Abstract
Objective
Associations between vascular disease and depression in late life, including increased white matter hyperintensities (WMHs), have been reported. Whether depression is an etiology or a consequence of vascular disease is still unknown. We investigated the temporal relationship between depressive symptoms and WMHs in older men and women.
Methods
We utilized data from 90 dementia-free older adults (39 women, 51 men), 57 years of age and older at baseline, from the neuroimaging substudy of the Baltimore Longitudinal Study of Aging. Participants were followed for up to 8 years. Ratings of white matter disease burden were available for the first, last, and at least one interim visit, and participants completed the Center for Epidemiologic Studies Depression Scale (CES-D) annually. Statistical models, performed separately in men and women, examined whether depressive symptoms predicted subsequent WMH ratings or WMHs predicted subsequent depressive symptoms.
Results
The total CES-D score was not associated with WMHs in men or women. In men, the CES-D depressed mood subscale predicted accelerating longitudinal increases in WMHs at older ages, but WMHs did not predict subsequent depressive symptoms. In women, there were no significant associations between the CES-D depressed mood subscale and WMHs.
Conclusions
White matter disease may be a consequence of depressed mood in men but not in women. Intervention strategies for depression may slow the progression of white matter disease in older men. These results add to previous findings documenting sex differences in the correlates of depressive disorders in late life.
Keywords: depression, aging, elderly, vascular disease, sex differences, longitudinal studies
A relationship between late-life depression and vascular disease has been widely reported (Kales et al., 2005; Musselman et al., 1998; Thomas et al., 2004). Patients with vascular diseases such as hypertension, diabetes, and coronary artery disease have disproportionately higher rates of depression compared to the general population and individuals with other medical conditions (Katz et al., 1994; Krishnan et al., 2002; Kumar et al., 1997; Lyness et al., 1996; Miller et al., 1996; Tiemeier et al., 2004). High rates of depression following stroke (Angelelli et al., 2004; Burvill et al., 1995; Folstein et al., 1977; Pohjasvaara et al., 1998; Whyte et al., 2004) and coronary artery bypass grafting (Reichenberg et al., 2007) also suggest a link between vascular disease and depression. Moreover, neuroimaging studies have documented evidence of compromised white matter integrity (Bae et al., 2006; Krishnan and McDonald, 1995; Taylor et al., 2004; Nobuhara et al., 2006) in older depressed adults, including the presence of increased white matter hyperintensities (WMHs) in subcortical, cortical, and periventricular regions (Chen et al., 2006; Hickie et al., 1997; Krishnan, 2002; MacFall et al., 2006; Nebes et al., 2001; O'Brien et al., 1996; Simpson et al., 1997; Taylor et al., 2005). Consistent with these in vivo observations, autopsy studies reveal increased vascular lesions in the brains of deceased older depressed patients compared to non-depressed older adults (Thomas et al., 2001). These associations are not surprising given the evidence that vascular disease disrupts frontal-subcortical neural circuits (Schmahmann et al., 2008), which are thought to be involved in the etiology of depression (Drevets, 2000; Mayberg, 1997).
These findings support the vascular depression hypothesis (Alexopoulos et al., 1997; Krishnan et al., 1997), which proposes that cerebrovascular diseases, particularly those involving fronto-striatal circuitry, can predispose, precipitate, or perpetuate depression. Although this hypothesis posits that vascular disease precedes depression, there is also evidence that depression may predispose individuals to vascular disease or exacerbate pre-existing vascular disease (Baldwin, 2005; Everson et al., 1998; Frasure-Smith et al., 1999; Kilbourne et al., 2005; Lenze et al., 1999; Musselman et al., 1996; Pratt et al., 1996; Wassertheil-Smoller et al., 2004; Ziegelstein et al., 2000; Jones et al., 2003). Moreover, vascular disease and depression may share etiologies, such as atherosclerosis or genetic risk (Alexopoulos, 2003; Krishnan et al., 1996; Steffens et al., 2003). Consequently, there is increasing recognition that the relationship between depression and vascular disease may be bidirectional.
Previous studies have found sex differences in the correlates of depression, including cognitive deficits, risk for dementia, and structural and functional brain changes (Dal Forno et al., 2005; Dotson et al., 2009a; Fuhrer et al., 2003; Taki et al., 2005; Videbech et al., 2002; Lavretsky et al., 2004; Ng et al., 2009). DS were found to have a significant cross-sectional association with WMHs and silent infarcts in women but not in men in one study (Wendell et al., 2010). Sex differences in the longitudinal association between DS and WMHs have not been determined, but based on the preponderance of evidence suggesting that men are more vulnerable to the adverse impact of depression than are women, we would expect to have stronger associations in men than in women. To address this issue, we use longitudinal data from the neuroimaging substudy of the Baltimore Longitudinal Study of Aging (BLSA) to test the bidirectionality hypothesis in older men and women by examining whether subthreshold depressive symptoms (DS) precede or follow increases in white matter disease as measured by WMHs on magnetic resonance imaging (MRI) scans.
Methods
Participants
Participants were drawn from the neuroimaging substudy of the BLSA, a prospective longitudinal study of brain changes in normal and pathological aging (Resnick et al., 2000). Beginning in 1994, participants received annual neuroimaging, cognitive, and clinical evaluations through the ninth evaluation. Participants were ages 55–85 at baseline and at enrollment were without CNS disease (epilepsy, stroke, bipolar illness, previous diagnosis of dementia), severe cardiovascular disease (myocardial infarction, coronary artery disease requiring angioplasty or bypass surgery), severe pulmonary disease or metastatic cancer. Medical diagnoses were based on medical record review, medication review, and self-report.
Of the 158 participants initially enrolled in the study, 102 had available WMH ratings. Because our DS variable was based on averaging two or more successive scores on the Center for Epidemiologic Studies Depression (CES-D) scale, we excluded participants who did not have at least 3 WMH ratings based on visits in nonconsecutive years (n = 9). We also excluded individuals who developed dementia during the study interval (n = 3). As described in detail elsewhere (Kawas et al., 2000), diagnoses of Alzheimer’s disease and other dementias are determined at consensus conferences using Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised criteria for dementia and the National Institute of Neurological and Communication Disorders-Alzheimer’s Disease and Related Disorders Association criteria for Alzheimer’s disease (McKhann et al., 1984). Thus, we present data on 90 dementia-free individuals (mean age at baseline = 69.10, SD = 6.54; 51 men, 39 women), including up to 8 years of follow-up (mean = 7.82, SD = 0.59). Nine participants (5 women, 4 men) reported a history of major depression. No other major psychiatric disorder was reported. Participant demographic information is provided in Table 1.
Table 1.
Sample Characteristics
| Women (N = 39) | Men (N = 51) | Total (N = 90) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Baseline age (years) | 68.85 | 6.94 | 69.29 | 6.27 | 69.10 | 6.54 |
| Education (years) | 16.26 | 2.42 | 16.55 | 2.66 | 16.42 | 2.55 |
| Total follow-up interval (years) | 7.92 | .59 | 7.75 | .59 | 7.82 | .59 |
| Interval between WMH ratings (years) | 3.96 | .29 | 3.88 | .29 | 3.91 | .30 |
| CES-D score | 8.73 | 3.88 | 7.83 | 3.91 | 8.22 | 3.90 |
| Mood subscale score | 1.65 | 2.34 | 1.42 | 2.19 | 1.52 | 2.26 |
| Somatic complaints subscale score | 3.97 | 3.78 | 3.49 | 2.86 | 3.70 | 3.29 |
| Lack of positive wellbeing subscale score | 2.53 | 3.91 | 2.41 | 3.58 | 2.46 | 3.72 |
| Interpersonal problems subscale score* | .60 | 1.21 | .48 | 1.07 | .53 | 1.14 |
| WMH rating | 1.82 | 1.17 | 1.98 | 1.20 | 1.91 | 1.19 |
| N | % | N | % | N | % | |
| Handedness (Right) | 37 | 94.87 | 50 | 98.04 | 87 | 96.67 |
| Race (White) | 33 | 84.62 | 47 | 92.16 | 80 | 88.89 |
| Diabetesa | 1 | .03 | 9 | 17.65 | 10 | 11.11 |
| Hypertension*a | 16 | 41.03 | 34 | 66.67 | 50 | 55.56 |
| Heart Diseaseb | 5 | 12.82 | 18 | 35.29 | 23 | 25.56 |
Note. WMH = white matter hyperintensity; CES-D = Center for Epidemiologic Studies Depression Scale. Heart disease included myocardial infarction, coronary artery disease, and congestive heart failure.
p <.05
Value represents the number of cases at baseline and incident cases.
Values represent the number of participants who developed medical conditions during the study interval.
Depressive Symptom Measurement
At each annual visit, participants completed the 20-item CES-D (Radloff, 1977), a measure of the severity of DS reported during the past week. This scale has been validated in older adults (Beekman et al., 1997; Haringsma et al., 2004) and is commonly used in longitudinal and epidemiological studies. Factor analytic studies have elucidated four subscales within the CES-D: depressed mood, somatic complaints, lack of positive well-being, and interpersonal problems (Hertzog et al., 1990; Radloff, 1977). Depending on the statistical analysis, the mean of each participant’s CES-D scores in the interval preceding or following each WMH rating was used as a continuous predictor variable, as described in more detail below.
Image Acquisition and Ratings of WMHs
As described in detail elsewhere (Resnick et al., 2000), MRI scanning was performed on a GE Signa 1.5 Tesla scanner (Milwaukee, WI) and included oblique axial interleaved spin echo proton density and T2-weighted images (repetition time (TR) = 3000, echo time (TE) = 34/100, field of view (FOV) = 24 cm, matrix = 256×192, number of excitations (NEX) = 0.5, 5-mm slice thickness; oriented parallel to the anterior–posterior intercommissural line), and axial and coronal three-dimensional spoiled gradient refocused images. The proton density and T2-weighted images were used to evaluate white matter disease burden. WMH ratings as a measure of vascular lesion burden were based on a validated protocol from the Cardiovascular Health Study (CHS; Manolio et al., 1994; Yue et al., 1997). WMH ratings based on this scale range from 0 (virtually normal white matter) to 9 (presence of a very thick rind of confluent periventricular white matter disease along with marked subcortical disease). Ratings were made by an experienced board certified neuroradiologist (MAK), who was masked to participant identity, demographic characteristics, and year of scan, and had extensive experience using the WMH scale under the auspices of the CHS.
All participants had at least 3 MRI scans rated for WMH severity. Because we used an average measure of CES-D scores that preceded or followed WMH ratings, we only included ratings that were at least two years apart in time. For most participants, ratings were performed on MRI scans from visits 1, 5, and 9, though the interval between WMH ratings varied somewhat across subjects. Ratings were on average 3.91 (SD = 0.30) years apart and the interval between the first and last rating ranged from 5.98 to 8.39 years (mean = 7.82, SD = 0.59).
Statistical Analyses
Data were analyzed using mixed-effects regression models performed in SAS 9.1 (SAS Institute, Cary, NC). Separate models were performed in men and women to determine whether there are sex differences in the relationship between DS and WMHs. Two sets of models were performed separately in men and women.
Model 1
In one model, WMH ratings predicted DS in the subsequent interval, i.e., visits between that rating and the next rating. For example, the WMH rating at year 1 would predict the mean of the CES-D scores at years 2, 3, 4 and 5, and the WMH rating at year 5 would predict the mean of the CES-D scores at years 6, 7, 8, and 9. This analysis tested whether vascular disease precedes DS, as would be predicted by the vascular depression hypothesis. Thus, mean CES-D scores were the dependent variable and WMH ratings were an independent variable. Other independent variables in this analysis included baseline age, which captures cross-sectional age differences, and interval (time since baseline), which indexes longitudinal changes over time. Interval2 was included as an independent variable to capture nonlinear changes over time. All two-way and three-way interactions between independent variables were also entered into the model. All variables were treated as continuous variables. Cerebrovascular disease (subarachnoid or intracerebral hemorrhage, occlusion or stenosis of the cerebral arteries), vascular risk factors (hypertension, diabetes, and heart disease) and self-reported history of major depression were initially included in the models but these variables were eliminated because they were found to be non-significant predictors in all analyses. All independent variables and their interactions were modeled as fixed effects while intercept and interval were modeled as random effects. A backward elimination procedure was employed in which all lower order terms remained in the model while non-significant interaction terms (p > 0.05) were eliminated from the model in stages until a final solution was reached (Morrell et al., 1997).
Model 2
In the second model, DS predicted the WMH rating that followed. For example, the mean of the CES-D scores at visits 1, 2, 3, and 4 would predict the white matter rating at year 5. This analysis tested whether DS precede vascular disease. In contrast to Model 1, in which WMH ratings were an independent variable and CES-D scores were the dependent variable, in Model 2, CES-D scores were an independent variable and WMH ratings were the dependent variable. Otherwise, model parameters were identical in Models 1 and 2.
Depressed mood subscale analyses
In addition to the total CES-D score, we were interested in the relationship between white matter disease and scores on the depressed mood subscale of the CES-D. This subscale includes items such as “I felt sad,” or “I had crying spells,” and provides a measure of negative mood independent of somatic complaints that are common in older adults. Scores range from 0–21 on this subscale. We performed identical analyses as those described for Models 1 and 2, replacing the total CES-D score with the depressed mood subscale score.
Results
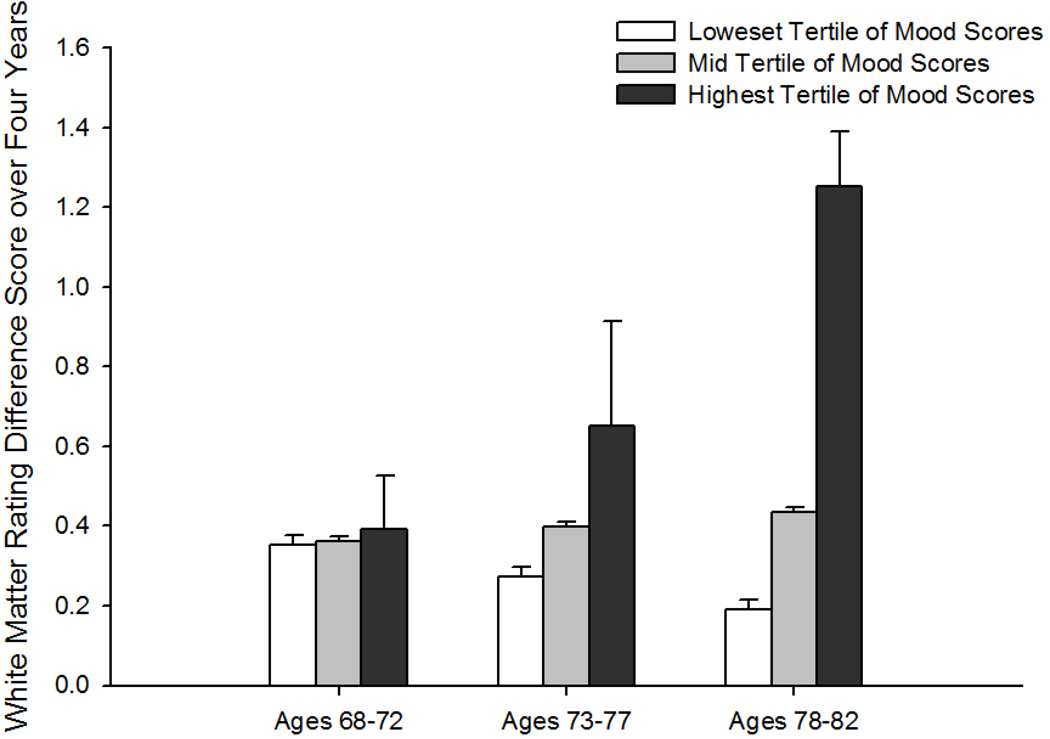
Neither men nor women showed a relationship between total CES-D scores and WMHs in either model (Table 2). When the depressed mood subscale of the CES-D was analyzed (Table 3), WMH ratings did not predict subsequent depressed mood for either sex. However, in men, higher mean mood symptoms were associated with subsequent increases in WMH ratings (mood × interval effect [F(1, 92) = 7.23, p = .01]; Figure) that accelerated over time (mood × interval2 effect [F(1, 92) = 6.86, p = .01]). Moreover, baseline age × mood × interval [F(1, 92) = 6.93, p = .01] and baseline age × mood × interval2 [F(1, 92) = 6.57, p = .01] effects reflected a tendency for these associations to be greater at older ages. In contrast, depressed mood did not predict WMH ratings in women.
Table 2.
Regression Coefficients in the Final Mixed-Effects Regression Models with CES-D Total Score
| Women (N = 39) | Men (N = 51) | |||
|---|---|---|---|---|
| b | SE | b | SE | |
| Model 1: WMHs predicting CES-D | ||||
| Baseline Age | 0.13 | 0.10 | −0.16 | 0.11 |
| Interval | −0.01 | 0.11 | −2.56* | 0.98 |
| Baseline Age × Interval | -- | -- | 0.04** | 0.01 |
| WMH Rating | −0.06 | 0.57 | −0.27 | 0.42 |
| Model 2: CES-D predicting WMHs | ||||
| Baseline Age | 0.05* | 0.02 | 0.04* | 0.02 |
| Interval | −0.97** | 0.31 | 0.11** | 0.01 |
| Interval2 | 0.10* | 0.04 | -- | -- |
| Baseline Age × Interval | 0.01** | 0.00 | -- | -- |
| Baseline Age × Interval2 | −0.00* | 0.00 | -- | -- |
| CES-D | 0.01 | 0.01 | −0.01 | 0.01 |
CES-D = Center for Epidemiologic Studies Depression Scale.
WMHs = white matter hyperintensity ratings.
-- indicates variables that were dropped from the model by backward elimination.
indicates significance at p <.05.
indicates significance at p <.01.
Table 3.
Regression Coefficients in the Final Mixed-Effects Regression Models with the CES-D Depressed Mood Subscale Score
| Women (N = 39) | Men (N = 51) | |||
|---|---|---|---|---|
| b | SE | b | SE | |
| Model 1: WMHs predicting Mood | ||||
| Baseline Age | 0.01 | 0.04 | −0.023 | 0.03 |
| Interval | −0.02 | 0.04 | 0.02 | 0.04 |
| WMH Rating | 0.02 | 0.20 | −0.02 | 0.15 |
| Model 2: Mood predicting WMHs | ||||
| Baseline Age | 0.05* | 0.02 | 0.03 | 0.02 |
| Interval | −0.93** | 0.31 | 0.33 | 0.45 |
| Interval2 | 0.09* | 0.04 | −0.03 | 0.05 |
| Baseline Age × Interval | 0.01** | 0.00 | −0.00 | 0.00 |
| Baseline Age × Interval2 | −0.00* | 0.00 | 0.00 | 0.00 |
| Mood | 0.01 | 0.02 | −.22 | 0.46 |
| Baseline Age × Mood | -- | -- | 0.00 | 0.01 |
| Interval × Mood | -- | -- | −.84* | 0.42 |
| Interval2 × Mood | -- | -- | 0.12* | 0.05 |
| Baseline Age × Interval × Mood | -- | -- | 0.01* | 0.01 |
| Baseline Age × Interval2 × Mood | -- | -- | −0.00* | 0.00 |
CES-D = Center for Epidemiologic Studies Depression Scale.
Mood = Center for Epidemiologic Studies Depression Scale Depressed Mood subscale.
WMHs = white matter hyperintensity ratings.
-- indicates variables that were dropped from the model by backward elimination.
indicates significance at p <.05.
indicates significance at p <.01.
Figure.
Change in white matter hyperintensity rating over 4 years as a function of Center for Epidemiologic Studies Depression Scale (CES-D) depressed mood subscale scores. Depressed mood groups are based on tertile cutoffs. White bars = lowest CES-D depressed mood subscale tertile (average score <.25); gray bars = middle CES-D depressed mood subscale tertile (average score <1.5); black bars = highest CES-D depressed mood subscale tertile (average subscale score >=1.5). The depressive symptoms and age groupings depicted in the figure are for ease of display only as depressive symptoms and age were continuous variables in all analyses.
Discussion
Our findings confirm a predicted association between DS related to depressed mood and the progression of white matter disease in older men. Contrary to our hypothesis, WMHs did not predict subsequent DS in men or women. However, consistent with our hypothesis, subthreshold symptoms of depressed mood predicted longitudinal progression of white matter disease in older men but not women.
Numerous studies have shown that individuals with vascular disease are at an increased risk of developing depression (Katz et al., 1994; Krishnan et al., 2002; Kumar et al., 1997; Lyness et al., 1996; Miller et al., 1996; Tiemeier et al., 2004). Longitudinal neuroimaging studies have shown that WMHs are associated with response to treatment, chronicity of symptoms, cognitive deficits, and functional outcomes in depressed older adults (Kales et al., 2005; Lavretsky et al., 1999; O'Brien et al., 1998; Steffens et al., 2002; Teodorczuk et al., 2010; Teodorczuk et al., 2007; Steffens et al., 2007; Taylor et al., 2003b; Hickie et al., 1997). Nonetheless, because previous research has also suggested that depressive disorders may cause or exacerbate vascular disease (Baldwin, 2005; Everson et al., 1998; Frasure-Smith et al., 1999; Jones et al., 2003; Kilbourne et al., 2005; Lenze et al., 1999; Musselman et al., 1996; Pratt et al., 1996; Wassertheil-Smoller et al., 2004; Ziegelstein et al., 2000) and that the two conditions may be related to common etiological contributors (Alexopoulos, 2003; Krishnan et al., 1996; Steffens et al., 2003), there is increasing recognition that the relationship between depression and vascular disease may be bidirectional (Alexopoulos, 2003; Baldwin, 2005; Camus et al., 2004; Chen et al., 2006; Thomas et al., 2004).
Similar to the present results, baseline WMHs were not related to DS severity approximately 3 years later in the PROSPER study (Versluis et al., 2006). Moreover, progression of WMHs was not related to progression of DS in that sample. However, the investigators did not examine whether DS at baseline predicted WMHs at follow-up. In contrast, we examined both the possibility that WMHs precede DS and that DS precede increased white matter disease in the present study, which allowed us to test a bidirectional relationship. Our results did not support the vascular depression hypothesis or a bidirectional relationship between DS and white matter disease. Rather, we only found a significant relationship between depressed mood symptoms and subsequent WMHs that was limited to men.
There are a number of possible explanations for the lack of association between WMHs and subsequent DS in this study. First, there is evidence that the location of WMHs (e.g., cortical, deep, or periventricular locations) impacts the association with DS (Greenwald et al., 1998; O'Brien et al., 2000; Steffens et al., 1999; Taylor et al., 2003a), presumably because not all WMHs reflect damage to neural circuits involved in emotion and mood regulation. Although the evidence is inconclusive, many studies suggest that DS are more strongly related to deep white matter lesions than to periventricular lesions (de Groot et al., 2000; Krishnan et al., 2006; Taylor et al., 2003a). Moreover, there is evidence that the interaction between sex and age affects lesion location (Dalby et al., 2010), which may have impacted the WMHs in our sample. Because the rating scale used in the present study is based on total WMHs in the brain, we were not able to examine possible differential effects of WMHs in different locations. This is a limitation of the present study that will be addressed in future studies as quantitative white matter lesion volume data become available for the BLSA.
Another possible contributor to our findings is the severity of DS. We examined individuals with low levels of depressive symptomatology rather than individuals with and without a diagnosis of major depression. Although participants had a fairly wide range of CES-D scores (0 to 34), on average the level of DS was relatively low (mean CES-D score = 8.22). Moreover, the CES-D is based on self-report of symptoms experienced during the previous week, rather than a structured diagnostic interview that would be more comprehensive and include symptoms experienced throughout the interval between annual study visits. It is possible that the relationship between white matter disease and DS differs for individuals with clinical depression and those with subthreshold DS, and that stronger associations would have been found if depressive symptoms between visits had been quantified.
Additionally, longitudinal studies of WMHs and depression generally examine associations of DS severity or depression diagnosis at baseline with WMH severity at follow-up or trajectories of WMH change. In contrast, we averaged DS scores over multiple years during each interval between WMH ratings. Since DS can be transient in nature, this procedure not only resulted in multiple DS data points for each participant but also provided a more stable measurement of DS preceding and following the WMH ratings. Indeed, previous research by our group and others has shown that associations of DS with cognitive and neural outcomes differ when chronic or persistent symptoms are examined rather than baseline symptoms (Dotson et al., 2008; Paterniti et al., 2002). The contrast between the present results and a previous report of no differences in rates of change in white matter lesions over time in depressed and nondepressed older adults (Chen et al., 2006) may be at least partially attributable to our examination of more chronic symptoms.
Finally, characteristics of our study sample may have affected our results. Our sample comprises high functioning, healthy community dwelling elderly with relatively low WMH burden in addition to low levels of DS. Results may have differed in a more representative sample. Nonetheless, given the strengths of our well-characterized sample, including the fairly large sample size and long follow-up period, our results provide valuable preliminary data that can inform further research in more representative samples.
The specificity of our findings to analyses that used the depressed mood subscale of the CES-D is an important consideration when interpreting our findings as well as the results of other studies. Depression is a multifaceted syndrome that is characterized by a heterogeneous group of symptoms. The frequency of somatic complaints in older adults complicates the assessment of depression in this population, particularly in investigations of depression-vascular disease relationships since we would expect individuals with vascular problems to have more somatic complaints. Using the depressed mood subscale of the CES-D allowed us to distinguish between affective and somatic symptoms. Indeed, the observation that the depressed mood score was associated with WMHs while the total CES-D was not may reflect greater precision in capturing true depressed mood when physical symptoms that may not be related to depression are not considered. Conflicting findings in previous studies may be in part related to mischaracterization of individuals with primarily somatic symptoms that in fact are not related to depressed mood.
We found a relationship between WMHs and DS in men but not in women, and this association only existed at older ages. Other cognitive and neuroimaging studies have also found evidence that the adverse impact of depression increases with advancing age (Dotson et al., 2009b; Dotson et al., 2008; Konarski et al., 2007; Lockwood et al., 2002; Sackeim et al., 1990). Older adults may be more vulnerable to the adverse impact of depression due to accumulating cognitive and neural changes that already occur with normal aging. Our finding that only men showed a relationship between WMHs and DS is in accord with previous findings that risk factors for WMHs may differ in men and women (Sachdev et al., 2009) and is consistent with findings that the risk of cognitive deficits and dementia increases as a function of depression status or depression severity in men, but not in women, (Dal Forno et al., 2005; Dotson et al., in press; Fuhrer et al., 2003; Ng et al., 2009) and that men show greater depression-related decreases in brain volumes than women (Lavretsky et al., 2004; Taki et al., 2005). Additional research is needed to clarify the nature of these sex differences. Because men may be less willing to acknowledge experiencing depressive symptoms (Williams, et al., 1995), symptoms may be more severe among those men who actually report having them, and thus are more strongly associated with negative outcomes. Additionally, given the higher rates of vascular diseases in men than in women (Halbreich and Lumley, 1993; Khaw, 1996), which was also found in the current study, vascular disease may be a more likely correlate of DS in men, while other factors may predominate in women.
As summarized by other authors (Baldwin, 2005; Kales et al., 2005; Musselman et al., 1998), a variety of mechanisms could explain depression-related increases in vascular disease risk. Proposed mechanisms include hypercortisolemia, sympathoadrenal hyperactivity, diminished heart rate variability, ventricular instability and myocardial ischemia in reaction to mental stress, depression-related platelet aggregation and/or reactivity leading to increased thrombosis, depression-induced impairment of arterial endothelial functioning, or abnormal folate or homocysteine metabolism. Although the present results do not address mechanisms of action, the finding that DS predict white matter disease in older men has important clinical implications. The presence of WMHs is associated with poor outcomes, greater disability, greater risk of persistent cognitive impairment and dementia, and mortality. Our results suggest that depressed mood in older men may increase the risk for white matter disease or exacerbate existing white matter disease. As a result, intervention strategies for depression may slow the progression of white matter disease and subsequent negative sequelae in this group. Future studies that clarify individual differences that may impact whether DS precede or follow vascular disease are essential to translating research findings into clinical interventions.
Key points.
Depressed mood symptoms predicted accelerating longitudinal increases in white matter hyperintensities in men, but not in women.
Overall depressive symptom severity was not associated with white matter hyperintensities in either men or women.
Acknowledgements
The National Institute on Aging Intramural Research Program of the National Institutes of Health supported this research. The authors wish to thank Yang An, M.S. for assistance with statistical analyses.
Footnotes
The authors have no potential conflicts of interest to be disclosed.
Presented in part at the 39th annual meeting of the Society for Neuroscience, Chicago, IL, October 17–21, 2009
References
- Alexopoulos GS. Vascular disease, depression, and dementia. J Am Geriatr Soc. 2003;51:1178–1180. doi: 10.1046/j.1532-5415.2003.51373.x. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- Angelelli P, Paolucci S, Bivona U, Piccardi L, Ciurli P, Cantagallo A, Antonucci G, Fasotti L, Di Santantonio A, Grasso MG, Pizzamiglio L. Development of neuropsychiatric symptoms in poststroke patients: a cross-sectional study. Acta Psychiatr Scand. 2004;110:55–63. doi: 10.1111/j.1600-0447.2004.00297.x. [DOI] [PubMed] [Google Scholar]
- Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry. 2006;60:1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Baldwin RC. Is vascular depression a distinct sub-type of depressive disorder? A review of causal evidence. Int J Geriatr Psychiatry. 2005;20:1–11. doi: 10.1002/gps.1255. [DOI] [PubMed] [Google Scholar]
- Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Burvill PW, Johnson GA, Jamrozik KD, Anderson CS, Stewart-Wynne EG, Chakera TM. Prevalence of depression after stroke: the Perth Community Stroke Study. Br J Psychiatry. 1995;166:320–327. doi: 10.1192/bjp.166.3.320. [DOI] [PubMed] [Google Scholar]
- Camus V, Kraehenbuhl H, Preisig M, Bula CJ, Waeber G. Geriatric depression and vascular diseases: what are the links? J Affect Disord. 2004;81:1–16. doi: 10.1016/j.jad.2003.08.003. S0165032703002052 [pii] [DOI] [PubMed] [Google Scholar]
- Chen PS, McQuoid DR, Payne ME, Steffens DC. White matter and subcortical gray matter lesion volume changes and late-life depression outcome: a 4-year magnetic resonance imaging study. Int Psychogeriatr. 2006;18:445–456. doi: 10.1017/S1041610205002796. [DOI] [PubMed] [Google Scholar]
- Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer's disease. Ann Neurol. 2005;57:381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- Dalby RB, Chakravarty MM, Ahdidan J, Sorensen L, Frandsen J, Jonsdottir KY, Tehrani E, Rosenberg R, Ostergaard L, Videbech P. Localization of white-matter lesions and effect of vascular risk factors in late-onset major depression. Psychol Med. 2010;40:1389–1399. doi: 10.1017/S0033291709991656. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57:1071–1076. doi: 10.1001/archpsyc.57.11.1071. yoa8384 [pii] [DOI] [PubMed] [Google Scholar]
- Dotson VM, Beason-Held L, Kraut MA, Resnick SM. Longitudinal study of chronic depressive symptoms and regional cerebral blood flow in older men and women. Int J Geriatr Psychiatry. 2009a;24:809–819. doi: 10.1002/gps.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009b;34:367–375. [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Resnick SM, Zonderman AB. Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. Am J Geriatr Psychiatry. 2008;16:318–330. doi: 10.1097/JGP.0b013e3181662a9c. 16/4/318 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Zonderman AB, Davatzikos C, Kraut MA, Resnick SM. Frontal Atrophy and Attention Deficits in Older Adults with a History of Elevated Depressive Symptoms. Brain Imaging Behav. doi: 10.1007/s11682-009-9078-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med. 1998;158:1133–1138. doi: 10.1001/archinte.158.10.1133. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Maiberger R, McHugh PR. Mood disorder as a specific complication of stroke. J Neurol Neurosurg Psychiatry. 1977;40:1018–1020. doi: 10.1136/jnnp.40.10.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Fuhrer R, Dufouil C, Dartigues JF. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J Am Geriatr Soc. 2003;51:1055–1063. doi: 10.1046/j.1532-5415.2003.51352.x. [DOI] [PubMed] [Google Scholar]
- Greenwald BS, Kramer-Ginsberg E, Krishnan KR, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke. 1998;29:613–617. doi: 10.1161/01.str.29.3.613. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Lumley LA. The multiple interactional biological processes that might lead to depression and gender differences in its appearance. J Affect Disord. 1993;29:159–173. doi: 10.1016/0165-0327(93)90030-n. 0165-0327(93)90030-N [pii] [DOI] [PubMed] [Google Scholar]
- Haringsma R, Engels GI, Beekman AT, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Van Alstine J, Usala PD, Hultsch DF, Dixon R. Measurement properties of the center for epidemiological studies depression scale (CES-D) in older populations. Psychological Assessment. 1990;2:64–72. [Google Scholar]
- Hickie I, Scott E, Wilhelm K, Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression--a longitudinal evaluation. Biol Psychiatry. 1997;42:367–374. doi: 10.1016/S0006-3223(96)00363-0. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Bromberger JT, Sutton-Tyrrell K, Matthews KA. Lifetime history of depression and carotid atherosclerosis in middle-aged women. Arch Gen Psychiatry. 2003;60:153–160. doi: 10.1001/archpsyc.60.2.153. yoa10332 [pii] [DOI] [PubMed] [Google Scholar]
- Kales HC, Maixner DF, Mellow AM. Cerebrovascular disease and late-life depression. Am J Geriatr Psychiatry. 2005;13:88–98. doi: 10.1176/appi.ajgp.13.2.88. [DOI] [PubMed] [Google Scholar]
- Katz IR, Striem J, Parmelee P. Psychiatric-medical comorbidity: implications for health services delivery and for research on depression. Biol Psychiatry. 1994;36:141–145. doi: 10.1016/0006-3223(94)91219-x. [DOI] [PubMed] [Google Scholar]
- Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- Khaw KT. Gender and cardiovascular risk. J Hum Hypertens. 1996;10:403–407. [PubMed] [Google Scholar]
- Kilbourne AM, Reynolds CF, 3rd, Good CB, Sereika SM, Justice AC, Fine MJ. How does depression influence diabetes medication adherence in older patients? Am J Geriatr Psychiatry. 2005;13:202–210. doi: 10.1176/appi.ajgp.13.3.202. [DOI] [PubMed] [Google Scholar]
- Konarski JZ, Kennedy SH, McIntyre RS, Rafi-Tari S, Soczynska JK, Mayberg HS. Relationship between regional brain metabolism, illness severity and age in depressed subjects. Psychiatry Res. 2007;155:203–210. doi: 10.1016/j.pscychresns.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Krishnan KR. Biological risk factors in late life depression. Biol Psychiatry. 2002;52:185–192. doi: 10.1016/s0006-3223(02)01349-5. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Delong M, Kraemer H, Carney R, Spiegel D, Gordon C, McDonald W, Dew M, Alexopoulos G, Buckwalter K, Cohen PD, Evans D, Kaufmann PG, Olin J, Otey E, Wainscott C. Comorbidity of depression with other medical diseases in the elderly. Biol Psychiatry. 2002;52:559–588. doi: 10.1016/s0006-3223(02)01472-5. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, McDonald WM. Arteriosclerotic depression. Med Hypotheses. 1995;44:111–115. doi: 10.1016/0306-9877(95)90081-0. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Tupler LA, Ritchie JC, Jr, McDonald WM, Knight DL, Nemeroff CB, Carroll BJ. Apolipoprotein E-epsilon 4 frequency in geriatric depression. Biol Psychiatry. 1996;40:69–71. doi: 10.1016/0006-3223(95)00424-6. [DOI] [PubMed] [Google Scholar]
- Krishnan MS, O'Brien JT, Firbank MJ, Pantoni L, Carlucci G, Erkinjuntti T, Wallin A, Wahlund LO, Scheltens P, van Straaten EC, Inzitari D. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The LADIS Study. Int J Geriatr Psychiatry. 2006;21:983–989. doi: 10.1002/gps.1596. 10.1002/gps.1596. [DOI] [PubMed] [Google Scholar]
- Kumar A, Miller D, Ewbank D, Yousem D, Newberg A, Samuels S, Cowell P, Gottlieb G. Quantitative anatomic measures and comorbid medical illness in late-life major depression. Am J Geriatr Psychiatry. 1997;5:15–25. [PubMed] [Google Scholar]
- Lavretsky H, Kurbanyan K, Ballmaier M, Mintz J, Toga A, Kumar A. Sex differences in brain structure in geriatric depression. Am J Geriatr Psychiatry. 2004;12:653–657. doi: 10.1176/appi.ajgp.12.6.653. 12/6/653 [pii] [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry. 1999;7:309–316. [PubMed] [Google Scholar]
- Lenze E, Cross D, McKeel D, Neuman RJ, Sheline YI. White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. Am J Psychiatry. 1999;156:1602–1607. doi: 10.1176/ajp.156.10.1602. [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- Lyness JM, Bruce ML, Koenig HG, Parmelee PA, Schulz R, Lawton MP, Reynolds CF., 3rd Depression and medical illness in late life: report of a symposium. J Am Geriatr Soc. 1996;44:198–203. doi: 10.1111/j.1532-5415.1996.tb02440.x. [DOI] [PubMed] [Google Scholar]
- MacFall JR, Taylor WD, Rex DE, Pieper S, Payne ME, McQuoid DR, Steffens DC, Kikinis R, Toga AW, Krishnan KR. Lobar distribution of lesion volumes in late-life depression: the Biomedical Informatics Research Network (BIRN) Neuropsychopharmacology. 2006;31:1500–1507. doi: 10.1038/sj.npp.1300986. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Rifai AH, Mazumdar S, Pollock B, Perel JM, Frank E, Reynolds CF., III Chronic medical illness in patients with recurrent major depression. Am J Geriatr Psychiatry. 1996;4:281–290. doi: 10.1097/00019442-199622440-00002. [DOI] [PubMed] [Google Scholar]
- Morrell CH, Pearson JD, Brant LJ. Linear transformations of linear mixed-effects models. Am Stat. 1997;51:338–343. [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Tomer A, Manatunga AK, Knight BT, Porter MR, Kasey S, Marzec U, Harker LA, Nemeroff CB. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153:1313–1317. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Vora IJ, Meltzer CC, Fukui MB, Williams RL, Kamboh MI, Saxton J, Houck PR, DeKosky ST, Reynolds CF., 3rd Relationship of deep white matter hyperintensities and apolipoprotein E genotype to depressive symptoms in older adults without clinical depression. Am J Psychiatry. 2001;158:878–884. doi: 10.1176/appi.ajp.158.6.878. [DOI] [PubMed] [Google Scholar]
- Ng TP, Niti M, Zaw MH, Kua EH. Depressive symptoms and incident cognitive impairment in cognitively well-functioning older men and women. J Am Geriatr Soc. 2009;57:1058–1063. doi: 10.1111/j.1532-5415.2009.02262.x. [DOI] [PubMed] [Google Scholar]
- Nobuhara K, Okugawa G, Sugimoto T, Minami T, Tamagaki C, Takase K, Saito Y, Sawada S, Kinoshita T. Frontal white matter anisotropy and symptom severity of late-life depression: a magnetic resonance diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2006;77:120–122. doi: 10.1136/jnnp.2004.055129. 77/1/120 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Ames D, Chiu E, Schweitzer I, Desmond P, Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. BMJ. 1998;317:982–984. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Desmond P, Ames D, Schweitzer I, Harrigan S, Tress B. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer's disease. Br J Psychiatry. 1996;168:477–485. doi: 10.1192/bjp.168.4.477. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Perry R, Barber R, Gholkar A, Thomas A. The association between white matter lesions on magnetic resonance imaging and noncognitive symptoms. Ann N Y Acad Sci. 2000;903:482–489. doi: 10.1111/j.1749-6632.2000.tb06403.x. [DOI] [PubMed] [Google Scholar]
- Paterniti S, Verdier-Taillefer MH, Dufouil C, Alperovitch A. Depressive symptoms and cognitive decline in elderly people. Longitudinal study. Br J Psychiatry. 2002;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T, Leppavuori A, Siira I, Vataja R, Kaste M, Erkinjuntti T. Frequency and clinical determinants of poststroke depression. Stroke. 1998;29:2311–2317. doi: 10.1161/01.str.29.11.2311. [DOI] [PubMed] [Google Scholar]
- Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaton WW. Depression, psychotropic medication, and risk of myocardial infarction. Prospective data from the Baltimore ECA follow-up. Circulation. 1996;94:3123–3129. doi: 10.1161/01.cir.94.12.3123. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Reichenberg A, Dahlman KL, Mosovich S, Silverstein JH. Neuropsychiatric consequences of coronary artery bypass grafting and noncardiovascular surgery. Dialogues Clin Neurosci. 2007;9:85–91. doi: 10.31887/DCNS.2007.9.1/areichenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30:946–956. doi: 10.1016/j.neurobiolaging.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prohovnik I, Moeller JR, Brown RP, Apter S, Prudic J, Devanand DP, Mukherjee S. Regional cerebral blood flow in mood disorders. I. Comparison of major depressives and normal controls at rest. Arch Gen Psychiatry. 1990;47:60–70. doi: 10.1001/archpsyc.1990.01810130062009. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SW, Jackson A, Baldwin RC, Burns A. Subcortical hyperintensities in late-life depression: acute response to treatment and neuropsychological impairment. Int Psychogeriatr. 1997;9:257–275. doi: 10.1017/s1041610297004432. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Helms MJ, Krishnan KR, Burke GL. Cerebrovascular disease and depression symptoms in the cardiovascular health study. Stroke. 1999;30:2159–2166. doi: 10.1161/01.str.30.10.2159. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Krishnan KR, Crump C, Burke GL. Cerebrovascular disease and evolution of depressive symptoms in the cardiovascular health study. Stroke. 2002;33:1636–1344. doi: 10.1161/01.str.0000018405.59799.d5. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Norton MC, Hart AD, Skoog I, Corcoran C, Breitner JC. Apolipoprotein E genotype and major depression in a community of older adults. The Cache County Study. Psychol Med. 2003;33:541–547. doi: 10.1017/s0033291702007201. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Potter GG, McQuoid DR, MacFall JR, Payne ME, Burke JR, Plassman BL, Welsh-Bohmer KA. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2007;15:839–849. doi: 10.1097/JGP.0b013e318048a1a0. [DOI] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Awata S, Inoue K, Sato K, Ito H, Goto R, Uchida S, Tsuji I, Arai H, Kawashima R, Fukuda H. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. J Affect Disord. 2005;88:313–320. doi: 10.1016/j.jad.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293–1296. doi: 10.1176/appi.ajp.161.7.1293. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan RR. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Steffens DC, Payne ME, Provenzale JM, Krishnan KR. Localization of age-associated white matter hyperintensities in late-life depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003a;27:539–544. doi: 10.1016/S0278-5846(02)00358-5. S0278-5846(02)00358-5 [pii] [DOI] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, MacFall JR, McQuoid DR, Payne ME, Provenzale JM, Krishnan KR. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003b;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- Teodorczuk A, Firbank MJ, Pantoni L, Poggesi A, Erkinjuntti T, Wallin A, Wahlund LO, Scheltens P, Waldemar G, Schrotter G, Ferro JM, Chabriat H, Bazner H, Visser M, Inzitari D, O'Brien JT. Relationship between baseline white-matter changes and development of late-life depressive symptoms: 3-year results from the LADIS study. Psychol Med. 2010;40:603–610. doi: 10.1017/S0033291709990857. [DOI] [PubMed] [Google Scholar]
- Teodorczuk A, O'Brien JT, Firbank MJ, Pantoni L, Poggesi A, Erkinjuntti T, Wallin A, Wahlund LO, Gouw A, Waldemar G, Schmidt R, Ferro JM, Chabriat H, Bazner H, Inzitari D. White matter changes and late-life depressive symptoms: longitudinal study. Br J Psychiatry. 2007;191:212–217. doi: 10.1192/bjp.bp.107.036756. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Ferrier IN, Kalaria RN, Perry RH, Brown A, O'Brien JT. A neuropathological study of vascular factors in late-life depression. J Neurol Neurosurg Psychiatry. 2001;70:83–87. doi: 10.1136/jnnp.70.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79:81–95. doi: 10.1016/S0165-0327(02)00349-X. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, van Dijck W, Hofman A, Witteman JC, Stijnen T, Breteler MM. Relationship between atherosclerosis and late-life depression: the Rotterdam Study. Arch Gen Psychiatry. 2004;61:369–376. doi: 10.1001/archpsyc.61.4.369. [DOI] [PubMed] [Google Scholar]
- Versluis CE, van der Mast RC, van Buchem MA, Bollen EL, Blauw GJ, Eekhof JA, van der Wee NJ, de Craen AJ. Progression of cerebral white matter lesions is not associated with development of depressive symptoms in elderly subjects at risk of cardiovascular disease: The PROSPER Study. Int J Geriatr Psychiatry. 2006;21:375–381. doi: 10.1002/gps.1477. 10.1002/gps.1477. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen TH, Hartvig H, Egander A, Clemmensen K, Rasmussen NA, Andersen F, Gjedde A, Rosenberg R. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand. 2002;106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Shumaker S, Ockene J, Talavera GA, Greenland P, Cochrane B, Robbins J, Aragaki A, Dunbar-Jacob J. Depression and cardiovascular sequelae in postmenopausal women. The Women's Health Initiative (WHI) Arch Intern Med. 2004;164:289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- Wendell CR, Hosey MM, Lefkowitz DM, Katzel LI, Siegel EL, Rosenberger WF, Waldstein SR. Depressive symptoms are associated with subclinical cerebrovascular disease among healthy older women, not men. Am J Geriatr Psychiatry. 2010;18:940–947. doi: 10.1097/JGP.0b013e3181d57a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte EM, Mulsant BH, Vanderbilt J, Dodge HH, Ganguli M. Depression after stroke: a prospective epidemiological study. J Am Geriatr Soc. 2004;52:774–778. doi: 10.1111/j.1532-5415.2004.52217.x. [DOI] [PubMed] [Google Scholar]
- Yue NC, Arnold AM, Longstreth WT, Jr, Elster AD, Jungreis CA, O'Leary DH, Poirier VC, Bryan RN. Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the cardiovascular health study. Radiology. 1997;202:33–39. doi: 10.1148/radiology.202.1.8988189. [DOI] [PubMed] [Google Scholar]
- Ziegelstein RC, Fauerbach JA, Stevens SS, Romanelli J, Richter DP, Bush DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med. 2000;160:1818–1823. doi: 10.1001/archinte.160.12.1818. [DOI] [PubMed] [Google Scholar]