Abstract
Cancer progression has been associated with the presence of tumor-associated M2-macrophages (M2-TAMs) able to inhibit anti-tumor immune responses. It is also often associated with metastasis-induced bone destruction mediated by osteoclasts. Both cell types are controlled by the CD115 (CSF-1R)/colony-stimulating factor-1 (CSF-1, M-CSF) pathway, making CD115 a promising target for cancer therapy. Anti-human CD115 monoclonal antibodies (mAbs) that inhibit the receptor function have been generated in a number of laboratories. These mAbs compete with CSF-1 binding to CD115, dramatically affecting monocyte survival and preventing osteoclast and macrophage differentiation, but they also block CD115/CSF-1 internalization and degradation, which could lead to potent rebound CSF-1 effects in patients after mAb treatment has ended. We thus generated and selected a non-ligand competitive anti-CD115 mAb that exerts only partial inhibitory effects on CD115 signaling without blocking the internalization or the degradation of the CD115/CSF-1 complex. This mAb, H27K15, affects monocyte survival only minimally, but downregulates osteoclast differentiation and activity. Importantly, it inhibits monocyte differentiation to CD163+CD64+ M2-polarized suppressor macrophages, skewing their differentiation toward CD14-CD1a+ dendritic cells (DCs). In line with this observation, H27K15 also drastically inhibits monocyte chemotactic protein-1 secretion and reduces interleukin-6 production; these two molecules are known to be involved in M2-macrophage recruitment. Thus, the non-depleting mAb H27K15 is a promising anti-tumor candidate, able to inhibit osteoclast differentiation, likely decreasing metastasis-induced osteolysis, and able to prevent M2 polarization of TAMs while inducing DCs, hence contributing to the creation of more efficient anti-tumor immune responses.
Keywords: CD115, CSF-1R, FcγR, M-CSFR, M2-macrophages, cancer immunotherapy, dendritic cells, immunomodulation, osteolysis, tumor microenvironment
Introduction
CD115 (macrophage colony-stimulating factor receptor, or CSF-1 receptor) is the sole cell-surface receptor identified for CSF-1, the predominant growth factor regulating the survival, growth and differentiation of myeloid lineage cells comprising monocytes, macrophages, DCs and osteoclasts.1-5 It is encoded by the c-fms proto-oncogene and belongs to the class III receptor tyrosine kinase family.5 CD115 overexpression has been reported in a wide variety of human tumors (notably breast, ovary, endometrium, cervix, prostate and kidney cancers6-9), where it has been correlated with more aggressive disease. Circulating CSF-1 is found at elevated concentrations in the plasma of patients with epithelial cancers and constitutes a poor prognosis marker, especially in breast, cervical or ovary cancers.8,10
Signaling through the CD115 pathway mediates monocyte survival and differentiation.11 Interleukin (IL)-6 can upregulate autocrine CSF-1 consumption by monocytes, stimulating their survival and differentiation into macrophages rather than DCs.11-13 Skewing of monocyte differentiation from DCs to macrophages has been proposed to contribute to tumor-induced immunosuppression.13 Results from murine models have shown that the CD115/CSF-1 pathway plays a central role in tumor progression through its effects on the differentiation of tumor-associated macrophages (TAMs).3,14 TAM infiltration into tumors has been linked with poor prognosis in many cancers.15 In breast cancer models, CSF-1 was shown to be an important chemoattractant for macrophages and to enhance their infiltration into the primary tumor, contributing to progression.14,16 Once at the tumor site, TAMs mediate the angiogenic switch,17 and they facilitate tumor cell extravasation and metastasis.18,19 It is now recognized that TAMs can represent the most abundant immunosuppressive cell population in the tumor microenvironment, recruited by CSF-1 and MCP-1 (CCL2).15 CSF-1 is known to polarize macrophages toward M2-type.20-25 M2-type macrophages that express the hemoglobin scavenger receptor (CD163)25-28 are characterized by high FcR-mediated phagocytic capacity associated with regulatory functions.29-32 Duluc et al.22 suggested that human monocytes are skewed to a M2d subtype through autocrine CSF-1 consumption, facilitated by tumor-induced IL-6 production.
CSF-1 is also a main cytokine regulating osteoclast differentiation, as evidenced by the osteopetrotic phenotypes of CSF-1 or CD115-deficient mice.2,3,33 Tumor cells metastatic to bone and producing CSF-1 stimulate the differentiation of osteoclasts that induce bone degradation and pain in cancer patients. Not only the differentiation but also the bone-resorption activity of human osteoclasts is dependent on CSF-1/CD115 in addition to receptor activator of NF-kappaB (RANK)/RANKL.34 Both cell-surface and secreted CSF-1 expressed by bone-metastatic tumor cells can contribute to osteoclast formation.35
The CD115 pathway is therefore implicated at multiple levels during cancer progression and its inhibition represents a promising therapeutic strategy. MAbs to CD115 have been previously described to block the receptor signaling (ref. 36 and patent WO2009/026303); however, one difficulty in the clinical use of anti-CD115 mAbs is the ubiquitous expression and function of CD115 in normal myeloid cells, evidenced by the severe phenotype of CD115-knockout mice.3 Moreover, the use of mAbs that block the formation of the CSF-1/CD115 complex affects the physiological degradation pathway of CSF-1 and results in massively elevated plasma CSF-1 levels, which may lead to rebound effects in treated patients.1,4
The development of new anti-CD115 mAbs is needed to overcome these important drawbacks. We have therefore selected a new mAb to CD115 (patent WO2009/112245), H27K15, that exhibits inhibitory effects on the receptor function. In contrast to other anti-CD115 mAbs (ref. 36 and patent WO2009/026303), H27K15 does not compete with ligand binding and exhibits different effects on signal transduction and cellular trafficking. This mAb shows interesting properties that may make it suitable for clinical use as a cancer therapy. First, H27K15 downregulates osteoclast differentiation and activity, which could block metastasis-induced bone degradation. Second, it inhibits monocyte differentiation into CD163+CD64+ M2-polarized suppressor macrophages, rather driving their differentiation toward CD14-CD1a+ DCs. Third, this antibody differs from other anti-CD115 mAbs by affecting only marginally the survival of monocytes. Thus, mAb H27K15 is a promising candidate for cancer immunotherapy that could help avoid rebound effects and toxicity in treated patients.
Results
Anti-CD115 mAbs differently affect CSF-1 binding
We generated a new mAb directed against human CD115, referred to as CXIIG6, which stained a CD115-transfected NIH/3T3 cell line, but not untransfected cells (Supplementary Methods and Fig. S1). The mAb is an IgG2a,κ recognizing an epitope located in the N-terminal domain of human CD115 (data not shown) and does not cross-react with murine CD115. It was found to dose-dependently decrease MMP-9 production by monocyte-derived osteoclasts differentiated with CSF-1 and RANKL (Fig. S2). It also decreased the CSF-1-dependent phosphorylation of CD115 intracellular tyrosine (Tyr) 708 in NIH/3T3-CD115 cells (data not shown). To evaluate the potential effects of this mAb as a tool for cancer immunotherapy, we derived a humanized IgG1 version, H27K15, as described in the Supplementary Methods. Like the parental mAb, H27K15 decreased the CSF-1-dependent phosphorylation of CD115 Tyr708 in NIH/3T3-CD115 cells (data not shown). ELISA showed that H27K15 did not cross-react with other TK receptors sharing sequence homologies with CD115 (data not shown), suggesting that the mAb would selectively target CD115 in humans.
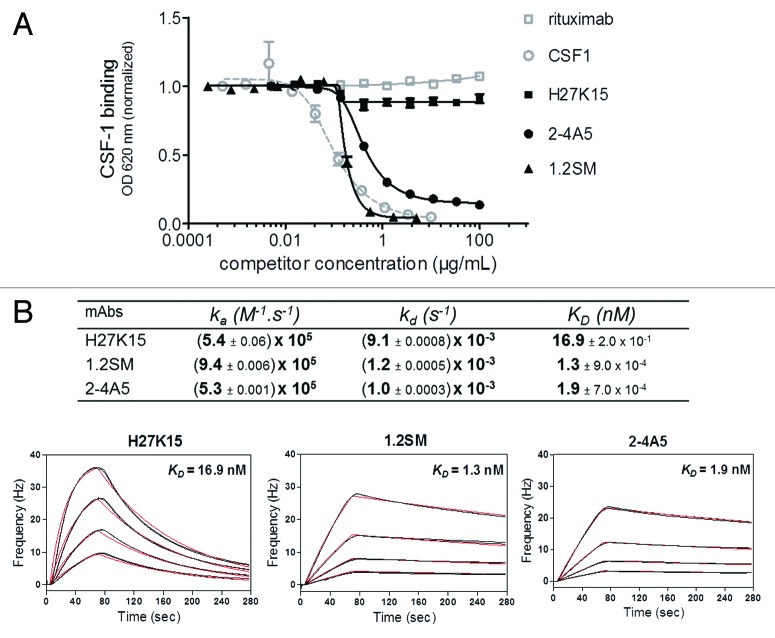
MAbs 2–4A536,37 and 1.2SM (WO2009/026303) are other anti-CD115 mAbs previously shown to inhibit the CSF-1/CD115 pathway. We aimed to analyze and compare the modes of action and biological effects of these two anti-CD115 mAbs with those of H27K15. mAb 1.2SM was produced on a similar human IgG1 backbone as H27K15, while mAb 2–4A5 was available only as a rat IgG1. Their competition with CSF-1 binding was studied by ELISA on immobilized recombinant CD115 extracellular domain (ECD)-Fc. As shown on Figure 1A, mAb H27K15 had a minimal effect on CSF-1 binding to CD115, with only around 10% inhibition at highest concentrations, reflecting the fact that its epitope is located outside of the CSF-1-binding site (data not shown). In contrast, mAbs 1.2SM and 2–4A5 totally prevented CSF-1 binding to CD115, with a slightly lower EC50 for mAb 1.2SM than for 2–4A5 in this assay (0.17 and 0.40 µg/ml, respectively).
Figure 1. Effects of anti-CD115 mAbs on CSF-1 binding to its receptor and affinity studies. (A) Binding of biotinylated CSF-1 incubated at 0.06 µg/ml on immobilized CD115 ECD-Fc in the presence of increasing concentrations of anti-CD115 mAbs, isotype control mAb (rituximab) or unlabeled CSF-1. MAbs 1.2SM and 2–4A5 block CSF-1/CD115 binding, which is only minimally (~10%) affected by H27K15. (B) Affinities of anti-CD115 mAbs for human CD115 ECD measured by QCM. Four different concentrations of CD115 were injected on each immobilized mAb. Rates and affinity constants (ka, kd and KD) were calculated from fits of two sets of sensorgrams obtained for each of the anti-CD115 mAbs using a simple model 1:1.
Using Quartz Crystal Microbalance (QCM), mAbs 1.2SM and 2–4A5 showed high affinities for recombinant CD115 ECD, with KD of 1.3 and 1.9 nM respectively (Fig. 1B). H27K15 differed from 1.2SM and 2–4A5 by its faster dissociation rate. Its KD in this setting was of 16.9 nM, one-log higher than for mAbs 1.2SM or 2–4A5.
These results show that mAbs 1.2SM and 2–4A5 are high affinity anti-CD115 mAbs that block CSF-1 binding to its receptor, whereas H27K15 has intermediate affinity to CD115 and does not prevent CSF-1/CD115 binding.
Diverging effects of anti-CD115 mAbs on receptor signaling and trafficking
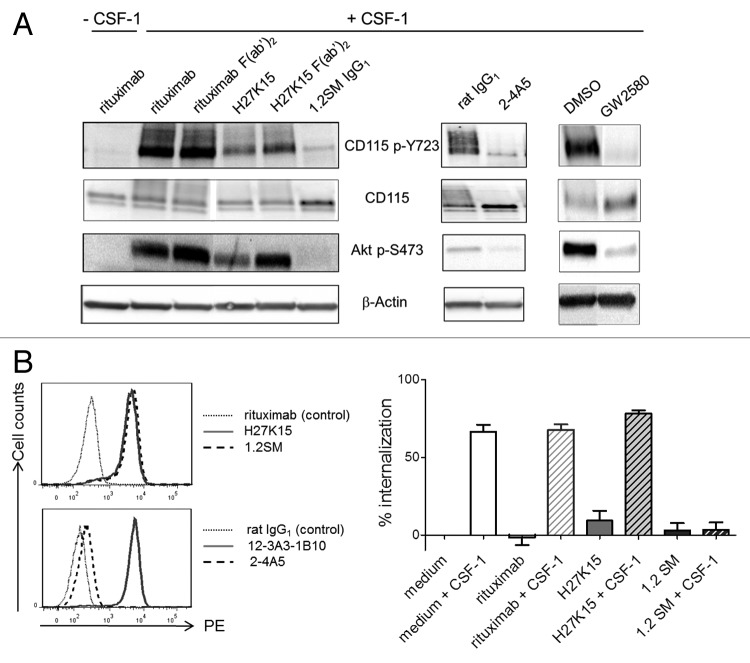
The anti-CD115 mAbs were then tested in a phosphorylation assay using the myeloid leukemia cell line OCI-AML5,38 expressing detectable surface levels of CD115 (Fig. S3). In the absence of CSF-1, the mAbs had no effect on CD115 phosphorylation, showing that they had no agonistic activity (data not shown). Stimulation by CSF-1 induced CD115 phosphorylation on Tyr723 and activation of the PI3 kinase pathway, as indicated by Akt phosphorylation (Fig. 2A).5,39,40 Compared with the control IgG1 rituximab, H27K15 decreased the CSF-1-dependent phosphorylation of CD115 Tyr723 and Akt Ser473 (Fig. 2A). This effect was partially dependent on the presence of H27K15 Fc region, since F(ab’)2 at the same molar concentration appeared less potent. In contrast, mAbs 1.2SM or 2–4A5 completely inhibited CD115 and Akt phosphorylation. F(ab’)2 derived from 1.2SM had the same effect (not shown), indicating that this inhibitory effect was independent from the mAb Fc. The small-molecule CD115 tyrosine kinase inhibitor GW258041 also potently inhibited CD115 and Akt phosphorylation.
Figure 2. Effects of anti-CD115 mAbs on CSF-1-dependent signal transduction and receptor trafficking. (A) Effects on CD115-mediated signal transduction in AML5 cells. CSF1-deprived AML5 were treated with the indicated anti-CD115 mAbs, isotype controls, the TK inhibitor GW2580 or vehicle (DMSO) during 1h at 37°C. Cells were then stimulated for 3 min at 37°C with CSF-1 (100 ng/ml) and cell lysates were subjected to western blotting with anti-phospho-Tyr723 CD115, anti-CD115, anti-phospho-Ser473 Akt. Membranes were also probed with the anti-β actin antibody as a loading control. The results shown are representative from at least two independent experiments. (B) Effects on CSF-1-dependent CD115 internalization. Left panels: Immunostaining of EL4-CD115 cells with mAbs H27K15, 1.2SM or isotype control rituximab (top panel), or with mAbs 2–4A5, 12–3A3–1B10 or isotype control rat IgG1 (bottom panel). Right panel: CD115 internalization in EL4-CD115 cells stimulated or not with CSF-1 for 30 min in the presence or absence of mAbs. Remaining cell surface CD115 was measured by FC using detection mAb 12–3A3–1B10. Mean percentages of CD115 internalization +/− SD were calculated from 3 independent experiments as described in the Methods.
After CSF-1 binding, the cytokine-receptor complex is normally internalized and degraded.39 The band corresponding to total CD115 was more intense in the presence of mAbs 1.2SM and 2–4A5 compared with their respective isotype controls, suggesting that these anti-CD115 mAbs inhibited the receptor degradation. CD115 degradation was also inhibited in the presence of GW2580 (Fig. 2A). In contrast, in the presence of mAb H27K15, total CD115 was not increased, suggesting that degradation of the receptor-ligand complex could still occur.
We further investigated whether the CSF-1-dependent internalization of CD115 was modified in the presence anti-CD115 mAbs. This was analyzed using transfected EL4-CD115 cells, devoid of detectable surface FcγR (data not shown). These cells were brightly stained by mAbs H27K15 or 1.2SM (Fig. 2B, upper left panel). In contrast, mAb 2–4A5 was inefficient in staining CD115 on these cells (Fig. 2B, lower left panel), possibly due to a poor accessibility of its membrane-proximal epitope. For this reason, only mAbs H27K15 and 1.2SM were tested. EL4-CD115 cells were stimulated with CSF-1 in the presence of anti-CD115 mAbs before incubation at 37°C for 30 min, after which remaining cell-surface CD115 was measured by flow cytometry (FC) using another mAb (clone 12–3A3–1B10, Figure 2B, lower left panel). This mAb did not compete, or only minimally, with the binding of either CSF-1 or the other anti-CD115 mAbs tested (data not shown). As shown in Figure 2B (right panel), when EL4-CD115 cells were stimulated with CSF-1, the level of surface CD115 rapidly diminished, reflecting the receptor internalization. This occurred in the presence or absence of irrelevant IgG1 rituximab. mAb H27K15 did not modify the CSF-1-induced disappearance of CD115 from the cell surface, suggesting that the receptor had been internalized after binding both CSF-1 and the mAb. In sharp contrast, CSF-1-dependent CD115 internalization was completely inhibited in the presence of mAb 1.2SM.
These results indicate that the ligand-blocking mAbs 1.2SM and 2–4A5 have a drastic inhibitory effect on CD115 signaling. They also prevent CSF-1-dependent CD115 degradation, in line with the capacity of mAb 1.2SM to immobilize CD115 on the cell surface. In contrast, mAb H27K15, which does not block CSF-1 binding, partially inhibits CD115 signaling, but still allows internalization and degradation of the CSF-1/CD115 complex.
Effects of anti-CD115 mAbs on human osteoclast differentiation and activity
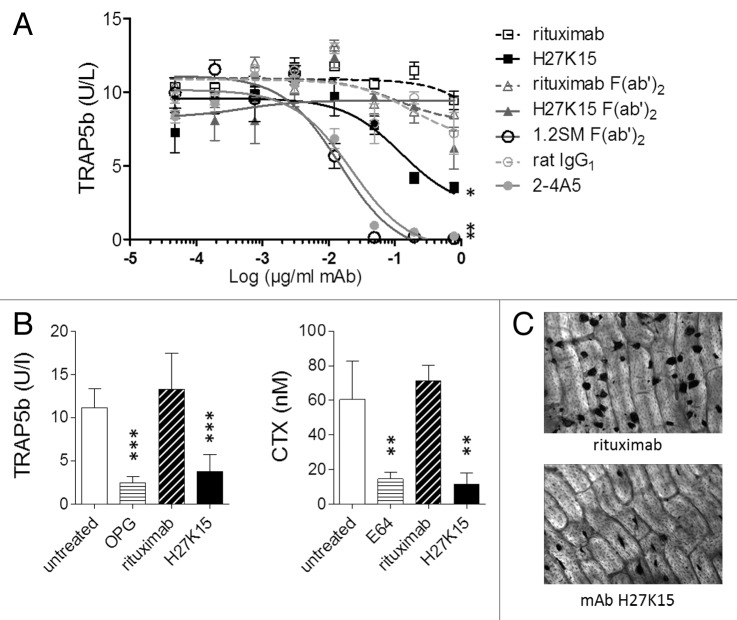
To investigate their activities on human osteoclast differentiation, anti-CD115 mAbs were added to purified blood monocytes cultured for 7 to 8 d in the presence of CSF-1 and RANKL. Osteoclast formation, measured by titration of secreted tartrate-resistant acid phosphatase 5b (TRAP5b), was drastically inhibited in cultures treated either with mAb 2–4A5 or with 1.2SM F(ab’)2 at concentrations above 0.1 µg/ml (Fig. 3A). With H27K15, osteoclast numbers were reduced less dramatically. Its inhibitory effect was Fc-dependent, since H27K15-derived F(ab’)2 showed much weaker activity in this assay. Fab fragments alone did not have any significant inhibitory effect (data not shown).
Figure 3. Effects of anti-CD115 mAbs on human osteoclast differentiation and activity. (A) Model of osteoclast differentiation from human monocytes. TRAP5b was titrated in culture supernatants from primary monocytes cultured for 8 d with CSF-1 and RANKL in the presence of graded mAb or F(ab’)2 concentrations. Three-parameter fit curves were calculated by GraphPad Prism using means +/− SEM from quadruplicate wells. Results shown were obtained from one blood donor representative of 3 tested. *: p < 0.05 using Mann-Whitney’s 2-tailed test. (B) Humanized anti-CD115 mAb H27K15 inhibits osteoclast differentiation from human CD34+ precursors and their bone resorption activity. Levels of TRAP5b (left) and CTX (right) measured following culture with 1 µg/ml H27K15 (means from 6 wells +/− SD) were compared with those obtained with 1 µg/ml of control IgG1 rituximab. Osteoprotegerin (OPG) and the cysteine protease E64 were used as reference inhibitors of osteoclast differentiation and bone resorption activity, respectively, and their effects were analyzed by comparison with untreated cultures. Results shown are from one donor representative of 4. Statistical analysis was performed using one-way ANOVA followed by t-test for comparing TRAP5b concentrations between H27K15- and rituximab-treated cultures, or with Kruskall-Wallis followed by Mann-Whitney for comparing CTX levels. **p < 0.01 and ***p < 0.001 vs. control, n = 6. (C) Representative microscopic images of the effect of mAb H27K15 on cultures from the same donor as in (B), compared with control IgG1. TRAP staining of osteoclasts differentiated on bone slices was performed after 10 d of culture with CSF-1 and RANKL in presence of the mAbs. Original microscope magnification × 100.
Since mAb H27K15 could downregulate osteoclast differentiation, its effects were tested in another model measuring both osteoclast differentiation and their bone resorption activity, using human CD34+ cells as precursors.42 These were cultured for 10 d on bone slices in the presence of CSF-1 and RANKL, and of mAb H27K15 or irrelevant IgG1 rituximab, added at 1 µg/ml from the first day of culture. In line with the previous results, significant reduction of secreted TRAP5b was observed with mAb H27K15 (Fig. 3B). In addition, bone resorption measured by titration of C-terminal cross-linked telopeptides of type I collagen (CTX) was inhibited. Microscopic images of TRAP staining at day 10 (Fig. 3C) illustrate the reduced osteoclast numbers in cultures treated with H27K15.
In summary, the ligand-blocking mAbs had a dramatic effect on osteoclast differentiation, which was totally inhibited after culture at concentrations above 0.1 µg/ml. In contrast, mAb H27K15 did not eradicate osteoclasts but diminished their number and osteolytic activity.
Complete blockade of CD115 signaling affects cell survival in macrophage differentiation cultures
We then studied the effects of the anti-CD115 mAbs or F(ab’)2 in a model of macrophage differentiation from blood monocytes. CD14+ monocytes from different blood donors were allowed to differentiate in the presence of both GM-CSF and CSF-1, known to induce macrophage differentiation toward M1- and M2-polarized populations, respectively.15,23,31,43 The CD115 tyrosine kinase inhibitor GW2580, known to inhibit the CSF-1-dependent proliferation of human monocytes and the differentiation of murine macrophages in vitro,41,44 was tested in the same assay.
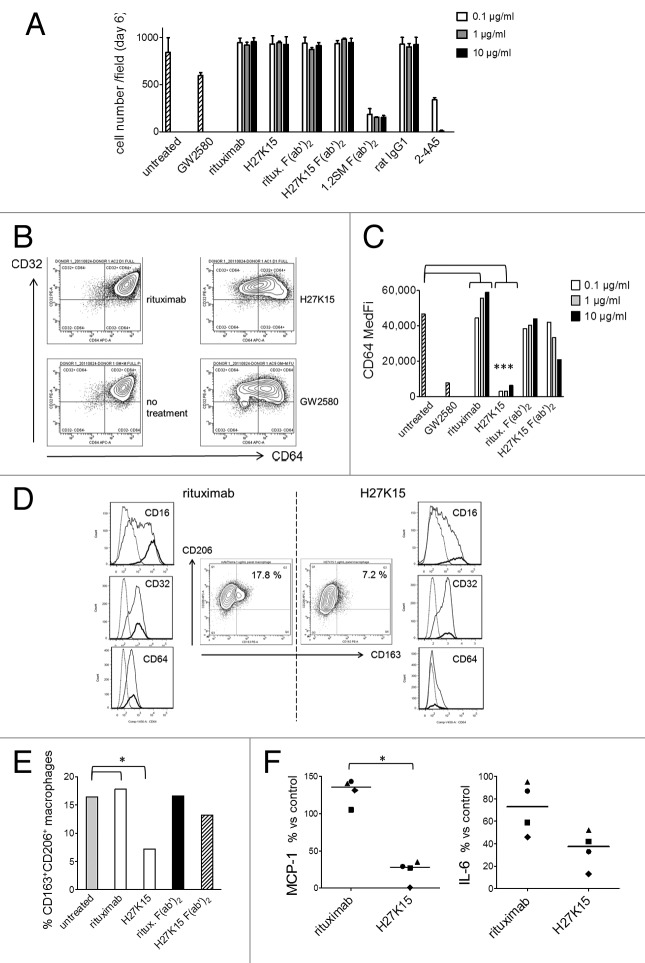
Observation of day-6 cultures showed that cells with mononuclear phagocyte-like morphologies were able to differentiate in cultures treated with H27K15. In contrast, the CD115-blocking mAbs 2–4A5 and F(ab’)2 derived from 1.2SM induced almost total cell death. Results from one representative donor are shown on Figure 4A. Cell counts at day 6 were comparable between H27K15- and control IgG1-treated cultures, but drastically reduced in the presence of 2–4A5 and 1.2SM F(ab’)2. F(ab’)2 from mAb 1.2SM showed similar high cytotoxicity at all concentrations tested (equimolar to 0.1–10 µg/ml full IgG) while mAb 2–4A5 induced total cell death at 1 and 10 µg/ml. With GW2580, cytotoxicity was less drastic at the dose tested (1 µM), but only 70% of the cells remained alive at day 6 compared with untreated cultures. Thus, the anti-CD115 mAbs differently affected cell viability in this monocyte-to-macrophage differentiation model: the CSF-1-blocking mAbs prevented cell survival, while mAb H27K15 was not cytotoxic to differentiating cells.
Figure 4. H27K15 is not cytotoxic to monocytes cultured in GM-CSF and CSF-1 but inhibits M2-type macrophage differentiation. CD14+ monocytes were cultured with GM-CSF alone (from day 0 to day 3) and GM-CSF plus CSF-1 (from day 3 to day 6), in the presence of anti-CD115 or control mAbs at the indicated concentrations, F(ab’)2 at corresponding equimolar concentrations (0.06, 0.6 or 6 µg/ml) or the CD115 tyrosine kinase inhibitor GW2580 at 1 µM. (A) Macrophage viability after 6 d. Shown are the mean cell counts in 3 wells ± SD obtained in each culture condition from one representative blood donor. (B) Inhibition of CD64 expression after monocyte differentiation in the presence of mAb H27K15 (10 µg/ml) or GW2580, compared with their respective negative controls rituximab or no treatment. Dot plots represent CD64 and CD32 staining of live cells derived from one blood donor representative of 4 tested. (C) Medians of CD64 fluorescence intensities in monocyte differentiated from the same donor in the presence or absence of GW2580, H27K15 or rituximab (both at 0.1, 1 or 10 µg/ml), or their derived F(ab’)2 at equimolar concentrations. * Mann-Whitney’s two-tailed test p = 0.029 (n = 4 donors) between CD64 MFI with mAb H27K15 / CD64 MFI in untreated culture compared with rituximab. (D) Middle panels: Dot plots represent CD163 and CD206 surface expression in monocytes differentiated with GM-CSF and CSF-1 in the presence of either H27K15 or control rituximab (1 µg/ml). Resulting percentages of CD163+ CD206+ cells are indicated. Histogram overlays of CD16, CD32 and CD64 fluorescence in the live cell population (plain lines) and in the CD163+ CD206+ (bold lines) subpopulation are shown for H27K15- (right) or rituximab-treated (left) cultures. Dotted lines: isotype controls. Data obtained from one blood donor representative of 4 tested. (E) Percentages of CD163+ CD206+ macrophages in cultures from the same donor after differentiation in the presence of H27K15 or rituximab (1 µg/ml), or the corresponding F(ab’)2 (0.6 µg/ml), or no reagent. * Mann-Whitney’s two-tailed test p = 0.029 (n = 4 donors) between % CD163+ cells with mAb H27K15 / % CD163+ cells in untreated culture compared with rituximab. (F) MCP-1/CCL2 and IL-6 were titrated in culture supernatants from monocyte-derived cells from 4 different blood donors after a 6-d culture with GM-CSF and CSF-1 in the presence of mAb H27K15 or isotype control rituximab (both at 1 µg/ml). Shown are the median percentages of variation in MCP-1/CCL2 (left) or IL-6 (right panel) produced in rituximab- or H27K15-treated cultures vs untreated cultures. *Mann-Whitney’s two-tailed test p < 0.05 between MCP-1 production with mAb H27K15 / MCP-1 production in untreated culture compared with rituximab.
mAb H27K15 inhibits macrophage polarization toward the M2 type
Because H27K15, but not the other anti-CD115 mAbs, maintained cell viability in the monocyte-to-macrophage differentiation model, we studied its effects on the cell phenotypes and on cytokine production. Day-6 cultures were analyzed by FC for cell-surface expression of the human IgG Fc receptors CD64 (FcγRI), CD32 (FcγRII) and CD16 (FcγRIII). Among those receptors, CD16 and CD64 are expressed at high levels by M2-polarized macrophages induced by CSF-111,27. Surface expression of CD64 was drastically reduced after treatment with H27K15 or with the CD115 TK inhibitor GW2580, compared with rituximab or untreated cultures, as shown in Figure 4B and C). CD64 downregulation by H27K15 occurred in cultures from all blood donors tested (p = 0.029, n = 4). CD16 expression was found to be concomitantly reduced (Fig. 4D and data not shown). Downregulation of CD16 and CD64 were also observed with GW2580, although not in all donors tested (data not shown), suggesting that FcγR downregulation was not caused by the binding of the mAb Fc region to CD16 or CD64 followed by internalization of the complex, but rather a consequence of CD115 function blockade. While H27K15 potently inhibited expression of CD64 and CD16, CD32 was hardly affected (Fig. 4B and data not shown), suggesting that the mAb had led to the emergence of a cell population likely related to DCs, which are CD32+ but barely express CD16 and CD64.45 F(ab’)2 derived from H27K15 showed only a weak, non-statistically significant effect on CD64 expression (Fig. 4C), as on CD16 expression (data not shown), showing that this effect of H27K15 was Fc-dependent.
We then analyzed the expression of CD163 and CD206, two phenotypic markers of M2-polarized macrophages.26-28,31 After differentiation in GM-CSF and CSF-1, the vast majority of cells were CD206-positive and a smaller proportion of cells also expressed CD163 (Fig. 4D). Cells exhibiting both surface markers represented from 7.1 to 16.4% of the total population without treatment (data not shown). In control cultures, CD163+ cells were CD16-bright and CD64-positive (Fig. 4D), a phenotype previously described for CSF-1-induced M2 macrophages.23,25,27 Culture with mAb H27K15 inhibited the differentiation of this CD163+CD16brightCD64+ macrophage population, as shown in Figure 4D and E) for one representative donor. The percentages of CD206+CD163+ cells decreased from 2.5- to 4-folds in cultures treated with 1 µg/ml H27K15 compared with rituximab (Fig. 4E and data not shown, p = 0.029, n = 4). F(ab’)2 derived from H27K15 had weak or no effect on CD163 expression, again indicating that the Fc region of the anti-CD115 mAb was involved in its mode of action.
The chemokine MCP-1/CCL2 and IL-6 are two soluble factors implicated in M2 macrophage polarization.22,46 Strikingly, in all donors tested, production of MCP-1 was drastically suppressed when monocytes differentiated with GM-CSF and CSF-1 in the presence of mAb H27K15 (Fig. 4F). MCP-1 levels ranged from 317 to 17,021 pg/ml in the supernatants from control rituximab-treated cultures, depending on the blood donor. They were reduced to levels of 81 to 172 pg/ml after differentiation in the presence of H27K15, representing a decrease from 4-folds to 2-log. MCP-1 inhibition by H27K15 was Fc-dependent, since F(ab’)2 were less potent in similar conditions (Fig. S4) and Fab fragments did not decrease MCP-1 levels compared with untreated control cultures (data not shown). We investigated the contributions of FcγR in H27K15 mode of action, by adding blocking F(ab’)2 to CD16, CD32 and CD64 alone or in combinations to the monocyte cultures (Fig. S5). Only the combination of the 3 F(ab’)2 significantly inhibited the effect of H27K15 on MCP-1 production, suggesting that each FcγR may contribute by binding the mAb Fc region.
IL-6 levels between 19 and 134 pg/ml were found in the supernatants from control rituximab-treated cultures. They were also decreased in all donors upon culture with H27K15 (from 1.8- to 4.4-fold, Figure 4F), although to a lesser extent than MCP-1 levels. Thus, mAb H27K15 is a potent inhibitor of MCP-1 secretion and downregulates IL-6 production by differentiating monocytes. As evidenced by these changes in cytokine/chemokine production and in cell phenotypes, targeting CD115 with mAb H27K15 inhibits the differentiation of M2-polarized macrophages.
mAb H27K15 skews monocyte differentiation from macrophages toward DCs
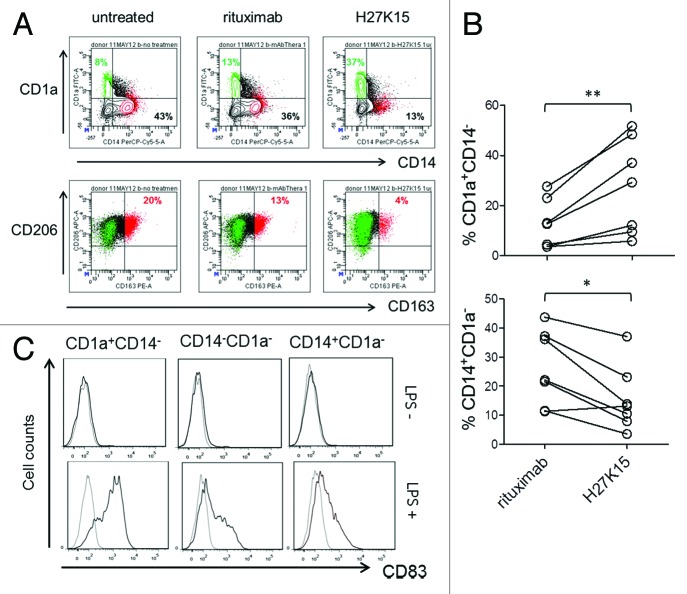
It has been reported that both CSF-1 and IL-6 produced by carcinoma cells inhibit the differentiation of DCs, which may contribute to cancer-induced immunosuppression.13 IL-6 alone can switch monocyte differentiation from DC to macrophages, by upregulating CD115 expression and facilitating CSF-1 consumption.12 We therefore analyzed the expression of CD1a and CD14, as DC and monocyte/macrophage markers, respectively, in addition to CD163 and CD206 in cultures from monocytes stimulated for 6 d with GM-CSF and CSF-1 (Fig. 5A). In control cultures, depending on the blood donors, the majority of cells were either CD14+CD1a- macrophages, or CD14-CD1a- cells of immature or intermediate differentiation stage. A population of CD1a+CD14-CD163- DCs was also present, representing 3% to 28% of the live cell population in control cultures (Fig. 5A and data not shown). In the presence of anti-CD115 mAb H27K15, DC percentages were increased in all donors tested (Fig. 5B, p = 0.0006, n = 7), reaching up to 52% of the total cell population generated in the presence of H27K15. The percentages of CD14+CD1a- macrophages were concomitantly decreased (Fig. 5B, p = 0.007, n = 7).
Figure 5. mAb H27K15 skews monocyte differentiation from macrophages toward DCs. (A) Dot plots represent CD1a and CD14 expression (upper panel) or CD206 and CD163 expression (lower panel) after 6 d of monocyte differentiation with GM-CSF and CSF-1 in the presence or absence of mAb H27K15 or control IgG1 rituximab. CD1a+CD14- cells (green) are CD163-CD206+, while the CD163-positive population (red) is CD14+CD1a-. Percentages of CD1a+CD14- and CD14+CD1a- cells are indicated in the corresponding quadrants and percentages of CD206+CD163+ cells are shown in red. (B) Increase in DCs (CD1a+CD14-, upper panel) and decrease in macrophages (CD14+CD1a-, lower panel) within the live cell population in monocyte from 7 different blood donors differentiated with GM-CSF and CSF-1 in presence of 1 µg/ml H27K15, compared with control rituximab. **p = 0.015 and *p = 0.03 using Wilcoxon’s paired test. (C) CD83 expression was analyzed by FC in monocytes cultured for 6 d with GM-CSF and CSF-1 in presence of mAb H27K15, without (upper panels) or after LPS stimulation (lower panels) for an additional 24 h. Histograms showing CD83 staining (black lines) compared with isotype control (gray lines) in the CD1a+CD14-, CD1a-CD14- and CD14+CD1a- populations. Results from one blood donor representative of 2 tested in independent experiments.
To verify that the CD1a+CD14- cells induced by mAb H27K15 were bona fide DCs, we analyzed the expression of CD83, maturation marker of human myeloid DCs,47 in day-6 cultures stimulated with LPS. After 24 h, LPS-treated CD1a+CD14- cells strongly expressed surface CD83, while the double-negative or the remaining CD1a-CD14+ populations expressed only low levels or no CD83 (Fig. 5C). These results show that targeting CD115 with mAb H27K15 induced monocytes to differentiate into DCs rather than macrophages.
Ligand-blocking anti-CD115 mAbs induce rapid monocyte death
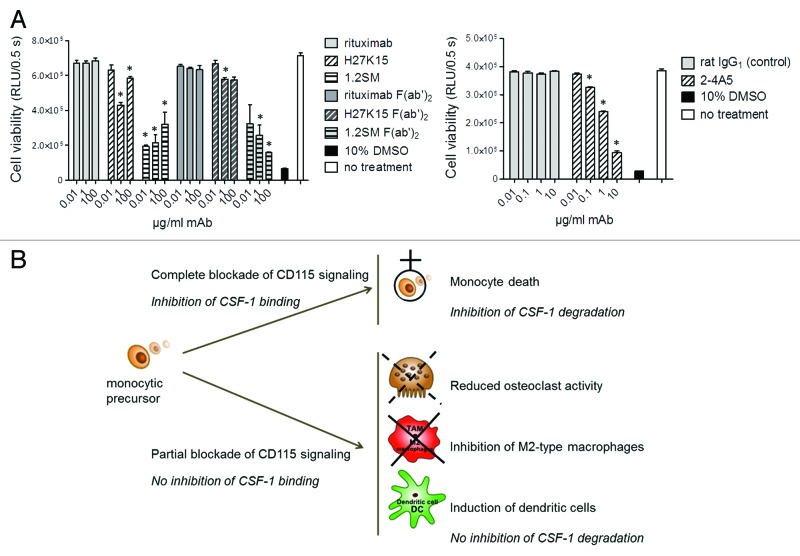
Models of osteoclast or macrophage differentiation described above utilized blood monocytes as precursor cells. In all cases, ligand-blocking anti-CD115 mAbs 2–4A5 or 1.2SM drastically prevented cell differentiation. Monocyte survival is known to require signaling through CD115, which can be mediated by autocrine CSF-1.11,12 We therefore studied the effects of the different mAbs on the viability of primary blood monocytes, cultured in medium containing FCS, but in the absence of exogenous human CSF-1. Figure 6A (left panel) shows that after 1-d culture, only a minor decrease in monocyte viability was observed in the presence of mAb H27K15 (mean ± SEM: 6% ± 2% at 0.01 µg/ml and 21 ± 5% at 1 µg/ml, n = 5 donors, data not shown). In contrast, culture with mAb 1.2SM even at low concentrations resulted in monocyte death within 24 h (52 ± 6% at 0.01 µg/ml and 61 ± 5% at 1 µg/ml, n = 5, data not shown). This cytotoxic effect was also observed with 1.2SM-derived F(ab’)2 and thus independent from its Fc fragment. mAb 2–4A5 appeared less cytotoxic than 1.2SM at low concentrations (Fig. 6A, right panel), but it showed dose-dependent toxicity and up to 73% mortality (n = 2) was observed with 10 µg/ml mAb.
Figure 6. Effects of anti-CD115 mAbs on monocyte viability. (A) Viability of primary blood monocytes analyzed by titration of cellular ATP after 1-d culture in the absence of exogenous human CSF-1. Culture in 10% DMSO was used as a positive control for toxicity. Left: mAbs H27K15, 1.2SM or rituximab were added to the cultures at 0.01, 1 or 100 µg/ml. Their derived F(ab’)2 were used at the corresponding equimolar concentrations. Right: anti-CD115 mAb 2–4A5 or control rat IgG1 were added to the cultures at 0.01, 0.1, 1 or 10 µg/ml. Each of the results shown is representative of those obtained from 2 different donors. * p < 0.05 vs. control using Mann-Whitney’s two-tailed test. (B) Schematic illustration of the different modes of action of anti-CD115 mAbs and their corresponding biological effects. MAbs which block ligand binding to CD115 and completely inhibit CD115 signaling induce rapid monocyte death and prevent CSF-1 degradation. In contrast, an anti-CD115 mAb which does not block CSF-1 binding but down-modulates CD115 signaling reduces osteoclast differentiation and activity and skews monocyte differentiation from M2-macrophages toward DCs, without blocking CSF-1 degradation.
In summary, as shown schematically in Figure 6B, mAbs that block ligand binding to CD115 and totally inhibit CD115 signaling also induce rapid monocyte death. This effect alone might explain the complete lack of monocytic differentiation observed toward either osteoclasts (with CSF-1 and RANKL) or macrophages (with GM-CSF and CSF-1) in the presence of mAbs 2–4A5 or 1.2SM. In contrast, the anti-CD115 mAb H27K15, which does not prevent CSF-1 binding and only reduces CD115 signaling via Akt, is minimally cytotoxic to monocytes, while re-directing CSF-1-dependent myeloid cell differentiation, inhibiting M2-type macrophages and inducing dendritic cells.
Discussion
We have shown that different mAbs directed against the same target, CD115, could have diverse and even diverging effects at the molecular and cellular levels. These appear directly related to their differential effects on CSF-1 binding to its receptor. Two mAbs that potently blocked CSF-1/CD115 binding, even through distant epitopes on CD115 (D1-D2 for 1.2SM, vs D4-D5 for 2–4A5; patent WO2009/026303 and data not shown), totally inhibited CD115 phosphorylation and signaling. As expected based on the receptor biology,39 they also prevented the receptor internalization and blocked CD115 on the cell surface. This had drastic consequences on myeloid lineage cells, as neither macrophages nor osteoclasts could differentiate from cultured monocytes. Monocytes rapidly died in the presence of ligand-blocking mAbs, with mAb 1.2SM showing the strongest effect, possibly reflecting its highest affinity to CD115. Thus, the complete blockade of CD115 signaling in the absence of exogenous human CSF-1 resulted in rapid monocyte death. When monocytes were cultured with CSF-1 and RANKL or with CSF-1 and GM-CSF, signaling through either the RANK or GM-CSF receptor pathways could not compensate for the lack of CD115 stimulation to sustain osteoclast or macrophage differentiation. Osteoclast differentiation is dependent on CD115, upstream of the RANK/RANKL pathway.33,34 GM-CSF has been shown to induce CSF-1 production in monocytes.48 Since the total blockade of CD115 signaling resulted in cell death even in the presence of GM-CSF, our results, which are in line with former studies,48 suggest that GM-CSF mediates monocyte survival through the sole induction of CD115 ligand.
mAb H27K15 showed a different mode of action because it did not block CSF-1 binding and inhibited only partially CD115-mediated signaling. Importantly, internalization and degradation of the receptor-ligand complex still occurred. Our results suggest that the trimeric H27K15/CD115/CSF-1 complex can be internalized from the cell surface and degraded, concomitant with a decrease in receptor-mediated signal transduction. mAb H27K15 had only a weak effect on monocyte viability, suggesting that low intensity signaling through CD115 is sufficient to support monocyte survival, but not their full differentiation into either M2-type macrophages or bone-resorbing osteoclasts.
The elimination of circulating CSF-1 is physiologically mediated through CD115 binding, internalization and degradation.49 One major issue in the clinical use of anti-CD115 mAbs is the dramatic several-log increase of plasma CSF-1 that has been observed in preclinical models upon use of ligand-blocking mAbs.1,4 Such elevation of circulating CSF-1 may lead to severe rebound effects following withdrawal of the treatment or in organs not accessible to mAbs. Our results show that ligand-blocking mAbs inhibit CSF-1-dependent CD115 degradation and that mAb 1.2SM immobilizes the receptor on the cell surface. mAb H27K15, in contrast, did not block CD115 internalization or degradation, suggesting that in vivo treatment may not result in the accumulation of plasmatic CSF-1. This remains to be verified in a relevant animal model, still lacking because mAb H27K15 recognizes only human and chimpanzee CD115, and in clinical trials. A transgenic mouse strain is currently being generated to provide a model for preclinical proof-of-concept.
When present during monocyte differentiation, in the presence of GM-CSF and CSF-1, mAb H27K15 inhibited the generation of trophic- or M2-type macrophages. This anti-CD115 mAb drastically inhibited the expression of CD163, the scavenger receptor marker of M2-polarized macrophages. CD163 expression is induced during differentiation with CSF-1, while the expression of the mannose receptor CD206 is rather dependent on GM-CSF.25 In line with these results, CD115 inhibition affected CD163, but not CD206 expression. M2-type macrophages are also characterized by their high phagocytic and IgG binding capacities, related to surface expression of several FcγRs.23,25-27,30,43 The expression of CD64/FcγRI and CD16/FcγRIII was drastically downregulated on differentiating cells treated with H27K15, while CD32/FcγRII was hardly affected. This modulation of FcγR expression reflects monocyte differentiation into CD1a+CD14-CD163- DCs instead of CD163+CD14+CD1a- macrophages. Indeed, monocyte-derived DCs are known to express CD32, but barely CD16 and CD64, if any.45
The Fc region of mAb H27K15 participates in its mode of action since the F(ab’)2 alone displayed much weaker biological activities in both CSF-1-dependent signal transduction and monocyte differentiation assays. Monomeric Fabs did not show any CD115 inhibitory activity, suggesting that dimerization of CD115 through H27K15 F(ab’)2 might perturbate the receptor function, possibly by preventing conformational changes required for signal transduction. The role of FcγRs can be central for the effect of therapeutic mAbs.50,51 An antibody may simultaneously bind to a cell-surface antigen with its variable region and to an activating or inhibitory FcR via its Fc region on the same cell, a phenomenon originally described by R.J. Kurlander52 and more recently termed “Scorpio effect.”53 Our results indicate that co-engagement of cell-surface CD115 with an FcγR expressed on the same cell or on a neighboring cell is required for the (full) function-blocking effect of mAb H27K15. OCI-AML5 cells are CD16-CD32+CD64- (Fig. S3), suggesting that the anti-CD115 mAb may cross-link CD32 with CD115 on their surface. In monocytes, which express a range of FcγR including CD32a and CD64,45 only the combination of blocking F(ab’)2 to CD16, CD32 and CD64 significantly affected H27K15 activity on MCP-1 production, suggesting that several FcγR may be involved through binding the mAb Fc region.
The cross-linking of FcγR with CD115 through mAb H27K15 may not only be involved in the mAb mode of action on the receptor inhibition, but also directly contribute to its effects on myeloid cell differentiation. Indeed, cross-linking FcγR on monocytes has been shown to favor their differentiation toward DCs.54 Together with the downregulation of the CD115 pathway, FcγR cross-linking may explain the DC-inducing effect of mAb H27K15.
A striking result was the potent suppression of MCP-1/CCL2 production by H27K15 in monocytes stimulated with GM-CSF and CSF-1. MCP-1/CCL2 has a main role in the recruitment of M2-polarized TAM and represents a promising target for cancer immunotherapy.15,55 The secretion of IL-6 was also reduced upon CD115 inhibition by H27K15 or GW2580 in all donors. MCP-1 and IL-6 are inducible by each other and their combination is known to induce M2-type macrophage polarization.46 CSF-1 has formerly been shown to stimulate the production of both IL-6 and MCP-1.56 In turn, IL-6 can stimulate M2 macrophage generation by facilitating autocrine CSF-1 consumption.22 Thus, one mechanism by which mAb H27K15 may block M2-macrophage differentiation is through inhibition of MCP-1/CCL2 and IL-6. Inhibition of IL-6 may also be directly involved in the skewing of macrophage differentiation toward DCs.12,13 The inhibition of DC development by CSF-1 and IL-6 may represent a frequent mechanism by which tumor cells escape immune recognition because macrophages or M2-polarized TAMs are poorly immunostimulatory compared with DCs.22 DCs generated in the presence of mAb H27K15 express CD83 after LPS stimulation, suggesting that they may be capable of stimulating immune responses in treated patients. Through its effects on the tumor microenvironment, mAb H27K15 may inhibit cancer host myeloid cells involved in disease progression, and also have immunostimulatory activity when used to treat cancer patients.
Methods
Antibodies
mAb H27K15 (patent application WO2009/112245) is a humanized anti-CD115 mAb derived from murine mAb CXIIG6 as described in the Supplementary Methods. mAb 1.2SM is a human anti-CD115 mAb described in patent application WO2009/026303. The variable regions of each of these mAbs were fused with a human IgG1,κ constant region (GeneBank accession numbers J00241 and J00228). MAbs H27K15, 1.2SM and rituximab (http://www.drugbank.ca/drugs/DB00073) were produced in CHO cells and purified as described in the Supplementary Methods. Rituximab was also kindly provided by Roche. The rat anti-human CD115 IgG1 2–4A5 (Neomarker, Santa Cruz or GeneTex) was generated and characterized by C.J. Sheer et al.36 Absence of endotoxin contamination in the mAb preparations was assessed using a L.A.L. test (Endosafe™ PTS, Charles River). F(ab’)2 were produced by pepsin digestion of H27K15, 1.2SM or rituximab using Pierce® F(ab’)2 preparation kit. Their purity was between 96% and 100% after gel filtration.
Competition experiments by ELISA
Serial dilutions of antibodies or human CSF-1 (GeneArt) were incubated with a fixed concentration of biotinylated CSF-1 in 96-well plates (Maxisorp, Nunc) coated with recombinant human CD115 ECD-Fc (R&D Systems). Plate-bound CSF-1 was revealed with streptavidin-HRP (Southern biotech) followed by 3,3′,5,5′-tetramethylbenzidine (TMB) Substrate (Sigma). Optical densities (OD) were recorded on a Tecan plate reader.
Affinity measurements by QCM
The affinities of anti-CD115 mAbs for human CD115 ECD were measured using the QCM technology on Attana 200 (Attana). Monoclonal antibodies were immobilized on LNB chips (Attana) and recombinant CD115 ECD (D1-D5, GeneArt) was injected for 70 sec, before a post-injection phase of 180 sec. Buffer was used as a reference and corresponding values subtracted for each hCD115 concentrations tested. Data were analyzed using Evaluation Software (Attana) and a simple 1:1 model for data fitting.
CD115 internalization in EL4-CD115 cells
EL4-CD115 cells were pre-incubated in ice-cold medium containing 10 µg/ml of each mAb or 100 ng/ml CSF-1 (ImmunoTools). The temperature was raised to 37°C. After 30 min, cells were transferred on ice and surface CD115 was detected by FC using mAb 12–3A3–1B10 (eBioscience). Median fluorescence intensities (MFI) were immediately measured on a FACS CANTO II flow cytometer (BD Bioscience). The percentages of CD115 internalization were calculated as follows: 100–100*(((Test MFI– Isotype control MFI) / (untreated control MFI– Isotype control MFI)) / ((Test MFI at t = 0 – Isotype control MFI at t = 0) / (untreated control MFI at t = 0 – Isotype control MFI at t = 0))).
CD115 phosphorylation assay
OCI-AML5 cells (DSMZ) were treated with 100 ng/ml CSF-1 (ImmunoTools) during 3 min at 37°C in the presence of anti-CD115 or control mAbs (1 µg/ml) added to the culture medium 1h before stimulation, or of the CD115 kinase inhibitor GW2580 (1 µM, LC Laboratories) or vehicle. Protein extracts were analyzed by western blot using antibodies to CD115 (C-20, Santa Cruz Biotechnology), phospho-Tyr708 CD115, phospho-Ser473 Akt (Cell Signaling Technology) and β-actin (Sigma).
Osteoclast differentiation from human monocytes
CD14+ monocytes from blood donors having given informed consent (EFS Alsace) were cultured in 96-well plates with serial dilutions of mAbs or F(ab’)2. CSF-1 (ImmunoTools) and RANKL (PeproTech) were added at respectively 25 and 40 ng/ml and cells were allowed to differentiate in the presence of mAbs or F(ab’)2 for 7 to 8 d. Secreted TRAP5b was titrated using the MicroVueTM TRAP5b EIA kit (Quidel).
Osteoclast differentiation from human CD34+ cells and activity assay
Human CD34+ cells (Lonza) were cultured on bovine bone slices (IDS Ltd) with 8.25 ng/ml CSF-1 and 16.5 ng/ml RANKL (OCP BulletKit®, Lonza). At day 7, medium was replaced and cells were cultured for 3 additional days, allowing them to resorb bone. mAb H27K15 or rituximab were added at days 0, 2, 4, 7, 8 and 9, OPG (PeproTech) at day 0 and E64 (Sigma-Aldrich) at day 7. Secreted TRAP5b and CTX were titrated by ELISA (BoneTRAP® and CrossLaps® kits, IDS Ltd).
Human macrophage differentiation assay
CD14+ monocytes were cultured in complete RPMI-Glutamax™ medium containing GM-CSF alone (10 ng/ml, PeproTech) from day 0 to day 3 and GM-CSF (2 ng/ml) plus CSF-1 (10 ng/ml, ImmunoTools) from day 3 to day 6. Antibodies, F(ab’)2 or the CD115 TK inhibitor GW2580 (LC Labs) were added at day 0 and day 3. At day 6, cells were counted (5 microscope fields /well), harvested and pools of triplicate wells were analyzed by FC using antibodies from BD Bioscience. For staining surface FcγR, anti-CD16 clone 3G8, anti-CD32 clone 3D3 (recognizing both FcγRIIa and FcγRIIb57) and anti-CD64 clone 10.1 were used. The fact that clones 3G8 and 10.1 can compete with Fc fragments for binding CD16 and CD64, respectively,58,59 did not prevent immunostaining after macrophage differentiation in the presence of IgG1. Secreted cytokines were titrated by multiplex (Bioplex, Bio-Rad).
Monocyte viability assay
CD14+ monocytes were cultured for 1 d in RPMI-1640 medium (Sigma) with 10% FCS and serial dilutions of mAbs or F(ab’)2. Cell survival was assessed using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega) and light emission was recorded on a TriStar LB 941 reader (Berthold Technologies).
Additional methods
Detailed methodologies are provided in the Supplementary Methods.
Supplementary Material
Acknowledgments
We thank Guillaume Fichet and Christine Kreuz for their contribution in the macrophage differentiation experiments, Pascal Mercier from Roche for the gift of rituximab, and Pascale Jeannin, Jean-Pierre Abastado, Bruce Acres and Jean-Luc Teillaud for critical reading of the manuscript.
Glossary
- Abbreviations: CSF-1
colony-stimulating factor-1
- CTX
C-terminal cross-linked telopeptides of type I collagen
- DCs
dendritic cells
- ECD
extra-cellular domain
- FC
flow cytometry
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- IL-6
interleukin-6
- MCP-1/CCL2
macrophage chemotactic protein-1/CC-chemokine ligand-2
- MFI
median fluorescence intensity
- QCM
quartz crystal microbalance
- RANKL
receptor activator of nuclear factor kappa-B ligand
- TAMs
tumor-associated macrophages
- TRAP
tartrate-resistant acid phosphatase
Disclosure of Potential Conflicts of Interest
Most authors are employees of Transgene, the company that patented mAb H27K15.
Supplemental Materials
All supplemental materials can be found here: www.landesbioscience.com/journals/mabs/article/25743.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/25743
References
- 1.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–20. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- 2.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 3.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–20. doi: 10.1182/blood.V99.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–9. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Sherr CJ. Colony-stimulating factor-1 receptor. Blood. 1990;75:1–12. [PubMed] [Google Scholar]
- 6.Ide H, Seligson DB, Memarzadeh S, Xin L, Horvath S, Dubey P, et al. Expression of colony-stimulating factor 1 receptor during prostate development and prostate cancer progression. Proc Natl Acad Sci U S A. 2002;99:14404–9. doi: 10.1073/pnas.222537099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith HO, Anderson PS, Kuo DY, Goldberg GL, DeVictoria CL, Boocock CA, et al. The role of colony-stimulating factor 1 and its receptor in the etiopathogenesis of endometrial adenocarcinoma. Clin Cancer Res. 1995;1:313–25. [PubMed] [Google Scholar]
- 8.Chambers SK. Role of CSF-1 in progression of epithelial ovarian cancer. Future Oncol. 2009;5:1429–40. doi: 10.2217/fon.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammes LS, Tekmal RR, Naud P, Edelweiss MI, Kirma N, Valente PT, et al. Up-regulation of VEGF, c-fms and COX-2 expression correlates with severity of cervical cancer precursor (CIN) lesions and invasive disease. Gynecol Oncol. 2008;110:445–51. doi: 10.1016/j.ygyno.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Scholl SM, Lidereau R, de la Rochefordière A, Le-Nir CC, Mosseri V, Noguès C, et al. Circulating levels of the macrophage colony stimulating factor CSF-1 in primary and metastatic breast cancer patients. A pilot study. Breast Cancer Res Treat. 1996;39:275–83. doi: 10.1007/BF01806155. [DOI] [PubMed] [Google Scholar]
- 11.Becker S, Warren MK, Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol. 1987;139:3703–9. [PubMed] [Google Scholar]
- 12.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–4. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 13.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–91. [PubMed] [Google Scholar]
- 14.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 18.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 20.Smith W, Feldmann M, Londei M. Human macrophages induced in vitro by macrophage colony-stimulating factor are deficient in IL-12 production. Eur J Immunol. 1998;28:2498–507. doi: 10.1002/(SICI)1521-4141(199808)28:08<2498::AID-IMMU2498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 22.Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–30. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 23.Verreck FA, de Boer T, Langenberg DM, van der Zanden L, Ottenhoff TH. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol. 2006;79:285–93. doi: 10.1189/jlb.0105015. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 25.Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puig-Kröger A, Sierra-Filardi E, Domínguez-Soto A, Samaniego R, Corcuera MT, Gómez-Aguado F, et al. Folate receptor beta is expressed by tumor-associated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69:9395–403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 27.Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375:196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Beck AH, Espinosa I, Edris B, Li R, Montgomery K, Zhu S, et al. The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin Cancer Res. 2009;15:778–87. doi: 10.1158/1078-0432.CCR-08-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 30.Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182:4415–22. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 31.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101:4560–5. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmieder A, Michel J, Schönhaar K, Goerdt S, Schledzewski K. Differentiation and gene expression profile of tumor-associated macrophages. Semin Cancer Biol. 2012;22:289–97. doi: 10.1016/j.semcancer.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–54. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodge JM, Collier FM, Pavlos NJ, Kirkland MA, Nicholson GC. M-CSF potently augments RANKL-induced resorption activation in mature human osteoclasts. PLoS One. 2011;6:e21462. doi: 10.1371/journal.pone.0021462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yagiz K, Rittling SR. Both cell-surface and secreted CSF-1 expressed by tumor cells metastatic to bone can contribute to osteoclast activation. Exp Cell Res. 2009;315:2442–52. doi: 10.1016/j.yexcr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherr CJ, Ashmun RA, Downing JR, Ohtsuka M, Quan SG, Golde DW, et al. Inhibition of colony-stimulating factor-1 activity by monoclonal antibodies to the human CSF-1 receptor. Blood. 1989;73:1786–93. [PubMed] [Google Scholar]
- 37.Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K, et al. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ. 2010;17:1917–27. doi: 10.1038/cdd.2010.60. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Koistinen P, Yang GS, Williams DE, Lyman SD, Minden MD, et al. Mast cell growth factor, a ligand for the receptor encoded by c-kit, affects the growth in culture of the blast cells of acute myeloblastic leukemia. Leukemia. 1991;5:493–9. [PubMed] [Google Scholar]
- 39.Li W, Stanley ER. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991;10:277–88. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacquel A, Benikhlef N, Paggetti J, Lalaoui N, Guery L, Dufour EK, et al. Colony-stimulating factor-1-induced oscillations in phosphatidylinositol-3 kinase/AKT are required for caspase activation in monocytes undergoing differentiation into macrophages. Blood. 2009;114:3633–41. doi: 10.1182/blood-2009-03-208843. [DOI] [PubMed] [Google Scholar]
- 41.Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A. 2005;102:16078–83. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rissanen JP, Ylipahkala H, Fagerlund KM, Long C, Väänänen HK, Halleen JM. Improved methods for testing antiresorptive compounds in human osteoclast cultures. J Bone Miner Metab. 2009;27:105–9. doi: 10.1007/s00774-008-0002-1. [DOI] [PubMed] [Google Scholar]
- 43.Akagawa KS. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int J Hematol. 2002;76:27–34. doi: 10.1007/BF02982715. [DOI] [PubMed] [Google Scholar]
- 44.Paniagua RT, Chang A, Mariano MM, Stein EA, Wang Q, Lindstrom TM, et al. c-Fms-mediated differentiation and priming of monocyte lineage cells play a central role in autoimmune arthritis. Arthritis Res Ther. 2010;12:R32. doi: 10.1186/ar2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boruchov AM, Heller G, Veri MC, Bonvini E, Ravetch JV, Young JW. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J Clin Invest. 2005;115:2914–23. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–54. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 48.Horiguchi J, Warren MK, Kufe D. Expression of the macrophage-specific colony-stimulating factor in human monocytes treated with granulocyte-macrophage colony-stimulating factor. Blood. 1987;69:1259–61. [PubMed] [Google Scholar]
- 49.Bartocci A, Mastrogiannis DS, Migliorati G, Stockert RJ, Wolkoff AW, Stanley ER. Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci U S A. 1987;84:6179–83. doi: 10.1073/pnas.84.17.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abès R, Dutertre CA, Agnelli L, Teillaud JL. Activating and inhibitory Fcgamma receptors in immunotherapy: being the actor or being the target. Expert Rev Clin Immunol. 2009;5:735–47. doi: 10.1586/eci.09.57. [DOI] [PubMed] [Google Scholar]
- 51.Woof JM. Insights from Fc receptor biology: a route to improved antibody reagents. MAbs. 2012;4:291–3. doi: 10.4161/mabs.20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurlander RJ. Blockade of Fc receptor-mediated binding to U-937 cells by murine monoclonal antibodies directed against a variety of surface antigens. J Immunol. 1983;131:140–7. [PubMed] [Google Scholar]
- 53.Hogarth PM, Pietersz GA. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat Rev Drug Discov. 2012;11:311–31. doi: 10.1038/nrd2909. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka M, Krutzik SR, Sieling PA, Lee DJ, Rea TH, Modlin RL. Activation of Fc gamma RI on monocytes triggers differentiation into immature dendritic cells that induce autoreactive T cell responses. J Immunol. 2009;183:2349–55. doi: 10.4049/jimmunol.0801683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eda H, Zhang J, Keith RH, Michener M, Beidler DR, Monahan JB. Macrophage-colony stimulating factor and interleukin-34 induce chemokines in human whole blood. Cytokine. 2010;52:215–20. doi: 10.1016/j.cyto.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 57.Vely F, Gruel N, Moncuit J, Cochet O, Rouard H, Dare S, et al. A new set of monoclonal antibodies against human Fc gamma RII (CD32) and Fc gamma RIII (CD16): characterization and use in various assays. Hybridoma. 1997;16:519–28. doi: 10.1089/hyb.1997.16.519. [DOI] [PubMed] [Google Scholar]
- 58.Stroncek DF, Skubitz KM, Plachta LB, Shankar RA, Clay ME, Herman J, et al. Alloimmune neonatal neutropenia due to an antibody to the neutrophil Fc-gamma receptor III with maternal deficiency of CD16 antigen. Blood. 1991;77:1572–80. [PubMed] [Google Scholar]
- 59.Dougherty GJ, Selvendran Y, Murdoch S, Palmer DG, Hogg N. The human mononuclear phagocyte high-affinity Fc receptor, FcRI, defined by a monoclonal antibody, 10.1. Eur J Immunol. 1987;17:1453–9. doi: 10.1002/eji.1830171011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.