Abstract
Purpose
Polymicrogyria (PMG) is an epileptogenic malformation of cortical development. We describe the clinical epilepsy and imaging features of a large cohort with PMG-related epilepsy.
Methods
Participants were recruited through the Epilepsy Phenome/Genome Project, a multi-center collaborative effort to collect detailed phenotypic data on individuals with epilepsy. We reviewed phenotypic data from participants with epilepsy and PMG.
Key Findings
We identified 87 participants, 43 female and 44 male, with PMG and epilepsy. Median age of seizure onset was 3 years (range <1 month-37 years). Most presented with focal epilepsy (87.4%), some in combination with seizures generalized from onset (23.0%). Focal seizures with dyscognitive features were most common (54.3%). Of those presenting with generalized seizure types, infantile spasms were most prevalent (45.2%). The most common topographic pattern was perisylvian PMG (77.0%), of which the majority was bilateral (56.7%). Generalized PMG presented with an earlier age of seizure onset (median age of 8 months) and an increased prevalence of developmental delay prior to seizure onset (57.1%). Of the focal, unilateral and asymmetric bilateral groups where PMG was more involved in one hemisphere, the majority (71.4%) of participants had seizures that lateralized to the same hemisphere as the PMG or the hemisphere with greater involvement.
Significance
Participants with PMG had both focal and generalized onset of seizures. Our data confirm the involvement of known topographic patterns of PMG and suggest that more extensive distributions of PMG present with an earlier age of seizure onset and increased prevalence of developmental delay prior to seizure onset.
Keywords: Epilepsy, polymicrogyria, perisylvian, EPGP
Malformations of cortical development are increasingly recognized as the basis for epilepsy, intellectual disability, autism, and a spectrum of neurological deficits (Guerrini et al., 1999; Wegiel et al., 2012). Polymicrogyria (PMG) refers to the excessive gyration or microfolding of the cerebral cortex and is a common clinically encountered cortical malformation, especially in patients with epilepsy (Leventer et al., 1999). PMG may be bilateral or unilateral and may occur in a variety of topographic regions, the most common of which is the perisylvian region (Hayashi et al., 2002; Leventer et al., 2010). Other topographic patterns have previously been described (Chang et al., 2003; Chang et al., 2004; Guerrini et al., 2000; Hayashi et al., 2002; Leventer et al., 2010). The etiology of PMG is likely heterogeneous because multiple environmental and genetic factors may play critical roles.
In a large past series, epilepsy was present in roughly 78% of cases of PMG, suggesting that PMG is a highly epileptogenic lesion (Leventer et al., 2010). However, detailed clinical characteristics of epilepsy related to PMG have not previously been reported in large numbers of patients. Furthermore, few descriptions of epilepsy lateralization and localization in regards to PMG distribution have been published. In this study, we report the clinical features of a cohort of 87 individuals with epilepsy in the setting of PMG. We evaluated whether the suspected region of seizure onset, based on ictal semiology and EEG data, corresponds to the location of PMG seen on MRI. Finally, we report outcomes in the small subset of participants who underwent epilepsy surgery.
Methods
Ascertainment
Participants were recruited through the Epilepsy Phenome/Genome Project (EPGP), a multi-center collaborative effort to collect detailed phenotypic and DNA on a large number of individuals with epilepsy, including a cohort with symptomatic epilepsy related to PMG, with the ultimate goal of establishing genotype-phenotype correlations in epilepsy (The EPGP Collaborative, in press). We retrospectively reviewed phenotypic data, including EEG and MRI data, from participants with epilepsy and PMG from 26 centers in the US, Argentina, Australia, and New Zealand. Each site’s local institutional review board approved the study, and site-specific screening procedures identified prospective participants. Participants with PMG and epilepsy were among those recruited from the clinical centers participating in EPGP.
Inclusion and exclusion criteria
Participants were consented and enrolled in the EPGP study if they met general inclusion and exclusion criteria. All participants had epilepsy (≥2 unprovoked seizures, or one unprovoked seizure with epileptiform EEG confirming epilepsy diagnosis), were at least 4 weeks of age at time of enrollment, and had high quality clinical, imaging, and laboratory data. Specific criteria for PMG participants included having an MRI showing PMG of any type reviewed by the EPGP MRI Core, as well as the absence of a confirmed genetic syndrome or metabolic disease. Participants with mild developmental delay prior to seizure onset, defined as any delay 50% or less of expected milestones in one area (motor, social, language, cognition, or activities of living), or 30% or less of milestones across more than one area, were included in the study. Participants with more severe developmental delay prior to seizure onset were excluded from the EPGP study. Individuals with PMG reported to have been caused by cytomegalovirus or other acquired etiologies were also excluded. According to the developmental and genetic classification for malformations of cortical development (Barkovich et al., 2012), this cohort of sporadic PMG thus comprises a subset of group IIIB, which includes cases of presumed genetic or disruptive PMG without clefts or calcifications. Of the 100 PMG participants enrolled within the EPGP cohort, 13 were excluded from our analysis due to insufficient records or poor quality MRI.
Clinical data
Participants underwent structured interviews to collect demographic information, as well as characteristics of their seizure history and additional medical conditions. Data were also abstracted from participants’ medical records to support the epilepsy diagnosis and provide additional phenotypic information, including details about seizure semiology and laterality, clinical diagnostic evaluation, and presurgical evaluation when applicable. Information on the number of anti-epileptic drug (AED) trials was obtained from the participants’ medical records. At each EPGP Clinical Center, the site principal investigator reviewed all collected clinical data (participant interviews, medical record abstraction, and EEG data) to classify participants’ epilepsy according to seizure type, seizure semiology, and epilepsy syndrome derived from the 2010 International League Against Epilepsy classification criteria (Berg et al., 2010). For this report, sites were specifically queried for additional data regarding surgery evaluation. Presurgical evaluation data were available for 61 participants.
MRI review
After the initial standard EPGP MRI Core review, participant MRIs were reviewed by three MRI Core reviewers (ZF, RK, and Ruben Kuzniecky) independently and later as a group to arrive at a consensus for eligibility, localization of PMG, and additional findings. Topographic extent of the PMG, and symmetry and laterality when applicable, were assessed for each participant, as well as details about white matter, basal ganglia, posterior fossa, and other abnormalities that might be relevant to future genotype-phenotype correlation. Any incidental findings were also documented.
Data analysis
Descriptive statistics were calculated for demographic and clinical data. Data are presented as frequencies and percentages for categorical data, and as means and medians for continuous variables including age at enrollment and age at seizure onset. We compared the location of PMG on MRI to the lateralization of epilepsy based on a synopsis of clinical and EEG data. The relationship between age of seizure onset and extent of PMG found on MRI was examined using a Wilcoxon Two-Sample Test. Epilepsy classification, seizure semiology, and presence of developmental delay were also compared by extent of PMG found on MRI using frequency distributions and Chi Square Test of Independence. For the 9 participants who underwent epilepsy surgery, the Engel scoring system was used to characterize surgical outcome (Engel et al., 1993).
Results
Descriptive results
One hundred participants constituted the EPGP PMG cohort. We describe 87 cases for which good quality MRI data were available. Forty-three participants (49.4%) were female and 44 male. The median age at enrollment was 10 years, with age ranging from less than 1 year to 55 years. The majority was of non-Hispanic ethnicity (81.6%) and Caucasian (73.6%). Head circumference data were available for 56 participants, among whom the majority being normocephalic (40/56, 71.4%); the remaining participants were divided equally among microcephalic and macrocephalic (n=8 for each). Developmental delay prior to the onset of seizures was present in 32 participants (36.8%). Further details regarding descriptive data are presented in Table 1.
Table 1.
Clinical and demographic history of the EPGP PMG cohort.
| Number of participants | 87 | |
| Number of females | 43 | |
| Number of males | 44 | |
| Median age at enrollment | 10 years (1 – 55) | |
| Head circumference data (n=56) | ||
| Microcephalic* | 8 (14.3%) | |
| Normocephalic | 40 (71.4%) | |
| Macrocephalic* | 8 (14.3%) | |
| Developmental delay prior to seizure onset | ||
| Present | 32 (36.8%) | |
| Absent | 46 (52.9%) | |
| Unknown | 9 (10.3%) | |
| Epilepsy-related data | ||
| Median age of seizure onset (range) | 3 years (<1 month – 37 years) | |
| Mean age of seizure onset | 6 years | |
| Number of participants with febrile seizures | 10 (11.5%) | |
| Broad epilepsy classification | participants | percent |
| Focal | 56 | 64.4% |
| Generalized | 6 | 6.9% |
| Both focal and generalized | 20 | 23.0% |
| Unclassified | 5 | 5.7% |
| Epilepsy laterality | participants | percent (out of n=76) |
| Left | 20 | 26.3% |
| Right | 28 | 36.8% |
| Bilateral | 11 | 14.5% |
| Multifocal | 7 | 9.2% |
| Focal, unknown laterality | 10 | 13.2% |
| Seizure type, generalized** | participants | percent (out of n=31)*** |
| Generalized tonic-clonic | 6 | 19.4% |
| Generalized tonic | 7 | 22.6% |
| Generalized convulsive**** | 6 | 19.4% |
| Absence | 2 | 6.5% |
| Atypical absence | 3 | 9.7% |
| Myoclonic | 13 | 41.9% |
| Infantile spasm | 14 | 45.2% |
| Atonic | 2 | 6.5% |
| Generalized nonconvulsive, unclassifiable | 3 | 9.7% |
| Seizure type, focal** | participants | percent (out of n=81)*** |
| Focal evolving to 2° GTC | 9 | 11.1% |
| Focal seizures with dyscognitive features, evolving to 2° GTC | 16 | 19.8% |
| Focal evolving to focal with dyscognitive features, evolving to 2° GTC | 3 | 3.7% |
| 2° GTC, evolution unclassifiable | 25 | 30.9% |
| Focal with dyscognitive features, no evolution | 44 | 54.3% |
| Focal, no evolution | 20 | 24.7% |
| Partial, nonconvulsive, unknown whether focal or focal with dyscognitive features | 18 | 22.2% |
| GTC, unclear whether generalized from onset or 2° generalized | 2 | 6.5% |
| Nonconvulsive seizure, unclear whether partial or generalized from onset | 2 | 2.5% |
| Antiepileptic Medications | ||
| Median number of AED trials, n=83 (range) | 3 (0–14) | |
± >2 standard deviations from the norm
Some participants may have multiple seizure semiologies. Participants with “unclassified” seizure onsets may have contributed to both focal and generalized semiologies.
Generalized seizure group does not include participants with focal epilepsy only. Focal group does not include participants with generalized epilepsy only.
Generalized convulsive indicates a tonic and/or clonic semiology which cannot be further classified.
The median age of seizure onset was 3 years, ranging from less than 1 month to 37 years. Focal seizure semiology, either exclusive or in conjunction with seizures generalized from onset, was the most common form of presentation (64.4%). Focal seizures with dyscognitive features, defined as a focal seizure with impairment of consciousness or awareness, were most common and documented in half of the entire cohort. Of the participants presenting with generalized seizure types, infantile spasms was the most prevalent (45.2%). Six participants had epilepsy that could not be definitively classified as focal or generalized. A history of febrile seizures was present in 11.5% of participants. Further details regarding epilepsy localization and seizure semiology are presented in Table 1. The proportion of participants with developmental delay did not differ significantly by gender (34.1% in females and 39.5% in males). We also did not find any significant differences between female and male participants for age of seizure onset or the extent of PMG on MRI.
Imaging results
MRI findings in this cohort are presented in Table 2. Many participants presented with bilateral PMG (49.4%) and had a perisylvian topographic pattern (77.0%).
Table 2.
Topographic description of polymicrogyria in EPGP cohort.
| Topographic description of polymicrogyria | |
|---|---|
| Perisylvian | 67 (77.0%) |
| Bilateral | 38 (56.7%) |
| Symmetric | 20 (52.6%) |
| Asymmetric | 18 (47.4%) |
| Unilateral | 29 (43.3%) |
| Generalized | 7 (8.05%) |
| Frontal | 4 (4.6%) |
| Frontal only | 3 (75.0%) |
| Fronto-parietal | 1 (25.0%) |
| Parasagittal parieto-occipital, bilateral | 2 (2.3%) |
| Other | 7 (8.05%) |
| Total | 87 |
Unilateral PMG
Thirty-seven participants had unilateral PMG lesions. We divided these into cases with only a single lobe involved versus multi-lobar involvement. There were 11 participants with focal PMG involving a single lobe. All but one of these participants presented with focal epilepsy (n=10, 90.9%); this individual had a combined focal and generalized epilepsy presentation. One participant had unclassified epilepsy localization. The median age of seizure onset in this series was 11 years (range 1 month – 28 years.) Of the six participants with lesions in the left hemisphere, four had concordant epilepsy lateralization. Of the five lesions on the right, three had concordant lateralization. Lesions were localized to the perisylvian region in five, dorsolateral parietal lobe in two, dorsolateral frontal lobe in one, fronto-parietal lobe in one, mesial temporal lobe in one, and lateral temporal lobe in one. Focal seizures with dyscognitive features predominated (n=7, 63.6%). Fewer participants had nonconvulsive focal seizures with unknown evolution (n=4, 36.4%) or focal seizures without dyscognitive features (n=3, 27.3.%). Developmental delay prior to seizure onset was noted in two participants (18.2%).
Of the 37 unilateral cases, 26 had multi-lobar PMG lesions. Twenty-five (96.2%) had focal epilepsy, of whom four also had seizures generalized from onset. One participant had unclassified seizures. The median age of onset was 3 years (range <1 month to 21 years). Nineteen participants had right-sided lesions (73.1%), of whom 16 had concordant laterality of seizure onsets (84.2%). Seven had left-sided lesions (26.9%), of which six were concordant (85.7%). Again, focal seizures with dyscognitive features predominated (n=14, 53.9%). Other seizure types observed included secondary generalized seizures with unknown evolution (n=9, 34.6) and focal seizures without dyscognitive features (n=8, 30.8%). Of note, the most common generalized seizure type was infantile spasms (n=3). Developmental delay prior to seizure onset was present in 6 participants (23.1%).
Bilateral PMG
Of the forty-three participants with bilateral lesions, 37 had focal epilepsy (86.0%). Thirteen of these participants also presented with seizures generalized from onset. Four participants had generalized epilepsy (9.3%) and two had unclassified seizure onsets. The median age of onset was 3 years (range <1 month – 37 years.) Twenty-four participants had symmetric lesions (55.8%). Of note, ten of these participants presented with focal epilepsy. Of the nineteen with asymmetric lesions, eleven (57.9%) had more extensive PMG on the right side. Six (54.5%) of these participants had concordant epilepsy laterality. Eight (42.1%) had more extensive PMG on the left. Epilepsy was localized to the same side in five (62.5%) of these participants. Focal seizures with dyscognitive features were most common (n=20, 46.5%), followed by secondarily generalized seizures with unknown evolutions (n=15, 34.9%). The most common generalized seizure types were epileptic spasms and myoclonic (n=7 for each type.) Twenty participants had developmental delay prior to seizure onset (46.5%).
Generalized PMG
Seven participants had generalized PMG, among whom one had white matter abnormalities. Four had generalized epilepsy (57.1%), two of whom also presented with seizures with focal onset. Two participants had focal epilepsy (28.6%) and one had unclassified epilepsy. The median seizure onset was 8 months (range 1 month – 7 years). Myoclonic seizures were noted in four, infantile spasms were noted in three, and focal seizures with dyscognitive features were noted in three participants. Developmental delay prior to seizure onset was documented in four of the seven participants (57.1%).
Other MRI findings
Additional subcortical findings included unilateral (n=16) and bilateral (n=14) enlarged ventricles, small or hypoplastic brainstem (n=3), small or hypoplastic cerebellum (n=2), hypoplastic basal ganglia (n=1), and corpus callosum abnormalities (n=3). A proportion of PMG malformations included relative hypoplasia of cortical volume in the cortex of affected regions (n=10). Other MRI findings noted were white matter abnormalities (n=4) and cavum septum pellucidum (n=3). Two participants had periventricular heterotopia (n=2), one of whom also had subcortical heterotopia. Non-periventricular subcortical heterotopia was noted in two. One participant had evidence of encephalomalacia.
Epilepsy concordance and surgery
We defined “concordance” as an agreement between epilepsy laterality based on seizure semiology and EEG (with most weight given to ictal EEG, when available) and cerebral hemisphere with PMG based on MRI. Of the focal, unilateral and asymmetric bilateral groups where PMG was lateralized to one hemisphere, the majority (71.4%) of participants had seizures with laterality concordant with the laterality of the PMG involved exclusively or predominantly.
Of the participants with data available regarding surgery evaluation (n=61), 25 underwent formal presurgical evaluation. Four of these participants had focal PMG, for which surgery was completed in three. One participant did not undergo surgery as adequate medical control of seizures was obtained. Nine participants had unilateral PMG, five of whom underwent surgery. Of those who did not, surgery was not done due to extensive malformation and lack of anticipated efficacy (n=3), or because evaluation was underway at time of data collection (n=1). Eleven participants had bilateral PMG, of whom one underwent surgery. Surgery was not done in this group due to bilateral/multifocal seizure onsets (n=5), adequate medical seizure control (n=2), extensive malformation without a single seizure focus (n=2), or concern for risk of deficits (n=1). One participant with generalized PMG underwent presurgical evaluation but was not considered a good candidate for surgery due to the extensive malformation and lack of a single seizure focus.
Nine participants underwent epilepsy surgery. In these participants, surgery consisted of partial or total lesionectomy. The median follow-up duration was 1 year (range 1 month-21 years). Among those who had surgery, seven had an outcome consistent with Engel score I, with significant post-surgery seizure reduction. One of these participants presented with bilateral PMG, but the source of ictal onset was sufficiently localized to a single region. One participant had no improvement of seizures after surgery (Engel score III). One, who underwent a minimal resection considering the extent of malformation on MRI, had worsening of seizures after surgery (Engel score IV). Additional data related to the presurgical evaluation of these participants is available in the Supplementary Table.
Discussion
In a large cohort of patients with PMG, we found that bilateral distributions were most common, with a perisylvian pattern predominating. There was a trend towards more right-sided involvement in unilateral and bilateral asymmetric cases. We found that most participants with lateralized PMG have seizure onset with concordant lateralization. Generalized PMG patterns tended to have an earlier seizure onset, and we observed a later age of seizure onset for focal PMG involving a single lobe. There was also a trend toward increasing prevalence of developmental delay prior to seizure onset as PMG distribution becomes more extensive. In contrast to a previously published case series of PMG, however, we did not find any overall gender differences in our series of epilepsy participants (Hayashi et al., 2002; Leventer et al., 2010).
PMG is strongly associated with epilepsy, with an incidence of 33% to 87% (Castano de la Mota et al., 2011; Kuzniecky et al., 1994a; Leventer et al., 2010; Teixeira et al., 2007). As observed in our study, epilepsy in PMG typically begins in mid-childhood, although it can also present in infancy or adulthood, as has been observed previously (Guerrini et al., 1998; Luders et al., 1998). In addition, our finding of an association of PMG with epileptic spasms and myoclonic seizures is consistent with prior reports (Brodtkorb et al., 1992; Luders et al., 1998). This constellation of seizure types has been specifically associated with congenital bilateral perisylvian PMG (Baykan-Kurt et al., 1997; De Coene et al., 2010; Kuzniecky et al., 1994b). The overall distribution of PMG in our cohort closely resembles that of previous descriptive studies of PMG, including the prevalence of previously described PMG patterns (Chang et al., 2003; Chang et al., 2004; Guerrini et al., 2000; Hayashi et al., 2002; Leventer et al., 2010). However, previous reports of PMG patients have not shown a trend in more right-sided involvement in unilateral and bilateral asymmetric cases.
There was a general trend in our cohort towards having a younger age of seizure onset in more extensive PMG distributions, although the differences did not reach statistical significance. The median age of seizure onset was younger in participants with generalized PMG than those with other forms of PMG. Similarly, participants with focal PMG showed a trend towards an older age of seizure onset. Developmental delay before seizure onset also tended to be more common in participants with more extensive PMG, although not statistically significant. In a previously published series of 26 patients with PMG, patients with bilateral involvement had a higher prevalence of motor delay, intellectual disability, and speech problems than those with unilateral involvement and were also noted to have an earlier age of epilepsy onset (Mavili et al., 2012).
Initial concerns regarding the efficacy of surgery in PMG emerged following case reports of surgery failure in participants with this malformation (Brodtkorb et al., 1998; Guerrini et al., 1998). One source of debate concerns whether the site of cortical malformation corresponds to epileptogenic foci (Sisodiya, 2000). In contrast to these findings, a number of case reports have shown more favorable outcomes following surgery (Chang et al., 2011; RamachandranNair et al., 2006). Factors postulated to result in seizure free outcome in surgical treatments of cortical malformations, obtained from a retrospective study of 143 patients, include grey-white blurring on MRI, smaller lesion size, and complete resection of both structural and electrographically abnormal areas (Chang et al., 2011).
We assessed the degree of concordance between laterality of seizure onset based on semiology and EEG data and laterality of PMG on MRI. As not all participants underwent formal presurgical evaluation mostly due to assumed adequate seizure control, extensive malformation with lack of a single seizure focus, or lack of the possibility of undertaking a pre-surgical evaluation at the clinical site; precise seizure onset localization information for many cases was not available. In most cases, the laterality of the visible lesion and the epilepsy laterality were concordant. Additionally, review of our surgical cases demonstrated that in carefully selected cases of epilepsy secondary to PMG, surgery may provide a favorable outcome. All but two participants who underwent surgery were seizure-free or had a major reduction in seizure frequency following surgery at their most recent follow-up. For all of these participants, the area of resection included the PMG lesion. Future attempts at genetically defining individuals with PMG may lead to better predictions of outcome from epilepsy surgery.
The data from this study were collected from multiple tertiary care centers and may be subject to referral bias, as well as information bias. Misclassification of epilepsy localization may have occurred in a small fraction of cases. Since all participants in our cohort had epilepsy, the clinical and imaging features of PMG without epilepsy can not be applied to all individuals with PMG. Because individuals with known genetic disorders were not included in the EPGP cohort, the PMG data thus acquired may not be applicable to these excluded participants.
In the future, these data should be correlated with genetic analysis of our PMG cohort to identify mutations that may correspond with specific phenotypes. In addition, prospective research should be conducted to evaluate the response of patients with PMG to medication based on both phenotypic and genotypic data. Further study is also needed to understand the specific association between the genetic mutations leading to PMG and their subsequent phenotypic expressions.
Supplementary Material
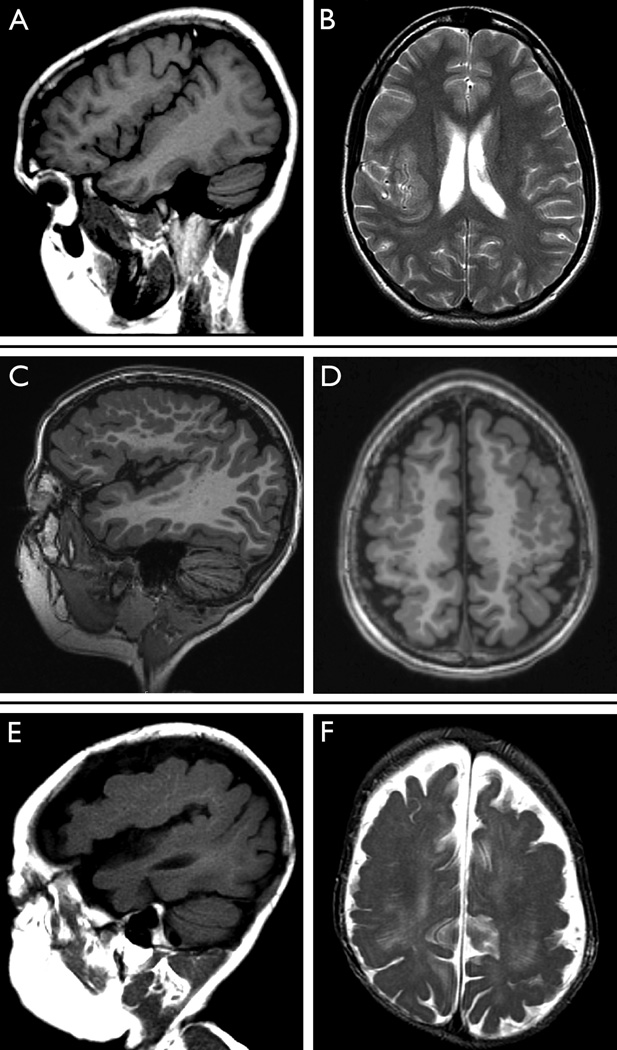
Figure. Polymicrogyria—focal, bilateral, and generalized.
We present representative images from the MRIs of three cases with PMG. First, sagittal T1-weighted (A) and axial T2-weighted (B) images from an 18-year-old young woman with unilateral right-sided PMG involving the perisylvian region are shown. Epilepsy began at 18 years with focal seizures with dyscognitive features. There had been no history of developmental delay prior to seizure onset.
Sagittal (C) and axial (D) images from a 5-year-old male with bilateral perisylvian PMG. Extensive involvement of the frontal lobes can be seen in the axial image (D), and bilateral enlargement of ventricles is also present. Seizures presented at 1 year of age with focal seizures with dyscognitive features and secondary generalization. The participant had developmental delay prior to seizure onset.
Finally, sagittal (E) and axial (F) images from an 18-month-old male illustrate generalized PMG. Ventricular enlargement and hypoplastic brainstem is also noted. Focal seizures with secondary generalization seizures presented at 1 month of age in the setting of severe chronic static encephalopathy.
ACKNOWLEDGEMENTS
Supported by NINDS U01 NS 053998, as well as planning grants from the Finding a Cure for Epilepsy and Seizures (FACES) Foundation and the Richard Thalheimer Philanthropic Fund. Annapurna Poduri is supported by the NINDS (K23NS069784).
We would like to acknowledge the recruitment contributions of the EPGP Community Referral Network (CRN). The CRN consists of healthcare professionals not paid by the EPGP grant who refer eligible families to EPGP. A list of individual contributors can be found at www.epgp.org. We are also grateful for the efforts of the clinical coordinators, the site principal investigators, neurologists, and support staff at our EPGP clinical centers who have contributed to the recruitment, data acquisition and storage, and extensive phenotyping. Finally, and most importantly, we extend our sincere appreciation to the patients with epilepsy and their families who have contributed to this research effort.
Appendix 1: The EPGP Investigators (Excluding authors listed above)
Bassel Abou-Khalil, MD; Data Review Core, Local PI; Vanderbilt University Medical Center
Brian Alldredge, PharmD; AED Core; University of California, San Francisco
Jocelyn Bautista, MD; Local PI; Cleveland Clinic
Sam Berkovic, MD; Local PI; The University of Melbourne
Judith Bluvstein, MD; Data Review Core; New York University School of Medicine
Alex Boro, MD; EEG Core; Albert Einstein College of Medicine
Gregory Cascino, MD; MRI Core, Local PI; Mayo Clinic College of Medicine Rochester, MN
Damian Consalvo, MD, PhD; Local PI; Hospital General de Agudos José Maria Ramos Mejía
Patricia Crumrine, MD; Local PI; Children's Hospital of Pittsburgh of UPMC
Orrin Devinsky, MD; Phenotyping Core, Local PI; New York University School of Medicine
Dennis Dlugos, MD, MCSE; EEG Core, Phenotyping Core, Local PI; The Children’s Hospital of Philadelphia
Michael Epstein, PhD; Data Analysis Core; Emory University School of Medicine
Miguel Fiol, MD; Referral Center PI; University of Minnesota Medical Center
Nathan Fountain, MD; Data Review Core, Local PI; University of Virginia Health System
Jacqueline French, MD; AED Core; New York University School of Medicine
Daniel Friedman, MD; Local Co-PI; New York University School of Medicine
Eric Geller, MD; Local Co-PI; St. Barnabas Health Care System
Tracy Glauser, MD; AED Core, Local PI; Cincinnati Children's Hospital Medical Center
Simon Glynn, MD; Local PI; University of Michigan
Kevin Haas, MD, Local PI; Vanderbilt University Medical Center
Sheryl Haut, MD, MS; Local PI; Albert Einstein College of Medicine
Jean Hayward, MD; Referral Center PI; Kaiser Permanente: Oakland Medical Center
Sandra Helmers, MD; Local PI; Emory University School of Medicine
Sucheta Joshi, MD; EEG Core; University of Michigan
Andres Kanner, MD; AED Core; Rush University Medical Center
Heidi Kirsch, MD, MS; Local PI; University of California, San Francisco
Eric Kossoff, MD; Local Co-PI; The Johns Hopkins University School of Medicine
Rachel Kuperman, MD; Local Referral Center PI; Children’s Hospital & Research Center Oakland
Ruben Kuzniecky, MD; Study PI; New York University School of Medicine
Daniel Lowenstein, MD; Study PI; University of California, San Francisco
Shannon McGuire, MD; Local PI; Louisiana State University Health Sciences Center
Paul Motika, MD; Local Co-PI; Rush University Medical Center
Edward Novotny, MD; Local PI; Seattle Children's Hospital
Ruth Ottman, PhD; Phenotyping Core, Data Analysis Core, Local PI; Columbia University
Juliann Paolicchi, MD; Local PI; Vanderbilt University Medical Center
Jack Parent, MD; Local Co-PI; University of Michigan
Kristen Park, MD; Local PI; The Children's Hospital Denver
Neil Risch PhD; Data Analysis Core; University of California, San Francisco
Lynette Sadleir, MBChB, MD; Local PI; Wellington School of Medicine and Health Sciences, University of Otago
Ingrid Scheffer, MBBS, PhD; Data Review Core, Local PI; The University of Melbourne
Renee Shellhaas, MD; EEG Core; University of Michigan
Elliot Sherr, MD, PhD; Phenotyping Core; University of California, San Francisco
Jerry Shih, MD; Data Review Core, Local PI; Mayo Clinic College of Medicine Jacksonville, FL
Shlomo Shinnar, MD, PhD; Phenotyping Core; Albert Einstein College of Medicine
Rani Singh, MD; Local Co-PI; University of Michigan
Joseph Sirven, MD; Local PI; Mayo Clinic College of Medicine Scottsdale, Arizona
Michael Smith, MD; Local PI; Rush University Medical Center
Joe Sullivan, MD; EEG Core; University of California, San Francisco
Liu Lin Thio, MD, PhD; Local PI; Washington University in St. Louis
Anu Venkat, MD; Local Co-PI; The Children’s Hospital of Philadelphia
Eileen Vining, MD; Local PI; The Johns Hopkins University School of Medicine
Gretchen Von Allmen, MD; Local PI; University of Texas Health Science Center at Houston
Judith Weisenberg, MD; Local PI; Washington University in St. Louis
Peter Widdess-Walsh, MD; Local PI and Data Review Core; St. Barnabas Health Care System
Melodie R. Winawer; Data Review Core, Data Analysis Core; Columbia University
Emily Acton; Local Study Staff; The Children’s Hospital of Philadelphia
Samantha Hagopian, RN; Local Study Staff; The Children’s Hospital of Philadelphia
Sarah Sanchez; Local Study Staff; The Children’s Hospital of Philadelphia
Administrative and Informatics Core Members contributing to the manuscript:
Catharine Freyer Karn, Project Director
Kristen Schardein Fox, RN, MS, Recruitment Director
Sabrina Cristofaro, RN, BSN, Phenotyping Director
Robyn Fahlstrom, MPH, Data Manager
Informatics Core Members:
Gerry Nesbitt, Director of Informatics
Footnotes
DISCLOSURES:
Catherine Shain, Sriram Ramgopal, Zianka Fallil, Isha Parulkar, Richard Alongi, Robert Knowlton, and Annapurna Poduri have nothing to disclose.
ETHICS STATEMENT: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykan-Kurt B, Sarp A, Gokyigit A, Tuncay R, Caliskan A. A clinically recognizable neuronal migration disorder: congenital bilateral perisylvian syndrome. Case report with long-term clinical and EEG follow-up. Seizure. 1997;6:487–493. doi: 10.1016/s1059-1311(97)80026-x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Brodtkorb E, Andersen K, Henriksen O, Myhr G, Skullerud K. Focal, continuous spikes suggest cortical developmental abnormalities. Clinical, MRI and neuropathological correlates. Acta Neurol Scand. 1998;98:377–385. doi: 10.1111/j.1600-0404.1998.tb07317.x. [DOI] [PubMed] [Google Scholar]
- Brodtkorb E, Nilsen G, Smevik O, Rinck PA. Epilepsy and anomalies of neuronal migration: MRI and clinical aspects. Acta Neurol Scand. 1992;86:24–32. doi: 10.1111/j.1600-0404.1992.tb08049.x. [DOI] [PubMed] [Google Scholar]
- Castano de la Mota C, Rojas ML, Penas JJ, Gero ML, Rodriguez AD, Pino MA. [Polymicrogyria: epidemiology, neurological and anatomical factors and clinical outcome in a series of 34 cases] An Pediatr (Barc) 2011;75:358–364. doi: 10.1016/j.anpedi.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Chang BS, Piao X, Bodell A, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, Voit T, Grant PE, Barkovich AJ, Walsh CA. Bilateral frontoparietal polymicrogyria: clinical and radiological features in 10 families with linkage to chromosome 16. Ann Neurol. 2003;53:596–606. doi: 10.1002/ana.10520. [DOI] [PubMed] [Google Scholar]
- Chang BS, Piao X, Giannini C, Cascino GD, Scheffer I, Woods CG, Topcu M, Tezcan K, Bodell A, Leventer RJ, Barkovich AJ, Grant PE, Walsh CA. Bilateral generalized polymicrogyria (BGP): a distinct syndrome of cortical malformation. Neurology. 2004;62:1722–1728. doi: 10.1212/01.wnl.0000125187.52952.e9. [DOI] [PubMed] [Google Scholar]
- Chang EF, Wang DD, Barkovich AJ, Tihan T, Auguste KI, Sullivan JE, Garcia PA, Barbaro NM. Predictors of seizure freedom after surgery for malformations of cortical development. Ann Neurol. 2011;70:151–162. doi: 10.1002/ana.22399. [DOI] [PubMed] [Google Scholar]
- De Coene A, Van Coster R, Verhelst H. Perisylvian polymicrogyria, infantile spasms and arthrogryposis: the severe end of the spectrum of congenital bilateral perisylvian polymicrogyria. Eur J Paediatr Neurol. 2010;14:270–273. doi: 10.1016/j.ejpn.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Engel JJ, Van Ness P, Rasmussen T, Ojemann L. Outcome with respect to epileptic seizures. In: Engel JJ, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- Guerrini R, Barkovich AJ, Sztriha L, Dobyns WB. Bilateral frontal polymicrogyria: a newly recognized brain malformation syndrome. Neurology. 2000;54:909–913. doi: 10.1212/wnl.54.4.909. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Canapicchi R, Dobyns WB. Epilepsy and malformations of the cerebral cortex. Neurologia. 1999;14(Suppl 3):32–47. [PubMed] [Google Scholar]
- Guerrini R, Genton P, Bureau M, Parmeggiani A, Salas-Puig X, Santucci M, Bonanni P, Ambrosetto G, Dravet C. Multilobar polymicrogyria, intractable drop attack seizures, and sleep-related electrical status epilepticus. Neurology. 1998;51:504–512. doi: 10.1212/wnl.51.2.504. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Tsutsumi Y, Barkovich AJ. Polymicrogyria without porencephaly/schizencephaly. MRI analysis of the spectrum and the prevalence of macroscopic findings in the clinical population. Neuroradiology. 2002;44:647–655. doi: 10.1007/s00234-002-0793-z. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Andermann F, Guerrini R. The epileptic spectrum in the congenital bilateral perisylvian syndrome. CBPS Multicenter Collaborative Study. Neurology. 1994a;44:379–385. doi: 10.1212/wnl.44.3_part_1.379. [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Andermann F, Guerrini R. Infantile spasms: an early epileptic manifestation in some patients with the congenital bilateral perisylvian syndrome. J Child Neurol. 1994b;9:420–423. doi: 10.1177/088307389400900418. [DOI] [PubMed] [Google Scholar]
- Leventer RJ, Jansen A, Pilz DT, Stoodley N, Marini C, Dubeau F, Malone J, Mitchell LA, Mandelstam S, Scheffer IE, Berkovic SF, Andermann F, Andermann E, Guerrini R, Dobyns WB. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010;133:1415–1427. doi: 10.1093/brain/awq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventer RJ, Phelan EM, Coleman LT, Kean MJ, Jackson GD, Harvey AS. Clinical and imaging features of cortical malformations in childhood. Neurology. 1999;53:715–722. doi: 10.1212/wnl.53.4.715. [DOI] [PubMed] [Google Scholar]
- Luders H, Acharya J, Baumgartner C, Benbadis S, Bleasel A, Burgess R, Dinner DS, Ebner A, Foldvary N, Geller E, Hamer H, Holthausen H, Kotagal P, Morris H, Meencke HJ, Noachtar S, Rosenow F, Sakamoto A, Steinhoff BJ, Tuxhorn I, Wyllie E. Semiological seizure classification. Epilepsia. 1998;39:1006–1013. doi: 10.1111/j.1528-1157.1998.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Mavili E, Coskun A, Per H, Donmez H, Kumandas S, Yikilmaz A. Polymicrogyria: correlation of magnetic resonance imaging and clinical findings. Childs Nerv Syst. 2012;28:905–909. doi: 10.1007/s00381-012-1703-2. [DOI] [PubMed] [Google Scholar]
- RamachandranNair R, Otsubo H, Ochi A, Rutka J, Donner EJ. Mirror movements following cortical resection of polymicrogyria in a child with intractable epilepsy. Pediatr Neurol. 2006;34:135–138. doi: 10.1016/j.pediatrneurol.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Sisodiya SM. Surgery for malformations of cortical development causing epilepsy. Brain. 2000;123(Pt 6):1075–1091. doi: 10.1093/brain/123.6.1075. [DOI] [PubMed] [Google Scholar]
- Teixeira KC, Montenegro MA, Cendes F, Guimaraes CA, Guerreiro CA, Guerreiro MM. Clinical and electroencephalographic features of patients with polymicrogyria. J Clin Neurophysiol. 2007;24:244–251. doi: 10.1097/WNP.0b013e31803bb792. [DOI] [PubMed] [Google Scholar]
- The EPGP Collaborative. The Epilepsy Phenome/Genome Project. Clinical Trials: Journal of the Society for Clinical Trials. (in press) [Google Scholar]
- Wegiel J, Schanen NC, Cook EH, Sigman M, Brown WT, Kuchna I, Nowicki K, Imaki H, Ma SY, Marchi E, Wierzba-Bobrowicz T, Chauhan A, Chauhan V, Cohen IL, London E, Flory M, Lach B, Wisniewski T. Differences between the pattern of developmental abnormalities in autism associated with duplications 15q11.2–q13 and idiopathic autism. J Neuropathol Exp Neurol. 2012;71:382–397. doi: 10.1097/NEN.0b013e318251f537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.