Abstract
Mast cells have been suggested to play key roles in adipogenesis. We herein show that the expression of preadipocyte, but not adipocyte, marker genes increases in the white adipose tissue of mast cell-deficient (KitW-sh/W-sh) mice under both obese and non-obese conditions. In vitro culturing with adipogenic factors revealed increased adipocytes differentiated from the KitW-sh/W-sh stromal vascular fraction, suggesting the accumulation of preadipocytes. Moreover, the increased expression of preadipocyte genes was restored by mast cell reconstitution in the KitW-sh/W-sh mice. These results suggest positive effects of mast cells on the preadipocyte to adipocyte transition under both physiological and pathological conditions.
Keywords: Mast cell, White adipose tissue, Adipogenesis, Obesity
Abbreviations: SVF, stromal vascular fraction; WAT, white adipose tissue; HFD, high-fat diet; ND, normal diet; BMMC, bone marrow-derived mast cell
Highlights
-
•
Mast cell-deficient mice are resistant to diet-induced obesity.
-
•
The expression of preadipocyte genes is increased in their white adipose tissue.
-
•
The content of preadipocytes is increased in the adipose stromal vascular fraction.
-
•
Mast cell reconstitution restores the enhanced expression of preadipocyte genes.
-
•
Mast cells may facilitate the preadipocyte–adipocyte transition in white adipose tissue.
1. Introduction
Adipose tissue is important for the maintenance of energy balance due to its ability to store large amounts of triglycerides during periods of energy excess and mobilize these stores during periods of nutritional starvation [1]. Obesity is defined as an increase in adipose tissue mass and is a major risk factor for the development of metabolic disorders, such as type 2 diabetes, atherosclerosis, dyslipidemia and hypertension [2,3]. In the adipose tissue of obese individuals, the stromal vascular fraction (SVF) within white adipose tissue (WAT) is characterized by chronic low-grade inflammation with infiltration of immune cells, such as macrophages and T-cells [4,5]. In particular, macrophages in obese WAT have been shown to play key roles in the progression of inflammation [6,7]. Recent studies have shown that, in addition to macrophages, mast cells are involved in the progression of obesity [8,9]. Mast cell-deficient (KitW-sh/W-sh) mice [10] fed a high-fat diet (HFD) exhibit less body weight gain than wild-type (Kit+/+) mice [8]. The production of inflammatory cytokines and proteases in WAT is reduced in KitW-sh/W-sh mice [8]. WAT has recently been defined as an important source of functional mast cell progenitors regardless of the nutritional status [11]. Therefore, we consider that the mast cell functions involved in adipogenesis may not be limited to the progression of obesity. However, the role of mast cells in adipogenesis under physiological conditions has not yet been elucidated.
We herein provide evidence for a novel role of mast cells in adipogenesis under both obese and nonobese conditions. We examined the expression of adipocyte-specific genes in the WAT of KitW-sh/W-sh and Kit+/+ mice fed either a HFD or ND using quantitative RT-PCR. Consistent with the findings of previous reports, body weight gain induced by HFD was suppressed in the KitW-sh/W-sh mice compared to that observed in the Kit+/+ mice. However, in contrast to body weight changes, the expression levels of preadipocyte markers (Pref-1, AEBP1 and GATA2), but not mature adipocyte markers (aP2, PPARγ, Acsl1 and adipsin), were significantly higher in the epididymal WAT and SVF of the KitW-sh/W-sh mice compared to those observed in the Kit+/+ mice. Notably, an enhanced expression of preadipocyte markers in the KitW-sh/W-sh WAT was observed even in mice fed an ND. The in vitro culture of SVF with adipogenic factors resulted in a greater number of adipocytes differentiated from the KitW-sh/W-sh WAT, suggesting the accumulation of preadipocytes. Finally, the increased expression of preadipocyte markers in the WAT and SVF was restored when the KitW-sh/W-sh mice were reconstituted with bone marrow-derived mast cells (BMMCs). Collectively, these data indicate that mast cell deficiency results in the accumulation of preadipocytes in the WAT of both lean and obese mice, suggesting positive effects of mast cells on the preadipocyte to adipocyte transition in WAT.
2. Materials and methods
2.1. Mice
C57BL/6-Kit+/+and C57BL/6-KitW-sh/W-sh mice were purchased from Japan SLC and RIKEN BRC, respectively. The mice were maintained in the animal facility of Takasaki University of Health and Welfare in accordance with institutional guidelines. The mice were given ad libitum access to food, either ND (CLEA Rodent diet CE-2; 12.6% of calories from fat) or HFD (PMI TestDiet 58Y1; 60.0% of calories from fat), and water. To create a model of diet-induced obesity, male mice were fed an HFD from 6 to 22 weeks of age.
2.2. RT-PCR
Total RNA was isolated from cells and tissues using Isogen (Nippon Gene) and treated with DNase I (Takara). The reverse transcription reactions were performed using the ReverTra Ace qPCR RT Kit (Toyobo) according to the manufacturer's instructions. Synthesized cDNAs were analyzed using an Mx3000P real-time PCR system (Stratagene) with the GoTaq qPCR Master Mix (Promega), as previously described [12]. The results were normalized to the GAPDH levels. The primers used for real-time PCR are shown in Table 1.
Table 1.
Primer sequences for RT-PCR.
| Gene product | Primer | Sequence |
|---|---|---|
| Pref-1 | 5′ | CTT GCT CCT GCT GGC TTT CG |
| 3′ | TGT CAC AGA GGG GAC CCT CC | |
| AEBP1 | 5′ | GGA ACG GTA GCC TGT GCA TG |
| 3′ | CTG GCG CAT GTC CTT GTA GC | |
| GATA2 | 5′ | GCA GAG AAG CAA GGC TCG C |
| 3′ | CAG TTG ACA CAC TCC CGG C | |
| aP2 | 5′ | AAA TCA CCG CAG ACG ACA GG |
| 3′ | TCC ACC ACC AGC TTG TCA CC | |
| PPARγ | 5′ | TCG CTG ATG CAC TGC CTA TG |
| 3′ | GGT CCA CAG AGC TGA TTC CG | |
| Acsl1 | 5′ | TGG TAT TCG AAG ATC AGC AG |
| 3′ | TTC CGA GAA CCT AAA CAA GG | |
| Adipsin | 5′ | CCT GAA CCC TAC AAG CGA TG |
| 3′ | GGT TCC ACT TCT TTG TCC TCG | |
| Adiponectin | 5′ | TCC TGG AGA GAA GGG AGA GAA AG |
| 3′ | TCA GCT CCT GTC ATT CCA ACA T | |
| MCCPA | 5′ | GCA TTG GCA CTG ACC TCA AC |
| 3′ | GCC TTG ATT GAG TTC AGA TG | |
| GAPDH | 5′ | TGT GTC CGT CGT GGA TCT GA |
| 3′ | CCT GCT TCA CCA CCT TCT TGA |
2.3. Isolation of SVF and in vitro adipocyte differentiation
Epididymal WAT (eWAT) was dissected and minced in PBS, followed by digestion with 1 mg/ml of collagenase (Sigma) for 30 min at 37 °C. After digestion, the cell suspension was filtered through a 40-μm nylon mesh (BD Biosciences), followed by centrifugation at 1500 rpm for 5 min to separate floating adipocytes from the SVF pellets. The SVF pellets were treated with a hypotonic buffer to lyse red blood cells and resuspended in DMEM containing 10% fetal bovine serum (FBS), penicillin and streptomycin. The isolated SVF was seeded in 24-well plates and cultured at 37 °C in a 5% CO2 incubator. As the cells reached confluency, they were cultured with the medium supplemented with 0.5 mM of isobutylmethylxanthine (IBMX), 1 μM of dexamethasone and 10 μg/ml of insulin for 2 days, followed by culturing with 10 μg/ml of insulin for 2 days and without insulin for 3 days. The degree of lipid accumulation in the cells was examined using Oil Red O staining according to a standard procedure [21]. To quantify retention of Oil red O, the lipids were extracted using 150 μl of isopropanol for 30 min, and the absorbance was measured at 540 nm.
2.4. Flow cytometry
The SVF cells were stained with allophycocyanin (APC)-conjugated rat anti-mouse CD117 (BD Pharmingen; clone 2B8) and phycoerythrin (PE)-conjugated mouse anti-mouse Fc epsilon receptor I alpha (eBioscience; clone MAR-1) antibodies that recognize c-Kit and the high-affinity IgE receptor alpha subunit (FcεRIα), respectively. The staining was performed using PBS containing 2% FBS at 4 °C for 60 min. After washing with PBS, the cell fluorescence was measured using a FACSCanto II flow cytometer (BD Biosciences).
2.5. Reconstitution of mast cells in mast cell-deficient mice
BMMCs were prepared as previously described [13]. In brief, femoral bone marrow cells were harvested from 4- to 8-week-old male Kit+/+ mice and cultured with RPMI 1640 supplemented with 10% FBS and 10 ng/ml of recombinant murine interleukin-3 (IL-3) (Peprotech). After 2 weeks of culturing, 10 ng/ml of recombinant murine stem cell factor (SCF) (Peprotech) was added. After 4–6 weeks of culturing, 2.0 × 106 BMMCs in 200 μl of PBS were injected intraperitoneally (i.p.) into 4-week-old male KitW-sh/W-sh mice, and the mice were used for the experiments after 12–16 weeks of injection. The transferred BMMCs were confirmed using cytospin preparations of peritoneal cells (5 × 104 cells) stained with toluidine blue or alcian blue/safranin O.
2.6. Statistics
Comparisons between the two groups were made using Student's t-test. Unless otherwise specified, the data are presented as the mean ± SEM. For all analyses, statistical significance was defined as a P-value of <0.05.
3. Results
3.1. Mast cell-deficient mice are resistant to diet-induced obesity
Kit+/+ and KitW-sh/W-sh mice were fed an HFD or ND, and their body weight was monitored weekly during 16 weeks of feeding (Fig. 1A). In the HFD group, the body weight gain in the KitW-sh/W-sh mice was less than that observed in the Kit+/+ mice at 9 weeks and afterwards, whereas the body weights were comparable in the ND group throughout the observation period (Fig. 1A). The Kit+/+ mice fed an HFD weighed 39% more than those fed an ND after 16 weeks of feeding (44.04 ± 3.22 and 31.65 ± 1.78 g, respectively, n = 12, Fig. 1B). In contrast, the KitW-sh/W-sh mice fed an HFD weighed only 8% more than those fed an ND (34.78 ± 2.83 and 32.20 ± 1.49 g, respectively, n = 12, Fig. 1B). Consistent with this finding, the eWAT weight of the KitW-sh/W-sh mice was significantly less than that of the Kit+/+ mice in the HFD group (1.29 ± 0.53 and 2.15 ± 0.44 g, respectively, n = 12, Fig. 1C), whereas these values were comparable in the KitW-sh/W-sh and Kit+/+ mice in the ND group (0.42 ± 0.12 and 0.46 ± 0.17 g, respectively, n = 12, Fig. 1C). These results are consistent with the findings of previous reports [8,14] and suggest the contribution of mast cells to the development of HFD-induced obesity.
Fig. 1.
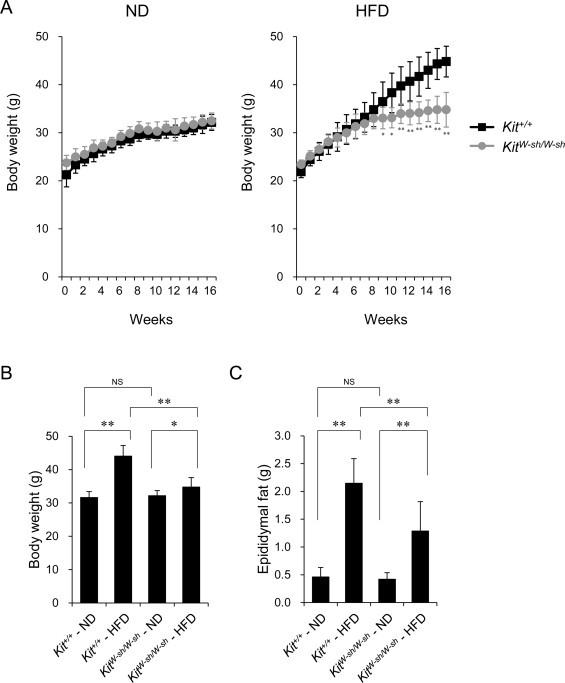
The body weight and epididymal fat weight of the KitW-sh/W-sh mice fed an HFD were less than those of the Kit+/+ mice. (A) Body weight gain in the Kit+/+ mice (black square) and KitW-sh/W-sh mice (gray circle) fed an ND (left panel) or HFD (right panel) for 16 weeks. The data are presented as the mean ± SD. *P < 0.05, **P < 0.01 versus the Kit+/+ mice. (B and C) Body weight (B) and epididymal fat weight (C) of the Kit+/+ and KitW-sh/W-sh mice that consumed an ND or HFD for 16 weeks. The data are presented as the mean ± SD. *P < 0.05, **P < 0.01 for the comparisons indicated. NS, not significant. n = 12 for each group.
3.2. Mast cell deficiency results in an increased expression of preadipocyte marker genes in WAT
To examine the molecular basis of the resistance to HFD-induced obesity observed in the KitW-sh/W-sh mice, we evaluated the expression of genes involved in adipogenesis using quantitative RT-PCR. In the HFD group, the expression levels of adipocyte marker genes [15–17], including adipocyte protein 2 (aP2), peroxisome proliferator-activated receptor γ (PPARγ), acyl-CoA synthetase long-chain family member 1 (Acsl1) and adipsin, were comparable in the eWAT prepared from the KitW-sh/W-sh mice compared to those observed in the eWAT prepared from the Kit+/+ mice (Fig. 2A). In contrast, the expression levels of preadipocyte marker genes [18–20], including preadipocyte factor-1 (Pref-1), adipocyte enhancer binding protein 1 (AEBP1) and GATA2, were significantly greater in the eWAT prepared from the KitW-sh/W-sh mice than that observed in the controls (Fig. 2A). Interestingly, the increased expression of preadipocyte markers in the KitW-sh/W-sh eWAT was also observed in the ND group, even though the weight of the eWAT was comparable between the KitW-sh/W-sh and Kit+/+ mice in these groups (Fig. 2B). Consistent results were obtained in the SVF composed of immune cells, fibroblasts, preadipocytes, endothelial cells and mesenchymal stem cells (Fig. 2C).
Fig. 2.
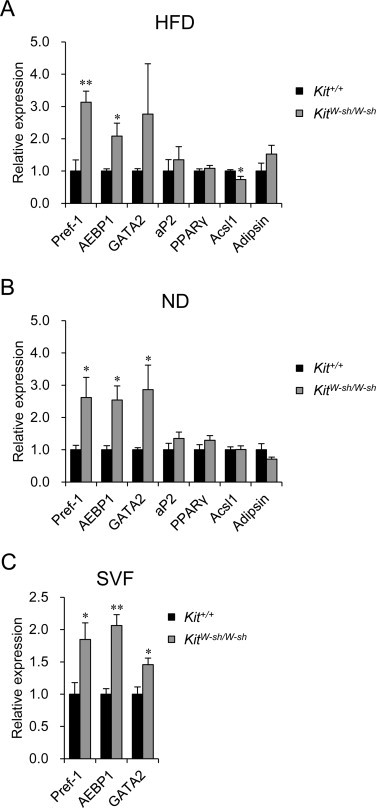
The mRNA expression levels of preadipocyte, but not mature adipocyte, marker genes were greater in the eWAT of the KitW-sh/W-sh mice than in those of the Kit+/+ mice. (A and B) The mRNA levels of preadipocyte markers (Pref-1, AEBP1 and GATA2) and adipocyte markers (aP2, PPARγ, Acsl1 and adipsin) were examined in the eWAT of the Kit+/+ (black bars) and KitW-sh/W-sh mice (gray bars) fed either an HFD (A) or ND (B) for 16 weeks using quantitative RT-PCR. n = 7 for each group. (C) The mRNA levels of the preadipocyte markers were examined in the SVF derived from the Kit+/+ (black bars) and KitW-sh/W-sh mice (gray bars) fed an ND using quantitative RT-PCR. n = 6 for each group. The data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 versus the Kit+/+ mice.
3.3. Adipogenic differentiation is more prevalent in the KitW-sh/W-sh SVF than in the Kit+/+ SVF in vitro
We next examined whether preadipocytes obtained from KitW-sh/W-sh SVF are able to differentiate into mature adipocytes in vitro. Since SVF cells are comprised of heterogeneous cell populations, they may contain a considerable amount of hematopoietic progenitor cells positive for the c-Kit expression. Therefore, in order to exclude the possibility that the Kit mutation in non-mast cells intrinsically affects adipocyte differentiation in the SVF of KitW-sh/W-sh mice, we examined the expression of c-Kit and FcɛRI in freshly isolated SVF cells of Kit+/+ and KitW-sh/W-sh mice using a flow cytometric analysis (Fig. 3A). While mast cells express both c-Kit and FcɛRI, other cell types are generally negative for the FcɛRI expression. We found a minor fraction of c-Kit-positive cells at a frequency of 0.43 ± 0.18% in the SVF isolated from the Kit+/+, but not the KitW-sh/W-sh, mice. Notably, all c-Kit-positive cells in the Kit+/+ SVF were FcɛRI-positive mast cells, suggesting that the effects of the Kit mutation are negligible in non-mast cells (Fig. 3A). Consistent with this finding, a quantitative RT-PCR analysis showed that the expression of the mast cell-specific gene cpa3, which encodes mast cell carboxypeptidase A (MCCPA), was detected in the freshly isolated SVF obtained from the Kit+/+, but not the KitW-sh/W-sh mice. The MCCPA mRNA level in the Kit+/+ SVF was significantly lower (approximately one seventy-fifth) than that observed in the BMMCs prepared from the Kit+/+ mice (data not shown). The low level of the MCCPA mRNA expression was no longer detectable when the cells reached confluency. Given that the BMMCs are isolated and maintained in the presence of IL-3 and SCF, the mast cells in the Kit+/+ SVF were unable to survive in the absence of these cytokines and were eliminated by that time (Fig. 3B). When the SVF cells reached confluency, they were supplemented with adipogenic factors to examine their ability to differentiate into adipocytes. After 7 days of culture with adipogenic factors, the number of cells stained with Oil red O was markedly increased in the SVF obtained from the KitW-sh/W-sh eWAT compared to that observed in the controls (Fig. 3C). A spectrophotometric quantitative analysis also showed greater lipid accumulation in the KitW-sh/W-sh SVF compared to that observed in the controls (Fig. 3D). Consistent with this finding, quantitative RT-PCR revealed that the mRNA levels of adipocyte markers, aP2, PPARγ and adiponectin, were significantly higher in the SVF obtained from the KitW-sh/W-sh mice than in the SVF obtained from the controls (Fig. 3E). These results indicate that in vitro adipogenic differentiation is more prevalent in KitW-sh/W-sh SVF than in Kit+/+ SVF. Given that the gain of body weight in response to an HFD was suppressed in the KitW-sh/W-sh mice, it is likely that the transition of preadipocytes to adipocytes in vivo is attenuated in KitW-sh/W-sh mice, although KitW-sh/W-sh preadipocytes are capable of responding to exogenous adipogenic factors and differentiating into mature adipocytes in vitro.
Fig. 3.
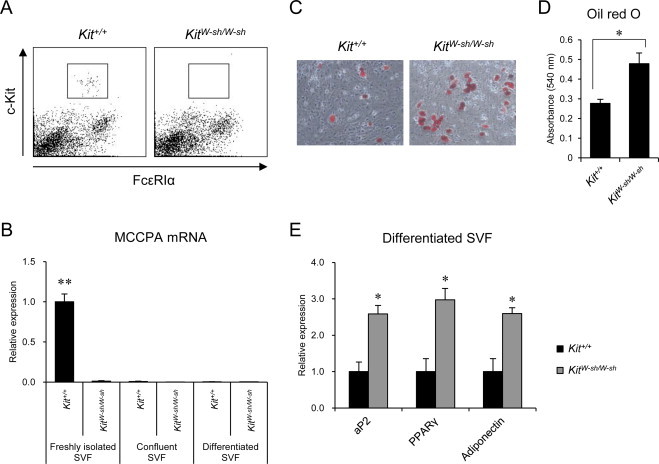
Adipogenic differentiation is more prevalent in KitW-sh/W-sh SVF than in Kit+/+ SVF in vitro. (A) A flow cytometric analysis of the SVF isolated from the Kit+/+ and KitW-sh/W-sh mice fed an ND. c-Kit/FcεRIα double-positive cells were observed within the gates. Representative data from three independent experiments are shown. (B) The mRNA level of MCCPA was evaluated in the Kit+/+ and KitW-sh/W-sh SVF using quantitative RT-PCR. The SVF cells obtained at isolation (freshly isolated), confluence (confluent) and day seven of adipogenic induction (differentiated) were examined (n = 4 for each group). The data are presented as the mean ± SEM. **P < 0.01 versus the KitW-sh/W-sh mice. (C) The SVF cells derived from the Kit+/+ and KitW-sh/W-sh mice fed an ND were cultured in the presence of adipogenic factors, then fixed and stained with Oil red O. Oil red O-positive differentiated adipocytes were observed under a microscope (×100). (D) The stained lipids were extracted, and the absorbance was measured at 540 nm. n = 5 for each group. The data are presented as the mean ± SEM. *P < 0.05 for the comparisons indicated. (E) The mRNA levels of the adipocyte marker genes were examined in the differentiated SVF obtained from the Kit+/+ (black bars) and KitW-sh/W-sh (gray bars) mice using quantitative RT-PCR (n = 3 for each group). The data are presented as the mean ± SEM. *P < 0.05 versus the Kit+/+ mice.
3.4. The enhanced expression of preadipocyte marker genes is restored by reconstituting mast cells in the KitW-sh/W-sh WAT
To examine whether mast cell deficiency results in impaired maturation of preadipocytes in KitW-sh/W-sh mice, BMMCs prepared from Kit+/+ mice were transferred into the peritoneal cavity of KitW-sh/W-sh mice. After 12–16 weeks of transplantation, toluidine blue- and alcian blue/safranin O-positive cells were detected in the peritoneal cavity in the KitW-sh/W-sh mice reconstituted with BMMCs, but not the KitW-sh/W-sh mice (Fig. 4A and B). Furthermore, the expression of MCCPA, a mast cell-specific marker, was detected in the eWAT of the BMMC-transferred, but not PBS-injected, KitW-sh/W-sh mice using quantitative RT-PCR (Fig. 4C). Collectively, these results suggest that the transferred BMMCs were terminally differentiated in the peritoneal cavity and infiltrated into the eWAT of the recipient mice.
Fig. 4.
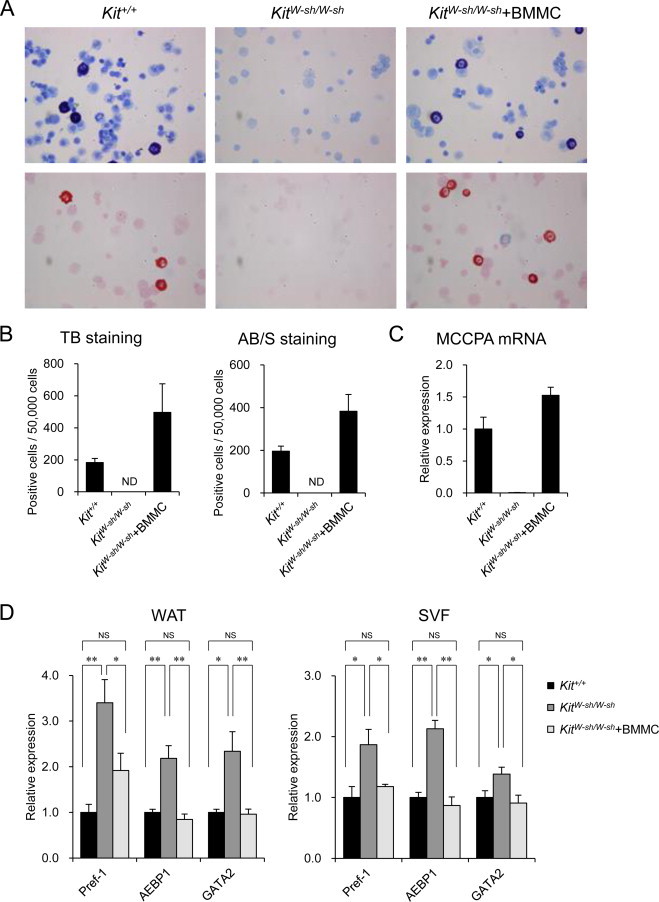
Mast cell reconstitution in the KitW-sh/W-sh mice restored the enhanced mRNA expression of preadipocyte marker genes in the eWAT. BMMCs prepared from Kit+/+ mice (2.0 × 106 cells) were injected intraperitoneally into KitW-sh/W-sh mice and examined after 12–16 weeks of injection. (A) Photomicrographs of peritoneal cells (×400) stained with toluidine blue (upper panels) or alcian blue/safranin O (lower panels) on cytospin preparations. Cells prepared from Kit+/+ and KitW-sh/W-sh mice with or without reconstitution are shown. (B) Quantification of the mast cells in the cytospin preparations shown in (A). n = 3 for each group. ND, not detected. (C) The mRNA expression of the mast cell-specific gene Cpa3 (MCCPA) in the eWAT was evaluated using quantitative RT-PCR (n = 5). (D) The mRNA levels of the preadipocyte marker genes were examined in the eWAT (n = 7) and SVF (n = 6) of the Kit+/+ (black bars) and KitW-sh/W-sh mice with (light gray bars) or without (dark gray bars) reconstitution using quantitative RT-PCR. The data are presented as the mean ± SEM. *P < 0.05, **P < 0.01 for the comparisons indicated. NS, not significant.
We next examined whether the increased expression levels of preadipocyte markers in the KitW-sh/W-sh eWAT were restored by mast cell reconstitution (Fig. 4D). Indeed, the expression levels of Pref-1, AEBP1 and GATA2 were significantly lower in the eWAT and SVF obtained from the mast cell-reconstituted mice than in the controls (Fig. 4D). Taken together, these results suggest that mast cells possibly facilitate the transition of preadipocytes to mature adipocytes in murine WAT.
4. Discussion
Recent studies have demonstrated that a greater number of mast cells reside in the WAT of obese mice receiving an HFD than in the WAT of lean mice and that the body weight gain induced by an HFD is reduced in mast cell-deficient mice [8,14]. Several mechanisms have been proposed to explain how mast cells promote diet-induced obesity. Liu et al. demonstrated that mast cells promote WAT angiogenesis in response to a Western diet by maintaining a high proteolytic activity of cathepsin, which generates angiogenic factors in vascular cells [8]. They showed that mast cells stimulate the cathepsin activity by producing two common cytokines, interleukin-6 and interferon-γ [8]. In a separate study, Tanaka et al. demonstrated that mast cells activate PPARγ, a key regulator of adipogenesis [14]. PPARγ is a ligand-dependent transcription factor that induces the expression of various adipogenic genes and is essential for adipocyte differentiation and fat tissue formation [15]. The authors showed that mast cells activate PPARγ by producing prostaglandin D2 (PGD2), which is metabolized to deoxy-delta-12,14-PGJ2, a natural ligand for PPARγ [14].
The current study provides evidence for a novel role of mast cells in adipogenesis. We demonstrated that the mRNA expression levels of preadipocyte markers were significantly higher in the eWAT and SVF of the KitW-sh/W-sh mice than in the controls and that the increased expression of these genes was restored by mast cell reconstitution. We selected three genes, Pref-1, AEBP1 and GATA2, as representative preadipocyte markers in this study. Notably, these genes are highly expressed in immature preadipocytes and downregulated during adipocyte maturation. Furthermore, the products of these genes are known to repress adipocyte maturation via distinct mechanisms. Pref-1 is a transmembrane protein that contains tandem epidermal growth factor-like repeats [18]. Pref-1 is activated when enzymatically cleaved into a soluble form, which stimulates MAPK kinase/ERK signaling [22], thus resulting in the upregulation of SRY (sex determining region Y)-box 9 (Sox9), an inhibitor of adipogenesis. AEBP1 is a unique transcriptional repressor with carboxypeptidase activity. It has been shown that AEBP1 directly binds to the aP2 gene regulatory sequence, repressing its transcription [19]. GATA2 is a zinc finger transcription factor that is known to be essential for hematopoietic and vascular development [23]. The constitutive expression of GATA2 suppresses the preadipocyte–adipocyte transition through, at least in part, direct repression of the PPARγ gene [20]. Collectively, our data suggest that mast cell deficiency results in the sustained expression of a set of genes that maintain preadipocytes in an immature state.
The important point of our findings is that an increased expression of preadipocyte marker genes was observed in the KitW-sh/W-sh eWAT and SVF, even in the mice fed an ND, thus suggesting that mast cells play a novel role in the homeostatic regulation of adipogenesis in nonobese individuals. Interestingly, despite the fact that the body weights were similar between the ND groups, more lipid accumulating cells were differentiated from the SVF of the KitW-sh/W-sh mice than from the controls. Our findings indicate that mast cells in Kit+/+ SVF are unable to survive, likely due to the absence of IL-3 and SCF, and are eliminated by the time of adipogenic differentiation. Therefore, it is unlikely that the increased adipogenic potential in the KitW-sh/W-sh SVF results from the lack of direct effects of mast cells during adipocyte maturation in vitro. Rather, the increased adipogenic potential may result from the expansion of adipocyte progenitor pools or the activation of the adipogenic gene expression program in KitW-sh/W-sh preadipocytes. Further studies are needed to determine the precise roles of mast cells in the homeostatic regulation of adipogenesis.
Taken together with previous results showing that a greater number of mast cells are present in WAT in obese humans and mice than in lean controls [8,9,14], our present findings suggest that mast cells may play a gate-keeper role by facilitating the preadipocyte to adipocyte transition under both physiological and pathological conditions. Thus far, a number of mediators released from mast cells, such as cytokines, chemokines, proteases and prostaglandins, have been suggested to play a role in adipogenesis [24]. As mast cells play diverse roles in adipogenesis, further studies are therefore needed to identify the mediators responsible for each function.
Acknowledgements
We appreciate Toshiya Hachisu for providing technical assistance. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (K.O.) and a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (K.O.).
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Spiegelman B.M., Flier S. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Gregoire F.M., Smas C.M., Sul H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 3.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H., Ghosh S., Perrard X.D., Feng L., Garcia G.E., Perrard J.L., Sweeney J.F., Peterson L.E., Chan L., Smith C.W., Ballantyne C.M. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 6.Xu H., Barnes G.T., Yang Q., Tan G., Yang D., Chou C.J., Sole J., Nichols A., Ross J.S., Tartaglia L.A., Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Divoux A., Sun J., Zhang J., Clément K., Glickman J.N., Sukhova G.K., Wolters P.J., Du J., Gorgun C.Z., Doria A., Libby P., Blumberg R.S., Kahn B.B., Hotamisligil G.S., Shi G.P. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altintas M.M., Azad A., Nayer B., Contreras G., Zaias J., Faul C., Reiser J., Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J. Lipid Res. 2011;52:480–488. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimbaldeston M.A., Chen C.C, Piliponsky A.M., Tsai M., Tam S.Y., Galli S.J. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poglio S., Toni-Costes D., Arnaud E., Laharrague P., Espinosa E., Casteilla L., Cousin B. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28:2065–2072. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- 12.Ishijima Y., Ohmori S., Uenishi A., Ohneda K. GATA transcription factors are involved in IgE-dependent mast cell degranulation by enhancing the expression of phospholipase C-γ1. Genes Cells. 2012;17:285–301. doi: 10.1111/j.1365-2443.2012.01588.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohmori S., Takai J., Ishijima Y., Suzuki M., Moriguchi T., Philipsen S., Yamamoto M., Ohneda K. Regulation of GATA factor expression is distinct between erythroid and mast cell lineages. Mol. Cell. Biol. 2012;32:4742–4755. doi: 10.1128/MCB.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka A., Nomura Y., Matsuda A., Ohmori K., Matsuda H. Mast cells function as an alternative modulator of adipogenesis through 15-deoxy-delta-12, 14-prostaglandin J2. Am. J. Physiol. Cell Physiol. 2011;301 doi: 10.1152/ajpcell.00514.2010. C1360-C1367. [DOI] [PubMed] [Google Scholar]
- 15.Tontonoz P., Hu E., Graves R.A., Budavari A.I., Spiegelman B.M. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 16.Oikawa E., Iijima H., Suzuki T., Sasano H., Sato H., Kamataki A., Nagura H., Kang M.J., Fujino T., Suzuki H., Yamamoto T.T. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J. Biochem. 1998;124:679–685. doi: 10.1093/oxfordjournals.jbchem.a022165. [DOI] [PubMed] [Google Scholar]
- 17.Cook K.S., Min H.Y., Johnson D., Chaplinsky R.J., Flier J.S., Hunt C.R., Spiegelman B.M. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402–405. doi: 10.1126/science.3299705. [DOI] [PubMed] [Google Scholar]
- 18.Smas C.M., Sul H.S. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 19.He G.P., Muise A., Li A.W., Ro H.S. A eukaryotic transcriptional represser with carboxypeptidase activity. Nature. 1995;378:92–96. doi: 10.1038/378092a0. [DOI] [PubMed] [Google Scholar]
- 20.Tong Q., Dalgin G., Xu H., Ting C.N., Leiden J.M., Hotamisligil G.S. Function of GATA transcription factors in preadipocyte–adipocyte transition. Science. 2000;290:134–138. doi: 10.1126/science.290.5489.134. [DOI] [PubMed] [Google Scholar]
- 21.Rodeheffer M.S., Birsoy K., Friedman J.M. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Sul H.S. Pref-1: role in adipogenesis and mesenchymal cell fate. Mol. Endocrinol. 2009;23:1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chlon T.M., Crispino J.D. Combinatorial regulation of tissue specification by GATA and FOG factors. Development. 2012;139:3905–3916. doi: 10.1242/dev.080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi M.A., Shi G.P. Different roles of mast cells in obesity and diabetes: lessons from experimental animals and humans. Front. Immunol. 2012;3:7. doi: 10.3389/fimmu.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


