Summary
Malignant migrating partial seizures in infancy (MMPEI) is an early onset epileptic encephalopathy with few known etiologies. We sought to identify a novel cause of MMPEI in a child with MMPEI whose healthy parents were consanguineous. We used array comparative genomic hybridization (CGH) to identify copy number variants (CNVs) genome-wide and long-range PCR to further delineate the breakpoints of a deletion found by CGH. The proband had an inherited homozygous deletion of chromosome 20p13, disrupting the promoter region and first three coding exons of the gene PLCB1. Additional MMPEI cases were screened for similar deletions or mutations in PLCB1 but did not harbor mutations. Our results suggest that loss of PLCβ1 function is one cause of MMPEI, consistent with prior studies in a Plcb1 knockout mouse model that develops early onset epilepsy. We provide novel insight into the molecular mechanisms underlying MMPEI and further implicate PLCB1 as a candidate gene for severe childhood epilepsies. This work highlights the importance of pursuing genetic etiologies for severe early onset epilepsy syndromes.
Keywords: Focal epilepsy, migrating partial seizures in infancy, genetics, phospholipase C beta 1 (PLCB1)
Introduction
Malignant migrating partial seizures in infancy (MMPEI) is a rare, severe early infantile onset epileptic encephalopathy (Coppola et al., 1995). The syndrome is associated with virtually continuous multifocal seizures on EEG that migrate during seizures between cortical regions and hemispheres. MRI and standard neuro-metabolic evaluations do not reveal an etiology. Seizures in MMPEI are refractory to conventional treatment with anti-epileptic drugs (AEDs), and overall developmental prognosis is poor.
MMPEI is a genetically heterogeneous disorder with few known etiologies. Both deletion and point mutation of the voltage-gated sodium channel gene SCN1A are associated with MMPEI (Freilich et al., 2011; Carranza Rojo et al., 2011). A case of MMPEI is also described with duplication of 16p11.2 (Bedoyan et al., 2010). Here we identify an inherited homozygous deletion of the gene phospholipase C beta 1 (PLCB1) in a child with MMPEI.
Subjects and Methods
The proband was evaluated at Children’s Hospital Boston (CHB). Additional MMPEI cases were ascertained from Australia, the United Kingdom, Saudi Arabia, Sweden, the United States, and New Zealand. All subjects had MMPEI as described by Coppola and colleagues (Coppola et al, 1995). Research was performed in accordance with the Institutional Review Board of CHB; written informed consent was obtained from all participants/their guardians.
Blood samples were collected, and DNA was extracted using standard methods. DNA from the proband and parents was fragmented, labeled, and hybridized to an oligonucleotide-based array for chromosomal microarray analysis (CMA) (CHB DNA Diagnostic Lab version 1.4, Agilent 244K platform). which detects CNVs as small as 150kB.
Delineation of the PLCB1 deletion was performed using long-range polymerase chain reaction (PCR) (Kurian et al., 2010). Additional MMPEI cases were evaluated for PLCB1 mutations and intragenic deletions by direct sequencing using PCR primers directed against each exon of PLCB1. Primers were designed using Primer 3 software (Rozen and Skaletsky, 2000) (sequences available on request).
Results
Clinical Presentation
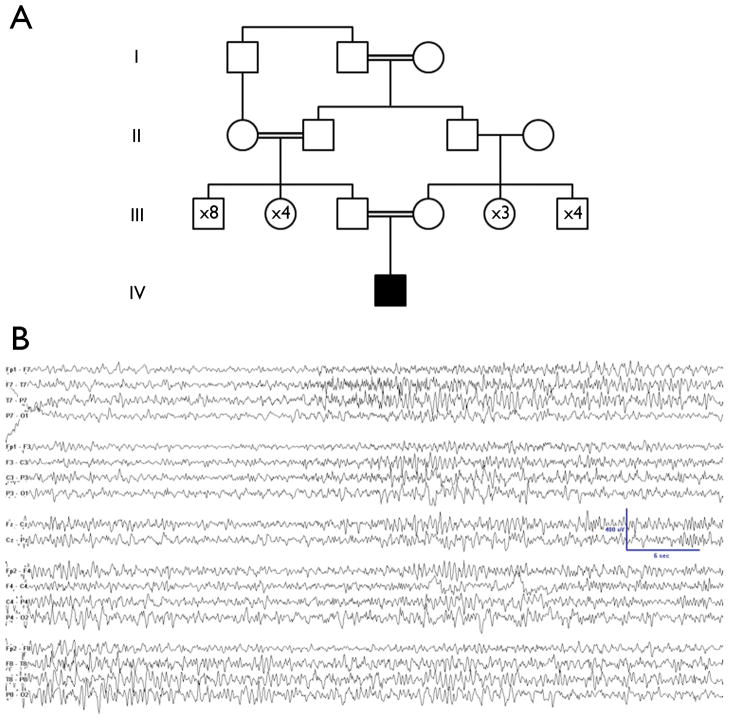
The patient was born at 42 weeks’ gestation after a normal pregnancy. He is the first child of healthy parents who are first cousins of Palestinian descent (pedigree shown in Figure 1A). Seizures began at 6 months with perioral cyanosis, limpness, mouth automatisms, eyelid fluttering, and at times desaturation with oxygen levels as low as 35 to 55%; seizures lasted from 10 seconds to 2 minutes. Eventually, some seizures consisted only of staring and activity arrest with eye deviation to the right or to the left. Before seizure onset, development was delayed but progressing; he was babbling and bringing objects together but not yet rolling or sitting. Once seizures began, he made only guttural sounds, did not fix or follow objects, and had limited voluntary movements of the limbs. At presentation at 6 months of age, his neurological examination was notable for marked truncal and appendicular hypotonia. Electroencephalogram (EEG) showed multifocal interictal spikes and abundant seizures arising from the right and left temporal lobes independently, at times with migration from one hemisphere to the other within a seizure (Figure 1B). In the first four months of hospitalization, from age 6 months to 10 months, he had an average of 27 electrographic seizures per day (about half with clinical symptoms) when EEG recordings were performed.
Figure 1.
A. The four-generation pedigree is notable for multiple consanguineous relationships. The proband’s parents are first cousins.
B. An example of the ictal EEG from the proband demonstrates the migrating nature of the seizures typical of MMPEI. On the left side of the page, a right temporal seizure is shown. On the right, migration of the seizure to include the left temporal region is shown.
Treatment was attempted with multiple AEDs, including fos-phenytoin, phenobarbital, pyridoxine, benzodiazepines (lorazepam, clonazepam, diazepam, clobazam, and infusion of midazolam), levetiracetam, rufinamide, topiramate, lacosamide, triple bromide solution (ammonium bromide, potassium bromide, and sodium bromide), stiripentol, prednisolone, the ketogenic diet (3.5:1 ratio).
Diagnostic Evaluation, including CMA
Magnetic resonance (MR) imaging at 6, 7, 8, and 9 months revealed mildly prominent cerebrospinal fluid (CSF) spaces. MR spectroscopy (MRS) performed at 9 months was normal. Laboratory investigations for inborn errors of metabolism, neurotransmitter disorders, and SCN1A mutations were unrevealing.
CMA of the proband identified three CNVs: (1) homozygous ~476kb deletion of chromosome 20p12.3 (0 copies, chr20: 8,099,741–8,575,520 in Human Genome build hg19) (Figure 2A); (2) heterozygous ~109kb duplication of chromosome 7p21.3 (3 copies); and (3) heterozygous ~125kb duplication of chromosome 12q24.12 (3 copies). Both parents were found to be heterozygous for the 20p12.3 deletion. Of more than 6600 patients who have been assessed by CGH in our DNA Diagnostic Laboratory, this is the only family bearing the 20p12.3 deletion. The 7p21.3 and 12q24.12 duplications were maternally inherited variants.
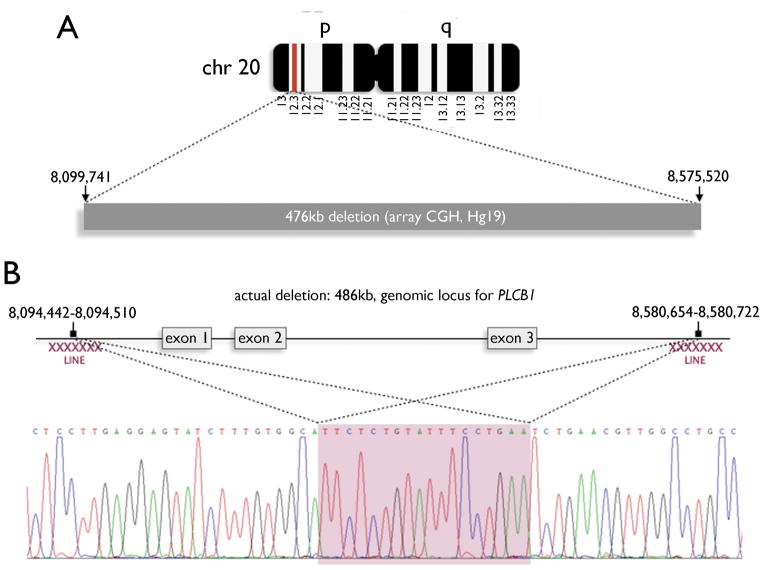
Figure 2.
A. Proband, paternal, and maternal genomic DNA were isolated from peripheral blood and then fragmented, labeled, and hybridized for targeted array comparative genomic hybridization (array CGH). The proband’s study revealed a homozygous 476kb deletion on chromosome 20p12.3 as illustrated in the schematic (red band). This deletion corresponds to coordinates 8,099,741–8,575,520 on chromosome 20 (human genome build hg19). Parental studies revealed that each of the proband’s parents is heterozygous for the 20p12.3 deletion.
B. The deletion occurs within the locus of the gene phospholipase C beta 1 (PLCB1). Comparison of genomic DNA with sequenced PLCB1 cDNAs revealed that the deleted region encompasses the first three coding exons of the gene. More precise deletion breakpoints were identified by long-range PCR of genomic DNA (8,094,049–8,094,072 to 8,580,261–8,580,284) and found to be flanked by repetitive long interspersed elements (LINE, red Xs). The exact deletion boundaries could not be resolved due to 23 nucleotides of 100% sequence homology between 5′ and 3′ breakpoints (shaded red).
The 20p12.3 deletion includes the first three coding exons of PLCB1 and 65.6 kilobases (kb) of 5′ upstream genomic DNA (Figure 2B). No other annotated genes or known non-coding RNAs were identified. Using long-range PCR, we localized the. deletion breakpoints to chr20: 8,094,049–8,094,072 and chr20: 8,580,261–8,580,284, defining a 486kb deletion (Figure 2B). The breakpoints could not be more precisely defined due to a 23-nucleotide sequence with 100% homology for both the upstream and the intron 3 sequence (Figure 2B). The breakpoints lie within two L1 family long interspersed nuclear elements (LINE) L1PA3 and L1PA2 occurring at chr20: 8,089,514–8,095,564 and chr20: 8,575,749–8,581,774.
Screening of Additional MMPEI Cases
We screened a consanguineous family from Saudi Arabia and 2 non-consanguineous families from New Zealand and Sweden, each with 2 children affected with MMPEI, for additional PLCB1 mutations or deletions. We also screened 12 MMPEI simplex cases from Australia, 2 from the United States, and one from the United Kingdom. None exhibited a mutation in PLCB1.
Discussion
We identified a homozygous deletion of PLCB1 in a patient with MMPEI. Loss of the first three coding exons of the PLCB1 cDNA and possibly important 5′ regulatory elements likely resulted in loss of wild type PLCβ1 protein expression and the MMPEI phenotype. This deletion is flanked by repetitive sequences and thus likely arose in the heterozygous state as a result of non-allelic homologous recombination.
PLCB1 is a novel gene for MMPEI, a rare epilepsy with few identifiable etiologies. Kurian and colleagues previously described a case of early onset epileptic encephalopathy (EOEE) associated with deletion of PLCB1 (Kurian et al., 2010). Epilepsy onset for our proband occurred at 6 months with focal seizures, an EEG characteristic of MMPEI, and developmental regression. In contrast, the prior case of PLCB1-associated epilepsy had onset of tonic seizures at 10 weeks, recurrence at 6 months, and infantile spasms at 8 months; this case had an initially normal EEG and normal development and later developed hypsarrhythmia on EEG and developmental regression (Kurian et al., 2010). Thus these two patients with PLCB1 deletions fall into two distinct electroclinical syndromes, with the previous case representing EOEE and our case representing MMPEI.
We demonstrate two phenomena that have become recurring themes in epilepsy genetics: (1) the heterogeneity of the phenotypic presentations of genes associated with early onset epileptic encephalopathies, including PLCB1, and (2) the heterogeneity of the genetic etiologies of a well-defined epileptic encephalopathy, namely MMPEI. The lack of PLCB1 mutations in our additional MMPEI cases further illustrates this genetic heterogeneity.
The enzyme encoded by PLCB1, phospholipase C isoform β1 (PLCβ1) generates the intracellular second messengers diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (Ins-1,4,5,-P3, also called IP3) from phosphatidylinositol-4,5,-bisphosphonate (PtdIns-4,5-P2, also called PIP2). A murine model for homozygous Plcb1 deletion presented with early generalized seizures and death, underscoring the role of PLCβ1 in normal neuronal development and function (Kim et al., 1997). In wild-type rats, the β1 and δ1 isoforms of the PLC enzyme are expressed postnatally, whereas the γ1 isoform is expressed prenatally (Shimohama et al., 1998). These observations suggest that disruption of PLCβ1 function might not affect the nervous system until after birth, consistent with the observation that both patients with PLCB1 deletion had normal development reported for several weeks to months. We postulate that this temporal pattern reflects one or more of the following phenomena: (1) a limited role for human PLCβ1 in prenatal and early postnatal neuronal development and function; (2) functional redundancy among different neuronal phospholipases expressed during development; and/or (3) the maturation of a neuronal pathway sensitive to PLCβ1 deficiency.
Together with other studies, our findings underscore the importance of genome-wide copy number assessment for all unexplained cases of epileptic encephalopathy (Heinzen et al., 2010; Mefford et al., 2010; Mefford et al., 2011). The identification of PLCB1 as the gene associated with MMPEI in our proband opens the possibility of addressing his molecular defect by modification of PLCβ1-related pathways. We are optimistic that continued efforts to unravel the molecular mechanisms of early onset epilepsies such as MMPEI may one day translate into clinical interventions to improve the lives of children afflicted with these devastating disorders.
Acknowledgments
We thank the families of the children with MMPEI who participated in this research study. Annapurna Poduri was supported by the NINDS (K23NS069784). Manju Kurian’s research is funded by Action Medical Research and Great Ormond Street Children’s Charities. Mustafa A. Salih was supported by the College of Medicine Research Center (CMRC Project No. 07-581), College of Medicine, King Saud University, Riyadh, Saudi Arabia. Mustafa Sahin is an investigator of the Children’s Hospital Boston Translational Research Program and was also supported by John Merck Fund, Nancy Lurie Marks Family Foundation, and Manton Center for Orphan Disease Research. Ingrid E. Scheffer receives/has received research support from the National Health and Medical Research Council of Australia, Health Research Council of New Zealand, The University of Melbourne, American Epilepsy Society, the Jack Brockhoff Foundation, the Shepherd Foundation and the Perpetual Charitable Trustees. Eamonn Maher’s research was funded by Action Medical Research. Bai-Lin Wu is supported by the Chinese National “973” project on Population and Health (2010CB529601) and the Science and Technology Council of Shanghai (09JC1402400). Christopher A. Walsh is an investigator of the Howard Hughes Medical Institute and was also supported by the NINDS (R01NS035129, R01NS035129S), the NIMH (NIMH1RC2MH089952), the Manton Center for Orphan Disease Research, the Dubai Harvard Foundation for Medical Research, the Simons Foundation, and the Nancy Lurie Marks Family Foundation.
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures of Conflicts of Interest
Dr. Neilan was supported by Pharming Group and the Luke O’Brien Foundation.
Dr. Scheffer has served on scientific advisory boards for UCB and Janssen-Cilag EMEA, received speaker honoraria from Athena Diagnostics, UCB, Biocodex and Janssen-Cilag EMEA, and received funding for travel from Athena Diagnostics, UCB, Biocodex and Janssen-Cilag EMEA.
Dr. Sahin’s research has been supported by Novartis (NCT00789828).
The other authors report no disclosures.
References
- Bedoyan JK, Kumar RA, Sudi J, Silverstein F, Ackley T, Iyer RK, Christian SL, Martin DM. Duplication 16p11.2 in a child with infantile seizure disorder. Am J Med Genet A. 2010;152A:1567–1574. doi: 10.1002/ajmg.a.33415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carranza Rojo D, Hamiwka L, McMahon JM, Dibbens LM, Arsov T, Suls A, Stödberg T, Kelley K, Wirrell E, Appleton B, Mackay M, Freeman JL, Yendle SC, Berkovic SF, Bienvenu T, De Jonghe P, Thorburn DR, Mulley JC, Mefford HC, Scheffer IE. De novo SCN1A mutations in migrating partial seizures of infancy. Neurology. 2011;77:380–383. doi: 10.1212/WNL.0b013e318227046d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G, Plouin P, Chiron C, Robain O, Dulac O. Migrating partial seizures in infancy: a malignant disorder with developmental arrest. Epilepsia. 1995;36:1017–1024. doi: 10.1111/j.1528-1157.1995.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Freilich ER, Jones JM, Gaillard WD, Conry JA, Tsuchida TN, Reyes C, Dib-Hajj S, Waxman SG, Meisler MH, Pearl PL. Novel SCN1A mutation in a proband with malignant migrating partial seizures of infancy. Arch Neurol. 2011;68:665–671. doi: 10.1001/archneurol.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzen EL, Radtke RA, Urban TJ, Cavalleri GL, Depondt C, Need AC, Walley NM, Nicoletti P, Ge D, Catarino CB, Duncan JS, Kasperaviciūte D, Tate SK, Caboclo LO, Sander JW, Clayton L, Linney KN, Shianna KV, Gumbs CE, Smith J, Cronin KD, Maia JM, Doherty CP, Pandolfo M, Leppert D, Middleton LT, Gibson RA, Johnson MR, Matthews PM, Hosford D, Kälviäinen R, Eriksson K, Kantanen AM, Dorn T, Hansen J, Krämer G, Steinhoff BJ, Wieser HG, Zumsteg D, Ortega M, Wood NW, Huxley-Jones J, Mikati M, Gallentine WB, Husain AM, Buckley PG, Stallings RL, Podgoreanu MV, Delanty N, Sisodiya SM, Goldstein DB. Rare deletions at 16p13.11 predispose to a diverse spectrum of sporadic epilepsy syndromes. Am J Hum Genet. 2010;86:707–718. doi: 10.1016/j.ajhg.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Jun KS, Lee SB, Kang NG, Min DS, Kim YH, Ryu SH, Suh PG, Shin HS. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- Kurian MA, Meyer E, Vassallo G, Morgan NV, Prakash N, Pasha S, Hai NA, Shuib S, Rahman F, Wassmer E, Cross JH, O’Callaghan FJ, Osborne JP, Scheffer IE, Gissen P, Maher ER. Phospholipase C beta 1 deficiency is associated with early-onset epileptic encephalopathy. Brain. 2010;133:2964–2970. doi: 10.1093/brain/awq238. [DOI] [PubMed] [Google Scholar]
- Mefford HC, Muhle H, Ostertag P, von Spiczak S, Buysse K, Baker C, Franke A, Malafosse A, Genton P, Thomas P, Gurnett CA, Schreiber S, Bassuk AG, Guipponi M, Stephani U, Helbig I, Eichler EE. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6:e1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Yendle SC, Hsu C, Cook J, Geraghty E, McMahon JM, Eeg-Olofsson O, Sadleir LG, Gill D, Ben-Zeev B, Lerman-Sagie T, Mackay M, Freeman JL, Andermann E, Pelakanos JT, Andrews I, Wallace G, Eichler EE, Berkovic SF, Scheffer IE. Rare copy number variants are an important cause of epileptic encephalopathies. Ann Neurol. 2011;70:974–985. doi: 10.1002/ana.22645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Sumida Y, Fujimoto S, Matsuoka Y, Taniguchi T, Takenawa T, Kimura J. Differential expression of rat brain phospholipase C isozymes in development and aging. Biochem Biophys Res Commun. 1998;243:210–216. doi: 10.1006/bbrc.1998.8090. [DOI] [PubMed] [Google Scholar]