Abstract
Purpose: This study aimed to assess the safety, pharmacokinetic and activity profiles of the human-mouse chimeric monoclonal anti-disialoganglioside GD2 antibody ch14.18 produced in Chinese hamster ovary (CHO) cells (ch14.18/CHO).
Methods: Sixteen children with recurrent/refractory neuroblastoma (median age 7.6 y) were enrolled in this Phase 1 dose-finding study. Patients received ch14.18/CHO courses of 10, 20 or 30 mg/m2/day as an eight-hour infusion over five consecutive days. Three courses at the same dose level were allowed unless disease progressed. Clearance and biodistribution of radiolabelled ch14.18/CHO in Balb/c and A/J mice were analyzed.
Results: A total of 41 ch14.18/CHO courses were given (10 × 3 courses, 5 × 2 courses, 1 × 1 course). Side effects were similar in expectedness, frequency and magnitude to those reported for ch14.18/SP2/0. The dose level of 20 mg/m2/day was confirmed. Toxicity was reversible and no treatment-related deaths occurred. In children, the peak plasma concentration was 16.51 µg/ml ± 5.9 µg/ml and the half-life was 76.91 h ± 52.5 h. A partial response following ch14.18/CHO was observed in 2/7 patients with residual disease. In mice, the half-lives were 22.7 h ± 1.9h for ch14.18/CHO and 25.0 h ± 1.9 h for ch14.18/SP2/0. The biodistribution of 125I-ch14.18/CHO in mice with neuroblastoma was identical to 125I-ch14.18/SP2/0, indicating GD2 targeting activity in vivo.
Ch14.18 produced in CHO cells showed an unchanged toxicity profile and pharmacokinetics in neuroblastoma patients compared with ch14.18 produced in SP2/0 cells, and evidence of clinical activity was observed. In mice, analysis of pharmacokinetics and biodistribution showed comparable results between ch14.18/CHO and ch14.18/SP2/0. Based on these results, ch14.18/CHO was accepted for prospective clinical evaluation.
Keywords: neuroblastoma, immunotherapy, anti GD2, ch14.18/CHO, monoclonal antibody
Introduction
Children with high-risk neuroblastoma diagnosed after 18 mo of age have a poor prognosis despite treatment with high-dose chemotherapy (HDT) and peripheral blood stem cell rescue (PBSCR) followed by differentiation therapy with isotretinoin.3 Given the success of monoclonal antibodies (mAb) in cancer therapy,4 passive immunotherapy targeting GD2 on neuroblastoma cells provides a promising strategy to improve outcome.5,6
Disialoganglioside GD2 is expressed at high density in neuroblastoma tumors with limited expression on normal tissue.7 The effector functions of anti-GD2 monoclonal antibodies (mAbs), including antibody-dependent cell-mediated cytotoxicity (ADCC), complement dependent cytotoxicity (CDC)8,9 and possibly the anti-idiotypic network,10,11 support using passive immunotherapy in neuroblastoma. A variety of anti-GD2 antibodies have been evaluated in the clinical setting, including ch14.18. Ch14.18 is a human/mouse chimeric antibody consisting of variable regions derived from the murine anti-GD2 antibody 14G2a and constant regions from a human IgG1 molecule.6,12-16
The ch14.18 antibody generated in non-secreting murine myeloma cells SP2/0 contains murine retroviruses and is unavailable in Europe. Therefore, the International Society of Paediatric Oncology European Neuroblastoma Group (SIOPEN) commissioned a Good Manufacturing Practice (GMP) production of ch14.18 antibody in cells of hamster origin (Chinese hamster ovary, CHO),1 the most commonly used mammalian host for industrial production of recombinant protein therapeutics. One of the advantages of selecting CHO cells for mAB expression is also a favorable glycosylation pattern that includes only minor amounts of the N-glycolylneuraminic acid (Neu5Gc) forms of sialic acid,17 which circumvents rapid clearance by xeno-autoantibodies against Neu5Gc that develop in humans in early childhood.18
An identical protein sequence was assured because the plasmid used was the same employed to produce the mAb evaluated in earlier clinical trials. The production change helped to avoid murine xenotropic retrovirus contamination.19 The European Medicines Agency (EMA) guidelines required a Phase 1 bridging study to assess the safety, pharmacokinetic and activity profiles of the recloned antibody ch14.18/CHO.20
Ch14.18/CHO was demonstrated to mediate ADCC and CDC and to suppress experimental liver metastasis in a preclinical neuroblastoma model as effectively as ch14.18 controls.1 We report here the results of pharmacokinetic and biodistribution analysis in mice and the Phase 1 bridging study in neuroblastoma patients.
Results
Patient characteristics
Three European centers enrolled a total of 16 patients (Table 1), nine of whom were females. At initial diagnosis, 14 patients had stage 4, one stage 2b and one stage 3 disease. Thirteen patients had measurable disease at study entry. Prior therapies included chemotherapy (16 patients), surgery (13 patients), radiotherapy (9 patients) and high-dose therapy (HDT) followed by peripheral blood stem cell rescue (PBSCR; 14 patients); six received meta-iodo-benzyl-guanidine (mIBG) therapy preceding HDT.
Table 1. Demographic data, treatments, response and outcome.
| Pt N |
Country | Sex | Age (yrs) | Stage | 1st Line Study |
Sx | CTH | RT | HDT | 2nd Line mIBG | Rel Treat |
Ch14.18/CHO start months after Dx | INSS Status prior ch14.18/CHO | Dose level | No. courses received | Centrally reviewed mIBG response | FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 |
AT |
f |
8.5 |
4 |
HRNBL1 |
yes |
yes |
yes |
yes |
yes |
n.d. |
16 |
PR |
10mg |
3 |
NE |
DOD |
|
2 |
IT |
m |
4.4 |
4 |
HRNBL1 |
yes |
yes |
yes |
yes |
yes |
n.d. |
28 |
PR |
10mg |
2 |
NE |
CR |
|
3 |
IT |
m |
13.1 |
4 |
HRNBL1 |
no |
yes |
yes |
yes |
yes |
n.d. |
34 |
PR |
10mg |
2 |
Progression |
DOD |
|
4 |
AT |
f |
6.6 |
4 |
HRNBL1 |
yes |
yes |
no |
yes |
no |
n.d. |
13 |
PR |
20mg |
3 |
Progression |
DOD |
|
5 |
AT/FR |
f |
5.3 |
4 |
HRNBL1 |
yes |
yes |
yes |
yes |
yes |
TVD |
31 |
CR |
20mg |
3 |
Continuous CR |
DOD |
|
6 |
IT |
m |
17.3 |
4 |
HRNBL1 |
no |
yes |
no |
no |
no |
TVD |
34 |
PR |
20mg |
1 |
> 90% response |
DOD |
|
7 |
AT |
f |
13.8 |
4 |
HRNBL1 |
yes |
yes |
no |
yes |
no |
n.d. |
20 |
CR |
30mg |
3 |
NE |
CR |
|
8 |
IT |
f |
10.8 |
4 |
HRNBL1 |
yes |
yes |
yes |
yes |
no |
TVD |
29 |
PR |
30mg |
3 |
Stable* |
DOD |
|
9 |
AT |
f |
3.8 |
4 |
HRNBL1 |
yes |
yes |
yes |
yes |
no |
n.d. |
15 |
PR |
30mg |
3 |
> 50% response |
CR |
|
10 |
IT |
f |
15.5 |
4 |
AIEOP NB 92 |
yes |
yes |
no |
yes |
no |
TVD |
131 |
PR |
20mg |
2 |
Stable |
DOD |
|
11 |
AT |
m |
7.4 |
4 |
HRNBL1 |
yes |
yes |
yes |
yes |
no |
TVD |
32 |
PR |
20mg |
3 |
NE |
DOD |
|
12 |
AT |
f |
5.8 |
4 |
HRNBL1 |
no |
yes |
no |
yes |
no |
n.d. |
8 |
CR |
20mg |
3 |
Continuous CR |
LTF |
|
13 |
DE |
f |
7.5 |
4 |
NB97 |
yes |
yes |
yes |
yes |
yes* |
TCE |
26 |
PR |
20mg |
3 |
NE |
DOD |
|
14 |
AT |
m |
15.3 |
2b |
NBL2000 |
yes |
yes |
no |
no |
no |
mIBG- Topo |
32 |
PR |
20mg |
3 |
Progression |
DOD |
|
15 |
IT |
m |
5.9 |
3 |
HRNBL1 |
yes |
yes |
yes |
yes |
no |
n.d. |
10 |
PR |
20mg |
2 |
NE |
CR |
|
16 |
IT |
m |
7.7 |
4 |
HRNBL1 |
yes |
yes |
no |
yes |
yes |
TVD |
8 |
PR |
20mg |
2 |
NE |
DOD |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total | 13 | 16 | 9 | 14 | 6 | 8 | 41 |
Legend: AT Austria, IT Italy, DE Germany, FR France, HRNBL1: High Risk Neuroblastoma 1 Study of the SIOPEN group; TVD: topotecan, vincristine, doxorubicin; TCE: topotecan cylophosphamide etoposide; NB97: German Pediatric Oncology-Hematology Society study trial for neuroblastoma 1997; AEIOP NB92: Assciazone Italiana Ematologica Oncologica Pediatrica Neuroblastoma Study Protocol 1992; Yrs: years; Sx:surgery, CTH: chemotherapy, RT: radiotherapy, HDT high dose therapy followed by stem cell rescue, 2nd Line mIBG: 2nd line meta-Iodobenzylguanidine treatment with and without Topotecan, Rel Treat: relapse treatment, No. courses received: number of courses received; FU follow up, Total: total number of patients having received the respective treatment modality; m: male, f: female; NE: not evaluable; CR: Complete remission, VGPR: very good partial remission, PR: partial remission; SD stable disease; PD: progressing disease; DOD: dead of disease; LTF: lost to follow-up; Cerebral metastases occurred during the third course in patients 1 and 11. Response: 2 of 7 patients reached improved disease status after immunotherapy. Median duration till start of ch14.18/CHO treatment: 27 mo (range, 8 to 131months). Mean interval was 38.9 mo/ * Response was assessed after two courses.
The median time from diagnosis to ch14.18/CHO therapy was 27 mo (range 8–131 mo). At study entry, the median age was 7.6 y (range 3.8‒17.3 y) and performance scores were ≥ 90 (Lansky or Karnofsky). The median follow-up is 39 mo.
Number of courses, dose level and toxicity
Forty-one courses (10 × 3 courses, 5 × 2 courses, 1 × 1 course) were administered; Patients received ch14.18/CHO courses of 10, 20 or 30 mg/m2/day, i.e., dose levels 1, 2 and 3 respectively, as an eight-hour infusion over five consecutive days. Sixteen patients completed the first course (level 1, 2 and 3 with 3, 10 and 3 patients each), 15 patients the second course (level 1, 2 and 3 with 3, 9 and 3 patients each) and 10 had a third course (level 1, 2 and 3 with 1, 6 and 3 patients each).
Toxicity was evaluable and as anticipated in all patients; details are listed in Table 2. Dose level 3 had a higher rate of fever, CRP and acute allergic reactions. Therefore, dose level 2 was chosen for the confirmatory phase.
Table 2. Summary of toxicities (days 0–4) according to CTC 2 over all courses.
| Toxicity per course | Toxicity according to dose level / courses | Toxicity per patient | |||||
|---|---|---|---|---|---|---|---|
|
Symptom |
Toxicity/ total courses |
Percent courses with toxicity (all) |
Dose level 1 10mg/m2 |
Dose level 2 20mg/m2 |
Dose level 3 30mg/m2 |
Toxicity/ total patients |
Percent patients with toxicity |
|
Fever |
31/41 |
76% |
5/7 |
17/25 |
9/9 |
15/16 |
94% |
|
Elevated C-reactive proteins |
20/41 |
49% |
2/7 |
12/25 |
6/9 |
8/16 |
50% |
|
Acute Allergic Reaction |
18/41 |
44% |
0/7 |
12/25 |
6/9 |
7/16 |
44% |
|
Symptoms of serum sickness |
0/41 |
0% |
0/7 |
0/25 |
0/9 |
0/16 |
0% |
|
Ocular symptoms |
0/41 |
0% |
0/7 |
0/25 |
0/9 |
0/16 |
0% |
|
Hypertension |
0/40 |
0% |
0/7 |
0/24 |
0/9 |
0/16 |
0% |
|
Pain despite analgesic therapy |
12/41 |
29% |
1/7 |
8/25 |
3/9 |
10/16 |
63% |
|
Urine retention |
14/41 |
34% |
1/7 |
10/25 |
3/9 |
8/16 |
50% |
|
Constipation |
21/41 |
51% |
2/7 |
14/25 |
5/9 |
8/16 |
50% |
|
Diarrhea |
3/41 |
7% |
0/7 |
1/25 |
2/9 |
3/16 |
19% |
|
Nausea or Vomiting |
14/41 |
34% |
1/7 |
9/25 |
4/9 |
7/16 |
44% |
|
Bilirubin |
22/41 |
54% |
3/7 |
13/25 |
6/9 |
10/16 |
63% |
|
SGOT/SGPT |
26/41 |
63% |
4/7 |
16/25 |
6/9 |
14/16 |
88% |
|
Renal toxicity |
0/40 |
0% |
0/7 |
0/24 |
0/9 |
0/16 |
0% |
|
Leukopenia |
25/41 |
61% |
4/7 |
21/25 |
6/9 |
14/16 |
88% |
|
Thrombopenia |
17/41 |
42% |
2/7 |
11/25 |
4/9 |
10/16 |
44% |
|
Anaemia |
36/41 |
88% |
6/7 |
22/25 |
8/9 |
14/16 |
63% |
|
Neutropenia |
29/41 |
71% |
5/7 |
17/25 |
7/9 |
15/16 |
94% |
| Infection | 8/41 | 20% | 2/7 | 4/25 | 2/9 | 6/16 | 38% |
Legend: Toxicities listed are for grades 1–4.
Fever and pain were less severe in subsequent courses (Fig. 1A). The supportive care guidelines allowed all patients to continue on the dose level and course (Fig. 1B and C). There were no treatment-related deaths, and all toxicities were reversible and similar to those reported for ch14.18/SP2/0.6,16 No DLT occurred.
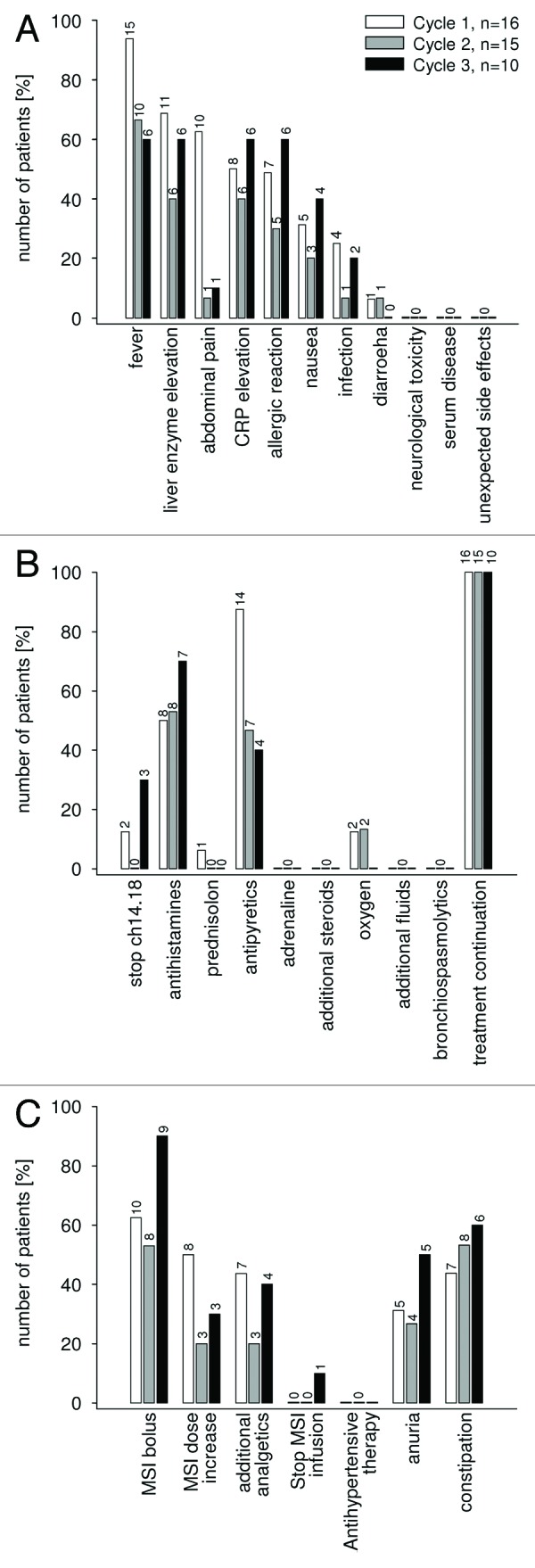
Figure 1. Antibody ch14.18/CHO therapy associated clinical symptoms, laboratory parameters frequency of co-medication and pain management. All 16 patients were evaluated for ch14.18/CHO associated side effects including clinical symptoms and laboratory changes (A), the use of supportive care medication (B) and pain management including pain management related side effects (C). The data are given in percentage of patients separated for each course (course 1: white bar, course 2: gray bar, course 3: black bar). The absolute number of patients is additionally provided for each column.
Outcome observations
Seven patients were evaluable for central review of mIBG response. Two of seven patients had improved disease by mIBG response (Table 1). A combination effect with 13-cis retinoid acid cannot be ruled out.
Eleven of 16 patients have died. All deaths were disease-related (Table 1). There were no deaths reported while on protocol therapy or within one month following completion of protocol therapy.
Pharmacokinetics of ch14.18/CHO in patients
Thirty-two courses in 14 neuroblastoma patients were evaluable for pharmacokinetic analyses. Tmax, Cmax, t½, AUC and Vdis were calculated (Table 3).
Table 3. Pharmacokinetics and HACA response of ch14.18/CHO treated neuroblastoma patients.
| Patient ID |
Course | Dosage (mg/m2/d) |
Tmax (h) |
Cmax (μg/ml) |
T 1/2 (h) |
AUC (μg/ml*h) |
V dis | HACA* | OD† (450nm) |
|---|---|---|---|---|---|---|---|---|---|
| | |||||||||
| 1 |
1 |
10 |
104.0 |
10.7 |
50.0 |
1483.4 |
2.5 |
- |
< 0.1 |
| |
2 |
10 |
106.0 |
7.6 |
1.3 |
362.4 |
0.2 |
+ |
0.46 |
| |
3 |
10 |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
+ |
1.61 |
| 2 |
1 |
10 |
104.5 |
9.6 |
5.1 |
360.9 |
0.9 |
- |
< 0.1 |
| |
2 |
10 |
104.0 |
2.5 |
208.5 |
91.8 |
121 |
- |
< 0.1 |
| 3 |
1 |
10 |
105.0 |
9.5 |
45.6 |
1236.6 |
2.8 |
+ |
0.26 |
| |
2 |
10 |
104.5 |
9.0 |
10.1 |
350.1 |
2.6 |
+ |
1.51 |
| 4 |
1 |
20 |
112.0 |
26.6 |
n.a. |
1874.8 |
n.a. |
- |
< 0.1 |
| 5 |
1 |
20 |
104.0 |
16.9 |
121.7 |
4242.2 |
4.0 |
- |
< 0.1 |
| |
2 |
20 |
10.0 |
23.0 |
140.1 |
6600.1 |
2.9 |
- |
< 0.1 |
| 6 |
1 |
20 |
108.0 |
15.0 |
102.5 |
2979.9 |
4.8 |
- |
< 0.1 |
| |
2 |
20 |
104.0 |
19.7 |
55.6 |
2698.5 |
2.9 |
- |
< 0.1 |
| 7 |
1 |
20 |
106.0 |
13.6 |
196.5 |
2579.2 |
9.6 |
- |
< 0.1 |
| |
2 |
20 |
104.5 |
14.2 |
203.6 |
1998.5 |
9.0 |
- |
< 0.1 |
| |
3 |
20 |
104.0 |
12.9 |
51.6 |
1399.4 |
5.3 |
- |
< 0.1 |
| 8 |
1 |
20 |
10.0 |
7.4 |
75.5 |
1287.1 |
8.3 |
- |
< 0.1 |
| |
2 |
20 |
104.0 |
14.4 |
38.1 |
1628.6 |
3.6 |
- |
< 0.1 |
| 9 |
1 |
20 |
8.5 |
21.3 |
40.0 |
2145.7 |
2.3 |
- |
< 0.1 |
| |
2 |
20 |
104.0 |
12.8 |
23.7 |
1163.0 |
2.7 |
- |
< 0.1 |
| 10 |
1 |
20 |
104.5 |
19.8 |
25.6 |
1972.2 |
2.3 |
- |
< 0.1 |
| |
2 |
20 |
104.0 |
9.7 |
4.9 |
182.7 |
5.8 |
+ |
0.14 |
| |
3 |
20 |
104.0 |
9.7 |
4.5 |
259.8 |
3.4 |
+ |
1.28 |
| 11 |
1 |
20 |
96.5 |
11.6 |
135.7 |
4293.3 |
4.2 |
- |
< 0.1 |
| |
2 |
20 |
104.0 |
5.6 |
n.a. |
46.5 |
n.a. |
- |
< 0.1 |
| 12 |
1 |
30 |
105.0 |
17.0 |
33.0 |
1302.2 |
3.6 |
- |
< 0.1 |
| |
2 |
30 |
104.0 |
18.3 |
18.6 |
1356.0 |
2.3 |
- |
< 0.1 |
| |
3 |
30 |
104.0 |
6.3 |
119.8 |
2163.9 |
11.7 |
- |
< 0.1 |
| 13 |
1 |
30 |
106.0 |
22.8 |
66.1 |
3375.3 |
4.2 |
- |
< 0.1 |
| |
2 |
30 |
104.5 |
17.0 |
27.7 |
1623.1 |
3.7 |
- |
< 0.1 |
| |
3 |
30 |
104.0 |
27.9 |
19.7 |
5801 |
3.1 |
- |
< 0.1 |
| 14 |
1 |
30 |
104.0 |
27.4 |
30.1 |
3527.9 |
1.9 |
- |
< 0.1 |
| |
2 |
30 |
104.0 |
24.4 |
21.6 |
2260.4 |
2.2 |
- |
< 0.1 |
| 3 | 30 | 106.0 | 22.6 | 24.3 | 1772.7 | 2.7 | - | < 0.1 | |
Legend: * positive (+) human anti-chimeric response corresponds with an OD 450 reading of > 0.1. † OD 450 values were given in HACA positive patients in subsequent courses. n.a. not available.
The peak serum concentrations in patients receiving 20 mg/m2 ch14.18/CHO (n = 8) ranged from 7.4‒26.6 µg/ml and the mean during the first course was 16.5 ± 5.9 µg/ml (Table 4).
Table 4. Peak Serum levels (Cmax) and half-life (t ½) of ch14.18/CHO in patients.
| ch14.18/ CHO: Cmax (1st courses only) * | ch14.18/ SP2/0(11): Cmax * | ||||||
|---|---|---|---|---|---|---|---|
| Dose level (mg/m2/d) |
Range (µg/ml) |
Mean § (µg/ml) |
n |
Dose level (mg/m2/d) |
Range (µg/ml) |
Mean § (µg/ml) |
n |
| 10 |
9.5 – 10.7 |
9.9 ± 0.6 |
3 |
10 |
2.4–15.6 |
9.5 ± 6.0 |
5 |
| 20 |
7.4 – 26.6 |
16.5 ± 5.9 |
8 |
20 |
12.3–23.7 |
19.3 ± 6.0 |
3 |
| 30 |
17.0 – 27.4 |
22.4 ± 5.1 |
3 |
50 |
16.6–57.0 |
34.8 ± 20.5 |
3 |
| |
|
||||||
| ch14.18/ CHO: t½ † |
ch14.18/ SP2/011: t½ † |
||||||
| Dose level (mg/m2/d) |
Range (h) |
Mean § (h) |
n |
Dose level (mg/m2/d) |
Range (h) |
Mean § (h) |
n |
| 10–30 |
25.6 - 196.6 |
76.9 ± 52.5 |
12 |
10–50 |
29.1 - 110.8 |
66.6 ± 27.4 |
9 |
| |
|
||||||
| 1st course ch14.18/ CHO: t½ |
2nd course ch14.18/ CHO: t½ |
||||||
| Dose level (mg/m2/d) |
Range (h) |
Mean § (h) |
n |
Dose level (mg/m2/d) |
Range (h) |
Mean § (h) |
n |
| 20 |
25.6 - 196.6 |
99.7 ± 58.8 |
7 |
20 |
23 - 203.6 |
92.1 ± 77.0 |
5 |
| 30 | 30.2 - 66.2 | 43.1 ± 20.0 | 3 | 30 | 18.7 - 27.8 | 22.7 ± 4.6 | 3 |
Legend: * Cmax (µg/ml) and † t½ (h) in patients treated with ch14.18/CHO are compared with results reported for ch14.18/SP2/0.(11) § Data represent mean values ± standard deviation.
The half-life was measured at two periods within each course. The first period was from the first ch14.18/CHO infusion until the second ch14.18/CHO infusion (24 h). The second period was from the last ch14.18/CHO infusion (day 4) until the end of the course (day 28). Only antibody levels from the second period were used for half-life determinations.
A comparison of half-lives between courses indicated that there was a tendency toward accelerated half-lives in subsequent courses (Table 3). Therefore, to compare half-lives of ch14.18/CHO with ch14.18/SP2/0, only the first courses were used (Table 4).
The analysis of ch14.18/CHO pharmacokinetics revealed a one-compartment model with t½ of 76.9 h ± 52.5 h for ch14.18/CHO, which is comparable to t½ of ch14.18/SP2/0 at 66.6 h ± 27.4 h (Table 4).
Induction of anti-chimeric antibodies and anti-idiotypic antibodies
Sera from 14 patients were screened for the development of HACA against ch14.18 or antibodies directed specifically to the antigen binding variable regions of ch14.18 (anti-idiotypic antibodies) by ELISA.21 Three patients developed HACA. The three HACA positive patients also tested positive for anti-idiotypic antibodies.
Clearance and biodistribution of ch14.18/CHO in mice
The clearance and biodistribution of 125I-labeled ch14.18/CHO were analyzed in Balb/c and A/J mice and the results were compared with those obtained with ch14.18/SP2/0, ch14.18-IL-2, 14G2a and rituximab as controls (Fig. 2A). The clearance of ch14.18/CHO in the blood of Balb/c mice was comparable to that of ch14.18/SP2/0 (Fig. 2A). In mice, the AUC observed with ch14.18/CHO was 18% lower than with ch14.18/SP2/0 (ch14.18/CHO: 1524566.8 ± 108471 CPM*h /10µl, ch14.18/SP2/0: 1844177.8 ± 201916 CPM*h /10µl). Thus, in Balb/c mice, exposure to ch14.18/CHO was somewhat lower than ch14.18/SP2/0 at equivalent dosing, possibly related to known differences in the glycosylation pattern of proteins manufactured in CHO and SP2/0 cells.
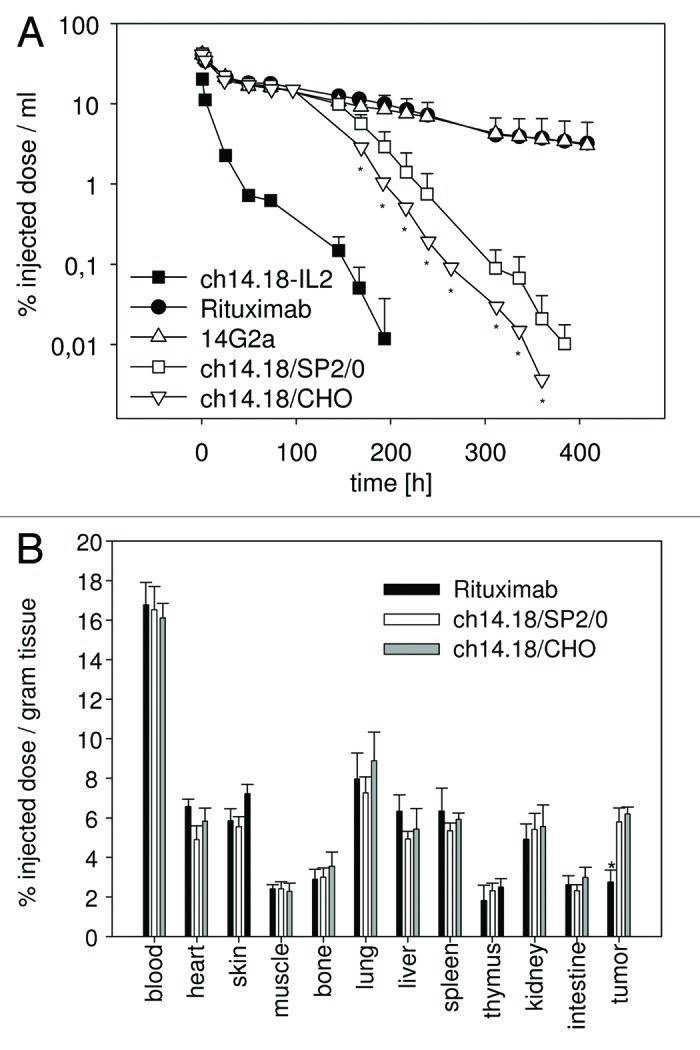
Figure 2. Clearance and biodistribution of ch14.18/CHO in mice. 125I-labeled ch14.18/CHO antibody (25 µg; 5 × 10E6 cpm) was injected into groups (n = 5) of Balb/c mice and neuroblastoma bearing A/J mice (average tumor weight 53 ± 12 mg) and clearance (A) as well as biodistribution (B) was analyzed and compared with indicated control antibodies. (A) The clearance of 125I-ch14.18/CHO from the blood of Balb/c mice was analyzed at indicated time points and compared with 125I-ch14.18/SP2/0. 125I-ch14.18-IL2, 125I-14G2a and 125I-rituximab are shown as controls. Blood levels of antibodies investigated are given as percent injected dose per milliliter of blood. Each value represents mean and standard deviation (n = 5). The t ½ α and β values were 25.0 h ± 1.9 h and 134.6 h ± 29.9 h for ch14.18 SP2/0 and 22.7 h ± 1.8 h and 136.2 h ± 12.2 h for ch14.18/CHO (p > 0.05). The rapid clearance of ch14.18-IL2 (human-mouse chimeric antibody interleukin-2 fusion protein) and the long half-life of 14G2 (murine IgG2a) and rituximab compared with ch14.18 (human-mouse chimeric antibody) are shown as controls. The difference between mice treated with ch14.18/CHO compared with controls was statistically significant (* p < 0.01). (B) Organ specific activity was detected 8 h after injection of 125I-ch14.18/CHO, 125I-ch14.18/SP2/0 and 125I-rituximab into neuroblastoma bearing A/J mice. Organ and tumor specific activity is given in percent of injected dose per gram of tissue. Each value represents the mean and standard deviation for five tumor bearing mice (* p < 0.01).
The analysis of the biodistribution of 125I-labeled ch14.18/CHO revealed targeting to a GD2-positive primary neuroblastoma tumor in vivo. This is indicated by a 2-fold higher activity in the tumor tissue compared with rituximab used as a negative control (Fig. 2B). There was no difference in radioactive signals in neuroblastoma tissue of tumor-bearing mice injected with 125I-ch14.18/CHO or 125I-ch14.18/SP2/0, indicating identical GD2 targeting activity of the two products in vivo. Overall, the targeting of GD2 in tumor tissue observed here comparing both versions of this low- to intermediate-affinity antibody explains the low absolute percentage of injected dose localizing to the tumor. The biodistribution in the tumor and in all other organs investigated, however, revealed no differences to results previously reported.22
Discussion
Antibodies directed against disialoganglioside GD2 emerged as an important therapeutic option for the treatment of neuroblastoma, and their continued development has been supported by encouraging response rates observed in clinical trials.5,12,13,16,23
Treatment of high-risk neuroblastoma patients with ch14.18/SP2/0 during maintenance has been investigated in two large clinical trials. Simon and colleagues conducted a nonrandomized retrospective study using ch14.18/SP2/0. Univariate analysis suggested a superior 3-y overall survival (OS) for patients treated with immunotherapy (p = 0.018).16 An updated analysis with a median follow-up of 10.3 y (range, 2.3 to 17.7) indicated that ch14.18/SP2/0 may prevent late relapses.24 The Children’s Oncology Group conducted a Phase 3 randomized trial using ch14.18/SP2/0 combined with aldesleukin (IL-2) and granulocyte-macrophage colony-stimulating factor (GM-CSF). A 20% increase in event-free survival after two years was reported (p = 0.01).6
Considering the early clinical data of ch14.18/SP2/0, SIOPEN aimed to make a stable, reliable and widely-used manufacturing process of ch14.18 using CHO cells available for European research studies. The recloned ch14.18/CHO antibody was characterized in vitro; preclinical models found ch14.18/CHO equally effective in mediating CDC, ADCC and anti-tumor efficacy in mice compared with ch14.18/SP2/0.1
We demonstrate for the first time that the safety profile of ch14.18/CHO is comparable to ch14.18/SP2/0 in humans. Anticipated toxicities specifically related to ch14.18/CHO included neuropathic pain due to GD2 binding to nerve fibers;7,25,26 this was well-controlled with prophylactic intravenous morphine. Other anticipated adverse reactions are fever and chills related to antibody-mediated cytokine release. Dose level 2, which reached peak levels and mAb half-lives as previously reported for ch14.18/SP2/0, was preferred over dose level 3 due to a more favorable toxicity profile in patients.2
The comparable safety profile is supported by pharmacokinetic data in patients and mice. The peak serum concentrations in patients treated with 20 mg/m2 ch14.18/CHO were similar to ch14.18/SP2/0 (Table 4). The faster clearance of ch14.18/CHO observed in mice did not translate into measurable differences in patients (Table 4).
A pharmacodynamic effect of human/mouse chimeric antibodies in patients is the development of HACA responses.27,28 In our cohort, we observed the induction of HACA and anti-idiotypic antibodies in 3/14 patients. The contribution of the anti-idiotypic network (i.e., anti-anti-Id responses to the anti-neuroblastoma effect of ch14.18/CHO) is not yet clear. Therefore, in future clinical trials monitoring of HACA and anti-Id responses is an important secondary endpoint. We observed objective responses in 2/7 patients. This indicates ch14.18/CHO is an active immunotherapeutic agent against neuroblastoma without alteration of the safety and pharmacokinetic profiles compared with other preparations. These findings provide an important rationale for further study of ch14.18/CHO-based immunotherapies in patients with high-risk neuroblastoma.
Materials and Methods
Patient eligibility
Patients with biopsy-proven neuroblastoma (> 1 y) with refractory or recurrent disease were eligible. Patients after first-line therapy had to have evaluable disease. Following second-line chemotherapies, patients were eligible without evidence of disease; treatment had to be discontinued three weeks prior to study entry. Patients with progression or previous antibody treatments were excluded. Treatment with isotretinoin, growth factor or other immunomodulatory therapy needed to be completed at least 7 d before study entry. A performance score above 70% and a life expectancy of at least 12 weeks were required. Participating institutions obtained ethics approval and written informed consent was given by patients or their parents or legal guardians.
Study design
This Phase 1 clinical trial (EudraCT Number: 2005–001267–63) was designed as an open-label, non-randomized study in order to assess the safety, efficacy and pharmacokinetics of ch14.18/CHO in three dose levels (levels 1, 2 and 3 using 10, 20 and 30 mg/m2/day respectively). One treatment cycle was planned. Patients were allowed to receive up to another two cycles provided they were progression-free.
The established dose for ch14.18/SP2/0 was 20 mg/m2/day as an eight-hour infusion over five consecutive days.12 A dose escalation design, based on Phase 1 rules, was used. In case the production change caused unexpected toxicities, a starting dose of 10 mg/m2/day was chosen. A maximum of three courses at 4–6 week intervals were allowed.
The ch14.18/CHO antibody
Recloning of ch14.18 antibody in CHO cells and GMP manufacturing of ch14.18/CHO was accomplished by Polymun. The recombinant ch14.18/CHO mAb is supplied as a final product in vials containing 20 mg (4.6 mg/ml in 10 mM sodium phosphate buffered saline, pH 7.0–7.4). During the filling by Sanochemia Pharmazeutika AG, the bulk purified product was filtered (0.2 µm Sartobran 300 sterile filter) prior to release testing and storage (5 ± 3°C).
Treatment schedule
Ch14.18/CHO was administered daily as an eight-hour infusion over five consecutive days (days 0‒4) in normal saline (0.25% human albumin). Adverse events and toxicities were graded using the National Cancer Institute Common Toxicity Criteria version 2.0. Dose-limiting toxicity (DLT) was defined as any unexpected grade 3 or 4 toxicity. Expected toxicities were: grade 3 pain requiring intravenous narcotics, fever lasting < 6 h and controllable with antipyretics, hypotension resolving within 48 h after resolution of capillary leak, allergic reactions controlled with non-steroidal treatments and hematologic, renal, hepatic or metabolic abnormalities reversing within 48 h.
For a DLT, the ch14.18/CHO infusion was to be discontinued and restarted at 50% once resolved. Patients with DLT not resolving to grade ≤ 2 within two-weeks were taken off-study. Patients with stabilization or regression of disease (partial or complete clinical response) and recovery of toxicities (grade ≤ 1) were eligible to continue on study.
Patients with progressive disease (≥ 20% increase, or the appearance of new lesions) were taken off-study.
Clinical assessment
Clinical assessment included ultrasounds, CT scans, MRIs and mIBG scintigraphy two weeks prior to treatment start and after the final course. MIBG response was evaluated by central review.29 Patients with prior bone marrow (BM) involvement had their BM assessed for immunohistochemistry of disialoganglioside GD2 before and after treatment. Complete blood counts, blood chemistry and human anti-chimeric antibodies (HACA) were assessed to ensure eligibility.
Between courses, tumor markers (LDH, NSE), urinary catecholamines and ultrasound were used to check for progression.
Supportive care
Supportive care included pain prophylaxis; morphine hydrochloride 0.5‒1.0 mg/kg bolus just before starting the ch14.18/CHO infusion followed by a 0.05 mg/kg/hour continuous infusion. Immunosuppressive agents (e.g., glucocorticoids) were only allowed for treating life-threatening symptoms. Anti-inflammatory drugs (e.g., metamizol, paracetamol, ibuprofen or indomethacin) were permitted. Pruritus and urticaria were treated with oral or intravenous diphenhydramine 0.5‒1.0 mg/kg. Hypotension was treated with a 20 ml/kg normal saline fluid challenge.
Clinical laboratory monitoring
Clinical, hematology and chemistry evaluations were done by standard methodology. Blood samples were obtained prior to ch14.18/CHO administration at designated days.
Immunological monitoring
Serum samples for HACA responses were obtained before ch14.18/CHO treatment and days 2, 7, 14, 21 and 28 of each course. Once drawn, the serum was placed on ice and kept refrigerated until freezing at -20°C.
Determination of ch14.18/CHO pharmacokinetics in patients
To determine pharmacokinetic parameters, serum samples were taken at 24 time points per course. Sampling points were pre-treatment (day < 0), day 0 (start), days 1, 2, 3, 4, 7, 14, 21 and 28. On days 0 and 4, seven additional samples were taken; 1 h after the infusion start, end of the infusion and 0.5 h, 1 h, 2 h, 4 h and 8 h after the end of infusion. Serum samples were stored at -80°C.
Pharmacokinetic analysis included time of maximal concentration (Tmax), maximal concentration (Cmax), half-life (t½), area under the curve (AUC) and volume of distribution (Vdis).
The serum concentration of ch14.18 was determined by enzyme linked immunosorbent assay (ELISA) using ganglidiomab as a capture reagent antibody, which is specific for the ch14.18/CHO idiotype.30
A standard curve in pooled human serum was used for the calculation of concentrations. Measurement of serum samples was performed in duplicate.
Clearance and biodistribution of ch14.18/CHO in mice
Female Balb/c mice and A/J mice (6‒8 weeks old) were obtained from Harlan Sprague Dawley, Sulzfeld, Germany. All animal experiments were performed according to the German guide for the care and use of laboratory animals (“Tierschutzgesetz”). Antibodies (ch14.18/CHO, ch14.18/SP2/0, 14G2a, rituximab) were iodinated with 125I using the iodogen method.22 Primary tumors were induced by subcutaneous (s.c.) injection of 2 × 106 NXS2 cells eight days before biodistribution analysis. Groups of mice (n = 5) were each injected with 25 µg of ch14.18 antibody (5 × 106 cpm). Clearance was determined in blood samples (10 µl) taken by serial retro-orbital eye bleeds. Biodistribution of iodinated ch14.18/CHO was analyzed eight hours after injection.22 Tumors (average weight 53 ± 12 mg) and major organs were removed, weighed and analyzed in a gamma counter.
Data analysis and statistics
To analyze the pharmacokinetic data, ch14.18/CHO serum concentrations were analyzed with the WINNONLIN NONPARAMETRICAL ANALYSIS PROGRAM, Version 5.2 (Pharsight, St. Louis, MO, USA). Pharmacokinetic variables were summarized using descriptive statistics on ch14.18/CHO serum levels obtained before and after the ch14.18/CHO infusions of each course. The AUC from time 0 to 24 h was calculated with the trapezoidal method with uniform weighting.
The statistical significance of differential findings of pharmacokinetic data in mice and patients was determined by two-tailed Student’s t-test. Findings were regarded as significant if the two-tailed p-values were < 0.05.
Acknowledgments
Funding sources: EU Grant SIOPEN-R-NET No. QLRI-CT-2002–01768 to R.L., Fördergesellschaft Kinderkrebs-Neuroblastom-Forschung e.V., German Federal Ministry of Education and Research (BMBF, NGFNplus, ENGINE) and Hector Stiftung to H.N.L. and grants from the Charité Forschungsförderung to M.B.. Further support was obtained from European charity organisations (list included in the online version). Financial support by the following European institutions, which are mainly charity organisations listed in alphabetical order according to the country of origin: Children’s Cancer Research Institute, Vienna, Austria; Télévie, Kinderkrankenfonds Gent, Kinderkrankenfonds KULeuven, Belgium; University Hospital of Aarhus, Denmark; L’Association pour la Recherche sur la Cancer (ARC), Paris, France; Hayim for children in cancer in Israel, Israel; Associazione Italiana Per La Lotta Al Neuroblastoma (Italian Neuroblastoma Association), Genova, Italy; The National Centre for Solid Tumours in Children, Norway; AMGEN GILEAD SCIENCES, Fundação Oriente, Portugal; Foundation of Friends of Children with Cancer (Fundacja Przyjaciół Dzieci z Chorobą Nowotworową “Wyspy Szczęśliwe”), Poland; Federaciòn Española de Padres de Niños con Cancer, Barcelona, Spain; Swedish Children’s Cancer Foundation, Sweden; FORCE (Fondation Recherche sur le Cancer de l’enfant), Switzerland; Cancer Research Campaign, London Neuroblastoma Society, United Kingdom. Without their support, GMP production of the ch14.18/CHO antibody, which is a “conditio sine qua non” for the HR-NBL-1/SIOPEN trial, would not have been possible. The authors also wish to thank Alisa Alspach for revising the English text.
Submitted
02/01/2013
Revised
05/29/2013
Accepted
05/29/2013
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/25215
References
- 1.Zeng Y, Fest S, Kunert R, Katinger H, Pistoia V, Michon J, et al. Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Mol Immunol. 2005;42:1311–9. doi: 10.1016/j.molimm.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Ladenstein R, Pötschger U, Siabalis D, Garaventa A, Bergeron C, Lewis IJ, et al. Dose finding study for the use of subcutaneous recombinant interleukin-2 to augment natural killer cell numbers in an outpatient setting for stage 4 neuroblastoma after megatherapy and autologous stem-cell reinfusion. J Clin Oncol. 2011;29:441–8. doi: 10.1200/JCO.2009.23.5465. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Children’s Cancer Group Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang RK, Sondel PM. Anti-GD2 Strategy in the Treatment of Neuroblastoma. Drugs Future. 2010;35:665. doi: 10.1358/dof.2010.035.08.1513490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svennerholm L, Boström K, Fredman P, Jungbjer B, Lekman A, Månsson JE, et al. Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim Biophys Acta. 1994;1214:115–23. doi: 10.1016/0005-2760(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 8.Mujoo K, Kipps TJ, Yang HM, Cheresh DA, Wargalla U, Sander DJ, et al. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 1989;49:2857–61. [PubMed] [Google Scholar]
- 9.Mueller BM, Romerdahl CA, Gillies SD, Reisfeld RA. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol. 1990;144:1382–6. [PubMed] [Google Scholar]
- 10.Cheung NK, Guo HF, Heller G, Cheung IY. Induction of Ab3 and Ab3′ antibody was associated with long-term survival after anti-G(D2) antibody therapy of stage 4 neuroblastoma. Clin Cancer Res. 2000;6:2653–60. [PubMed] [Google Scholar]
- 11.Riethmüller G, Holz E, Schlimok G, Schmiegel W, Raab R, Höffken K, et al. Monoclonal antibody therapy for resected Dukes’ C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol. 1998;16:1788–94. doi: 10.1200/JCO.1998.16.5.1788. [DOI] [PubMed] [Google Scholar]
- 12.Handgretinger R, Anderson K, Lang P, Dopfer R, Klingebiel T, Schrappe M, et al. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur J Cancer. 1995;31A:261–7. doi: 10.1016/0959-8049(94)00413-Y. [DOI] [PubMed] [Google Scholar]
- 13.Uttenreuther-Fischer MM, Huang CS, Yu AL. Pharmacokinetics of human-mouse chimeric anti-GD2 mAb ch14.18 in a phase I trial in neuroblastoma patients. Cancer Immunol Immunother. 1995;41:331–8. doi: 10.1007/BF01526552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushner BH, Kramer K, Cheung NK. Phase II trial of the anti-G(D2) monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–94. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 15.Cheung NK, Kushner BH, Cheung IY, Kramer K, Canete A, Gerald W, et al. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16:3053–60. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 16.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549–57. doi: 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 17.Borys MC, Dalal NG, Abu-Absi NR, Khattak SF, Jing Y, Xing Z, et al. Effects of culture conditions on N-glycolylneuraminic acid (Neu5Gc) content of a recombinant fusion protein produced in CHO cells. Biotechnol Bioeng. 2010;105:1048–57. doi: 10.1002/bit.22644. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010;207:1637–46. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shepherd AJ, Wilson NJ, Smith KT. Characterisation of endogenous retrovirus in rodent cell lines used for production of biologicals. Biologicals. 2003;31:251–60. doi: 10.1016/S1045-1056(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.European Agency for the Evaluation of Medicinal Products. Guideline on comparability of medicinal products containing biotechnology-derived proteins as active substance: quality issues. 1–11 (2003).
- 21.Albertini MR, Gan J, Jaeger P, Hank JA, Storer B, Schell K, et al. Systemic interleukin-2 modulates the anti-idiotypic response to chimeric anti-GD2 antibody in patients with melanoma. J Immunother Emphasis Tumor Immunol. 1996;19:278–95. doi: 10.1097/00002371-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Mueller BM, Reisfeld RA, Gillies SD. Serum half-life and tumor localization of a chimeric antibody deleted of the CH2 domain and directed against the disialoganglioside GD2. Proc Natl Acad Sci U S A. 1990;87:5702–5. doi: 10.1073/pnas.87.15.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu AL, Uttenreuther-Fischer MM, Huang CS, Tsui CC, Gillies SD, Reisfeld RA, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16:2169–80. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 24.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, et al. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer. 2011;11:21. doi: 10.1186/1471-2407-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuki N, Yamada M, Tagawa Y, Takahashi H, Handa S. Pathogenesis of the neurotoxicity caused by anti-GD2 antibody therapy. J Neurol Sci. 1997;149:127–30. doi: 10.1016/S0022-510X(97)05390-2. [DOI] [PubMed] [Google Scholar]
- 26.Sorkin LS, Otto M, Baldwin WM, 3rd, Vail E, Gillies SD, Handgretinger R, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149:135–42. doi: 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozkaynak MF, Sondel PM, Krailo MD, Gan J, Javorsky B, Reisfeld RA, et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: a Children’s Cancer Group Study. J Clin Oncol. 2000;18:4077–85. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 28.Johnson E, Dean SM, Sondel PM. Antibody-based immunotherapy in high-risk neuroblastoma. Expert Rev Mol Med. 2007;9:1–21. doi: 10.1017/S1462399407000518. [DOI] [PubMed] [Google Scholar]
- 29.Lewington V, Poetschger U, Boubaker A, Bar-Sever Z, Drake B, Staudenherz A, et al. The prognostic value of semi-quantitative 123I mIBG scintigraphy at diagnosis in high-risk neuroblastoma: Validation of the SIOPEN score method. J Clin Oncol. 2011;29:suppl;abstr 9511. [Google Scholar]
- 30.Lode HN, Schmidt M, Seidel D, Huebener N, Brackrock D, Bleeke M, et al. Vaccination with anti-idiotype antibody ganglidiomab mediates a GD2-specific anti-neuroblastoma immune response. Cancer Immunol Immunother. 2013;62:999–1010. doi: 10.1007/s00262-013-1413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


