Abstract
Voltage-gated sodium channels are responsible for the rising phase of the action potential in cardiac muscle. Previously, both TTX-sensitive neuronal sodium channels (NaV1.1, NaV1.2, NaV1.3, NaV1.4 and NaV1.6) and the TTX-resistant cardiac sodium channel (NaV1.5) have been detected in cardiac myocytes, but relative levels of protein expression of the isoforms were not determined. Using a quantitative approach, we analyzed z-series of confocal microscopy images from individual mouse myocytes stained with either anti-NaV1.1, anti-NaV1.2, anti-NaV1.3, anti-NaV1.4, anti-NaV1.5, or anti-NaV1.6 antibodies and calculated the relative intensity of staining for these sodium channel isoforms. Our results indicate that the TTX-sensitive channels represented approximately 23% of the total channels, whereas the TTX-resistant NaV1.5 channel represented 77% of the total channel staining in mouse ventricular myocytes. These ratios are consistent with previous electrophysiological studies in mouse ventricular myocytes. NaV1.5 was located at the cell surface, with high density at the intercalated disc, but was absent from the transverse (t)-tubular system, suggesting that these channels support surface conduction and inter-myocyte transmission. Low-level cell surface staining of NaV1.4 and NaV1.6 channels suggest a minor role in surface excitation and conduction. Conversely, NaV1.1 and NaV1.3 channels are localized to the t-tubules and are likely to support t-tubular transmission of the action potential to the myocyte interior. This quantitative immunocytochemical approach for assessing sodium channel density and localization provides a more precise view of the relative importance and possible roles of these individual sodium channel protein isoforms in mouse ventricular myocytes and may be applicable to other species and cardiac tissue types.
Introduction
Excitation-contraction coupling in the heart is initiated by voltage-gated sodium channels that participate in rapid propagation of the action potentials and the synchronous depolarization of cardiomyocyte membranes. The primary voltage-dependent sodium channel in the heart is NaV1.5 α subunit isoform, which defines the major electrophysiological and pharmacological properties of the sodium current recorded from ventricular myocytes [1, 2]. These sodium channels have a low sensitivity to tetrodotoxin (TTX) and are considered to be TTX-resistant [3–5]. Recent immunocytochemical work has demonstrated the presence of neuronal-type (NaV1.1, NaV1.2, NaV1.3, NaV1.4 and NaV1.6) sodium channels in cardiac tissue [6–11]. Unlike NaV1.5, these latter channels are TTX-sensitive and are responsible for a minor component of the sodium current. Electrophysiological studies suggest that TTX-sensitive channels account for 5–20% of the sodium current (INa) in cardiomyocytes, and TTX-resistant channels account for 80–95% of the total sodium current in ventricular myocytes [8, 10, 12]. Thus, both immunocytochemical and electrophysiological studies indicate that multiple sodium channels subtypes are important in cardiac function. However, previous immunocytochemical studies using standard approaches to localize the different sodium channel α subunit isoforms were not able to assess the relative levels of channel protein expressed in each location.
Here we have developed a method for assessing the relative expression of different sodium channel isoforms using a panel of sodium channel α subunit-specific antibodies. Quantification of immunocytochemical staining is inherently difficult due to differences in equipment, tissue preparation, inter-assay variability and analysis methods. However, considerable progress has been made in developing reliable methods for quantification of immunocytochemical staining [13], as well as in identifying variables that need to be considered and controlled [14]. Using such a quantitative approach, we have determined the localization and relative levels of sodium channel α subunit protein expression in mouse ventricular myocytes to glean further insights into their functional roles.
2. Materials and Methods
2.1. Antibodies
The specifications and the peptide sequences against which the antibodies are directed have been described [8, 9]. Antibodies recognizing Nav1.1, Nav1.2, and Nav1.3 were purchased from Chemicon (Temecula, CA) or Alomone Laboratories (Jerusalem). The antibodies recognizing Nav1.6 (anti-Scn8a) and rat Nav1.5 were obtained from Alomone Laboratories (Jerusalem). The mouse monoclonal antibodies against Nav1.4 and α-actinin were purchased from Sigma-Aldrich (St Louis). The mouse monoclonal antibodies against connexin 43 were purchased from Millipore (Billerica, MA).
2.1.1. Antibody Specificity
For tests of antibody specificity individual antibodies were pre-incubated with their respective antigenic peptide when possible and produced no specific staining. Further specificity of the anti-NaV1.6 antibody used in this study has been shown by using it in combination with NaV1.6 knockout animals in which no staining was observed [15]. The NaV1.1 antibody used in this study, along with other NaV1.1 antibodies, have been used in combination with NaV1.1 knockout mice [16, 17] to demonstrate specificity of the antibody via western blot and immunocytochemistry.
2.2. Expression in mammalian cells
As described previously [18], tsA-201 cells were grown in DMEM/F-12 medium (Invitrogen, Rockville, MD), supplemented with 10% fetal bovine serum (Invitrogen) and 100 U/ml penicillin and streptomycin. They were incubated at 37 °C in 10% CO2, grown to about 70% confluence, suspended with trypsin-EDTA, and plated onto 35mm culture dishes at 40% confluence and grown for 24 hour before transfection. The medium was then replaced with fresh medium before transient transfection with cDNAs using the calcium phosphate method [19, 20]. Cells were incubated at 37 °C in 3% CO2 for 12 to 16 hr, the medium was replaced, and then the cells were split and plated onto 35 mm dishes (for electrophysiology to verify expression) and 12 well microscope slides (for staining, Lab Tech) and allowed to stabilize for 9–24 hr before use. Electrophysiological recordings were done on each set of tsA201 cells, to verify expression of the sodium channel (unpublished data by Drs. Daniel Beacham and Chen-Yuan Pan).
2.3. cDNAs
cDNA (pCIN5/tIa) encoding the human NaV1.1 channel α subunit was a gift from Glaxo Wellcome. The cDNA (pVA200/rIIa) encoding rat NaV1.2 was a gift from Dr. VJ Auld while rat NaV1.3 cDNA (SP64T/rIII) was a gift from Dr. D. Ragsdale. NaV1.4 cDNA was obtained from Dr. P. Ruben. We generated cDNAs (SP64T/rH1.3) for rat NaV1.5 channels while mouse NaV1.6 cDNA (pRcCMV/mNav1.6) was a generous gift from Dr. A. Goldin. All cDNAs were sequenced and are now in pCDM8 mammalian expression vectors.
2.4. Isolation of myocytes
Using the methods described previously, myocytes were isolated from both ventricles of adult male wild-type (B6129F1) mice that were 8–10 weeks old [21].
2.5. Immunocytochemistry (myocytes)
Mouse ventricular myocytes were isolated and processed for immunocytochemistry as described previously [8, 9]. Briefly, the myocytes were incubated in either anti-NaV1.1, anti-NaV1.2, anti-NaV1.3, anti-NaV1.4, anti-NaV1.5 or anti-NaV1.6 diluted at 1:25 in a solution containing 0.1M Tris-buffered saline (TBS), 0.075% Triton X-100 and 1% normal goat serum. Following rinsing, the primary antibodies were detected using biotinylated goat anti-rabbit IgG (1:300) or goat anti-mouse IgG (1:300; for anti-NaV1.4 only) and Avidin D fluorescein (1:300). Following rinsing the myocytes were coverslipped with Vectashield and viewed using a Leica SL confocal microscope. For controls the antibodies were preincubated with their antigenic peptide (10–20 µM) before application to the tissue or no primary antibody was used during incubation. All myocytes stained with polyclonal anti-NaV1.1, NaV1.2, NaV1.3, NaV1.5, or NaV1.6 were double labeled with anti-α-actinin (1:1000) and detected with goat anti-mouse Alexa 555 (Invitrogen), as described previously [8]. Myocytes double-labeled with anti-connexin 43 antibodies (1:100) were incubated with goat anti-mouse Alexa 555 for detection of this antibody, as described previously [8].The health of individual myocytes in a given preparation can be variable. Myocytes used in this study were rod shaped, relaxed and with distinct z-lines as recognized by staining with α-actinin.
Staining of ventricular myocytes has several advantages over staining of intact tissue. Sectioning of intact tissue can result in cutting through individual cardiac cells unlike myocytes where the whole cell remains intack. With myocytes there is the added advantage of being able to tell the surface from the middle when z-sectioning is used.
2.6. Immunocytochemistry (tsA201 cells)
Following transfection, tsA201 cells were fixed with 4% paraforamaldehyde for 30 min, rinsed for 30 min, blocked in 2% Avidin in TBS for 30 min, rinsed in TBS for 30 min, blocked in 2% biotin in TBS and then rinsed in TBS for 30 min. The cells were then incubated in various dilutions of each of the isoform specific, polyclonal anti-sodium channel antibodies (1:25–1:2000) overnight at 4 °C. The primary antibodies were incubated in TBS containing 0.05% Triton X-100 and 1% normal goat serum. The primary antibodies were detected using biotinylated goat anti-rabbit IgG (1:300) and Avidin D-fluorescein (1:300). The cells were then rinsed, coverslipped with Vectashield, and viewed using a Leica SL confocal microscope. For control cells, primary antibodies were pre-incubated with their antigenic peptides, or no primary antibody was used.
2.7. Image collection and analysis
Images were collected using a Leica SL confocal microscope with a pinhole setting of 1.0. Specimens were excited using a wavelength of 488 nm (emission 494–539 nm) and/or a wavelength of 555 nm (emission 553–656 nm). Images were collected using a z-slice thickness of 1µm and stacked using Image J (NIH).
Digital images (.tif) were imported into Igor Pro 6.2 (Wavemetrics, Lake Oswego, OR). Using the Image Processing package in this software program, we outlined the cell or tissue region of interest stained by immunocytochemical methods and then used the region of interest function (ROI) in Igor to determine the mean pixel intensity of the selected area.
2.8. Line scan analysis
With the Igor computer program a 50-pixel-wide band was drawn across each myocyte, and the pixel intensity within the band was measured and plotted versus the distance across the myocyte. The cell widths were normalized to allow determination of pixel intensity as a function of distance across the myocyte cross section and to allow comparison of cross-sectional staining patterns between the antibodies.
2.9. Laser stability
We tested the stability of the Argon laser (used for 488 nm excitation) and the GrHeNe laser (used for 555nm excitation) on our Leica SL confocal microscope using a blank fluorescent slide (Chroma). Images of the fluorescent field were collected every five min over about 3.5 h for each laser. The offset and gain were initially set so the pixel intensity ranged from 0 to 256 to assure that no portion of the images was saturated. The gain, offset, and focal plane remained constant during the 3.5 h of image collection. Next, the average pixel intensity of the whole field image was determined at each time point using the Igor image analysis program as described in 2.7 above. Laser intensity fluctuated widely when the Argon laser was first turned on but became stable between 2 and 3 hr (Fig. 1A, green line). Similar initial fluctuations that stabilized at approximately 2 .5 hr were observed for the GrHeNe laser (Fig. 1A, red line). Thus, all images used for analysis were collected after the lasers had been on for at least 2.5 hr (vertical line in Fig. 1) to reduce variability introduced by laser instability.
Fig. 1.
Laser Stability and Analysis of Laser Intensities. (A) The stability of the Leica SL confocal microscope Arg laser (green line) or GrHeNe (red line) was measured over a period of more than three hours by collecting whole field images from a fluorescent slide. Wide fluctuations in laser stability dissipated by about 2.5 hr (verticle line) after startup of the lasers demonstrating the need to allow adequate warming up time of lasers before quantitative image collection. Mouse ventricular myocytes were stained with anti-NaV1.1 B and D) or anti-NaV1.5 (C and E) antibodies. Single confocal plane images were collected using two different laser intensities using the acousto optical tunable filter (AOTF) settings of 80% (B and C) or laser settings of 20% (D and E) demonstrating an over saturated image for anti-NaV1.5 with the laser setting of AOTF=80% while anti-NaV1.1 using this setting is in the linear range. In contrast, the image of the myocyte stained with anti-NaV1.1 (AOTF of 20%) is under saturated and undetectable while NaV1.5 is in the linear range for image collection. (F) Comparison of average pixel intensity for various AOTF settings collected from either a fluorescent slide (green line) or from ventricular myocytes (blue line) stained with anti-NaV1.1 antibodies illustrating a similar pattern of linearity for laser intensity settings using AOTF=20% to AOTF=80% using these two sample types. Scale bar = 35um
2.10. Intensity of excitation light
On the Leica SL confocal microscope, the individual laser lines are controlled by Acousto-Optical Tunable Filters (AOTF). The filters allow the user to select both the wavelength and intensity of the light used for excitation. The ability to adjust the laser intensity allows quantitative comparison of samples that are substantially different in their staining intensity. For the antibody that recognizes NaV1.5, the signal was very bright as NaV1.5 is the primary α subunit isoform in cardiac myocytes. Conversely, staining with the antibody recognizing NaV1.1 was far less intense. Thus, if gain, offset, and laser intensity appropriate for NaV1.1 are used, pixels in the NaV1.5 image are saturated (AOTF setting of 80%, Figs. 1B and C). In contrast, if gain, offset, and laser intensity appropriate for NaV1.5 are used, then the staining with the NaV1.1 antibody is barely visible (AOTF=20%; Figs. 1D and E).
For accurate quantification it is important that the entire image is within the measurable range of pixel intensity. This ensures that signal strength is not underestimated due to pixel saturation or not detected if the staining levels are too low for the AOTF setting. To achieve this, we determined the relationship between AOTF setting and signal intensity (Fig. 1F). We used a fluorescent standard slide (Chroma) and collected images at different laser intensities from three regions. Mean pixel intensity was plotted as a function of AOTF setting (Fig. 1F, green line). The intensity is nearly linear between 20% and 80% with slightly reduced slope between 80 and 90%. These values were used to normalize and compare staining intensities of images obtained at different AOTF settings.
The relationship between AOTF setting and laser intensity was determined for tsA-201 cells transfected with NaV1.1 α subunit cDNA and labeled with anti-NaV1.1 sodium channel antibodies. Images were collected at various AOTF settings using the Argon (488 nm) laser. Mean pixel intensity for each image was plotted versus AOTF setting (Fig. 1F, blue line; n=6). The relationship between AOTF setting and signal using these biological samples was similar to that obtained when using the fluorescent standard slide. Based on these findings, our images of mouse ventricular myocytes were collected using AOTF settings between 20% and 80% that were appropriate for the level of staining obtained with a particular antibody and avoided under-exposure or over-saturation of collected images. The resulting measurements were normalized to those for an AOTF setting of 80% using the data of Fig. 1F. This allowed us to directly compare images obtained using different AOTF settings.
3. Results
3.1. Standardization of immunocytochemical method for quantification
3.1.1 Strategy
To allow quantification of specific sodium channel alpha subunits in mouse cardiac myocytes, we made two important changes in the usual procedures for immunocytochemical staining. First, we standardized a series of microscope settings to be used throughout the study in order to achieve comparable resolution and sensitivity on all samples. Microscope variables investigated included the stability of the confocal lasers and a comparison of the intensity of the excitation light over a range of filter settings (see sections 2.9–2.10, Fig. 1). Second, we determined the relative affinities of the antibodies to be used in these studies in order to standardize them for use at saturating concentrations in these and future experiments. By saturating the antigenic site for each antibody, potential problems of quantification related to different antibody affinities are circumvented because each accessible antigenic site will be labeled with a single bound antibody molecule directed to one antigenic epitope.
3.1.2. Determination of antibody concentrations producing saturation of staining
Relative affinities of antibodies recognizing different sodium channel isoform proteins were determined by staining of tsA-201 cells transfected with the relevant α subunit isoform. Staining intensity was measured as a function of antibody concentration. Plots of mean fluorescence intensities versus antibody concentration (Fig. 2) showed some variation in affinity between antibodies. However, each antibody reached a saturating concentration at the higher concentrations with no evidence of nonspecific staining. Staining using the antibodies with lowest affinity (NaV1.6) gave saturating staining at a concentration of 0.008 mg/ml. The antibody concentraton used (Fig. 2, red vertical lines) for our studies in mouse myocytes was 2.5 fold higher than this saturating concentration for NaV1.4 and a greater multiple for the other antibodies. Thus each antibody is expected to be at saturating concentration. To test whether measured staining responded appropriately to altered level of antigen, we also analyzed the relationship between fluorescence intensity and the amount of cDNA transfected into the tsA-201 cells. We observed a linear relationship between transfected cDNA and fluorescence intensity for each of the sodium channels studied (Supplemental Fig. 1).
Fig. 2.
Antibody Dilution Series. TsA201 cells transfected with A.) NaV1.1 cDNA, B.) NaV1.2 cDNA, C.) NaV1.3 cDNA, D.) NaV1.4 cDNA, E.) NaV1.5 cDNA or F.) NaV1.6 cDNA were labeled with anti-NaV1.1-Nav1.6 antibodies, respectively. Average pixel intensity for each group of cells stained with different concentrations of a given antibody were analyzed in Igor and plotted to illustrate that when each antibody was used at high concentrations (red vertical line) it was saturating.
3.2. Relative staining of specific sodium channel isoforms in mouse ventricular myocytes
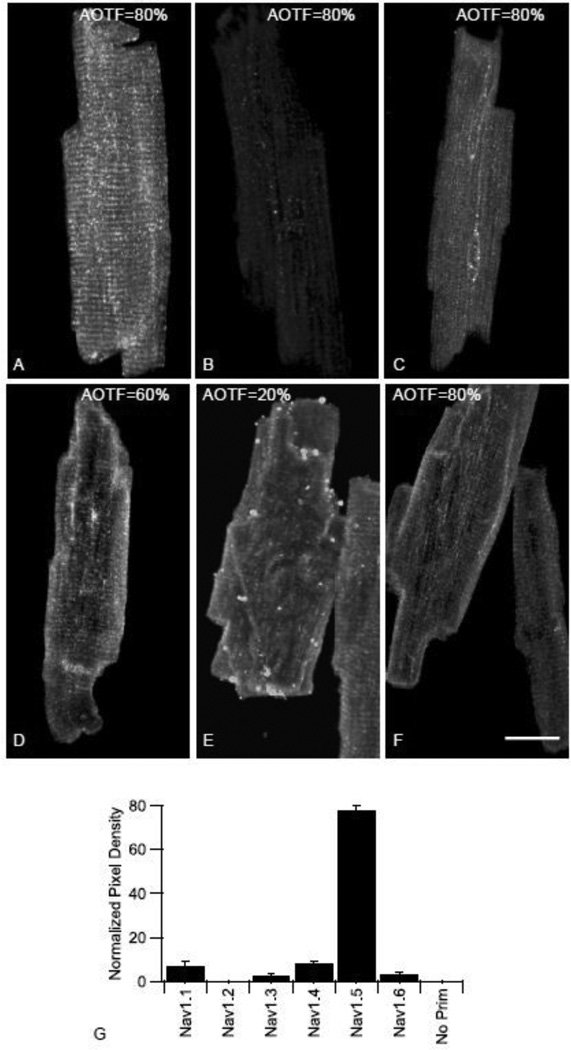
Using the standardized approach to antibody staining described above, we compared the relative staining of different sodium channel α subunits in mouse ventricular myocytes. Myocytes were isolated from single ventricles and individual aliquots from the isolation were stained with one of the subtype-specific sodium channel antibodies (NaV1.1–NaV1.6) and viewed at an appropriate AOTF setting that maximized staining without saturation as described above. Z-stacks of the optical sections through the myocytes were generated (Fig. 3) and used for quantification after correction for AOTF setting. Corrected overall staining intensity from Z-stacks of 4 to 6 myocytes per antibody from each of 3 isolations is shown in Fig. 3. Relative normalized pixel intensities from these immunocytochemical measurements were: NaV1.1=7.2 ± 2.0%, NaV1.2=0.04 ± 0%, NaV1.3=2.9 ± 0.5%, NaV1.4=8.7 ± 0.5%, NaV1.5=77.5 ± 1.3%, NaV1.6=3.7 ± 0.4%, and no primary antibody control=0.04 ± 0%. We also normalized the signal intensity of the isoform specific sodium channel antibodies to alpha-actinin and observed that the ratio of staining for each individual antibody did not change (data not shown). As in previous studies [8], staining with anti-NaV1.2 was indistinguishable from staining with no primary antibody. Thus, based on these methods, the TTX-sensitive channels represented 22.5% of total channel staining while TTX-resistant NaV1.5 channel represented 77.5% of the total channel staining. This value is similar to electrophysiological determinations of levels of TTX-sensitive versus TTX-resistant current in rodent ventricular myocytes [10]. For NaV1.5, intense levels of staining were observed at intercalated disc regions, as reported previously [8], compared to much lower levels of staining localized in a striated pattern that was not well characterized using previous methods [8].
Fig. 3.
Representative Staining Patterns of Ventricular Myocytes using Sodium Channel Antibodies. Images are z-stacks of ventricular myocyte images stained with (A) anti-NaV1.1 (B) anti-NaV1.2 (C) anti-NaV1.3 (D) anti-NaV1.4 (E) anti-NaV1.5 or (F) anti-NaV1.6 at the indicated AOTF setting illustrating typical myocytes used for the analysis of the relative sodium channel density. Note staining of z-lines with anti-NaV1.1, anti-NaV1.3, anti-NaV1.4 (G) Quantitative analysis of staining intensities of sodium channel antibodies in ventricular myocytes. The graph indicates the normalized pixel intensity for each subtype-specific sodium channel antibody used. Scale bar=25 um.
3.3. Surface and internal localization of sodium channel subtypes in mouse ventricular myocytes
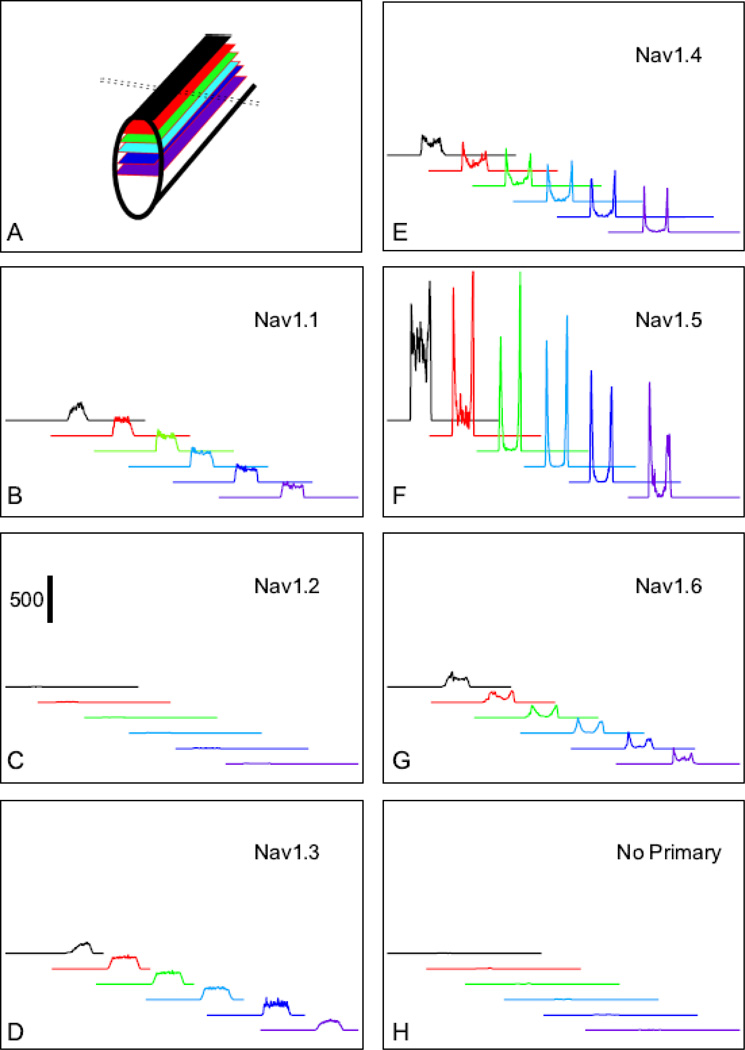
To quantify the relative levels and patterns of staining at different depths in the myocytes, single optical sections were examined (Fig. 4A). Transverse line-scans (50 pixels wide, thus extending 8 µm along the cell; dotted lines, Fig. 4A) were measured for each antibody at each depth and a representative series for each antibody were plotted (Fig. 4B–G). The width of the line scan included approximately 4 z-lines and their associated t-tubule bands. NaV1.1, NaV1.3, NaV1.4, NaV1.5 and NaV1.6 all stain the surface of the myocyte (Fig. 4B, D–G, black lines), but staining for NaV1.5 is more intense than for the other channels. Stain for NaV1.1 and NaV1.3 at each depth is approximately uniform across the width of the myocyte, suggesting similar expression level on the cell surface and internally (Fig. 4B, D). In contrast, line scans of NaV1.4, NaV1.5, and NaV1.6 staining exhibit peaks at the edges of the cell, indicating high levels of staining near the myocyte surface and lower levels inside the myocyte (Fig. 4E–F). As observed for overall staining (Fig. 3) and reported previously [8, 22], staining for NaV1.2 was virtually undetectable (Fig. 4C). Interestingly, we observed little detectable staining for NaV1.5 in the core of the myocytes, even when examined with our more quantitative approach. Thus, NaV1.5 appears to be most important sodium channel at the myocyte surface and intercalated disc, whereas other sodium channel isoforms are more important in the myocyte core.
Fig. 4.
Representative staining densities of NaV1.1-NaV1.6 antibodies at various levels throughout ventricular myocytes. Images (1 µm z- steps) of the staining in ventricular mouse myocytes were collected, analyzed using lines scans and the results were plotted as a function of their position within the myocyte. A) Schematic diagram of the image planes used to construct the plots in B–H. The dashed line represents the orientation of the line scan used for analysis. (BH) Staining densities at the surface of the myocyte is represented by the black line for each antibody. The red, green, light blue, dark blue and purple lines represent analysis from distances of 2, 4, 6, 8, 10, or 12 um, respectively below the surface image shown in black.
To quantify the relative staining at the cell surface versus the myocyte core for each antibody, 1-µm optical sections were analyzed at the cell surface and near the center of the cell, 7- µm from the surface (n= 8 cells/antibody). From each 50 pixel-wide line scan, the value for expression at the myocyte surface were taken as the most peripheral 3 pixel layer at each myocyte surface; the value for the center of the myocyte was the average of a 60 pixel-wide band at the midpoint of the line scan. Means of myocyte surface localization (red squares) and central localization (blue squares) for each alpha subunit are shown in Fig. 5. Consistent with the example data (Fig. 4), NaV1.5 is the primary isoform at the cell surface, with measurable staining of NaV1.4 as well (Fig. 5A). Mean density of sodium channels in the core of the myocyte is much lower, with NaV1.1, NaV1.3 and NaV1.4 having measurable staining (Fig. 5B).
Fig. 5.
Quantification of surface and internal staining patterns of Nav channels in mouse ventricular myocytes. A–B) Optical sections from the middle of mouse ventricular myocytes were analyzed using a line scan to compare the density of staining on the surface (edge) of the myocyte and internally (middle region) for each antibody investigated. Red represents surface staining averaged over 3 pixels. Blue is internal staining density levels averaged over 50 pixels.
3.4. NaV1.5 localization at intercalated discs
Previous studies found high-density clusters of NaV1.5 at or near intercalated discs [6, 8, 23, 24] as recognized by double-labeling and colocalization with connexin 43 [8, 23, 24]. Myocytes double labeled with NaV1.5 and connexin 43 antibodies also showed colocalization of these proteins in the intercalated disc regions in these experiments (Figs. 6A–C). Next, we compared NaV1.5 staining intensity at intercalated discs to staining elsewhere in the myocyte. Myocytes stained with antibodies recognizing NaV1.5 show a characteristic rim of staining at the cell membrane with little staining in the cell interior (Fig. 6A and 6D) as quantified above (Figs. 4 and 5). In addition, hot spots of intense staining are observed at intercalated disc, as indicated by colocalization with connexin-43 (Fig. 6A–C, see also hotspots in D; [8]). A colorized pixel density plot of Fig. 6D emphasizes the disposition of these sites across the myocyte and the intensity of the staining relative to the general myocyte surface (Fig. 6E). A line scan of the representative myocyte where the scanned region contains a hot spot near an intercalated disc (Fig. 6F; region indicated by dotted lines in Fig. 6D) indicates an approximately 5-fold higher density of staining in this hot spot at an intercalated disc relative to the two lower peaks that represent general immunostaining of NaV1.5 channels at the cell surface on the two sides of the cell. A similar finding is shown in the lower panels of Figs. 6A–C for a myocyte double labeled with connexin 43. Quantification of average pixel intensity of intercalated disc staining relative to general cell surface staining in the same set of cells (n=30 myocytes, 2–3 different planes/myocyte from 3 different preparations) is shown in Fig. 6G further illustrating an approximately 5-fold increase in NaV1.5 staining at intercalated discs.
Fig. 6.
Comparison of NaV1.5 staining at intercalated disc and surface membrane. (A–C) A representative myocyte is shown in the upper panels that was double labeled with anti-NaV1.5 antibodies (A) and anti connexin 43 antibodies (B) illustrating colocalization of these proteins in intercalated disc regions as shown in the merged image (C). The lower panels of A–C are the representative line scans taken from the regions between the dotted lines of their respective panels illustrating colocalization of NaV1.5 and connexin 43. D) Another representatative image of ventricular myocytes labeled with anti-NaV1.5 antibodies demonstrating staining at both intercalated disc and on surface membrane. E) Computer generated image of myocytes shown in (D) illustrating staining of hot spots at disc (red and yellow peaks) compared to less dense staining (blue) at the membrane. Image is tilted to align with the graph in panel F. F) Cross sectional line scan analysis of staining intensity through the region of the myocyte indicated by the white, dashed lines shown in (D). (G) Quantification of mean pixel intensity of fluorescence in disc compared to cell surface (edge) of myocytes. Scale bars: A–C = 10 um; D = 20 um.
4. Discussion
4.1. Differential expression and distribution of sodium channel isoforms in mouse ventricular myocytes
Here we have taken a quantitative approach to assessing the relative level of expression and the subcellular distribution of sodium channel α subunit subtypes in mouse ventricular myocytes. Previous reports addressed subcellular distribution of some sodium channel subtypes but did not quantify expression levels of particular α subunits in those locations [7, 8, 11]. Relative expression of α subunit isoform RNA was measured using RT-PCR but could not examine the levels of the expressed proteins or their subcellular locations [11]. Quantitative measurement of the density of sodium channel subtypes in different subcellular locations is important in order to relate channel localization to function measured with electrophysiological methods and to allow development of accurate cellular models of action potential generation and conduction in cardiac myocytes.
Our results show that approximately 80% of the expressed sodium channel α subunit protein molecules were of the NaV1.5 isoform. The remaining sodium channel protein consisted of TTX-sensitive NaV1.1, NaV1.3, NaV1.4 and NaV1.6 channels. NaV1.5 channels were found on the myocyte cylindrical surface with striking punctate concentrations near the intercalated discs, but these channels were largely absent from non-surface locations including the t-tubular system. The concentrations of NaV1.5 at intercalated discs are also seen in immunohistochemical analysis of intact tissue sections [6, 23, 24] and thus cannot result from the myocyte isolation procedure. NaV1.4 was the next most abundant channel and was also found on the myocyte surface. NaV1.1 and NaV1.6 were present at lower levels. The small amount of NaV1.6 was preferentially localized to the myocyte surface, whereas NaV1.1 and NaV1.3 were located in t-tubules approximately evenly distributed across the myocyte cross-sectional area. Localization of NaV1.1 and NaV1.3, but not NaV1.5, in t-tubular membranes is likely to be responsible for conducting signals into the center of the myocyte to initiate contraction. Blocking TTX-sensitive sodium currents had marked effects on excitation-contraction coupling [9, 25]. This role of t-tubular TTX-sensitive channels in excitation-contraction coupling has recently been confirmed and evaluated in detail in rat ventricular myocytes [25].
4.2. Quantitative differences in the levels of protein expression of sodium channels in mouse ventricular myocytes
Multiple reports indicated the presence of both TTX-sensitive (NaV1.1-NaV1.4 and NaV1.6) and TTX-resistant (NaV1.5) sodium channel proteins in rodent heart muscle using various methods of detection [6–11, 25, 26]; reviewed in [27]), though not all agree on the specific channel isoform. Our findings can be correlated with electrophysiological studies assessing the contribution of TTX-sensitive channels to total cardiac sodium current in rodent heart. Those studies utilized the TTX-resistance of the NaV1.5 sodium channel to estimate that TTX-sensitive currents contribute from 5% to 16–20% of peak sodium current in rodent ventricular myocytes [8–12, 25]. Using a detubulated preparation of rat ventricular cardiomyocytes, studies indicated that about 11% of the sodium current was due to TTX-sensitive channels [12]. A recent study recording sodium current from patches of membrane in the midsection (non-intercalated disc) of rat and mouse ventricular myocytes determined that approximately 20% of the current was due to TTX-sensitive sodium channels [28]. These findings in rodents are in line with levels of TTX-sensitive currents found in cardiac preparations of other species including humans. In canine ventricular myocytes, TTX-sensitive sodium current was estimated to be approximately 10% of total [11]. In human atrial mytocytes, the TTX-sensitive currents contribute up to 27% of the total cardiac sodium current [29]. Collectively, these various electrophysiological studies suggest a range from 10% to 27% TTX-sensitive current, a range bracketing our immunocytochemical estimates from mouse ventricular myocytes, supporting the accuracy of our immunocytochemical quantification.
Differences among studies may be due to differences between cardiac tissues and the species from which those tissues were isolated, but also may reflect differences in preparation method and technique [30]. Several issues may contribute to the generally lower level of TTX-sensitive sodium current in whole cell recordings relative to the protein measurements reported here. Much of the protein for TTX-sensitive sodium channels is not found on the cell-surface (Fig. 4). Some of this protein is localized in t-tubular membranes where it plays an important role in excitation-contraction coupling [9, 25]. Some also may be in truly intracellular compartments. The TTX-sensitive component of sodium current is preferentially up-regulated after exposure to TNFα, perhaps due to mobilization of synthesized, but not yet surface expressed α subunits [28]. These channels will be immunoreactive but will not contribute to measured current, thus overestimating the TTX-sensitive component. Even the channels that are functionally expressed on the cell membrane but within the t-tubules may contribute less to the peak current because of the relatively slow and incomplete voltage clamp of this membrane compartment. This too will reduce the contribution of TTX-sensitive sodium currents to the current measured in the whole cell mode or from cell-attached patches on the myocyte surface [28]. Thus, it is not surprising that the fractional level of TTX-sensitive α subunits is somewhat higher when assessed by immunocytochemistry than it is when measured by electrophysiology.
Our study did not include antibodies recognizing two TTX-insensitive sodium channels, NaV1.8 and NaV1.9. NaV1.8 has recently been reported to have significant expression in the heart [31]. Comparisons with electrophysiological measurements are expected to be unaffected because NaV1.8 and NaV1.9 activate slowly and are not expected to contribute to peak sodium current in electrophysiological studies.
While we standardized as many factors as possible when quantifying our immunocytochemistry results, additional variables can affect quantification. Both intra-assay and inter-assay variability must be considered and recognized as possible influences on immunohistochemical reactions and quantification. These variables include differences in antibody batches, fixation, and isolation of specimen, to name a few. Immunocytochemistry is a complement to other experimental approaches such as western blotting, in situ hybridization and electrophysiology but offers the unique advantage of subcellular localization.
4.3. Optical images reveal important differences between surface and internal membrane staining patterns for individual sodium channel α subunit isoforms
As expected from electrophysiological and biochemical studies NaV1.5 appears to be the predominant protein present in mouse ventricular myocytes compared to NaV1.1, NaV1.2, NaV1.3, NaV1.4, or NaV1.6 channels. This study provides new insight into the relative distribution of these channels throughout the entire myocyte, revealing important differences between surface membrane staining and internal staining. Our image analysis revealed that the sodium channel alpha subunit distributions fall into two general patterns. NaV1.4, NaV1.5, and NaV1.6 reside mainly on the cell surface membrane, with little internal staining (either t-tubular or intracellular). In contrast, NaV1.1 and NaV1.3 staining was similar at the surface and internally at all depths of the myocyte, representing staining of t-tubules and potentially other intracellular membrane compartments.
4.4 Conclusion
In summary, our findings suggest that the sodium channel antibodies and methods used in this study provide reasonable quantitative estimates of the relative density of individual isoforms of the sodium channel in the subcellular compartments of the mouse ventricular myocyte. By carefully controlling the variables in our immunocytochemistry experiments, we have revealed important quantitative relationships among the distributions of specific subtypes of voltage-gated sodium channels in mouse ventricular myocytes, which indicate that they serve different functional roles [9, 25]. Comparisons with myocytes from other cardiac regions and from different species will give further insight into the differential physiological roles of these sodium channel subtypes.
Supplementary Material
HIGHLIGHTS.
Methods for quantifying Na channel a subunit immunostaining are described.
Nav1.5 accounts for 77% of Na channels in mouse ventricular myocytes.
Nav1.1, Nav1.3, Nav1.4 and Nav1.6 account for the remaining 23%.
Nav1.5 is expressed on the cell surface and concentrated at intercalated discs.
Nav1.1 and Nav1.3 but not Nav1.5 are expressed in transverse tubules.
Acknowledgments
Sources of funding
This work was primarily supported by the Deutsche Forschungsgemeinschaft, DFG, (Ma2252) and the Interdisziplinäres Zentrum für klinische Forschung der Universität Würzburg (IZKF) to S.K.G. Maier. The authors also gratefully acknowledge financial support provided by the National Institutes of Health (P01 HL44948 and R01 HL085372) to W.A. Catterall.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
REFERENCES
- 1.Balser JR. The cardiac sodium channel: Gating function and molecular pharmacology. J Mol Cell Cardiol. 2001;33:599–613. doi: 10.1006/jmcc.2000.1346. [DOI] [PubMed] [Google Scholar]
- 2.Abriel H. Cardiac sodium channel Nav1.5 and its associated proteins. Arch Mal Coeur Vaiss. 2007;100:787–793. [PubMed] [Google Scholar]
- 3.Benndorf K, Boldt W, Nilius B. Sodium current in single myocardial mouse cells. Pflugers Arch. 1985;404:190–196. doi: 10.1007/BF00585418. [DOI] [PubMed] [Google Scholar]
- 4.Cribbs LL, Satin J, Fozzard HA, Rogart RB. Functional expression of the rat heart I Na+ channel isoform. Demonstration of properties characteristic of native cardiac Na+ channels. FEBS Lett. 1990;275:195–200. doi: 10.1016/0014-5793(90)81470-9. [DOI] [PubMed] [Google Scholar]
- 5.Chahine M, Deschene I, Chen LQ, Kallen RG. Electrophysiological characteristics of cloned skeletal and cardiac muscle sodium channels. Am J Physiol Heart Circ Physiol. 1996;271:H498–H506. doi: 10.1152/ajpheart.1996.271.2.H498. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SA. Immunocytochemical localization of rH1 sodium channel in adult rat heart atria and ventricle. Presence in terminal intercalated disks. Circulation. 1996;94:3083–3086. doi: 10.1161/01.cir.94.12.3083. [DOI] [PubMed] [Google Scholar]
- 7.Malhotra JD, Chen C, Rivolta I, Abriel H, Malhotra R, Mattei LN, et al. Characterization of sodium channel α- and β-subunits in rat and mouse cardiac myocytes. Circulation. 2001;103:1303–1310. doi: 10.1161/01.cir.103.9.1303. [DOI] [PubMed] [Google Scholar]
- 8.Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel α and β subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- 9.Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci U S A. 2002;99:4073–4078. doi: 10.1073/pnas.261705699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duclohier H. Neuronal sodium channels in ventricular heart cells are localized near T-tubules openings. Biochem Biophys Res Comm. 2005;334:1135–1140. doi: 10.1016/j.bbrc.2005.06.203. [DOI] [PubMed] [Google Scholar]
- 11.Haufe V, Camacho JA, Dumaine R, Gunther B, Bollensdorff C, von Banchet GS, et al. Expression pattern of neuronal and skeletal muscle voltage-gated Na+ channels in the developing mouse heart. J Physiol. 2005;564:683–696. doi: 10.1113/jphysiol.2004.079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brette F, Orchard CH. Density and sub-cellular distribution of cardiac and neuronal sodium channel isoforms in rat ventricular myocytes. Biochem Biophys Res Comm. 2006;348:1163–1166. doi: 10.1016/j.bbrc.2006.07.189. [DOI] [PubMed] [Google Scholar]
- 13.Grube D. Constants and variables in immunohistochemistry. Archiv Histol Cytol. 2004;67:115–134. doi: 10.1679/aohc.67.115. [DOI] [PubMed] [Google Scholar]
- 14.Pawley J. The 39 steps: a cautionary tale of quantitative 3-D fluorescence microscopy. Biotechniques. 2000;28:884–886. 8. doi: 10.2144/00285bt01. [DOI] [PubMed] [Google Scholar]
- 15.Noujaim SF, Kaur K, Milstein M, Jones JM, Furspan P, Jiang D, et al. A null mutation of the neuronal sodium channel NaV1.6 disrupts action potential propagation and excitation-contraction coupling in the mouse heart. FASEB J. 2012;26:63–72. doi: 10.1096/fj.10-179770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 17.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, et al. NaV1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beacham D, Ahn M, Catterall WA, Scheuer T. Sites and molecular mechanisms of modulation of NaV1.2 channels by Fyn tyrosine kinase. J Neurosci. 2007;27:11543–11551. doi: 10.1523/JNEUROSCI.1743-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CA, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 20.Qu Y, Rogers J, Tanada T, Scheuer T, Catterall WA. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Natl Acad Sci U S A. 1994;91:3289–3293. doi: 10.1073/pnas.91.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo W, Xu H, London B, Nerbonne JM. Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes. J Physiol. 1999;521:587–599. doi: 10.1111/j.1469-7793.1999.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier SK, Kirstein M. Beta-adrenergic stimulation modulates the sodium current block by propafenone in rat ventricular myocardium. J Electrocardiol. 2002;35:343–349. doi: 10.1054/jelc.2002.35849. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel b1 subunits are differentially localized in cardiac myocytes. J Biol Chem. 2004;279:40748–40754. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez JN, de la Rosa A, Navarro F, Franco D, Aranega AE. Tissue distribution and subcellular localization of the cardiac sodium channel during mouse heart development. Cardiovasc Res. 2008;78:45–52. doi: 10.1093/cvr/cvm118. [DOI] [PubMed] [Google Scholar]
- 25.Torres NS, Larbig R, Rock A, Goldhaber JI, Bridge JH. Na+ currents are required for efficient excitation-contraction coupling in rabbit ventricular myocytes: a possible contribution of neuronal Na+ channels. J Physiol. 2010;588:4249–4260. doi: 10.1113/jphysiol.2010.194688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brette F, Orchard CH. No apparent requirement for neuronal sodium channels in excitation-contraction coupling in rat ventricular myocytes. Circ Res. 2006;98:667–674. doi: 10.1161/01.RES.0000209963.02720.70. [DOI] [PubMed] [Google Scholar]
- 27.Haufe V, Chamberland C, Dumaine R. The promiscuous nature of the cardiac sodium current. J Mol Cell Cardiol. 2007;42:469–477. doi: 10.1016/j.yjmcc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Lin X, Liu N, Lu J, Zhang J, Anumonwo JM, Isom LL, et al. Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm. 2011;8:1923–1930. doi: 10.1016/j.hrthm.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufmann SG, Maas AH, Lange V, Renner A, Wischmeyer E, Bonz A, et al. Distribution and function of sodium channel subtypes in human atrial myocardium. J Mol Cell Cardiol. 2012 doi: 10.1016/j.yjmcc.2013.05.006. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blechschmidt S, Haufe V, Benndorf K, Zimmer T. Voltage-gated Na+ channel transcript patterns in the mammalian heart are species-dependent. Prog Biophys Mol Biol. 2008;98:309–318. doi: 10.1016/j.pbiomolbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe H, Yang T, Stroud DM, Lowe JS, Harris L, Atack TC, et al. Striking In vivo phenotype of a disease-associated human SCN5A mutation producing minimal changes in vitro. Circulation. 2011;124:1001–1011. doi: 10.1161/CIRCULATIONAHA.110.987248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.