scholar]
- Reiser P. J., Portman M. A., Ning X. H., Schomisch Moravec C. (2001). Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am. J. Physiol. 280, H1814-H1820 [DOI] [PubMed] [Google Scholar]
- Ritter O., Neyses L. (2003). The molecular basis of myocardial hypertrophy and heart failure. Trends Mol. Med. 9, 313-321 [DOI] [PubMed] [Google Scholar]
- Rossmanith G. H., Hoh J. F. Y., Kirman A., Kwan L. J. (1986). Influence of V1 and V3 isomyosins on the mechanical behaviour of rat papillary muscle as studied by pseudo-random binary noise modulated length perturbations. J. Muscle Res. Cell Motil. 7, 307-319 [DOI] [PubMed] [Google Scholar]
- Roth D. M., Swaney J. S., Dalton N. D., Gilpin E. A., Ross J., Jr (2002). Impact of anesthesia on cardiac function during echocardiography in mice. Am. J. Physiol. 282, H2134-H2140 [DOI] [PubMed] [Google Scholar]
- Rourke B. C., Qin A., Haddad F., Baldwin K. M., Caiozzo V. J. (2004a). Cloning and sequencing of myosin heavy chain isoform cDNAs in golden-mantled ground squirrels: effects of hibernation on mRNA expression. J. Appl. Physiol. 97, 1985-1991 [DOI] [PubMed] [Google Scholar]
- Rourke B. C., Yokoyama Y., Milsom W. K., Caiozzo V. J. (2004b). Myosin isoform expression and MAFbx mRNA levels in hibernating golden-mantled ground squirrels (Spermophilus lateralis). Physiol. Biochem. Zool. 77, 582-593 [DOI] [PubMed] [Google Scholar]
- Rourke B. C., Cotton C. J., Harlow H. J., Caiozzo V. J. (2006). Maintenance of slow type I myosin protein and mRNA expression in overwintering prairie dogs (Cynomys leucurus and ludovicianus) and black bears (Ursus americanus). J. Comp. Physiol. B 176, 709-720 [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. (1997). The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 59, 551-571 [DOI] [PubMed] [Google Scholar]
- Schaible T. F., Scheuer J. (1985). Cardiac adaptations to chronic exercise. Prog. Cardiovasc. Dis. 27, 297-324 [DOI] [PubMed] [Google Scholar]
- Schiller N. B. (1991). Two-dimensional echocardiographic determination of left ventricular volume, systolic function, and mass. Summary and discussion of the 1989 recommendations of the American Society of Echocardiography. Circulation 84 Suppl., I280-I287 [PubMed] [Google Scholar]
- Schoenmakers M., Ramakers C., van Opstal J. M., Leunissen J. D., Londoño C., Vos M. A. (2003). Asynchronous development of electrical remodeling and cardiac hypertrophy in the complete AV block dog. Cardiovasc. Res. 59, 351-359 [DOI] [PubMed] [Google Scholar]
- Schreiber S. S., Evans C. D., Oratz M., Rothschild M. A. (1981). Protein synthesis and degradation in cardiac stress. Circ. Res. 48, 601-611 [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Montgomery R. A., MacLeod K. T., Williams A. J. (1991). Sheep cardiac sarcoplasmic reticulum calcium-release channels: modification of conductance and gating by temperature. J. Physiol. 434, 469-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A. B., Tiwari S., Thomas P., Hunt G., Levent C., Stoddard M. F., Tang X. L., Bolli R., Dawn B. (2007). Effects of anesthesia on echocardiographic assessment of left ventricular structure and function in rats. Basic Res. Cardiol. 102, 28-41 [DOI] [PubMed] [Google Scholar]
- Stelzer J. E., Brickson S. L., Locher M. R., Moss R. L. (2007). Role of myosin heavy chain composition in the stretch activation response of rat myocardium. J. Physiol. 579, 161-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stypmann J., Engelen M. A., Epping C., van Rijen H. V. M., Milberg P., Bruch C., Breithardt G., Tiemann K., Eckardt L. (2006). Age and gender related reference values for transthoracic Doppler-echocardiography in the anesthetized CD1 mouse. Int. J. Cardiovasc. Imaging 22, 353-362 [DOI] [PubMed] [Google Scholar]
- Sugiura S., Kobayakawa N., Fujita H., Yamashita H., Momomura S., Chaen S., Omata M., Sugi H. (1998). Comparison of unitary displacements and forces between 2 cardiac myosin isoforms by the optical trap technique: molecular basis for cardiac adaptation. Circ. Res. 82, 1029-1034 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Schunkert H., Isoyama S., Wei J. Y., Nadal-Ginard B., Grossman W., Izumo S. (1992). Age-related differences in the expression of proto-oncogene and contractile protein genes in response to pressure overload in the rat myocardium. J. Clin. Invest. 89, 939-946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge R. J., Roy R. R. (1993). Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J. Appl. Physiol. 75, 2337-2340 [DOI] [PubMed] [Google Scholar]
- Tanamura A., Takeda N., Iwai T., Tuchiya M., Arino T., Nagano M. (1993). Myocardial contractility and ventricular myosin isoenzymes as influenced by cardiac hypertrophy and its regression. Basic Res. Cardiol. 88, 72-79 [DOI] [PubMed] [Google Scholar]
- Tang Y. D., Kuzman J. A., Said S., Anderson B. E., Wang X., Gerdes A. M. (2005). Low thyroid function leads to cardiac atrophy with chamber dilatation, impaired myocardial blood flow, loss of arterioles, and severe systolic dysfunction. Circulation 112, 3122-3130 [DOI] [PubMed] [Google Scholar]
- Tomasi T. E., Hellgren E. C., Tucker T. J. (1998). Thyroid hormone concentrations in black bears (Ursus americanus): hibernation and pregnancy effects. Gen. Comp. Endocrinol. 109, 192-199 [DOI] [PubMed] [Google Scholar]
- Verduyn S. C., Ramakers C., Snoep G., Leunissen J. D., Wellens H. J., Vos M. A. (2001). Time course of structural adaptations in chronic AV block dogs: evidence for differential ventricular remodeling. Am. J. Physiol. 280, H2882-H2890 [DOI] [PubMed] [Google Scholar]
- Wade M. E., Herb R. A., Powers S. K., Criswell D. (1999). Exercise and beta-adrenergic regulation of rat cardiac myosin isoforms. J. Sports Med. Phys. Fitness 39, 42-46 [PubMed] [Google Scholar]
- Walsh K. B., Begenisich T. B., Kass R. S. (1989). Beta-adrenergic modulation of cardiac ion channels. Differential temperature sensitivity of potassium and calcium currents. J. Gen. Physiol. 93, 841-854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Q., Lakatta E. G., Cheng H., Zhou Z. Q. (2002). Adaptive mechanisms of intracellular calcium homeostasis in mammalian hibernators. J. Exp. Biol. 205, 2957-2962 [DOI] [PubMed] [Google Scholar]
- Wickler S. J., Hoyt D. F., van Breukelen F. (1991). Disuse atrophy in the hibernating golden-mantled ground squirrel, Spermophilus lateralis. Am. J. Physiol. 261, R1214-R1217 [DOI] [PubMed] [Google Scholar]
- Woledge R. C., Barclay C. J., Curtin N. A. (2009). Temperature change as a probe of muscle crossbridge kinetics: a review and discussion. Proc. Biol. Sci. 276, 2685-2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C., Haddad F., Qin A. X., Baldwin K. M. (1997). Analysis of myosin heavy chain mRNA expression by RT-PCR. J. Appl. Physiol. 83, 1389-1396 [DOI] [PubMed] [Google Scholar]
- Yatani A., Kim S. J., Kudej R. K., Wang Q., Depre C., Irie K., Kranias E. G., Vatner S. F., Vatner D. E. (2004). Insights into cardioprotection obtained from study of cellular Ca2+ handling in myocardium of true hibernating mammals. Am. J. Physiol. 286, H2219-H2228 [DOI] [PubMed] [Google Scholar]