Abstract
The blood-brain barrier (BBB) is a physical and metabolic barrier that separates the CNS from the peripheral circulation. CNS drug delivery across the BBB is challenging, primarily due to the physical restriction of paracellular diffusion between the endothelial cells that comprise the microvessels of the BBB and the activity of efflux transporters that quickly expel back into the capillary lumen a wide variety of xenobiotics. Therapeutic manipulation of protein trafficking is emerging as a novel means of modulating protein function, and in this mini-review, the targeting of the trafficking of two key BBB proteins, P-glycoprotein and occludin, is presented as a novel, reversible means of optimizing CNS drug delivery.
Keywords: blood-brain barrier, CNS drug delivery, protein trafficking, protein-protein interaction, oxidative stress, peripheral inflammatory pain, P-glycoprotein, occludin
Dynamic nature of the BBB
The blood-brain barrier (BBB) is the critical boundary between the central nervous system (CNS) and the periphery. It is both friend and foe to the clinician. In safeguarding the CNS from exposure to potentially harmful substances in the systemic circulation, the BBB simultaneously presents a serious obstacle to CNS drug delivery. Anatomically, the BBB is a vast network of ~650 km of microcapillaries, each of which has a lumen diameter of ~6 mm and is encircled by a single, nonfenestrated endothelial cell whose luminal (blood-facing) and abluminal (brain-facing) membranes are separated by ~300-500 nm of cytoplasm.1-3 Substances attempting to pass from the systemic circulation to the brain must take either the paracellular route between - or the transcellular route through the microvascular endothelial cells. Paracellular diffusion of water-soluble substances and small ions is severely restricted by tight junctions (TJs) that connect apposing endothelial cell membranes to physically obliterate the interendothelial cleft. Blood-borne substances attempting to pass through the luminal membrane of microvascular endothelial cells are actively expelled back into the capillary lumen by embedded efflux transporters, or acted upon by a variety of metabolizing enzymes. The combined efforts of passive obstruction (TJs), active drug efflux (embedded transporters) and biochemical transformation (metabolism) create an obstacle to drug delivery that prevents approximately 98% of small molecule drugs and essentially 100% of large molecule drugs (e.g., monoclonal antibodies, antisense drugs) from entering the brain under normal conditions.4-7
The BBB is not a static anatomical boundary, but a dynamic interface capable of rapid response to stressors including hypoxia, inflammation, trauma and pain.3,4,8-11 Therapeutic targeting of the BBB is emerging as a critically relevant clinical goal3,8,9,11-13 because BBB dysfunction exacerbates (and in selected instances, perhaps initiates14), numerous diseases and pathologies including stroke,11,15-17 Alzheimer’s disease,18-23 acute liver failure,24 multiple sclerosis,25,26 meningitis,27,28 HIV,29-31 diabetes,32-34 depressive and psychotic disorders,35 cerebral malaria,36 Parkinson’s disease,22,26 traumatic10,23,37-40 and surgical41 brain injury, peripheral nerve injury,42 brain cancer,43-45 epilepsy46-49 and peripheral inflammatory pain.3,8,50 Loss of BBB integrity (i.e., leak) exposes the brain to potentially harmful concentrations of substances in the peripheral circulation (e.g., ions, amino acids, neurotransmitters, proteins and other macromolecules) that may disrupt brain homeostasis and adversely affect neuronal signaling. Inappropriate paracellular passage of therapeutic pharmaceuticals, nutraceuticals or xenobiotics into the brain following TJ disruption may result in significant drug side effects and/or adverse drug-drug interactions. Alternatively, BBB impairment may involve pathologically increased drug efflux across the microvascular luminal membrane that results in reduced drug uptake into the brain and diminished drug efficacy.
Protein trafficking as a therapeutic target to modulate BBB function
BBB integrity and function is critically influenced by what is now referred to as the “extended neurovascular unit”51 that incorporates not only microvascular endothelial cells and adjacent pericytes, astrocytes and neurons, but also neighboring smooth muscle cells and microglia in the brain, and blood cells in the capillary lumen such as polymorphonuclear cells, lymphocytes and monocytes.3,4,12,14,52 Given the multiplicity of cell types, intra- and extracellular signaling pathways, and interacting proteins, lipids and carbohydrates involved in the formation, maintenance and disruption of the different barrier functions performed by the BBB, there are a multitude of approaches for therapeutic manipulation of the BBB in both health and disease to optimize CNS drug delivery. At the molecular level, an established approach to influence the function of a particular protein important to BBB biochemistry (e.g., efflux transporter, TJ component) is direct modulation of its activity and/or gene transcription. An alternative approach for enhancing CNS drug delivery that is under study in our laboratory is the targeting of protein trafficking whereby altering the location of a protein is used as the means of modulating its activity. The unique advantage to therapeutic subcellular misdirection (or redirection) of a protein is that its physiological impact can be reversibly modified, despite pathology-induced changes in gene transcription.53 Discussed below are two examples from our laboratory, involving the drug efflux transporter P-glycoprotein, and the TJ transmembrane protein occludin, that demonstrate the potential of therapeutic modulation of pathology-induced changes in BBB protein trafficking to optimize CNS drug delivery in the presence of stressors (e.g., peripheral inflammatory pain, hypoxia).
Altered P-glycoprotein trafficking promotes increased drug efflux at the BBB
P-glycoprotein (ABCB1/MDR1, EC 3.6.3.44) is the pre-eminent molecular challenge to CNS drug delivery at the BBB.54-56 Strategically enriched at the luminal membrane of cerebral microvascular endothelial cells,57-59 P-glycoprotein uses energy from ATP-hydrolysis to expel an impressive variety of structurally divergent drugs back into the microcapillary lumen against steep concentration gradients. P-glycoprotein substrates range in mass from ~300-4000 Da and include analgesics, anti-cancer and immunosuppressive agents, psychotropics, antibiotics, anti-allergenics, anti-epileptics, beta-blockers, steroid hormones and HIV-1 protease inhibitors.54,55,60-64 Although intense research effort has focused on the development of P-glycoprotein inhibitors, clinical trials incorporating direct inhibition of P-glycoprotein have largely proved unsuccessful in improving therapeutic efficacy.65-70 High doses of inhibitor appear to be required, unfortunately giving rise to systemic toxicity. Moreover, complete inhibition of P-glycoprotein could be life-threatening due to the lack of protection against potentially dangerous blood-borne substances. Currently, research effort is focused on identifying therapeutic targets within multiple signaling pathways that promote disease-related changes in P-glycoprotein activity.45-47,54,55,62,71-73
In our laboratory, we discovered that the onset of peripheral inflammatory pain (experimentally induced by injection of λ-carrageenan in the rat hind paw) is followed within three hours by an increase in P-glycoprotein-associated efflux of morphine at the BBB. The important consequence of this was a corresponding decrease in morphine efficacy in vivo due to a reduction in morphine uptake into the brain.74 These data demonstrated that inflammatory pain itself hinders the ability of clinically relevant pain drugs such as morphine to gain entrance into the brain. Inflammation caused by tissue injury contributes to the severity of post-operative pain,75 and therefore our finding of increased morphine efflux by P-glycoprotein at the BBB may explain in part the reported difficulties with achieving post-operative opioid analgesia.76,77 To identify novel strategies to overcome significant decreases in CNS analgesic drug delivery that occur following the onset of peripheral inflammatory pain, we investigated the hypothesis that the rapid increase in P-glycoprotein efflux function was due to a dynamic redistribution of P-glycoprotein within the microvascular endothelial cell wherein P-glycoprotein stored within a putative reservoir was released from storage and trafficked to the luminal plasma membrane. Evolutionary selection of P-glycoprotein to be a critical “gatekeeper” at the BBB can be inferred by the fact that microvessels at the BBB contain the highest levels of P-glycoprotein within the body,78 and that in vivo dosing studies using P-glycoprotein substrates show that brain uptake is substantially increased in P-glycoprotein knockout animals.79,80 Given that diet, environmental exposure or external stressors can quickly raise the concentration of potentially harmful substances in the systemic circulation, we surmised that as P-glycoprotein evolved to perform so significant a barrier role at the BBB, a mechanism must also have evolved to ensure the timely trafficking of sufficient amounts of P-glycoprotein to the microvascular luminal membrane to meet the current threat.
Biosynthetic trafficking of P-glycoprotein in vivo at the BBB has not been studied. As an N-linked glycosylated protein, P-glycoprotein can be assumed within microvascular endothelial cells to follow the classical anterograde biosynthetic pathway, with co-translational insertion into the lumen of the endoplasmic reticulum being followed by glycosylation, folding, additional post-translational modification in the Golgi and finally, transport to the plasma membrane.81,82 Protein transport from one subcellular location to another directly results from specific protein-protein interactions that are governed by unique motifs encoded within a protein’s primary sequence. P-glycoprotein has a binding motif in its N-terminus for caveolin-1,83 a key trafficking protein capable of forming both caveolar and noncaveolar oligomeric scaffolds.84 Caveolin-1 colocalizes with P-glycoprotein in caveolae isolated from rat brain capillaries85 and at the luminal endothelial membrane and the border of the luminal/abluminal compartments in human brain capillaries.59,86 Studies using rat brain endothelial cells in vitro have demonstrated that the physical interaction between P-glycoprotein with caveolin-1 is enhanced by tyrosine-14-phosphorylation of caveolin-1, and that the binding of P-glycoprotein to caveolin-1 negatively regulates P-glycoprotein function.87,88
To investigate the constitutive and inflammation/pain-induced trafficking of P-glycoprotein within cerebral microvascular endothelial cells in vivo, we used the λ-carrageenan model of inflammatory pain (i.e. hyperalgesia), combined with confocal microscopy and subcellular fractionation of isolated cerebral microvessels.89 Quantitative microscopic global colocalization examination of intact cerebral microvessels revealed that under normal conditions there was a significant amount of colocalization between P-glycoprotein and caveolin-1. Subcellular fractionation of isolated cerebral microvessel homogenate revealed that P-glycoprotein trafficking is highly regulated, with the bulk of the transporter apparently being targeted to- and sequestered within high molecular weight (>250 kDa) “storage” structures enriched in caveolin-1 and maintained by disulfide-bonds. Biochemical analysis of isolated membrane domains enriched with P-glycoprotein-containing high molecular weight structures revealed that these apparent reservoirs of P-glycoprotein quickly disassembled in the presence of a hydrophilic reducing agent, indicating that the cysteine residues forming the structural disulfide bonds were readily accessible to the external milieu. Incorporation of P-glycoprotein within densely-packed, high molecular weight complexes would protect against both limited proteolysis and proteasomal or lysosomal degradation. Moreover, the sensitivity of the P-glycoprotein-containing high molecular weight structures to reduction by a hydrophilic reducing agent suggested that this manner of “storing” P-glycoprotein involved its sequestration within a structure that could readily be completely dismantled in order to release a large amount of monomeric, biologically active P-glycoprotein at one time. Peripheral inflammatory pain reduced the colocalization of P-glycoprotein with caveolin-1 within cerebral microvessels by approximately half within three hours of onset, and promoted a dramatic redistribution of P-glycoprotein and caveolin-1 between endothelial cell subcellular compartments. Disassembly of high molecular weight structures containing P-glycoprotein coincided with an increase in drug-stimulated P-glycoprotein-dependent ATPase activity associated with plasma membrane domains identified to be at the luminal surface of cerebral microvessels. These data are the first observation that peripheral inflammatory pain leads to altered trafficking of P-glycoprotein that is responsible for controlling analgesic drug delivery to the brain. Future biochemical analysis of isolated P-glycoprotein-containing high molecular weight “storage” complexes will allow identification of potential therapeutic targets for maintaining the integrity of high molecular weight complexes storing P-glycoprotein. Thus, optimizing CNS drug delivery to the brain during pathological states such as peripheral inflammatory pain could be achieved by preventing release of P-glycoprotein from subcellular storage compartments.
Inhibition of occludin trafficking away from TJs restores BBB integrity
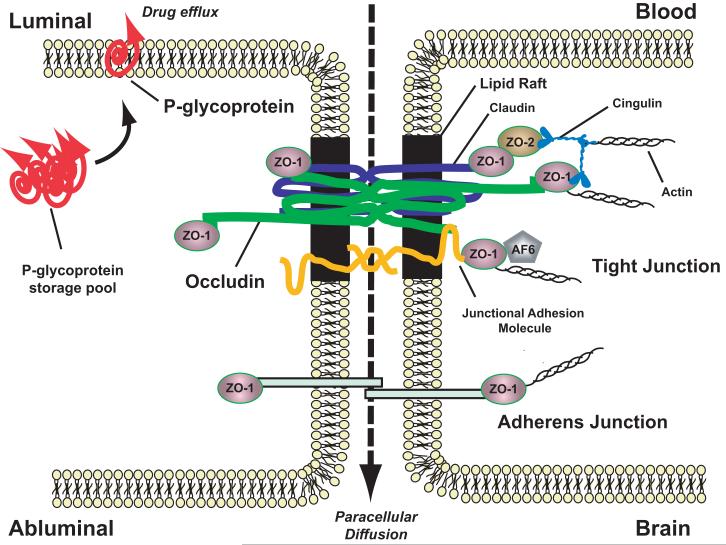
The transmembrane protein occludin is critical for barrier function at TJs between microvascular endothelial cells at the BBB,3,90 and trafficking of occludin away from TJs is a sensitive, early and reliable sign that of TJ opening and BBB dysfunction.91 The ability to rapidly seal BBB breaches (i.e., leaks) at TJs that occur during oxidative stress (e.g., stroke) would preclude the development of life-threatening cerebral edema and the inappropriate CNS delivery of neurotoxic blood-borne substances.9,11,16,51 In our laboratory, we discovered that peripheral inflammatory pain induced in three different experimental models (formalin, carrageenan and complete Freund’s adjuvant) promoted BBB dysfunction characterized by increased paracellular permeability to vascular markers such as sucrose.92-96 Peripheral inflammatory pain was also found to promote an increase in paracellular permeability of the opioid analgesic codeine.97 Codeine analgesia is centrally mediated, which requires it to accumulate within the brain, and it does so by passive paracellular diffusion.98,99 An uncontrolled increase in brain uptake of codeine during peripheral inflammatory pain due to a pathological increase in paracellular permeability may result in significant CNS side effects associated with opioids such as respiratory depression, addiction and tolerance. Alterations in BBB TJ barrier function have now been reported in experimental pain models incorporating chronic spinal nerve ligation100 and peripheral nerve injury42, highlighting the need to develop a means of targeting increases in paracellular permeability to optimize CNS drug delivery of clinically relevant pain drugs such as codeine that passage paracellularly across the BBB.
Occludin has an M-shaped topology, fashioned by four transmembrane domains, two extracellular loops and cytoplasmic N- and C-termini, that facilitates its performance of both structural and signaling roles at the TJ.90,101-103 Through its extracellular loops, occludin physically extends into the interendothelial space to interact with homologous segments of occludin molecules on adjacent microvascular endothelial cells to help fuse apposing cell membranes to form a tight seal that restricts paracellular diffusion.104,105 Occludin is capable of self-association,106-108 and occludin oligomerization is facilitated by the presence in its primary sequence of a motif of ~200 amino acids that has statistical similarity to the myelin and lymphocyte (MAL) and related proteins for vesicle trafficking and membrane link (MARVEL).109,110 Through its C-terminus, occludin interacts with TJ accessory proteins such as the zonula occludens proteins (ZO-1, ZO-2, and ZO-3) that anchor multiprotein TJ complexes to the underlying actin cytoskeleton.111,112 At different sites within the N- and C-termini and the intracellular loop that are exposed to the cytoplasm, occludin interacts with a variety of signaling, regulatory and vesicle trafficking proteins including numerous kinases and phosphatases,101,113 growth factor receptors,114,115 caveolin,116 rab13,117 the ubiquitin-protein ligase itch118 and proteins containing a ubiquitin interacting motif (Epsin, epidermal growth factor receptor pathway substrate 15 and hepatocyte growth factor-regulated tyrosine kinase ).119
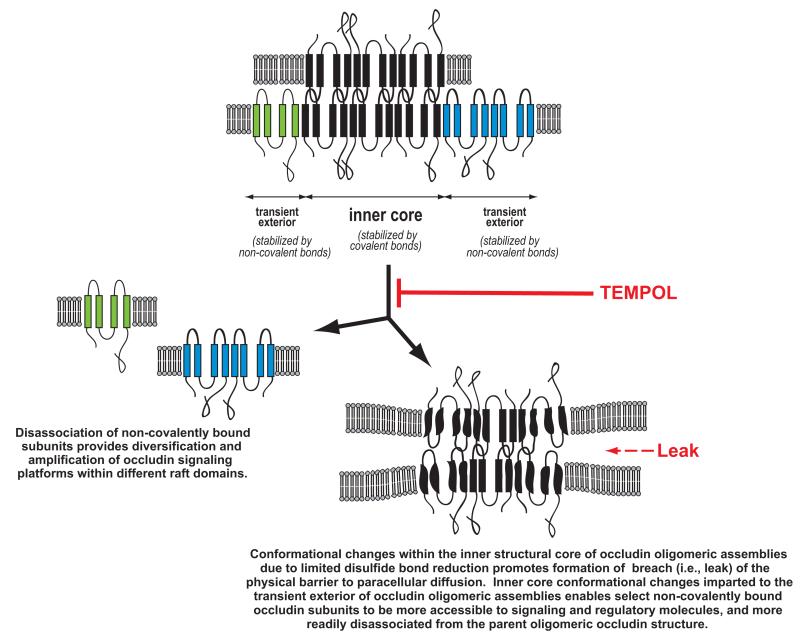
To understand, on a molecular level, how occludin performs both structural and signaling roles at BBB TJs, it was first necessary to develop a means of isolating native TJ complexes containing oligomeric assemblies of occludin. Early freeze-fracture replica electron microscopy studies revealed TJs to be continuous, anastomosing, intramembranous particle strands of ~10 nm thickness, giving rise to the belief that tightly packed oligomeric assemblies of integral membrane proteins is an essential architectural feature of the TJ.120-122 To avoid disruption of occludin oligomeric assemblies during their isolation, we incorporated the use of a novel detergent-free, density gradient method123 to fractionate cerebral microvessels. This made it possible, for the first time, to isolate occludin oligomeric complexes within the context of their normal lipid-enriched plasma membrane raft environment.124 Biochemical analysis of isolated occludin oligomeric assemblies derived from naive rats revealed the importance of disulfide bonds in maintaining the structural integrity of these large molecular weight structures, and provided an explanation for the evolutionary conservation of cysteine residues in the occludin molecule found within hydrophobic transmembrane regions, and hydrophilic regions such as the second extracellular loop and the cytoplasmic C-terminus.125 Occludin oligomeric assemblies were found to contain two sets of disulfide bonds, distinguished by the ease with which they could be reduced by either a hydrophilic or a hydrophobic reducing agent. Although high molecular weight occludin complexes were sensitive to a hydrophilic reducing agent that disrupts easily accessible disulfide bonds, complete disruption of occludin oligomeric assemblies could only be achieved by treatment with a stronger hydrophobic reducing agent capable of penetrating the hydrophobic, transmembrane regions of occludin multiprotein complexes to reach buried disulfide bonds. Our data generated the hypothesis that disassembly of oligomeric occludin complexes within “TJ-associated” plasma membrane lipid rafts could be initiated by selective reduction of readily accessible disulfide bonds involving cysteine residues within extracellular loops of occludin molecules on apposing cell membranes, or perhaps within the cytoplasmic C-termini of adjacent occludin molecules within the same cell. Conformational changes promoted by relaxation of structural restrictions invoked by disulfide bonds could then lead to altered protein–lipid interactions, a remodeling of the lipid raft environment, and a progressive dismantling of the oligomeric complex into component lower molecular weight occludin isoforms.
Biochemical analysis of occludin oligomeric complexes, isolated from cerebral microvessels from experimental animals subjected to either peripheral inflammatory pain or global hypoxia/reoxygenation, provided additional data to refine our model of protein isoform interaction within occludin oligomeric assemblies.96,126,127 Occludin oligomeric assemblies at BBB TJs were revealed to have an inner “structural core” of covalently bonded subunits that was associated through non-covalent, hydrophobic interactions with a variety of monomeric and dimeric subunits. Disruption of a disulfide bond(s) between occludin molecules on different sides of the paracellular cleft caused by an eternal stressor conceivably could lead to conformational changes in selected subunits within the inner core that resulted in a physical breach in the transmembrane protein diffusion barrier. The change in conformation of specific occludin isoforms within the center of the oligomeric complex would expectedly lead to a change in conformation of selected non-covalently bound subunits rendering the latter more accessible to signaling and regulatory molecules and/or more readily disassociated from the parent oligomeric occludin structure.
To provide evidence that limited disulfide reduction due to oxidative stress was a precipitating event leading to the trafficking of occludin away from “TJ-associated” occludin oligomeric assemblies, we investigated if TJ disruption and altered occludin trafficking could be modulated by an antioxidant. Using the non-invasive in vivo rat model of global ischemia, we found that the membrane-permeable, free radical scavenger TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) preserved the integrity of disulfide bonds within occludin oligomeric assemblies and prevented an increase in paracellular permeability to sucrose.11,127 TEMPOL also prevented carrageenan-induced peripheral inflammatory pain from inducing TJ disruption, altered trafficking of occludin and an associated increase in paracellular permeability to codeine.8,95 These data demonstrated that use of antioxidants such as TEMPOL to protect the “Achilles heel of occludin oligomeric assemblies”96 (i.e., sensitivity to disulfide-bond reduction during oxidative stress103) is a viable means of therapeutically manipulating occludin trafficking in vivo in order to optimize CNS drug delivery by preventing the dismantling of multiprotein TJ complexes and the associated changes in paracellular permeability at the BBB.
Conclusion
Protein trafficking and the development of pathological conditions and disease are closely linked.128-130 Although the development of pharmaceutical “relocators and mislocators”53 for the therapeutic modulation of protein trafficking is in its infancy, recent clinical trials incorporating the use of the lipid-based compound perifosine131 to prevent antiapoptotic kinase (AKT) activation by inhibiting its trafficking to the plasma membrane highlight the promise that clinical targeting of protein trafficking can offer to the treatment of colorectal cancer and multiple myeloma132 and possibly also neuroblastoma.133 Protein-protein interactions, and the changes in protein trafficking that they promote, are increasingly being recognized as an important means of regulating CNS proteins such as opioid receptors,134-137 AMPA receptors,138 nicotinic acetylcholine receptors,139 glutamate receptors,140 tyrosine kinase receptors,141 cannabinoid CB1 receptors142 and other GCPRs143,144 and vesicular neurotransmitter transporters.145 Methodology to identify motifs within primary sequences of proteins that regulate protein-protein interactions is rapidly advancing in sophistication and scope.146-153 In addition, research efforts to develop small molecules and peptides for clinical use as manipulators of the protein-protein interactions that drive changes in protein trafficking are greatly intensifying.53,147,153-161 By designing the protein-protein interaction inhibitor to preferentially inhibit the interaction of two specific proteins, as opposed to the interaction of one protein with a variety of substrates, then specificity of protein trafficking changes can be achieved within minimal side effects.154
Therapeutic targeting of protein-protein interactions that lead to changes in key BBB efflux transporter or TJ protein trafficking and localization is a relatively unexplored means of optimizing CNS drug delivery. In the example of P-glycoprotein, temporary reduction of its activity at the plasma membrane can be achieved by inhibiting its release from a storage reservoir, thus providing a short-term window for drug delivery at the BBB. Trafficking modulation can also be longer-term, as in the case of using a pharmacological reactive oxygen species scavenger such as TEMPOL to inhibit the oxidative stress-induced disruption of disulfide bonds within occludin oligomeric assemblies that promotes the trafficking of occludin isoforms away from the TJ and the development of increased paracellular permeability. As detailed understanding of the trafficking mechanisms involved in trafficking of key BBB efflux transporter- and TJ proteins increases, so will the identification of novel targets for therapeutic modulation of BBB integrity and function.
Using the parameters of effectiveness, safety and cost of application to rank a drug delivery strategy,162 enhancement of CNS drug delivery at the BBB through therapeutic manipulation of efflux transporter trafficking (e.g., P-glycoprotein) ranks favorably as it has the potential to provide a non-invasive and low cost means of increasing the entry of a wide variety of drugs into the brain under conditions of disease or stress without compromising basal BBB protection. This stands in contrast to the high cost of treatment and patient safety concerns associated with artificial opening of the BBB (e.g. with mannitol), which may led to uncontrolled CNS entry of potentially harmful substances present in the systemic circulation along with the target drug.163-165 CNS drug delivery strategies employing chemical delivery systems (e.g. liposomes, nanocarriers, conjugates) or endocytosis and transcytosis (adsorptive and receptor mediated) are similar in concept to therapeutic manipulation of P-glycoprotein trafficking in that they provide a means of circumventing P-glycoprotein drug efflux; but these approaches have potentially high formulation costs and significant patient safety concerns. However, with further development, these technologies have the potential to provide a targeted approach for the delivery of specific drugs to the CNS.162,164,166-172 In contrast, therapeutic manipulation of P-glycoprotein trafficking represents a relatively inexpensive and nonspecific approach for enhancing CNS drug delivery of a multitude of drugs and drug combinations.
Figure 1.
Basic molecular organization of blood-brain barrier tight junctions. Adapted from Hawkins and Davis, Pharmacological Rev 57:173-185 (2005).
Figure 2.
Proposed model of protein-protein interactions within occludin oligomeric assemblies that enables occludin to perform both structural and signaling roles at blood-brain barrier tight junctions. Limited disulfide bond reduction within the structural core of occludin oligomeric assemblies initiates conformational changes that facilitate tight junction disruption, and this can be pharmacologically targeted with the membrane-permeable, free radical scavenger TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) to prevent paracellular leak. Adapted from McCaffrey, Willis and Staatz et al., J Neurochem 110:58-71 (2009).
Acknowledgements
This symposium was supported in part by a grant from the National Center for Research Resources (R13 RR023236).
This work was supported by NIH grants R01-NS 39592, R01-NS42652, R01-DA12684 to TPD, and CA 09820-0251 to GM. The authors thank Dr. William D. Staatz for his helpful advice on this manuscript, and his expert assistance with illustration preparation.
Footnotes
The authors declare that they do not have a financial interest conflict related to this work.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zlokovic BV, Apuzzo ML. Strategies to circumvent vascular barriers of the central nervous system. Neurosurgery. 1998;43(4):877–8. doi: 10.1097/00006123-199810000-00089. [DOI] [PubMed] [Google Scholar]
- 2.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12(10):1395–406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 4.Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WM. Drug targeting to the brain. Pharm Res. 2007;24(9):1733–44. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 7.el-Bacha RS, Minn A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell Mol Biol (Noisy-legrand) 1999;45(1):15–23. [PubMed] [Google Scholar]
- 8.Ronaldson PT, Davis TP. Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery. Ther Deliv. 2011;2(8):1015–1041. doi: 10.4155/tde.11.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68(5):311–23. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- 10.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2(4):492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronaldson PT, Davis TP. Blood-Brain Barrier Integrity and Glial Support: Mechanisms that can be Targeted for Novel Therapeutic Approaches in Stroke. Curr Pharm Des. 2012 doi: 10.2174/138161212802002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vangilder RL, Rosen CL, Barr TL, et al. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol Ther. 2011;130(3):239–47. doi: 10.1016/j.pharmthera.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37(1):26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hom S, Fleegal MA, Egleton RD, et al. Comparative changes in the blood-brain barrier and cerebral infarction of SHR and WKY rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1881–92. doi: 10.1152/ajpregu.00761.2005. [DOI] [PubMed] [Google Scholar]
- 16.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32(2):200–19. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Fraser PA. The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med. 2011;51(5):967–77. doi: 10.1016/j.freeradbiomed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Banks WA. Drug delivery to the brain in Alzheimer’s disease: Consideration of the blood-brain barrier. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer AM. The role of the blood brain barrier in neurodegenerative disorders and their treatment. J Alzheimers Dis. 2011;24(4):643–56. doi: 10.3233/JAD-2011-110368. [DOI] [PubMed] [Google Scholar]
- 21.Humpel C. Chronic mild cerebrovascular dysfunction as a cause for Alzheimer’s disease? Exp Gerontol. 2011;46(4):225–32. doi: 10.1016/j.exger.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartels AL. Blood-brain barrier p-glycoprotein function in neurodegenerative disease. Curr Pharm Des. 2011;17(26):2771–7. doi: 10.2174/138161211797440122. [DOI] [PubMed] [Google Scholar]
- 23.Sivanandam TM, Thakur MK. Traumatic brain injury: A risk factor for Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36(5):1376–81. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen JH. Blood-brain barrier in acute liver failure. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller E. Multiple sclerosis. Adv Exp Med Biol. 2012;724:222–38. doi: 10.1007/978-1-4614-0653-2_17. [DOI] [PubMed] [Google Scholar]
- 26.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 27.van Sorge NM, Doran KS. Defense at the border: the blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012;7(3):383–94. doi: 10.2217/fmb.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mook-Kanamori BB, Geldhoff M, van der Poll T, et al. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev. 2011;24(3):557–91. doi: 10.1128/CMR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal. 2011;15(5):1285–303. doi: 10.1089/ars.2011.3929. [DOI] [PubMed] [Google Scholar]
- 30.Miller F, Afonso PV, Gessain A, et al. Blood-brain barrier and retroviral infections. Virulence. 2012;3(2) doi: 10.4161/viru.19697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strazza M, Pirrone V, Wigdahl B, et al. Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banks WA. The dam breaks: disruption of the blood-brain barrier in diabetes mellitus. Am J Physiol Heart Circ Physiol. 2006;291(6):H2595–6. doi: 10.1152/ajpheart.00751.2006. [DOI] [PubMed] [Google Scholar]
- 33.Wu KC, Pan HJ, Yin HS, et al. Change in P-glycoprotein and caveolin protein expression in brain striatum capillaries in New Zealand obese mice with type 2 diabetes. Life Sci. 2009;85(23-26):775–81. doi: 10.1016/j.lfs.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins BT, Lundeen TF, Norwood KM, et al. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50(1):202–11. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 35.De Klerk OL, Bosker FJ, Luurtsema G, et al. The role of p-glycoprotein in psychiatric disorders: a reliable guard of the brain? Cent Nerv Syst Agents Med Chem. 2011;11(3):197–209. doi: 10.2174/187152411798047744. [DOI] [PubMed] [Google Scholar]
- 36.Renia L, Wu Howland S, Claser C, et al. Cerebral malaria: Mysteries at the blood-brain barrier. Virulence. 2012;3(2) doi: 10.4161/viru.19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nag S, Kapadia A, Stewart DJ. Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol. 2011;37(1):3–23. doi: 10.1111/j.1365-2990.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Huang W. Non-impact, blast-induced mild TBI and PTSD: concepts and caveats. Brain Inj. 2011;25(7-8):641–50. doi: 10.3109/02699052.2011.580313. [DOI] [PubMed] [Google Scholar]
- 39.Helmy A, De Simoni MG, Guilfoyle MR, et al. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol. 2011;95(3):352–72. doi: 10.1016/j.pneurobio.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Pop V, Badaut J. A Neurovascular Perspective for Long-Term Changes After Brain Trauma. Transl Stroke Res. 2011;2(4):533–545. doi: 10.1007/s12975-011-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frontczak-Baniewicz M, Chrapusta SJ, Sulejczak D. Long-term consequences of surgical brain injury - characteristics of the neurovascular unit and formation and demise of the glial scar in a rat model. Folia Neuropathol. 2011;49(3):204–18. [PubMed] [Google Scholar]
- 42.Beggs S, Liu XJ, Kwan C, et al. Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Mol Pain. 2010;6:74. doi: 10.1186/1744-8069-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nico B, Ribatti D. Morphofunctional aspects of the blood-brain barrier. Curr Drug Metab. 2012;13(1):50–60. doi: 10.2174/138920012798356970. [DOI] [PubMed] [Google Scholar]
- 44.Kesari S. Understanding glioblastoma tumor biology: the potential to improve current diagnosis and treatments. Semin Oncol. 2011;38(Suppl 4):S2–10. doi: 10.1053/j.seminoncol.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal S, Hartz AM, Elmquist WF, et al. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des. 2011;17(26):2793–802. doi: 10.2174/138161211797440186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aronica E, Sisodiya SM, Gorter JA. Cerebral expression of drug transporters in epilepsy. Adv Drug Deliv Rev. 2011 doi: 10.1016/j.addr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Potschka H. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia. 2010;51(8):1333–47. doi: 10.1111/j.1528-1167.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- 48.Hartz AM, Notenboom S, Bauer B. Signaling to P-glycoprotein-A new therapeutic target to treat drug-resistant epilepsy? Drug News Perspect. 2009;22(7):393–7. doi: 10.1358/dnp.2009.22.7.1401354. [DOI] [PubMed] [Google Scholar]
- 49.Lazarowski A, Czornyj L, Lubienieki F, et al. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48(Suppl 5):140–9. doi: 10.1111/j.1528-1167.2007.01302.x. [DOI] [PubMed] [Google Scholar]
- 50.Wolka AM, Huber JD, Davis TP. Pain and the blood-brain barrier: obstacles to drug delivery. Adv Drug Deliv Rev. 2003;55(8):987–1006. doi: 10.1016/s0169-409x(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 51.Neuwelt EA, Bauer B, Fahlke C, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12(3):169–82. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mae M, Armulik A, Betsholtz C. Getting to know the cast - cellular interactions and signaling at the neurovascular unit. Curr Pharm Des. 2011;17(26):2750–4. doi: 10.2174/138161211797440113. [DOI] [PubMed] [Google Scholar]
- 53.Hung MC, Link W. Protein localization in disease and therapy. J Cell Sci. 2011;124(Pt 20):3381–92. doi: 10.1242/jcs.089110. [DOI] [PubMed] [Google Scholar]
- 54.Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60(2):196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31(6):246–54. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharom FJ. The P-glycoprotein multidrug transporter. Essays Biochem. 2011;50(1):161–78. doi: 10.1042/bse0500161. [DOI] [PubMed] [Google Scholar]
- 57.Roberts LM, Black DS, Raman C, et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155(2):423–38. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 58.Bendayan R, Ronaldson PT, Gingras D, et al. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54(10):1159–67. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Virgintino D, Robertson D, Errede M, et al. Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem. 2002;50(12):1671–6. doi: 10.1177/002215540205001212. [DOI] [PubMed] [Google Scholar]
- 60.Sun J, He ZG, Cheng G, et al. Multidrug resistance P-glycoprotein: crucial significance in drug disposition and interaction. Med Sci Monit. 2004;10(1):RA5–14. [PubMed] [Google Scholar]
- 61.Ueno M, Nakagawa T, Wu B, et al. Transporters in the brain endothelial barrier. Curr Med Chem. 2010;17(12):1125–38. doi: 10.2174/092986710790827816. [DOI] [PubMed] [Google Scholar]
- 62.Potschka H. Targeting regulation of ABC efflux transporters in brain diseases: a novel therapeutic approach. Pharmacol Ther. 2010;125(1):118–27. doi: 10.1016/j.pharmthera.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 63.Hartz AM, Bauer B. Regulation of ABC transporters at the blood-brain barrier: new targets for CNS therapy. Mol Interv. 2010;10(5):293–304. doi: 10.1124/mi.10.5.6. [DOI] [PubMed] [Google Scholar]
- 64.Hodges LM, Markova SM, Chinn LW, et al. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein) Pharmacogenet Genomics. 2011;21(3):152–61. doi: 10.1097/FPC.0b013e3283385a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmeira A, Sousa E, Vasconcelos MH, et al. Three Decades of P-gp Inhibitors: Skimming Through Several Generations and Scaffolds. Curr Med Chem. 2012 doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- 66.Kaye SB. Reversal of drug resistance in ovarian cancer: where do we go from here? J Clin Oncol. 2008;26(16):2616–8. doi: 10.1200/JCO.2008.16.2123. [DOI] [PubMed] [Google Scholar]
- 67.Szakacs G, Paterson JK, Ludwig JA, et al. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 68.Fletcher JI, Haber M, Henderson MJ, et al. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10(2):147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 69.Kannan P, John C, Zoghbi SS, et al. Imaging the function of P-glycoprotein with radiotracers: pharmacokinetics and in vivo applications. Clin Pharmacol Ther. 2009;86(4):368–77. doi: 10.1038/clpt.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choo EF, Kurnik D, Muszkat M, et al. Differential in vivo sensitivity to inhibition of P-glycoprotein located in lymphocytes, testes, and the blood-brain barrier. J Pharmacol Exp Ther. 2006;317(3):1012–8. doi: 10.1124/jpet.105.099648. [DOI] [PubMed] [Google Scholar]
- 71.Bauer B, Hartz AM, Fricker G, et al. Modulation of p-glycoprotein transport function at the blood-brain barrier. Exp Biol Med (Maywood) 2005;230(2):118–27. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- 72.Hawkins BT, Rigor RR, Miller DS. Rapid loss of blood-brain barrier P-glycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab. 2010;30(9):1593–7. doi: 10.1038/jcbfm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahringer A, Ott M, Reimold I, et al. The ABC of the blood-brain barrier - regulation of drug efflux pumps. Curr Pharm Des. 2011;17(26):2762–70. doi: 10.2174/138161211797440221. [DOI] [PubMed] [Google Scholar]
- 74.Seelbach MJ, Brooks TA, Egleton RD, et al. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102(5):1677–90. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- 75.Fujita I, Okumura T, Sakakibara A, et al. Involvement of inflammation in severe post-operative pain demonstrated by pre-surgical and post-surgical treatment with piroxicam and ketorolac. J Pharm Pharmacol. 2012;64(5):747–55. doi: 10.1111/j.2042-7158.2012.01468.x. [DOI] [PubMed] [Google Scholar]
- 76.Costantini R, Affaitati G, Fabrizio A, et al. Controlling pain in the post-operative setting. Int J Clin Pharmacol Ther. 2011;49(2):116–27. doi: 10.5414/cp201401. [DOI] [PubMed] [Google Scholar]
- 77.Brennan TJ. Pathophysiology of postoperative pain. Pain. 2011;152(3 Suppl):S33–40. doi: 10.1016/j.pain.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cordon-Cardo C, O’Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86(2):695–8. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang JS, Taylor R, Ruan Y, et al. Olanzapine penetration into brain is greater in transgenic Abcb1a P-glycoprotein-deficient mice than FVB1 (wild-type) animals. Neuropsychopharmacology. 2004;29(3):551–7. doi: 10.1038/sj.npp.1300372. [DOI] [PubMed] [Google Scholar]
- 80.Schinkel AH, Smit JJ, van Tellingen O, et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77(4):491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 81.Fu D, Arias IM. Intracellular trafficking of P-glycoprotein. Int J Biochem Cell Biol. 2011 doi: 10.1016/j.biocel.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Porcelli L, Lemos C, Peters GJ, et al. Intracellular trafficking of MDR transporters and relevance of SNPs. Curr Top Med Chem. 2009;9(2):197–208. doi: 10.2174/156802609787521562. [DOI] [PubMed] [Google Scholar]
- 83.Jodoin J, Demeule M, Fenart L, et al. P-glycoprotein in blood-brain barrier endothelial cells: interaction and oligomerization with caveolins. J Neurochem. 2003;87(4):1010–23. doi: 10.1046/j.1471-4159.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- 84.Lajoie P, Goetz JG, Dennis JW, et al. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185(3):381–5. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Demeule M, Jodoin J, Gingras D, et al. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000;466(2-3):219–24. doi: 10.1016/s0014-5793(00)01087-5. [DOI] [PubMed] [Google Scholar]
- 86.Guo Z, Zhu J, Zhao L, et al. Expression and clinical significance of multidrug resistance proteins in brain tumors. J Exp Clin Cancer Res. 2010;29:122. doi: 10.1186/1756-9966-29-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barakat S, Turcotte S, Demeule M, et al. Regulation of brain endothelial cells migration and angiogenesis by P-glycoprotein/caveolin-1 interaction. Biochem Biophys Res Commun. 2008;372(3):440–6. doi: 10.1016/j.bbrc.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 88.Barakat S, Demeule M, Pilorget A, et al. Modulation of p-glycoprotein function by caveolin-1 phosphorylation. J Neurochem. 2007;101(1):1–8. doi: 10.1111/j.1471-4159.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- 89.McCaffrey G, Staatz WD, Sanchez-Covarrubias L, et al. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem. 2012 doi: 10.1111/j.1471-4159.2012.07831.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv Drug Deliv Rev. 2005;57(6):883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 91.Dobrogowska DH, Vorbrodt AW. Immunogold localization of tight junctional proteins in normal and osmotically-affected rat blood-brain barrier. J Mol Histol. 2004;35(5):529–39. doi: 10.1007/10.1007/s10735-004-1318-3. [DOI] [PubMed] [Google Scholar]
- 92.Brooks TA, Hawkins BT, Huber JD, et al. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289(2):H738–43. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huber JD, Hau VS, Borg L, et al. Blood-brain barrier tight junctions are altered during a 72-h exposure to lambda-carrageenan-induced inflammatory pain. Am J Physiol Heart Circ Physiol. 2002;283(4):H1531–7. doi: 10.1152/ajpheart.00027.2002. [DOI] [PubMed] [Google Scholar]
- 94.Huber JD, Witt KA, Hom S, et al. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280(3):H1241–8. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- 95.Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L, et al. Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain. Am J Physiol Heart Circ Physiol. 2012;302(3):H582–93. doi: 10.1152/ajpheart.00889.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCaffrey G, Seelbach MJ, Staatz WD, et al. Occludin oligomeric assembly at tight junctions of the blood-brain barrier is disrupted by peripheral inflammatory hyperalgesia. J Neurochem. 2008;106(6):2395–409. doi: 10.1111/j.1471-4159.2008.05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hau VS, Huber JD, Campos CR, et al. Effect of lambda-carrageenan-induced inflammatory pain on brain uptake of codeine and antinociception. Brain Res. 2004;1018(2):257–64. doi: 10.1016/j.brainres.2004.05.081. [DOI] [PubMed] [Google Scholar]
- 98.Xie R, Hammarlund-Udenaes M. Blood-brain barrier equilibration of codeine in rats studied with microdialysis. Pharm Res. 1998;15(4):570–5. doi: 10.1023/a:1011929910782. [DOI] [PubMed] [Google Scholar]
- 99.Bradbury MW, Patlak CS, Oldendorf WH. Analysis of brain uptake and loss or radiotracers after intracarotid injection. Am J Physiol. 1975;229(4):1110–5. doi: 10.1152/ajplegacy.1975.229.4.1110. [DOI] [PubMed] [Google Scholar]
- 100.Gordh T, Sharma HS. Chronic spinal nerve ligation induces microvascular permeability disturbances, astrocytic reaction, and structural changes in the rat spinal cord. Acta Neurochir Suppl. 2006;96:335–40. doi: 10.1007/3-211-30714-1_70. [DOI] [PubMed] [Google Scholar]
- 101.Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32(2):242–50. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36(7):1206–37. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 103.Blasig IE, Bellmann C, Cording J, et al. Occludin protein family: oxidative stress and reducing conditions. Antioxid Redox Signal. 2011;15(5):1195–219. doi: 10.1089/ars.2010.3542. [DOI] [PubMed] [Google Scholar]
- 104.Tsukita S, Furuse M, Itoh M. Structural and signalling molecules come together at tight junctions. Curr Opin Cell Biol. 1999;11(5):628–33. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 105.Tsukita S, Furuse M, Itoh M. Molecular dissection of tight junctions. Cell Struct Funct. 1996;21(5):381–5. doi: 10.1247/csf.21.381. [DOI] [PubMed] [Google Scholar]
- 106.Blasig IE, Winkler L, Lassowski B, et al. On the self-association potential of transmembrane tight junction proteins. Cell Mol Life Sci. 2006;63(4):505–14. doi: 10.1007/s00018-005-5472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walter JK, Castro V, Voss M, et al. Redox-sensitivity of the dimerization of occludin. Cell Mol Life Sci. 2009;66(22):3655–62. doi: 10.1007/s00018-009-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Walter JK, Rueckert C, Voss M, et al. The oligomerization of the coiled coil-domain of occludin is redox sensitive. Ann N Y Acad Sci. 2009;1165:19–27. doi: 10.1111/j.1749-6632.2009.04058.x. [DOI] [PubMed] [Google Scholar]
- 109.Yaffe Y, Shepshelovitch J, Nevo I, et al. The MARVEL transmembrane motif of occludin mediates oligomerization and targeting to the basolateral surface in epithelia. J Cell Sci. 2012 doi: 10.1242/jcs.100289. [DOI] [PubMed] [Google Scholar]
- 110.Raleigh DR, Marchiando AM, Zhang Y, et al. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21(7):1200–13. doi: 10.1091/mbc.E09-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furuse M, Itoh M, Hirase T, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127(6 Pt 1):1617–26. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fanning AS, Little BP, Rahner C, et al. The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell. 2007;18(3):721–31. doi: 10.1091/mbc.E06-08-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dorfel MJ, Huber O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J Biomed Biotechnol. 2012;2012:807356. doi: 10.1155/2012/807356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barrios-Rodiles M, Brown KR, Ozdamar B, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307(5715):1621–5. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- 115.Ozdamar B, Bose R, Barrios-Rodiles M, et al. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307(5715):1603–9. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 116.Nusrat A, Parkos CA, Verkade P, et al. Tight junctions are membrane microdomains. J Cell Sci. 2000;113(Pt 10):1771–81. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- 117.Morimoto S, Nishimura N, Terai T, et al. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J Biol Chem. 2005;280(3):2220–8. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- 118.Traweger A, Fang D, Liu YC, et al. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J Biol Chem. 2002;277(12):10201–8. doi: 10.1074/jbc.M111384200. [DOI] [PubMed] [Google Scholar]
- 119.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284(31):21036–46. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279(2):G250–4. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 121.Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108(Pt 11):3443–9. doi: 10.1242/jcs.108.11.3443. [DOI] [PubMed] [Google Scholar]
- 122.Shivers RR, Betz AL, Goldstein GW. Isolated rat brain capillaries possess intact, structurally complex, interendothelial tight junctions; freeze-fracture verification of tight junction integrity. Brain Res. 1984;324(2):313–22. doi: 10.1016/0006-8993(84)90042-8. [DOI] [PubMed] [Google Scholar]
- 123.Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res. 2005;46(5):1061–7. doi: 10.1194/jlr.D400041-JLR200. [DOI] [PubMed] [Google Scholar]
- 124.McCaffrey G, Staatz WD, Quigley CA, et al. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. 2007;103(6):2540–55. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- 125.Hua VB, Chang AB, Tchieu JH, et al. Sequence and phylogenetic analyses of 4 TMS junctional proteins of animals: connexins, innexins, claudins and occludins. J Membr Biol. 2003;194(1):59–76. doi: 10.1007/s00232-003-2026-8. [DOI] [PubMed] [Google Scholar]
- 126.McCaffrey G, Willis CL, Staatz WD, et al. Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress. J Neurochem. 2009;110(1):58–71. doi: 10.1111/j.1471-4159.2009.06113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lochhead JJ, McCaffrey G, Quigley CE, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;30(9):1625–36. doi: 10.1038/jcbfm.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davis JR, Kakar M, Lim CS. Controlling protein compartmentalization to overcome disease. Pharm Res. 2007;24(1):17–27. doi: 10.1007/s11095-006-9133-z. [DOI] [PubMed] [Google Scholar]
- 129.Gonnord P, Blouin CM, Lamaze C. Membrane trafficking and signaling: Two sides of the same coin. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 130.De Matteis MA, Luini A. Mendelian disorders of membrane trafficking. N Engl J Med. 2011;365(10):927–38. doi: 10.1056/NEJMra0910494. [DOI] [PubMed] [Google Scholar]
- 131.Kondapaka SB, Singh SS, Dasmahapatra GP, et al. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2(11):1093–103. [PubMed] [Google Scholar]
- 132.Richardson PG, Eng C, Kolesar J, et al. Perifosine , an oral, anti-cancer agent and inhibitor of the Akt pathway: mechanistic actions, pharmacodynamics, pharmacokinetics, and clinical activity. Expert Opin Drug Metab Toxicol. 2012;8(5):623–33. doi: 10.1517/17425255.2012.681376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sun W, Modak S. Emerging treatment options for the treatment of neuroblastoma: potential role of perifosine. Onco Targets Ther. 2012;5:21–9. doi: 10.2147/OTT.S14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Whistler JL. Examining the role of mu opioid receptor endocytosis in the beneficial and side-effects of prolonged opioid use: From a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Georgoussi Z, Georganta EM, Milligan G. The other side of opioid receptor signalling: regulation by protein-protein interaction. Curr Drug Targets. 2012;13(1):80–102. doi: 10.2174/138945012798868470. [DOI] [PubMed] [Google Scholar]
- 136.Arttamangkul S, Lau EK, Lu HW, et al. Desensitization and trafficking of mu-opioid receptors in locus ceruleus neurons: modulation by kinases. Mol Pharmacol. 2012;81(3):348–55. doi: 10.1124/mol.111.076208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol. 2010;10(1):73–9. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Henley JM, Barker EA, Glebov OO. Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci. 2011;34(5):258–68. doi: 10.1016/j.tins.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones AK, Buckingham SD, Sattelle DB. Proteins interacting with nicotinic acetylcholine receptors: expanding functional and therapeutic horizons. Trends Pharmacol Sci. 2010;31(10):455–62. doi: 10.1016/j.tips.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 140.Mao LM, Guo ML, Jin DZ, et al. Post-translational modification biology of glutamate receptors and drug addiction. Front Neuroanat. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mukherjee S, Tessema M, Wandinger-Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ Res. 2006;98(6):743–56. doi: 10.1161/01.RES.0000214545.99387.e3. [DOI] [PubMed] [Google Scholar]
- 142.Smith TH, Sim-Selley LJ, Selley DE. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery? Br J Pharmacol. 2010;160(3):454–66. doi: 10.1111/j.1476-5381.2010.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bunnett NW, Cottrell GS. Trafficking and signaling of G protein-coupled receptors in the nervous system: implications for disease and therapy. CNS Neurol Disord Drug Targets. 2010;9(5):539–56. doi: 10.2174/187152710793361621. [DOI] [PubMed] [Google Scholar]
- 144.Xu ZQ, Zhang X, Scott L. Regulation of G protein-coupled receptor trafficking. Acta Physiol (Oxf) 2007;190(1):39–45. doi: 10.1111/j.1365-201X.2007.01695.x. [DOI] [PubMed] [Google Scholar]
- 145.Fei H, Grygoruk A, Brooks ES, et al. Trafficking of vesicular neurotransmitter transporters. Traffic. 2008;9(9):1425–36. doi: 10.1111/j.1600-0854.2008.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lise S, Buchan D, Pontil M, et al. Predictions of hot spot residues at protein-protein interfaces using support vector machines. PLoS One. 2011;6(2):e16774. doi: 10.1371/journal.pone.0016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fink A, Sal-Man N, Gerber D, et al. Transmembrane domains interactions within the membrane milieu: Principles, advances and challenges. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamem.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 148.Vazquez A. Protein Interaction Networks. 2010. [PubMed]
- 149.Davis FP, Sali A. The overlap of small molecule and protein binding sites within families of protein structures. PLoS Comput Biol. 2010;6(2):e1000668. doi: 10.1371/journal.pcbi.1000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rajasekaran S. Computational techniques for motif search. Front Biosci. 2009;14:5052–65. doi: 10.2741/3586. [DOI] [PubMed] [Google Scholar]
- 151.Zeytuni N, Zarivach R. Structural and functional discussion of the tetra-trico-peptide repeat, a protein interaction module. Structure. 2012;20(3):397–405. doi: 10.1016/j.str.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 152.Koes DR, Camacho CJ. PocketQuery: protein-protein interaction inhibitor starting points from protein-protein interaction structure. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koes DR, Camacho CJ. Small-molecule inhibitor starting points learned from protein-protein interaction inhibitor structure. Bioinformatics. 2012;28(6):784–91. doi: 10.1093/bioinformatics/btr717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arkin MR, Whitty A. The road less traveled: modulating signal transduction enzymes by inhibiting their protein-protein interactions. Curr Opin Chem Biol. 2009;13(3):284–90. doi: 10.1016/j.cbpa.2009.05.125. [DOI] [PubMed] [Google Scholar]
- 155.Strauss HM, Keller S. Pharmacological interference with protein-protein interactions mediated by coiled-coil motifs. Handb Exp Pharmacol. 2008;(186):461–82. doi: 10.1007/978-3-540-72843-6_19. [DOI] [PubMed] [Google Scholar]
- 156.Dietz GP, Bahr M. Synthesis of cell-penetrating peptides and their application in neurobiology. Methods Mol Biol. 2007;399:181–98. doi: 10.1007/978-1-59745-504-6_13. [DOI] [PubMed] [Google Scholar]
- 157.Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta. 2011;1816(2):232–46. doi: 10.1016/j.bbcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 158.Zhao TX, Martinko AJ, Le VH, et al. Development of agents that modulate protein-protein interactions in membranes. Curr Pharm Des. 2010;16(9):1055–62. doi: 10.2174/138161210790963878. [DOI] [PubMed] [Google Scholar]
- 159.Jubb H, Higueruelo AP, Winter A, et al. Structural biology and drug discovery for protein-protein interactions. Trends Pharmacol Sci. 2012 doi: 10.1016/j.tips.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 160.von Kleist L, Haucke V. At the Crossroads of Chemistry and Cell Biology: Inhibiting Membrane Traffic by Small Molecules. Traffic. 2011 doi: 10.1111/j.1600-0854.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 161.Mason JM. Design and development of peptides and peptide mimetics as antagonists for therapeutic intervention. Future Med Chem. 2010;2(12):1813–22. doi: 10.4155/fmc.10.259. [DOI] [PubMed] [Google Scholar]
- 162.Rajadhyaksha M, Boyden T, Liras J, et al. Current advances in delivery of biotherapeutics across the blood-brain barrier. Curr Drug Discov Technol. 2011;8(2):87–101. doi: 10.2174/157016311795563866. [DOI] [PubMed] [Google Scholar]
- 163.Bellavance MA, Blanchette M, Fortin D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008;10(1):166–77. doi: 10.1208/s12248-008-9018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104(1):29–45. doi: 10.1016/j.pharmthera.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 165.Banks WA. Drug delivery to the brain in Alzheimer’s disease: Consideration of the blood-brain barrier. Adv Drug Deliv Rev. 2012;64(7):629–39. doi: 10.1016/j.addr.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Krol S. Challenges in drug delivery to the brain: Nature is against us. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 167.Orthmann A, Fichtner I, Zeisig R. Improving the transport of chemotherapeutic drugs across the blood-brain barrier. Expert Rev Clin Pharmacol. 2011;4(4):477–90. doi: 10.1586/ecp.11.26. [DOI] [PubMed] [Google Scholar]
- 168.Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 2012;64(7):640–65. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 169.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Battaglia L, Gallarate M. Lipid nanoparticles: state of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Deliv. 2012;9(5):497–508. doi: 10.1517/17425247.2012.673278. [DOI] [PubMed] [Google Scholar]
- 171.Rollerova E, Scsukova S, Jurcovicova J, et al. Polymeric nanoparticles - targeted drug delivery systems for treatment of CNS disorders and their possible endocrine disrupting activities. Endocr Regul. 2011;45(1):49–60. [PubMed] [Google Scholar]
- 172.Craparo EF, Bondi ML, Pitarresi G, et al. Nanoparticulate systems for drug delivery and targeting to the central nervous system. CNS Neurosci Ther. 2011;17(6):670–7. doi: 10.1111/j.1755-5949.2010.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]