Abstract
P-glycoprotein (ABCB1/MDR1, EC 3.6.3.44), the major efflux transporter at the blood–brain barrier (BBB), is a formidable obstacle to CNS pharmacotherapy. Understanding the mechanism(s) for increased P-glycoprotein activity at the BBB during peripheral inflammatory pain is critical in the development of novel strategies to overcome the significant decreases in CNS analgesic drug delivery. In this study, we employed the λ-carrageenan pain model (using female Sprague–Dawley rats), combined with confocal microscopy and subcellular fractionation of cerebral microvessels, to determine if increased P-glycoprotein function, following the onset of peripheral inflammatory pain, is associated with a change in P-glycoprotein trafficking which leads to pain-induced effects on analgesic drug delivery. Injection of λ-carrageenan into the rat hind paw induced a localized, inflammatory pain (hyperalgesia) and simultaneously, at the BBB, a rapid change in colocalization of P-glycoprotein with caveolin-1, a key scaffolding/trafficking protein. Subcellular fractionation of isolated cerebral microvessels revealed that the bulk of P-glycoprotein constitutively traffics to membrane domains containing high molecular weight, disulfide-bonded P-glycoprotein-containing structures that cofractionate with membrane domains enriched with monomeric and high molecular weight, disulfide-bonded, caveolin-1-containing structures. Peripheral inflammatory pain promoted a dynamic redistribution between membrane domains of P-glycoprotein and caveolin-1. Disassembly of high molecular weight P-glycoprotein-containing structures within microvascular endothelial luminal membrane domains was accompanied by an increase in ATPase activity, suggesting a potential for functionally active P-glycoprotein. These results are the first observation that peripheral inflammatory pain leads to specific structural changes in P-glycoprotein responsible for controlling analgesic drug delivery to the CNS.
Keywords: blood–brain barrier, caveolin, inflammatory pain, P-glycoprotein, protein trafficking
Drug delivery across the blood–brain barrier (BBB) to the brain and CNS remains a major challenge in the treatment of numerous diseases and pathologies including epilepsy, Parkinson’s disease, Alzheimer’s disease, HIV-associated neurocognitive disease, mood and psychotic disorders, brain cancer, and peripheral inflammatory pain (Pardridge 2007; Hartz and Bauer 2010; Miller 2010; Potschka 2010b; Ronaldson and Davis 2011; Vangilder et al. 2011). Endothelial cells that line microvessels of the brain and form the BBB are non-fenestrated (Reese and Karnovsky 1967). This forces substances attempting passage from the peripheral circulation to the brain to take either the paracellular route (between endothelial cells) or the transcellular route. Paracellular diffusion of water-soluble substances and small ions is severely restricted by large, multiprotein tight junctional complexes that exist between apposing endothelial cell membranes and physically obliterate the interendothelial cleft. Transcellular passage of blood-borne substances through microvascular endothelial cells into the brain is actively thwarted by efflux transporters embedded in the luminal membrane (Hawkins and Davis 2005; Abbott et al. 2010).
P-glycoprotein, the predominant efflux transporter at the BBB, combines ATP hydrolysis with drug efflux to actively extrude drugs against steep concentration gradients (Miller 2010). Designated ‘P-glycoprotein’ because of its ability to alter the permeability of biological membranes to xenobiotics (Juliano and Ling 1976), P-glycoprotein is capable of effluxing an impressive variety of structurally divergent drugs that range in mass from approximately 300–4000 Da, including analgesics, anti-cancer and immunosuppressive agents, psychotropics, antibiotics, anti-allergenics, anti-epileptics, beta-blockers, steroid hormones, and HIV-1 protease inhibitors (Sun et al. 2004; Hartz and Bauer 2010; Miller 2010; Potschka 2010b; Ueno et al. 2010). To overcome multidrug resistance and increase drug distribution and therapeutic effect, intense research has focused on the development of P-glycoprotein inhibitors. Results of clinical trials using P-glycoprotein inhibitors have not been encouraging because the high inhibitor doses necessary for effective transporter inhibition are associated with systemic toxicity (Choo et al. 2006; Szakacs et al. 2006; Kaye 2008; Kannan et al. 2009; Fletcher et al. 2010).
P-glycoprotein expression and function are altered in many disease states (Roberts and Goralski 2008; Bartels 2011; De Klerk et al. 2011), creating greater challenges to CNS pharmacotherapy. To preserve the protection afforded by basal P-glycoprotein expression and function, research efforts currently focus on therapeutic regulation of disease-related increases in P-glycoprotein activity (Potschka 2010a,b). Pioneering work investigating transient, functional modulation of P-glycoprotein conducted by Miller and colleagues using a combination of in vivo and ex vivo studies on rat cerebral microvessels has identified multiple signaling pathways involved in regulation of P-glycoprotein (Bauer et al. 2005; Miller et al. 2008; Miller 2010). In addition, demonstration of a rapid reduction of P-glycoprotein efflux function at the BBB through endocytosis importantly highlighted the potential that therapeutic manipulation of a trafficking pathway may have in temporarily reducing P-glycoprotein efflux activity at the luminal membrane (Hawkins et al. 2010).
Previous studies by our group showed that functional expression of P-glycoprotein is significantly increased 3 h after onset of peripheral inflammatory hyperalgesia in vivo, an effect that leads to enhanced efflux transport of morphine at the BBB (Seelbach et al. 2007). The observed decrease in brain uptake of morphine corresponds with a decrease in morphine-induced analgesia. These findings importantly demonstrate a decreased ability for morphine, a clinically relevant drug used specifically in pain states, to gain entrance into the brain under conditions of inflammatory pain. The basis of this study is to propose that a rapid increase in P-glycoprotein activity at the BBB may arise from dynamic changes in intracellular trafficking of P-glycoprotein within cerebral microvascular endothelial cells. This could occur if P-glycoprotein is stored within a putative reservoir under steady-state conditions and then released during stressful conditions to be trafficked to the luminal plasma membrane.
Caveolin-1, a key trafficking protein capable of forming both caveolar and non-caveolar oligomeric scaffolds (Lajoie et al. 2009), colocalizes with P-glycoprotein in caveolae isolated from rat brain capillaries (Demeule et al. 2000), and at the luminal endothelial membrane and the border of the luminal/abluminal compartments in human brain capillaries (Virgintino et al. 2002; Guo et al. 2010). Pioneering work by Beliveau and colleagues identified a caveolin-1-binding motif within the N-terminus of P-glycoprotein, and showed that, in isolated rat brain capillary homogenate, a portion of P-glycoprotein associates with a high molecular weight caveolin-1-containing complex (Jodoin et al. 2003). Moreover, using rat brain endothelial cells in vitro, these researchers demonstrated that the physical interaction between P-glycoprotein with caveolin-1 is enhanced by tyrosine-14-phosphorylation of caveolin-1 and negatively regulates P-glycoprotein function (Barakat et al. 2007, 2008).
In this work, we hypothesized that a dynamic redistribution of P-glycoprotein between subcellular compartments of microvascular endothelial cells was associated with peripheral inflammatory pain. We investigated this hypothesis using the well-established and highly reproducible λ-carrageenan rat model of inflammatory pain (i.e. hyperalgesia), combined with confocal microscopy and subcellular fractionation of isolated cerebral microvessels, to investigate the constitutive and inflammation/pain-induced trafficking of P-glycoprotein within microvascular endothelial cells in vivo at the BBB. Our data demonstrate that under non-disease conditions P-glycoprotein trafficking is highly regulated, wherein the majority of P-glycoprotein is trafficked to membrane domains of a narrowly defined density that are enriched with caveolin-1, and that facilitates the sequestration of P-glycoprotein as a component of high molecular weight (> 250 kDa), disulfide-bonded structures. Our data show that peripheral inflammatory hyperalgesia leads to a dramatic redistribution of P-glycoprotein and caveolin-1 between endothelial cell subcellular compartments at the BBB, and a significant increase in drug-stimulated P-glycoprotein-dependent ATPase activity associated with plasma membrane domains identified to be at the luminal surface of cerebral microvessels.
Materials and methods
OptiPrep was purchased from Accurate Chemical (Westbury, NY, USA). EDTA-free Complete Proteinase Inhibitor was obtained from Roche (Indianapolis, IN, USA). Criterion TGX 7.5% and 4–20% gels, Tris/Glycine/SDS running buffer, reducing agent tris(2-carboxyethyl)phosphine hydrochloride (TCEP), and Precision Plus All Blue prestained molecular weight standards were obtained from Bio-Rad (Hercules, CA, USA). Western Lightning Chemiluminescence Reagent Plus was purchased from Perkin Elmer (Waltham, MA, USA) and blue autoradiography film was bought from ISC Bioexpress (Kaysville, UT, USA). The mouse monoclonal antibody against P-glycoprotein (C219) was purchased from ID Labs (London, ON, Canada). The rabbit polyclonal antibody against nucleoporin (sc-25523) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The rabbit polyclonal antibodies against caveolin-1 (3238) and tyrosine-14-phosphorylated caveolin-1 (pY14caveolin-1, 3251) were obtained from Cell Signaling (Danvers, MA, USA). The rat monoclonal antibody against multidrug resistance protein 4 (Mrp4, MC-273) was purchased from Kamiya Biomedical (Seattle, WA, USA). Pgp-Glo Assay Systems were purchased from Promega (Madison, WI, USA). All other chemicals and reagents were obtained from either Thermo-Scientific (Suwannee, GA, USA) or Sigma (St. Louis, MO, USA).
Animals and treatments
All animal protocols abide by National Institutes of Health guidelines and were approved by the University of Arizona Institutional Animal Care and Use Committee. Female Sprague–Dawley rats (250–300 g; Harlan Sprague Dawley, Indianapolis, IN, USA) were housed under standard 12-h light/12-h dark conditions and given food and water ad libitum. Animals were randomly assigned to each treatment group. In the conscious state, rats were gently restrained and at time t = 0 min, each received an injection (100 μL, s.c.) of either saline (0.9% NaCl) or λ-carrageenan (3% w/v in saline) into the plantar surface of the right hind paw. Paw injection sites were swabbed with 70% ethanol before injection. Following injection, animals were placed in clean cages and closely monitored. Plethysmography was used to measure paw edema, and the Hargreaves radiant heat test (Hargreaves et al. 1988) was used to assess thermal hyperalgesia, as previously described (Seelbach et al. 2007; McCaffrey et al. 2008).
Microvessel isolation and fractionation
Animals were anesthetized with sodium pentobarbital (64 mg/kg; i.p.), decapitated, and brains were immediately placed in ice-cold Buffer I (136.9 mM NaCl, 2.7 mM KCl, 1 mM CaCl2-2H20, 1.5 mM KH2PO4, 8.1 mM Na2HPO4, 0.5 mM MgCl2-6H20, 5 mM glucose, 1 mM sodium pyruvate, pH 7.4) supplemented with 2 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 1 mM Na4P2O7, Roche EDTA-free Complete Protease Inhibitor, and Sigma inhibitor cocktail. Cerebral microvessels were isolated as previously described (McCaffrey et al. 2007, 2008, 2009; Seelbach et al. 2007; Loch-head et al. 2010), with minor modifications. Briefly, following removal of choroid plexus and meninges, cerebral hemispheres (from four to six rats) were pooled, placed in 30 mL ice-cold Buffer I and homogenized on ice using a motor-driven Potter–Elvehjem homogenizer set at low speed (serrated PTFE pestle, clearance 0.13–0.18 mm, 30 up/down strokes). This initial brain homogenate was then transferred to a tight-fitting Dounce homogenizer, and homogenized on ice by hand (eight up/down strokes). Ten millilitre aliquots of homogenized brain tissue were then mixed with 15 mL of Buffer II (30% Ficoll in Buffer II) and centrifuged in a Sorvall SS-34 rotor for 20 min at 5800 g (4°C). Following careful removal of the supernatant, each pellet was supplemented with 10 mL of ice-cold Buffer III (1% bovine serum albumin in Buffer I), resuspended by gentle homogenization on ice (Potter–Elvehjem homogenizer, smooth PTFE pestle, clearance 0.13–0.18 mm, two up/down strokes), and filtered by gravity through a 300-μm mesh filter, and then collected on a 40-μm mesh filter. Brain microvessels retained on the 40-μm mesh filter were resuspended in ice-cold Buffer III, concentrated by benchtop centrifugation for 10 min at 1500 g (4°C), and washed twice in Buffer IV (20 mM Tris-HCl, 250 mM sucrose, pH 7.4) supplemented with 1 mM CaCl2, 1 mM MgCl2, 2 mM PMSF, 1 mM Na3VO4, 1 mM NaF, 1 mM Na4P2O7, Roche EDTA-free Complete Protease Inhibitor, and Sigma inhibitor cocktail. After removal of aliquots for confocal microscopy, final microvessel pellets were covered with 0.5–1.0 mL of Buffer IV and then stored at −80°C until use.
Rat cerebral microvessels were fractionated by an adaptation of the detergent-free method of Macdonald and Pike (2005), as previously described (McCaffrey et al. 2007, 2008, 2009; Lochhead et al. 2010), with minor modifications. Briefly, rat brain microvessel pellets were rapidly thawed, supplemented with 1–2 mL of Buffer IV (containing ions and proteinase/phosphatase inhibitors), and passed 20 times through a 21-gauge needle on ice. The protein concentration of each sample was determined using the Coomassie Plus Better Bradford Assay Kit. Aliquots of microvessel homogenate (containing equal amounts of protein) were adjusted to 2 mL with Buffer IV, mixed with 60% OptiPrep, and the resultant 30% OptiPrep-microvessel homogenate was overlayered with a discontinuous 5/10/15/20/25% OptiPrep gradient (prepared in Buffer IV without ions or proteinase/phosphatase inhibitors). The gradients were centrifuged in a Beckman SW-28.1 rotor for 90 min at 52 000 g (4°C). A Biocomp Gradient Station (Fredericton, NB, Canada) was used to collect 24 fractions (0.67 mL) from each gradient, starting from the top. Fractions were immediately aliquoted for measurement of refractive index and protein content, and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)/Western blot, and stored at −80°C.
SDS-PAGE/Western blot
Protein samples were mixed with 4X sample loading buffer (80 mM Tris-HCl. pH 6.8, 8% SDS, 32% glycerol, and 0.12% bromophenol blue) supplemented with either water or the reducing agent tris(2-carboxyethyl)phosphine hydrochloride (TCEP), heated for 10 min (70°C), and upon cooling, benchtop centrifuged at maximum speed for 5 min (21°C), separated by SDS-PAGE on 10-, 15- or 26-well Criterion TGX 7.5% or 4–20% precast gels using Tris-Glycine-SDS running buffer, and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes using Genie Electroblotters (Idea Scientific, Minneapolis, MN, USA). Blots were blocked for 1 h at 21°C in 5% non-fat milk in 0.1% Tween-20-TBS buffer, and probed overnight at 4°C with primary antibodies to P-glycoprotein, caveolin-1, pY14caveolin-1, Mrp4 and nucleoporin (1 : 1000). After washing, the blots were incubated for 1.5 h with the appropriate horseradish peroxidase-conjugated secondary antibodies (1 : 2000). Antibody specificity was routinely verified by control experiments in which the primary antibody was omitted and only secondary antibody was used. Protein bands were detected using Western Lightning Chemiluminescence Reagent Plus and blue autoradiography film; a series of exposures were taken to ensure that non-saturated blots were used for quantification. Blot images were electronically scanned at a resolution of 1200 dpi using an Epson Perfection 610 Scanner (Epson America, Inc., Long Beach, CA, USA) Mean pixel density of each band was determined using Adobe Photoshop and Image J software (Wayne Rasband, Research Services Branch, National Institutes of Mental Health, Bethesda, MD, USA).
Measurement of ATPase activity
Quadruplicate 20-μL aliquots from selected density gradient fractions were assayed for verapamil-stimulated P-glycoprotein-dependent ATPase activity measured using a Pgp-Glo Assay System from Promega (Madison, WI, USA) according to the manufacturer’s instructions.
Confocal microscopy
Immunofluorescence of rat brain microvessels (isolated as described above) was performed as previously described (Lochhead et al. 2010). Briefly, brain microvessels were incubated on glass slides at 21°C for 15 min to enable vessel adherence. This was followed by fixation in 4% paraformaldehyde for 10 min. The slides were blocked in 2% goat serum and 1% bovine serum albumin before incubation in mouse monoclonal anti-P-glycoprotein antibody C219 (1 : 100) and either rabbit polyclonal antibody directed against caveolin-1 (1 : 1000) or rabbit polyclonal antibody directed against pY14-caveolin-1 (1 : 100). Slides were incubated in the presence of both primary antibodies for 90 min at 21°C. All slides were then incubated with appropriate AlexaFluor-conjugated secondary antibodies (i.e., AlexaFluor 488 goat anti-mouse IgG, AlexaFluor 568 goat anti-rabbit IgG; Invitrogen, Carlsbad, CA, USA) for 60 min at 21°C in the dark. All slides from control and treated rats were collected and processed in parallel. Primary antibody was omitted from slides in each treatment group as a negative control. All slides were imaged sequentially using a Zeiss LSM 510 meta-NLO confocal microscope (Zeiss, Oberkochen, Germany) using 40X or 100X oil-immersion objective lenses. Filters were appropriately set to avoid bleed-through and to enhance the signal-to-noise ratio for each fluorophore. Images were obtained with a resolution of 2048 × 2048 and a pixel size of 0.11 μm (for images acquired using a 40X lens) or 0.044 μm (for images acquired using a 100X lens). For colocalization analysis, we utilized Manders’ overlap coefficient because fluorescence intensities between P-glycoprotein and caveolin-1, and between P-glycoprotein and pY14-caveolin-1, are likely to differ. Manders’ overlap coefficients, which indicate actual overlap of fluorescence signals and represent the true degree of colocalization, were calculated using LSM Image Browser software (Carl Zeiss, Hamburg, Germany). Manders’ coefficient ranged from 0 (non-overlapping images) to 1.0 (100% colocalization) between the two fluorophores. Background correction settings were the same for all images acquired to ensure that colocalization data could be accurately compared between treatment groups.
Statistical analysis
Data are expressed as mean ± SE and analyzed using Student’s t-test. Significance was defined as p < 0.05.
Results
Characterization of λ-carrageenan peripheral inflammatory pain model
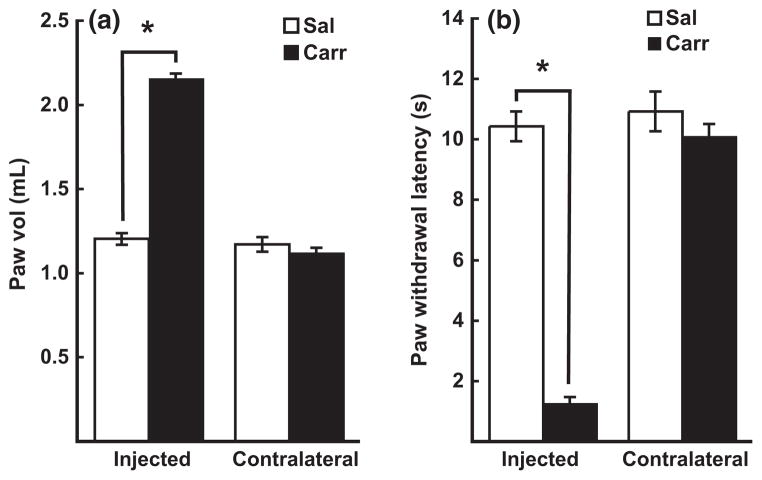
Using in situ brain perfusion, we previously showed that P-glycoprotein efflux of morphine increases 3 h after onset of λ-carrageenan-induced peripheral inflammatory hyperalgesia (Seelbach et al. 2007). In this study, we used identical animal treatment conditions for induction of peripheral inflammatory pain, to determine if these λ-carrageenan-induced increases in P-glycoprotein efflux function are associated with dynamic changes in intracellular trafficking of P-glycoprotein at the BBB. To confirm that injection of λ-carrageenan into the plantar surface of the rat hind paw evoked the expected localized inflammatory response and associated hyperalgesia (Huber et al. 2002; Ibuki et al. 2003), paw edema (indicator of inflammation), and thermal hyperalgesia (pain perceived via nociception), were measured using plethysmography and the Hargreaves radiant heat test (Hargreaves et al. 1988), respectively. Significant increases in paw swelling (Fig. 1a) and thermal hyperalgesia (Fig. 1b) were observed only in the hind paw that was injected with λ-carrageenan. No changes in either paw swelling or paw withdrawal latency were detected in either hind paw of saline-injected animals, or in the contralateral paw of λ-carrageenan-injected animals. Collectively, these data confirmed that the λ-carrageenan pain model used in this study produced a reproducible and consistent inflammatory hyperalgesia specifically restricted to the right hind paw. Furthermore, the behavioral and physiological data obtained for animals used in this study corresponded to that reported in our previous study in which in situ brain perfusions showed increased P-glycoprotein function in vivo at the BBB after λ-carrageenan treatment (Seelbach et al. 2007).
Fig. 1.
Physiological and behavioral characterization of peripheral inflammatory pain model. Female Sprague–Dawley rats received either saline or λ-carrageenan (3% in saline) into their right hind paws. Three hours post-injection, paw edema (a measure of inflammation) was determined by plethysmography, and paw withdrawal latency (a measure of inflammation-induced pain perceived by nociception) was measured using the Hargreaves radiant heat test. Injection of λ-carrageenan produced significant changes in (a) paw edema and (b) paw withdrawal latency. No changes in paw edema or paw withdrawal latency were observed in either the contralateral paw of λ-carrageenan-injected animals, or in the hind paws of saline-injected animals. Bar graphs show mean ± SE; n ≥ 20 per group, *p < 0.001. Sal, saline; Carr, λ-carrageenan.
Effect of λ-carrageenan on P-glycoprotein colocalization with caveolin-1 in isolated cerebral microvessels
To provide a global assessment of P-glycoprotein localization at the BBB, intact cerebral microvessels isolated from saline- and λ-carrageenan-injected animals were analyzed using confocal microscopy. The effect of λ-carrageenan on P-glycoprotein localization was characterized with respect to caveolin-1, a key scaffolding/trafficking protein that colocalizes with P-glycoprotein at the luminal membrane of brain microvessels (Demeule et al. 2000; Virgintino et al. 2002). Phosphorylation of caveolin-1 at tyrosine-14 in vitro in rat brain endothelial cells promotes its physical interaction with P-glycoprotein. This negatively regulates P-glycoprotein transport activity (Barakat et al. 2007, 2008). Therefore, in this study, double immunolabeling was performed. Micro-vessels from brains of either saline- or λ-carrageenan-injected animals were probed (side-by-side on the same slides) with antibodies directed against P-glycoprotein and caveolin-1 (Fig. 2a), or against P-glycoprotein and pY14caveolin-1 (Fig. 2b). Quantitative colocalization analysis was performed, in which the degree of protein overlap was determined by calculating a Manders’ colocalization coefficient, ranging from 0 (no overlap) to 1 (100% colocalization) (Bolte and Cordelieres 2006). The Manders’ coefficients for colocalization of P-glycoprotein and caveolin-1 in whole brain microvessels from saline- and λ-carrageenan-treated animals were 0.417 ± 0.038 and 0.203 ± 0.023, respectively (Fig. 2c). These data showed that under non-pain conditions there was a substantial degree of P-glycoprotein and caveolin-1 colocalization within cerebral microvessels, and that λ-carrageenan-induced peripheral inflammatory pain reduced this by 51.3%. In contrast, Manders’ coefficients for P-glycoprotein and pY14caveolin-1 in whole brain microvessels from saline- and λ-carrageenan-treated animals were calculated to be 0.216 ± 0.042 and 0.511 ± 0.036, respectively (Fig. 2c). These data demonstrated that hind paw injection of λ-carrageenan induced a 136.6% increase in colocalization of P-glycoprotein and pY14caveolin-1 at the BBB. Tyrosine-14-phosphorylated caveolin-1 comprises a very small proportion of the total caveolin-1 pool, estimated in NIH-3T3 cells to be less than 1% (del Pozo et al. 2005). In summary, our colocalization data demonstrate that peripheral inflammatory hyperalgesia differentially promoted significant changes in the colocalization within brain microvessels of P-glycoprotein with both caveolin-1, and the fraction of total caveolin that was phosphorylated at tyrosine-14.
Fig. 2.
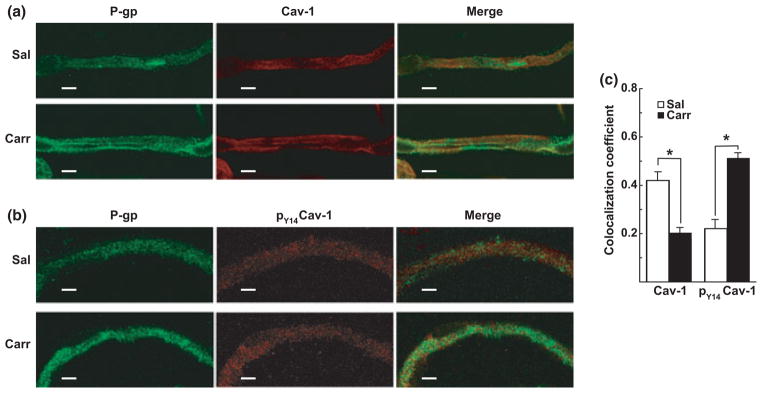
λ-Carrageenan modulated the colocalization of P-glycoprotein and caveolin-1. Intact cerebral microvessels isolated from saline- or λ-carrageenan-injected rats were imaged by confocal microscopy following immunostaining for (a) P-glycoprotein (P-gp, green) and caveolin-1 (cav-1, red), and (b) P-glycoprotein (green) and tyrosine-14 phosphorylated caveolin-1 (pY14caveolin-1) (pY14cav-1, red). Manders’ colocalization coefficients, determined using 50 randomly chosen microvessels from each treatment group, revealed that λ-carrageenan treatment promoted a decrease in P-glycoprotein and caveolin-1 colocalization, and an increase in P-glycoprotein and pY14caveolin-1 colocalization (c). Bar graphs show mean ± SE; n = 50 microvessels per group, *p < 0.001. Sal, saline; Carr, λ-carrageenan. Scale bar = 5 μm.
Effect of λ-carrageenan on P-glycoprotein cofractionation with caveolin-1-enriched membrane domains
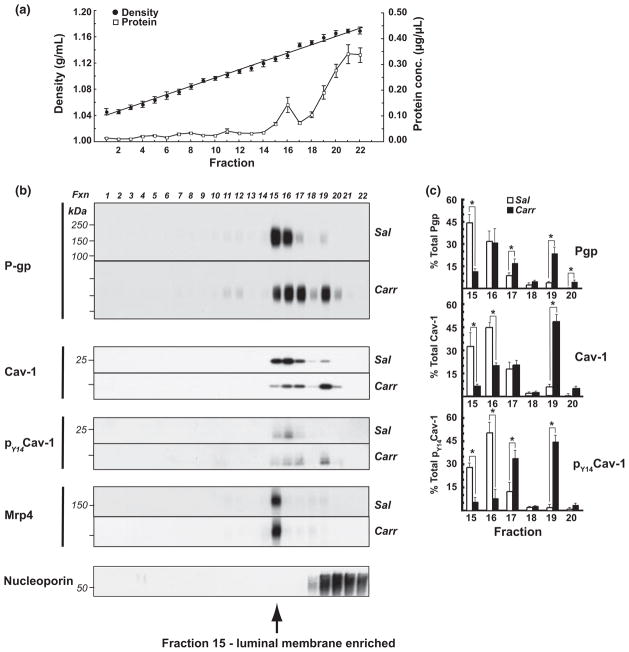
To investigate the effect of peripheral inflammatory hyperalgesia on the association of P-glycoprotein with different membrane domains within microvascular endothelial cells at the BBB, subcellular fractionation of isolated cerebral micro-vessels was performed (Macdonald and Pike 2005; McCaffrey et al. 2007, 2008, 2009; Lochhead et al. 2010). Brain microvessels were homogenized under detergent-free conditions in the presence of divalent cations to preserve native protein–protein and protein–lipid interactions. Equal volume aliquots of microvessel homogenates, containing equal amounts of protein, were adjusted to 30% OptiPrep and overlayered with a discontinuous 5/10/15/20/25% OptiPrep gradient. Following centrifugation, twenty-two 0.67 mL fractions were machine-collected from the top of the gradient and routinely assayed for refractive index (to determine fraction density, ρ) and protein content. The density gradient used in this study was previously optimized to allow detection of changes in P-glycoprotein cofractionation with membrane domains of steadily increasing density encompassing both low-density plasma membrane lipid rafts and caveolin-enriched membrane domains, and high-density, non-raft caveolin-enriched membrane domains (data not shown).
Fractionation of cerebral microvessels from saline- and λ-carrageenan-injected animals produced gradients with similar density and protein profiles (Fig. 3a). Fraction densities rose linearly from 1.046 g/mL (top of gradient, fraction 1) to 1.175 g/mL (bottom of gradient, fraction 22). Protein profiles for density gradients of fractionated cerebral microvessels obtained from saline- and λ-carrageenan-treated animals were similar, showing a lesser amount of protein in the low-density fractions and the bulk of protein at the bottom of the gradient, as expected. Equal volume aliquots of gradient fractions were subjected to Laemmli TGX 4–20% SDS-PAGE under reducing conditions to identify changes in protein cofractionation with membrane domains of different density. Western blots were probed using antibodies to P-glycoprotein, caveolin-1, and pY14-caveolin-1 (Fig. 3b), and band densities for each protein were calculated as a percentage of the sum of band densities across the gradient (Fig. 3c). Density gradients were screened using ‘equal volume’ aliquots of each fraction, as opposed to ‘equal protein’ aliquots, to avoid overestimation of proteins associated with low-density, lipid-enriched membrane domains (Rudajev et al. 2005). Moreover, pairs of SDS-PAGE gels (one gel loaded with samples from saline-injected animals, and one gel loaded with samples from λ-carrageenan-injected animals) were routinely electrophoresed in the same apparatus, and blotted side-by-side onto the same PVDF membrane, to ensure identical conditions for electrophoresis, blotting, and antibody probing.
Fig. 3.
λ-Carrageenan modulated P-glycoprotein trafficking. Intact cerebral microvessels from either saline- or λ-carrageenan-injected rats were homogenized in a neutral pH, detergent-free buffer (containing MgCl2 and CaCl2 to stabilize lipid raft structures), and fractionated in a discontinuous 5/10/15/20/25/30% OptiPrep gradient (in the absence of MgCl2 and CaCl2). Fractions (0.67 mL) were machine-collected from the top of the gradient. (a) Mean densities ± SE (circles) and protein distribution ± SE (squares) for all gradients. Western blots of the first 22 fractions from representative gradients (for each animal treatment) for (b) P-glycoprotein (P-gp), caveolin-1 (cav-1), and pY14caveolin-1 (pY14cav-1). Samples were electrophoresed using Laemmli TGX 4–20% SDS-PAGE gels under reducing conditions. (c) Bar graphs showing changes in protein band density as a percentage of the total immunostaining across the gradient for P-glycoprotein, caveolin-1, and pY14caveolin-1, as determined by densitometry using NIH Image J software. Data are from at least three separate gradients (per animal treatment), each performed on a different day (n = 12–24 rats), *p < 0.05. Sal, saline; Carr, λ-carrageenan.
Fractionation of cerebral microvessels from saline-injected animals revealed that, under normal conditions, the majority of P-glycoprotein immunostaining (77%) was associated with membrane domains of a narrowly defined density range (ρ = 1.127–1.132 g/mL, fractions 15 and 16). In these blots, a greater amount of P-glycoprotein immunostaining was typically associated with the lower density fraction 15 than with the higher density fraction 16 (45% vs. 32% of total P-glycoprotein immunostaining). Lesser amounts of P-glycoprotein were also detected in other parts of the gradient, in both higher density membrane domains and lower density membrane domains. Hind paw injection of λ-carrageenan dramatically altered the P-glycoprotein fractionation profile, most extensively within fractions 15–20, promoting a significant decrease in P-glycoprotein in fraction 15 (from 45% to 12%), and significant increases in P-glycoprotein in fraction 17 (from 9% to 17%), fraction 19 (from 4% to 24%), and fraction 20 (from 0.4% to 5%). Increased amounts of P-glycoprotein immunostaining were also discernible in lower density membrane domains upon λ-carrageenan-treatment, and this was especially evident upon longer film exposure (data not shown). Collectively, these data revealed that the previously characterized increase in P-glycoprotein efflux activity at the BBB 3 h following the onset of peripheral inflammatory pain (Seelbach et al. 2007) was associated with a dynamic redistribution of P-glycoprotein between micro-vascular endothelial cell subcellular compartments.
Analysis of the fractionation profiles of caveolin-1 obtained using cerebral microvessels from saline-treated animals showed that, similar to P-glycoprotein, the bulk of this scaffolding/trafficking protein was associated with just two fractions (15 and 16). However, in contrast to P-glycoprotein, maximum immunostaining for caveolin-1 typically was evident in the higher density fraction 16 (44%) with a lesser, but still prominent, amount of immunostaining (32%) associated with the lower density fraction 15. Injection of λ-carrageenan in the hind paw promoted a distinct alteration in the caveolin-1 fractionation profile, causing a significant decrease in fraction 15 (from 32% to 6%, a significant decrease in fraction 16 (from 44% to 20%) and a significant increase in the higher density fraction 19 (from 6% to 47%). Tyrosine-14-phosphorylated caveolin-1 (pY14-caveolin-1) in fractionated cerebral microvessels from saline-treated animals was also predominantly found in fraction 16 (51%), with the lower density fraction 15 containing 28% of the total immunostaining for this protein. λ-Carrageenan-injection promoted significant decreases in immunostaining for pY14-caveolin-1 in fraction 15 (from 28% to 6%) and fraction 16 (from 51% to 8%), and significant increases in fraction 17 (from 13% to 34%) and fraction 19 (from 2% to 45%). Comparison of relative increases/decreases in protein band densities for all three proteins revealed that the extent of trafficking changes of subcellular compartments was not identically modulated by λ-carrageenan-injection. To provide additional characterization of the OptiPrep gradient used in this study, Western blots of gradient fractions were also probed for Mrp4 (ABCC4) and nucleoporin. The brain efflux transporter Mrp4 localizes to the microvascular endothelial cell luminal membrane (Leggas et al. 2004; Nies et al. 2004). In human coronary artery smooth muscle cells, Mrp4 associates primarily with caveolin-1-enriched membrane domains (Sassi et al. 2008). In cerebral microvessels isolated from saline-injected animals, we found that Mrp4 cofractionated with the bulk of P-glycoprotein in fraction 15, suggesting that a considerable quantity of immunodetectable P-glycoprotein in brain endothelial cells is localized to the luminal plasma membrane. However, unlike P-glycoprotein, Mrp4 trafficking remained unchanged following hind paw injection of λ-carrageenan, although there was a non-significant trend for a slight increase in immunostaining for this efflux transporter. Immunostaining for the nuclear membrane marker nucleoporin was associated with high-density membrane domains, fractions 19–22, at the bottom of the gradient.
Effect of λ-carrageenan on integrity of high molecular weight, disulfide-bonded P-glycoprotein-containing complexes
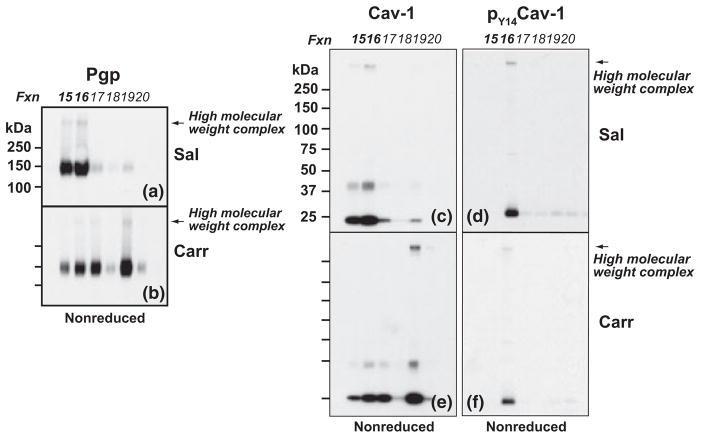
To investigate if high molecular weight protein complexes containing P-glycoprotein were present in density gradient fractions, equal volume aliquots of density gradient fractions for each treatment condition were subjected to either reducing or non-reducing SDS-PAGE/Western blot analysis. Electrophoresis was performed using a variety of gel compositions (Laemmli TGX 7.5%, 4–15% and 4–20%; Bis-Tris 10%, 4–12%; Tris-acetate 3–8%) to select optimal conditions for detecting high molecular weight, P-glycoprotein-containing protein complexes within density gradient fractions, and to facilitate comparative analysis of our data with previously reported studies examining high molecular weight P-glycoprotein structures (Poruchynsky and Ling 1994; Jodoin et al. 2003). Moreover, non-reducing and reducing SDS-PAGE, versus native PAGE alone, allowed determination of the susceptibility to disulfide-bond reduction of high molecular weight P-glycoprotein-containing structures. Studies using human wild-type P-glycoprotein expressed in P. pastoris show that, in the absence of reducing agent, higher order structures spontaneously form. However, in the presence of reducing agent, oligomeric structures disassemble and ATPase activity doubles (Urbatsch et al. 2001). In preliminary experiments (data not shown), Western blot screenings of density gradient fractions 1–22 subjected to non-reducing SDS-PAGE using Laemmli TGX, Bis-Tris, and Tris-acetate gels, demonstrated that, as a group, samples from saline- and λ-carrageenan-injected animals exhibited high molecular weight P-glycoprotein-containing structures only within the density range encompassed by fractions 15–19. Sharp resolution of the high molecular weight (> 250 kDa) protein band immunostained with the C219 antibody for P-glycoprotein was obtained using electrophoresis with Laemmli TGX 7.5% gels, and the validity of this protein complex was confirmed with two additional antibodies: Abbiotec 250820 (directed to the N-terminus of P-glycoprotein) and Santa Cruz sc-1517 (directed to the C-terminus of P-glycoprotein). Therefore, to directly compare effects of reducing agent, and of experimental animal treatment, on the intensity of high molecular weight P-glycoprotein-containing structures, sets of non-reducing and reducing Laemmli TGX 7.5% SDS-PAGE gels (loaded with samples from 15 to 20 from both saline- and λ-carrageenan-injected animals) were electrophoresed in the same apparatus, blotted side-by-side onto the same PVDF membrane and probed using the C219 antibody (Fig. 4).
Fig. 4.
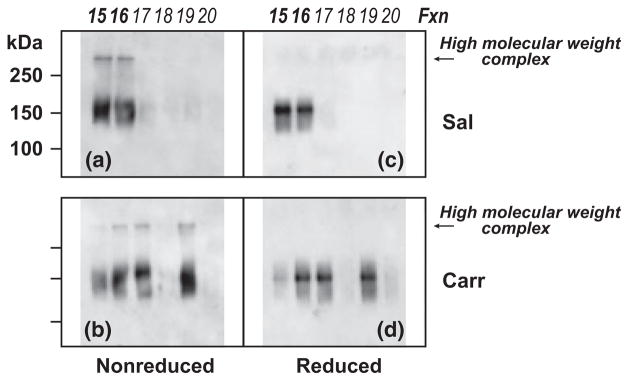
λ-Carrageenan modulated integrity of high molecular weight, disulfide-bonded P-glycoprotein-containing complexes. Intact cerebral microvessels from either saline- or λ-carrageenan-injected rats were homogenized in a neutral pH, detergent-free buffer (containing MgCl2 and CaCl2 to stabilize lipid raft structures), and fractionated in a discontinuous 5/10/15/20/25/30% OptiPrep gradient (in the absence of MgCl2 and CaCl2). Fractions (0.67 mL) were machine-collected from the top of the gradient. Western blots of fractions 15–20 from representative gradients (for each animal treatment) probed for P-glycoprotein. Samples from control (a, c) and experimental (b, d) animals were electrophoresed using Laemmli TGX 7.5% SDS-PAGE gels under non-reducing (a, b) and reducing (c, d) conditions. Sal, saline; Carr, λ-carrageenan.
Prominent staining for high molecular weight P-glycoprotein-containing structures (> 250 kDa) was evident only in the lower density caveolin-1-enriched membrane domains (fractions 15 and 16) from fractionated cerebral microvessels from saline-injected animals (Fig. 4a, non-reduced blot). λ-Carrageenan-injection dramatically reduced the amount of high molecular weight P-glycoprotein-containing structures in the low-density fraction 15 (Fig. 4b, non-reduced blot), shown to be enriched with the endothelial luminal membrane marker Mrp4 (Fig. 3b). High molecular weight P-glycoprotein-containing structures present in samples from both saline- and λ-carrageenan-injected animals could be reduced upon exposure of fraction aliquots to a hydrophilic reducing agent (Fig. 4c and d, reduced blots), indicating that the disulfide bonds stabilizing the high molecular weight complexes were readily accessible to the external environment. Collectively, these data revealed the presence, under non-pain conditions, of high molecular weight, disulfide-bonded P-glycoprotein-containing structures that cofractionate with a subset of caveolin-1-enriched membrane domains within microvascular endothelial cells at the BBB. Moreover, these data demonstrated that hind paw injection of λ-carrageenan disrupted the integrity of high molecular weight P-glycoprotein-containing structures that cofractionate with endothelial luminal membrane domains, and suggested that disulfide-bond reduction was involved.
Effect of λ-carrageenan on high molecular weight P-glycoprotein-containing and caveolin-1-containing complexes
Although sharp resolution of high molecular weight P-glycoprotein-containing structures was obtained using Laemmli TGX 7.5% gels, these gels were not as effective as the broader span 4–20% TGX gels in resolving high molecular weight complexes containing caveolin-1/pY14-caveolin-1 (data not shown). Therefore, to directly compare the effect of hind paw injection of λ-carrageenan on the extent of immunostaining for P-glycoprotein and caveolin-1 with high molecular weight structures associated with membrane domains present in the same density gradient sample, SDS-PAGE using 4–20% TGX gels was performed under non-reducing conditions wherein pairs of gels (one loaded with samples from saline-injected animals, and the other with samples from λ-carrageenan-injected animals) were electrophoresed in the same apparatus, blotted on the same PVDF membrane, and probed for pY14-caveolin-1, caveolin-1, and P-glycoprotein (Fig. 5).
Fig. 5.
λ-Carrageenan modulated integrity of high molecular weight P-glycoprotein-containing and caveolin-1-containing complexes. Intact cerebral microvessels from either saline- or λ-carrageenan-injected rats were homogenized in a neutral pH, detergent-free buffer (containing MgCl2 and CaCl2 to stabilize lipid raft structures), and fractionated in a discontinuous 5/10/15/20/25/30% OptiPrep gradient (in the absence of MgCl2 and CaCl2). Fractions (0.67 mL) were machine-collected from the top of the gradient. Samples were electrophoresed using Laemmli TGX 7.5% SDS-PAGE gels under non-reducing conditions. Western blots of fractions 15–20 from representative gradients (for each animal treatment) were probed for P-glycoprotein (P-gp) (a, b), caveolin-1 (cav-1) (c, e), and pY14caveolin-1 (pY14cav-1) (d, f). Sal, saline; Carr, λ-carrageenan.
In non-reduced density gradient samples from saline-injected animals, immunostaining for high molecular weight (> 250 kDa) structures containing P-glycoprotein was detected only in membrane fractions 15 and 16 (Fig. 5a). The absence of high molecular weight structures in fraction 19, which has considerably more protein than lower density fractions 15 and 16 (Fig. 3a), confirms that the appearance of higher mass bands in other fractions was not because of heavier sample loading. Blots of identical samples also exhibited discernible immunostaining for dimeric caveolin-1 in both fractions 15 and 16, but only fraction 16, which displayed the greater amount of dimeric caveolin-1, was found to contain appreciable immunostaining for high molecular weight caveolin-1-containing structures (Fig. 5c). Immunostaining for pY14-caveolin-1 was only detected in fraction 16 on these non-reduced blots from saline-injected animals (Fig. 5d). Hind paw injection of λ-carrageenan dramatically reduced the amount of high molecular weight structures containing caveolin-1 and pY14-caveolin-1 in fraction 16 (Fig. 5b, e and f).
Effect of λ-carrageenan on P-glycoprotein-ATPase activity within caveolin-1-enriched membrane domains
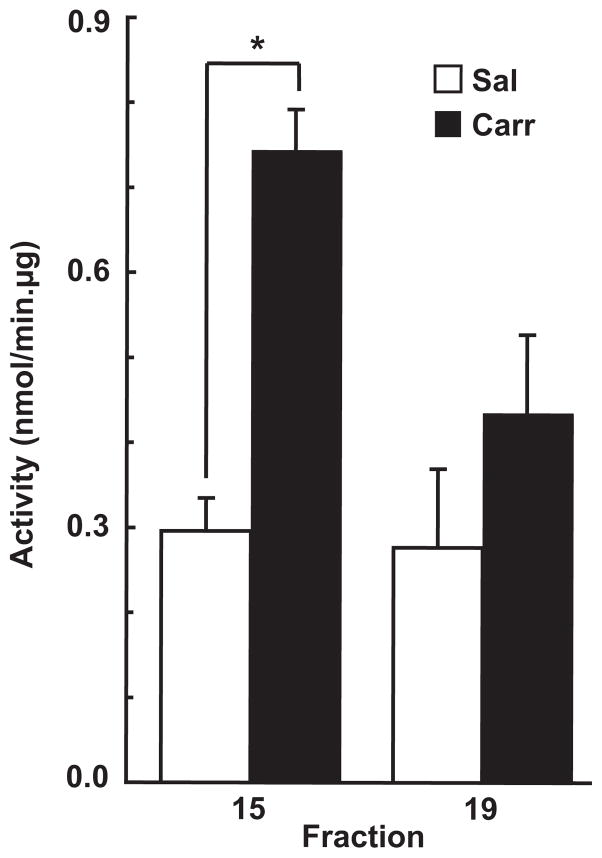
To determine if the disassembly of high molecular weight P-glycoprotein-containing structures within microvascular endothelial luminal membrane domains was accompanied by an increase in drug-transport capability of P-glycoprotein, density gradient samples from fraction15 (enriched with plasma membrane luminal domains) and from fraction 19 (enriched with nuclear membrane domains) were assayed for verapamil-stimulated P-glycoprotein-dependent ATPase activity. As shown in Fig. 6, hind paw injection of λ-carrageenan significantly increased the ATPase activity of P-glycoprotein associated with endothelial luminal membrane domains.
Fig. 6.
λ-Carrageenan modulated luminal membrane P-glycoprotein-ATPase activity. Intact cerebral microvessels from either saline- or λ-carrageenan-injected rats were homogenized in a neutral pH, detergent-free buffer (containing MgCl2 and CaCl2 to stabilize lipid raft structures), and fractionated in a discontinuous 5/10/15/20/25/30% OptiPrep gradient (in the absence of MgCl2 and CaCl2). Fractions (0.67 mL) were machine-collected from the top of the gradient. Quadruplicate 20-μL aliquots from density gradient fraction 15 (enriched with plasma membrane luminal domains) and fraction 19 (enriched with nuclear membrane domains) were assayed for verapamil-stimulated P-glycoprotein-dependent ATPase activity. Data are from at least three separate gradients (per animal treatment), each performed on a different day (n = 12–24 rats), *p < 0.05. Sal, saline; Carr, λ-carrageenan.
Discussion
In this study, we investigated the effect of peripheral inflammatory pain on P-glycoprotein trafficking at the BBB in vivo. Our hypothesis was that a dynamic redistribution of P-glycoprotein between subcellular compartments of micro-vascular endothelial cells was associated with the identical stressor (injection of λ-carrageenan into the hind paw) that we previously reported promotes a 40% increase in P-glycoprotein expression, a rapid increase in brain efflux of morphine by P-glycoprotein, and a corresponding decrease in morphine-induced analgesia (Seelbach et al. 2007). Although an increase in protein expression detected by Western blot analysis may reflect an actual increase in protein content because of an increase in transcription, it may also reflect a change in protein conformation wherein the antigenic site is made more accessible to the probing antibody. Such a change in conformation conceivably could occur following disassociation of monomeric P-glycoprotein from incorporation within a high molecular weight protein complex. A lack of correlation between not only mRNA and protein levels, but also between either mRNA or protein levels and activity, has been reported in many studies on P-glycoprotein analyzing the effect of inflammatory mediators or hypoxia (Robertson et al. 2009; von Wedel-Parlow et al. 2009; Pan et al. 2010; Poller et al. 2010). These studies emphasize the importance of analyzing trafficking and post-translational forms of regulation in the study of P-glycoprotein. Our current data show injection of λ-carrageenan induced, after 3 h, a localized, peripheral inflammatory pain (i.e., hyperalgesia) that was accompanied by a dramatic alteration in P-glycoprotein trafficking at the BBB. Quantitative colocalization analysis of isolated, intact cerebral microvessels demonstrated that hind paw injection of λ-carrageenan differentially promoted significant changes in the colocalization of P-glycoprotein with both caveolin-1 and tyrosine-14-phosphorylated caveolin-1 (pY14-caveolin-1). Subcellular fractionation of isolated cerebral microvessels, combined with non-reducing and reducing SDS-PAGE and Western blot analysis of density gradient samples, revealed a constitutive sequestration of P-glycoprotein to membrane domains of a narrowly defined density that facilitated incorporation of P-glycoprotein within high molecular weight (> 250 kDa) disulfide-bonded structures. These P-glycoprotein-rich domains cofractionated with membrane domains enriched in monomeric caveolin-1 and pY14-caveolin-1, and with high molecular weight, disulfide-bonded structures incorporating both caveolin-1 and pY14-caveolin-1. Furthermore, λ-carrageenan-treatment of the hind paw disrupted this pattern of P-glycoprotein sequestration. An important consequence of the extensive and complex changes in P-glycoprotein trafficking induced by peripheral inflammatory hyperalgesia was a significant increase in drug-stimulated ATPase activity by P-glycoprotein associated with membrane domains that cofractionated with membrane domains enriched with Mrp4, a marker of cerebral micro-vascular endothelial luminal membranes. Activation of P-glycoprotein-associated ATPase activity in the presence of a drug substrate has been previously shown to correlate with this transporter’s ability to translocate drugs across biological membranes (Gannon et al. 2009; Loo et al. 2010; Wei et al. 2011). Furthermore, the use of P-glycoprotein-associated ATPase activity as a means of assessing the potential for functionally active P-glycoprotein is particularly appropriate when analyzing subcellular fractions containing membrane vesicles (Sharom et al. 1999; Barakat et al. 2005; Lespine et al. 2007).
To examine P-glycoprotein trafficking at the BBB, we used confocal microscopy to provide a global assessment of P-glycoprotein localization within isolated, intact cerebral microvessels, and detergent-free, density gradient fractionation of cerebral microvessel homogenates to provide samples for a biochemical examination of P-glycoprotein structure and ATPase activity associated with different subcellular compartments. Important criteria for concluding a protein has undergone a significant trafficking alteration that we took under consideration in this study were: (a) evidence of a change in colocalization with a key trafficking protein; (b) evidence of a change in cofractionation of different isoforms of the protein (e.g., monomers, oligomers) with membrane domains of different densities; and (c) evidence of a change in protein functional activity associated with a membrane domain exhibiting altered protein content and/or higher order structure.
The main conclusion of the current work is that peripheral inflammatory pain promoted a significant change in P-glycoprotein trafficking at the BBB. This was based on the following principal observations: (a) quantitative colocalization analysis of intact cerebral microvessels showed significant changes in colocalization of P-glycoprotein and caveolin-1, and of P-glycoprotein and pY14-caveolin-1; (b) screening of density gradient fractions by reducing SDS-PAGE/Western blot revealed significant changes in the distribution of P-glycoprotein, caveolin-1 and pY14-caveolin-1 between membrane domains of different density; and (c) P-glycoprotein ATPase activity was increased in membrane domains that showed a dramatic reduction in high molecular weight P-glycoprotein-containing structures, and that cofractionated with membrane domains enriched in the endothelial luminal membrane transporter Mrp4.
Evidence that the constitutive trafficking of P-glycoprotein at the BBB involved its sequestration in a storage pool, and that dynamic modulation of P-glycoprotein sequestration contributed to the rapid increase in P-glycoprotein activity following the onset of peripheral inflammatory hyperalgesia, was provided by the following observations: (a) Quantitative microscopic colocalization data from intact microvessels revealed that during steady state there was 41.7% colocalization between staining for P-glycoprotein and for caveolin-1. (b) SDS-PAGE/Western blot analysis of density gradient samples from saline-injected animals showed that membrane domains enriched with P-glycoprotein cofractionated at the same density as membrane domains enriched with caveolin-1. These observations are in agreement with previous studies showing colocalization between these two proteins in cerebral microvessels under normal conditions (Demeule et al. 2000; Virgintino et al. 2002; Bendayan et al. 2006; Guo et al. 2010). Moreover, they are consistent with the fact that P-glycoprotein contains within its N-terminus a binding motif for caveolin-1 through which it physically interacts with caveolin-1 (Jodoin et al. 2003). (c) Quantitative microscopic examination showed that during steady state there was also approximately 21.6% colocalization of staining for P-glycoprotein and for pY14-caveolin-1. These data are consistent with the fact that tyrosine-14-phosphorylation of caveolin-1 promotes the interaction of P-glycoprotein with caveolin-1 (Barakat et al. 2007). (d) Density gradient fractionation profiles showed that under normal conditions, although P-glycoprotein could be detected across the density gradient, the bulk of P-glycoprotein was associated with plasma membrane domains of narrow density range, which cofractionated with membrane domains enriched in high molecular weight, disulfide-bonded structures containing P-glycoprotein, caveolin-1, and pY14-caveolin-1. These observations agree with previous studies showing P-glycoprotein is located not only at the plasma membrane but also in numerous subcellular locations including the nuclear envelope, cytoplasmic vesicles, Golgi complex, and rough endoplasmic reticulum (Bendayan et al. 2006). They are also consistent with the possibility that in a storage pool, the assembly of high molecular weight protein complexes could be used to increase the density of protein packing. Denser packing within a specific membrane domain would also allow a greater amount of stored P-glycoprotein to be released upon a particular signal. In addition, P-glycoprotein is reported to have a relatively long half-life (Yoshimura et al. 1989; Muller et al. 1995; Wei et al. 2011) and incorporation of this transporter within a high molecular weight structure may facilitate this by protecting it from proteolysis. (e) Quantitative microscopic colocalization data revealed that λ-carrageenan-treatment decreased the global colocalization of P-glycoprotein with caveolin-1 by approximately half within intact cerebral microvessels. This observation is in agreement with our previously reported finding of increased P-glycoprotein function following the onset of peripheral inflammatory hyperalgesia (Seelbach et al. 2007), and the fact that the binding of P-glycoprotein to caveolin-1 negatively affects P-glycoprotein function (Barakat et al. 2007). Consistent with this finding, density gradient fractionation profiles showed that the onset of peripheral inflammatory hyperalgesia was accompanied by a dramatic reduction of high molecular weight structures identified with antibodies to P-glycoprotein, caveolin-1, and pY14-caveolin-1, and a significant increase in P-glycoprotein ATPase activity, within the membrane domains in which these proteins are sequestered under normal conditions. The finding that high molecular weight structures incorporating P-glycoprotein, caveolin-1, and pY14-caveolin-1 are disassembled by disulfide-bond reduction reveals their susceptibility to a change in oxidative stress. Caveolin-1 oligomerization does not involve the formation of disulfide bonds between caveolin-1 molecules (Sargiacomo et al. 1995). However, disulfide bonding amongst the proteins to which caveolin-1 binds may indirectly influence its incorporation into high molecular weight structures. P-glycoprotein is capable of forming disulfide-bonded, higher order structures, and P-glycoprotein activity is potentiated by treatment with a reducing agent, suggesting that disulfide bonds may perform an inhibitory role (Loo and Clarke 2000; Urbatsch et al. 2001). Because the minimal functional unit of P-glycoprotein is believed to be a monomer (Loo and Clarke 1996), the disassembly of high molecular structures containing P-glycoprotein would be expected to coincide with an increase in P-glycoprotein activity.
The importance of P-glycoprotein as an obstacle to be overcome in drug delivery to the brain and CNS is underscored by the fact that this transporter is the preeminent molecular cause for pre-clinical and clinical drug failure (Miller et al. 2008). Microvessels at the BBB contain the highest levels of P-glycoprotein within the body (Cordon-Cardo et al. 1989). Enrichment of P-glycoprotein at the luminal microvascular endothelial membrane (Beaulieu et al. 1997; Virgintino et al. 2002), combined with evidence from in vivo dosing studies that brain uptake of P-glycoprotein substrates is substantially increased in P-glycoprotein knockout animals (Schinkel et al. 1994; Wang et al. 2004), strongly suggest that the primary biological role of this transporter is to act as “gatekeeper” to restrict entry into the brain and CNS of toxic substances and xenobiotics present in the systemic circulation. Evolutionary selection of P-glycoprotein to perform a critical “gatekeeper” role at the BBB implies development of regulatory mechanisms to control the expression and trafficking of P-glycoprotein to ensure that rapid increases in potentially harmful blood-borne substances caused by diet, environmental exposure, or external stressors would be countered with rapid increases in P-glycoprotein efflux activity at the brain microvascular luminal membrane. Energetically, an efficient and economical way to achieve selective augmentation of biologically active protein at the plasma membrane would be through the creation of a nearby storage pool from which appropriate amounts of post-translationally processed, mature protein can be readily accessed. If P-glycoprotein is incorporated in a storage pool within microvascular endothelial cells, then understanding mechanisms promoting its sequestration and release could lead to identifying novel therapeutic targets for improving CNS pharmacotherapy during a disease state, which involves peripheral inflammatory pain where there is a pathological increase in P-glycoprotein function at the BBB. As an initial step in this approach, we undertook the current study with the overall aim of determining if P-glycoprotein undergoes a significant trafficking change following the onset of peripheral inflammatory hyperalgesia induced by hind paw injection of λ-carrageenan.
Evidence in this article shows clearly that protein trafficking played an important role in the increase in potentially functional P-glycoprotein at the endothelial luminal membrane during pain conditions. These pivotal findings have now engendered our current studies analyzing the biochemistry and protein–protein interactions of high molecular weight, P-glycoprotein-containing structures within caveolin-1-enriched membrane domains. Identification of therapeutic targets for maintaining the integrity of high molecular weight complexes storing P-glycoprotein would greatly enhance drug delivery to the brain during disease states such as peripheral inflammatory pain by preventing release of P-glycoprotein from subcellular stores. These results are the first observation that peripheral inflammatory pain leads to specific structural changes in P-glycoprotein responsible for controlling analgesic drug delivery to the CNS.
Acknowledgments
This work was supported by NIH grants R01-NS 39592, R01-NS42652, R01-DA12684 to TPD, and CA 09820-0251 to GM.
Abbreviations used
- BBB
blood-brain barrier
- Mrp4
multidrug resistance protein 4
- PVDF
polyvinylidene difluoride
- pY14caveolin-1
tyrosine-14-phosphorylated caveolin-1
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Footnotes
We state that all authors declare that none of the authors have a financial interest conflict related to this work.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Barakat S, Gayet L, Dayan G, Labialle S, Lazar A, Oleinikov V, Coleman AW, Baggetto LG. Multidrug-resistant cancer cells contain two populations of P-glycoprotein with differently stimulated P-gp ATPase activities: evidence from atomic force microscopy and biochemical analysis. Biochem J. 2005;388:563–571. doi: 10.1042/BJ20041999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat S, Demeule M, Pilorget A, Regina A, Gingras D, Baggetto LG, Beliveau R. Modulation of p-glycoprotein function by caveolin-1 phosphorylation. J Neurochem. 2007;101:1–8. doi: 10.1111/j.1471-4159.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- Barakat S, Turcotte S, Demeule M, Lachambre MP, Regina A, Baggetto LG, Beliveau R. Regulation of brain endothelial cells migration and angiogenesis by P-glycoprotein/ caveolin-1 interaction. Biochem Biophys Res Commun. 2008;372:440–446. doi: 10.1016/j.bbrc.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Bartels AL. Blood-brain barrier p-glycoprotein function in neurodegenerative disease. Curr Pharm Des. 2011;17:2771–2777. doi: 10.2174/138161211797440122. [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Fricker G, Miller DS. Modulation of p-glycoprotein transport function at the blood-brain barrier. Exp Biol Med (Maywood) 2005;230:118–127. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326:539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54:1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224:213–232. doi: 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- Choo EF, Kurnik D, Muszkat M, et al. Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier. J Pharmacol Exp Ther. 2006;317:1012–1018. doi: 10.1124/jpet.105.099648. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Klerk OL, Bosker FJ, Luurtsema G, Nolte IM, Dierckx R, Den Boer JA, Potschka H. The role of p-glycoprotein in psychiatric disorders: a reliable guard of the brain? Cent Nerv Syst Agents Med Chem. 2011;11:197–209. doi: 10.2174/187152411798047744. [DOI] [PubMed] [Google Scholar]
- Demeule M, Jodoin J, Gingras D, Beliveau R. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000;466:219–224. doi: 10.1016/s0014-5793(00)01087-5. [DOI] [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- Gannon MK, II, Holt JJ, Bennett SM, et al. Rhodamine inhibitors of P-glycoprotein: an amide/thioamide “switch” for ATPase activity. J Med Chem. 2009;52:3328–3341. doi: 10.1021/jm900253g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhu J, Zhao L, Luo Q, Jin X. Expression and clinical significance of multidrug resistance proteins in brain tumors. J Exp Clin Cancer Res. 2010;29:122. doi: 10.1186/1756-9966-29-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hartz AM, Bauer B. Regulation of ABC transporters at the blood-brain barrier: new targets for CNS therapy. Mol Interv. 2010;10:293–304. doi: 10.1124/mi.10.5.6. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Rigor RR, Miller DS. Rapid loss of blood-brain barrier P-glycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab. 2010;30:1593–1597. doi: 10.1038/jcbfm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JD, Hau VS, Borg L, Campos CR, Egleton RD, Davis TP. Blood-brain barrier tight junctions are altered during a 72-h exposure to lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol. 2002;283:H1531–H1537. doi: 10.1152/ajpheart.00027.2002. [DOI] [PubMed] [Google Scholar]
- Ibuki T, Matsumura K, Yamazaki Y, Nozaki T, Tanaka Y, Kobayashi S. Cyclooxygenase-2 is induced in the endothelial cells throughout the central nervous system during carrageenan-induced hind paw inflammation; its possible role in hyperalgesia. J Neurochem. 2003;86:318–328. doi: 10.1046/j.1471-4159.2003.01848.x. [DOI] [PubMed] [Google Scholar]
- Jodoin J, Demeule M, Fenart L, Cecchelli R, Farmer S, Linton KJ, Higgins CF, Beliveau R. P-glycoprotein in blood-brain barrier endothelial cells: interaction and oligomerization with caveolins. J Neurochem. 2003;87:1010–1023. doi: 10.1046/j.1471-4159.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- Kannan P, John C, Zoghbi SS, Halldin C, Gottesman MM, Innis RB, Hall MD. Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther. 2009;86:368–377. doi: 10.1038/clpt.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye SB. Reversal of drug resistance in ovarian cancer: where do we go from here? J Clin Oncol. 2008;26:2616–2618. doi: 10.1200/JCO.2008.16.2123. [DOI] [PubMed] [Google Scholar]
- Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24:7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lespine A, Martin S, Dupuy J, Roulet A, Pineau T, Orlowski S, Alvinerie M. Interaction of macrocyclic lactones with P-glycoprotein: structure-affinity relationship. Eur J Pharm Sci. 2007;30:84–94. doi: 10.1016/j.ejps.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, McCaffrey G, Quigley CE, Finch J, DeMarco KM, Nametz N, Davis TP. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30:1625–1636. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. The minimum functional unit of human P-glycoprotein appears to be a monomer. J Biol Chem. 1996;271:27488–27492. doi: 10.1074/jbc.271.44.27488. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Drug-stimulated ATPase activity of human P-glycoprotein is blocked by disulfide cross-linking between the nucleotide-binding sites. J Biol Chem. 2000;275:19435–19438. doi: 10.1074/jbc.C000222200. [DOI] [PubMed] [Google Scholar]
- Loo TW, Bartlett MC, Clarke DM. Human P-glycoprotein is active when the two halves are clamped together in the closed conformation. Biochem Biophys Res Commun. 2010;395:436–440. doi: 10.1016/j.bbrc.2010.04.057. [DOI] [PubMed] [Google Scholar]
- Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res. 2005;46:1061–1067. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. 2007;103:2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Seelbach MJ, Staatz WD, Nametz N, Quigley C, Campos CR, Brooks TA, Davis TP. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem. 2008;106:2395–2409. doi: 10.1111/j.1471-4159.2008.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, Lochhead JJ, Davis TP. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110:58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Laurent G, Ling V. P-glycoprotein stability is affected by serum deprivation and high cell density in multidrug-resistant cells. J Cell Physiol. 1995;163:538–544. doi: 10.1002/jcp.1041630314. [DOI] [PubMed] [Google Scholar]
- Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, Keppler D. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129:349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu C, Hsuchou H, Kastin AJ. The role of cerebral vascular NFkappaB in LPS-induced inflammation: differential regulation of efflux transporter and transporting cytokine receptors. Cell Physiol Biochem. 2010;25:623–630. doi: 10.1159/000315081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- Poller B, Drewe J, Krahenbuhl S, Huwyler J, Gutmann H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell Mol Neurobiol. 2010;30:63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PubMed] [Google Scholar]
- Poruchynsky MS, Ling V. Detection of oligomeric and monomeric forms of P-glycoprotein in multidrug resistant cells. Biochemistry. 1994;33:4163–4174. doi: 10.1021/bi00180a009. [DOI] [PubMed] [Google Scholar]
- Potschka H. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia. 2010a;51:1333–1347. doi: 10.1111/j.1528-1167.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- Potschka H. Targeting regulation of ABC efflux transporters in brain diseases: a novel therapeutic approach. Pharmacol Ther. 2010b;125:118–127. doi: 10.1016/j.pharmthera.2009.10.004. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-Garcia A, Anderson RG, Schwartz MA. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DJ, Goralski KB. A critical overview of the influence of inflammation and infection on P-glycoprotein expression and activity in the brain. Expert Opin Drug Metab Toxicol. 2008;4:1245–1264. doi: 10.1517/17425255.4.10.1245. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Kania KD, Hladky SB, Barrand MA. P-glycoprotein expression in immortalised rat brain endothelial cells: comparisons following exogenously applied hydrogen peroxide and after hypoxia-reoxygenation. J Neurochem. 2009;111:132–141. doi: 10.1111/j.1471-4159.2009.06306.x. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Davis TP. Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery. Ther Deliv. 2011;2:1015–1041. doi: 10.4155/tde.11.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudajev V, Novotny J, Hejnova L, Milligan G, Svoboda P. Dominant portion of thyrotropin-releasing hormone receptor is excluded from lipid domains. Detergent-resistant and detergent-sensitive pools of TRH receptor and Gqalpha/G11alpha protein. J Biochem. 2005;138:111–125. doi: 10.1093/jb/mvi114. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M, Scherer PE, Tang Z, Kubler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA. 1995;92:9407–9411. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi Y, Lipskaia L, Vandecasteele G, et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102:1677–1690. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- Sharom FJ, Yu X, Lu P, et al. Interaction of the P-glycoprotein multidrug transporter (MDR1) with high affinity peptide chemo-sensitizers in isolated membranes, reconstituted systems, and intact cells. Biochem Pharmacol. 1999;58:571–586. doi: 10.1016/s0006-2952(99)00139-2. [DOI] [PubMed] [Google Scholar]
- Sun J, He ZG, Cheng G, Wang SJ, Hao XH, Zou MJ. Multidrug resistance P-glycoprotein: crucial significance in drug disposition and interaction. Med Sci Monit. 2004;10:RA5–RA14. [PubMed] [Google Scholar]
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Ueno M, Nakagawa T, Wu B, Onodera M, Huang CL, Kusaka T, Araki N, Sakamoto H. Transporters in the brain endothelial barrier. Curr Med Chem. 2010;17:1125–1138. doi: 10.2174/092986710790827816. [DOI] [PubMed] [Google Scholar]
- Urbatsch IL, Gimi K, Wilke-Mounts S, Lerner-Marmarosh N, Rousseau ME, Gros P, Senior AE. Cysteines 431 and 1074 are responsible for inhibitory disulfide cross-linking between the two nucleotide-binding sites in human P-glycoprotein. J Biol Chem. 2001;276:26980–26987. doi: 10.1074/jbc.M010829200. [DOI] [PubMed] [Google Scholar]
- Vangilder RL, Rosen CL, Barr TL, Huber JD. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol Ther. 2011;130:239–247. doi: 10.1016/j.pharmthera.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgintino D, Robertson D, Errede M, Benagiano V, Girolamo F, Maiorano E, Roncali L, Bertossi M. Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem. 2002;50:1671–1676. doi: 10.1177/002215540205001212. [DOI] [PubMed] [Google Scholar]
- Wang JS, Taylor R, Ruan Y, Donovan JL, Markowitz JS, Lindsay De Vane C. Olanzapine penetration into brain is greater in transgenic Abcb1a P-glycoprotein-deficient mice than FVB1 (wild-type) animals. Neuropsychopharmacology. 2004;29:551–557. doi: 10.1038/sj.npp.1300372. [DOI] [PubMed] [Google Scholar]
- von Wedel-Parlow M, Wolte P, Galla HJ. Regulation of major efflux transporters under inflammatory conditions at the blood-brain barrier in vitro. J Neurochem. 2009;111:111–118. doi: 10.1111/j.1471-4159.2009.06305.x. [DOI] [PubMed] [Google Scholar]
- Wei N, Sun H, Wang F, Liu G. H1, a novel derivative of tetrandrine reverse P-glycoprotein-mediated multidrug resistance by inhibiting transport function and expression of P-glycoprotein. Cancer Chemother Pharmacol. 2011;67:1017–1025. doi: 10.1007/s00280-010-1397-7. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Kuwazuru Y, Sumizawa T, Ikeda S, Ichikawa M, Usagawa T, Akiyama S. Biosynthesis, processing and half-life of P-glycoprotein in a human multidrug-resistant KB cell. Biochim Biophys Acta. 1989;992:307–314. doi: 10.1016/0304-4165(89)90089-5. [DOI] [PubMed] [Google Scholar]