Abstract
Background
Cancer can affect many dimensions of a patient’s life, and in turn, it should be targeted using a multimodal approach. We tested the extent to which an interdisciplinary nutrition–rehabilitation program can improve the well-being of patients with advanced cancer.
Methods
Between January 10, 2007, and September 29, 2010, 188 patients with advanced cancer enrolled in the 10–12-week program. Body weight, physical function, symptom severity, fatigue dimensions, distress level, coping ability, and overall quality of life were assessed at the start and end of the program.
Results
Of the enrolled patients, 70% completed the program. Patients experienced strong improvements in the physical and activity dimensions of fatigue (effect sizes: 0.8–1.1). They also experienced moderate reductions in the severity of weakness, depression, nervousness, shortness of breath, and distress (effect sizes: 0.5–0.7), and moderate improvements in Six Minute Walk Test distance, maximal gait speed, coping ability, and quality of life (effect sizes: 0.5–0.7) Furthermore, 77% of patients either maintained or increased their body weight.
Conclusions
Interdisciplinary nutrition–rehabilitation can be advantageous for patients with advanced cancer and should be considered an integrated part of standard palliative care.
Keywords: Neoplasms, rehabilitation, patient care team, symptom assessment, palliative care, quality of life
1. INTRODUCTION
The McGill Cancer Nutrition–Rehabilitation (cnr) Program was inaugurated in 2003. The program is based on the hypothesis that nutrition counselling, together with an exercise program and dedicated symptom control, will improve quality of life and functioning in advanced cancer patients. It was conceived as a model for the application of palliative care early in the course of a predictably fatal cancer. To date, benefits in patients with head-and-neck and gastroesophageal cancers have been recorded and published1,2.
The aim of the present study is to report the degree to which a cnr program improves symptom control, nutrition status, physical function, psychological well-being, and overall quality of life in patients with advanced cancer.
2. METHODS
2.1. Study Design and Population
This uncontrolled prospective intervention study looked at patients enrolled in the McGill University Health Centre cnr program between January 10, 2007, and September 29, 2010. During that time, patient data were maintained in a computerized database. Using an uncontrolled pre–post test design, we analyzed data collected at the start and end of the program. Here, we report the results for participants with advanced cancer (stages iii and iv) and for those with hematologic cancers who had not received a bone marrow transplant.
2.2. Intervention
The intervention consisted of a 10- to 12-week interdisciplinary outpatient program offered by a team including a physician, a clinical nurse specialist, a pivot nurse, a dietitian, a physical therapist, an occupational therapist, and as needed, a psychologist and a social worker. The global aim of the program was to teach and empower patients and families who are experiencing complex problems to become more independent in physical self-care and symptom management, and to improve their own quality of life.
The team collaboratively designed a care plan shaped by each patient’s individual goals. Each patient was followed regularly by the physical therapist and at least once every 2 weeks by the other specialists, based on need. The team interacted throughout the program and had a formal weekly interdisciplinary meeting to report on patient progress and fine-tune the program in accordance with patient goals. A detailed description of the program structure has been published3.
The nutrition counselling component of the program included dietary advice tailored to patient needs and concerns. The advice could range from a prescription for nutrition planning to discussions in response to patient queries.
The exercise component consisted of strength, endurance, and flexibility training incorporated into semi-weekly exercise sessions with the physical therapist, and a home exercise plan. The occupational therapist provided interventions that touched on the activity domains of self-care, productivity, and leisure4.
The physicians on the team were palliative care specialists who reviewed the medical condition of the patients, conducted thorough symptom assessments, and provided appropriate medical interventions. The two nurses on the team were trained in patient–family counselling, assessment, and symptom care, but also played complementary team roles. The pivot nurse was responsible for referral assessment, screening, coordination of care planning, and case management. The clinical nurse specialist was involved in program design, management, and evaluation, and maintenance of cohesion among team members. Consultation with social workers and psychologists was readily available and used frequently5.
2.3. Procedures
At their initial visit, patients were assessed by all team members, underwent routine oncology blood tests [with added serum C-reactive protein (crp) measurements], and completed several self-report questionnaires. When patients completed the program, the same evaluations were repeated during their final visit to the clinic. Patients provided written informed consent to have their demographic and clinical information and evaluation results recorded in an anonymous and secure computerized database. The study was approved by the McGill University Health Centre Research Ethics Board.
2.4. Measures
2.4.1. Patient Self-Administered Questionnaires
We used the modified Edmonton Symptom Assessment System (esas), adapted for palliative cancer patients from the original esas6, to assess quality of life and the severity of 8 common cancer-related symptoms. Items are rated on an 11-point scale ranging from 0 to 10 in ascending order of severity, based on the preceding 48 hours. The original esas questionnaire was validated against the Functional Assessment of Cancer Therapy and the Memorial Symptom Assessment Scale and shown to have good internal consistency7.
We used the Multidimensional Fatigue Inventory to assess 5 dimensions of fatigue: general fatigue, physical fatigue, decreased activity, decreased motivation, and mental fatigue8. The inventory consists of 20 statements that patients rate from 1 (yes, that is true) to 5 (no, that is not true). Accordingly, each fatigue dimension receives a score out of 20, with higher scores indicating greater fatigue. The Multidimensional Fatigue Inventory has been shown to be both a valid and reliable tool for assessing fatigue in cancer patients8.
Patients also completed the Distress Thermometer, a 1-item visual analogue scale developed as a rapid screening tool for distress in patients with cancer9. Patients rate their level of distress over the preceding week on an 11-point scale ranging from 0 (no distress) to 10 (extreme distress). The Distress Thermometer has been shown to be comparable to the Hospital Anxiety and Depression Scale and the Brief Symptom Inventory as a measure of distress in ambulatory cancer patients10.
The Coping Thermometer, another 1-item visual analog scale, was used to assess how well patients were coping with distress on a scale from 0 (I have no difficulty coping) to 10 (I have great difficulty coping).
2.4.2. Nutrition Status and Physical Functioning
Patients received complete nutrition assessments by the team dietitian. We extracted 6-month recall weight loss, body weight, and presence of alterations in taste or smell from the database. Poor nutrition status at baseline was defined as two or more of a weight loss of 10% or more in the preceding 6 months, a body mass index below 20 kg/m2, and a serum albumin concentration less than 38 g/L.
The Six Minute Walk Test was used to assess walking capacity11. Patients were instructed to cover as much distance as possible in 6 minutes by walking up and down a 15-m stretch of floor. After each minute, the patients were informed of the number of minutes completed in the test, but no encouragement was provided. The Five-Metre Walk Test was used to assess maximal gait speed. Patients were instructed to walk as quickly as possible over a total distance of 10 metres. Using a stopwatch, time for the middle 5 meters was recorded to minimize the effects of acceleration and deceleration.
2.5. Statistical Analysis
To compare the baseline characteristics of patients who did and did not complete the cnr program, we used the Pearson chi-square test for categorical variables and the independent t-test for continuous variables. Descriptive statistics were used to determine the percentage of patients who gained more than 2 kg, maintained their weight within 2 kg, or lost more than 2 kg from baseline to the end of the program. A McNemar test was used to determine if the proportion of patients with taste or smell alterations changed from the start to the end of the program. Questionnaire results and measures obtained before and after the cnr program were compared using paired t-tests. The pre–post differences in the results are expressed as means with 95% confidence intervals. Effect sizes were used to estimate the degree to which the outcomes changed. The sizes were calculated as the mean change divided by the standard deviation of the mean change and were interpreted using the Cohen classification12. To adjust for multiple testing per the Bonferroni method13, we divided p ≤ 0.05 by 20 to establish a significance level of p ≤ 0.0025 for the paired t-tests. For all other tests, a p value of 0.05 or less was used. All statistical analyses were performed using the SAS software application (version 9.2: SAS Institute, Cary, NC, U.S.A.).
3. RESULTS
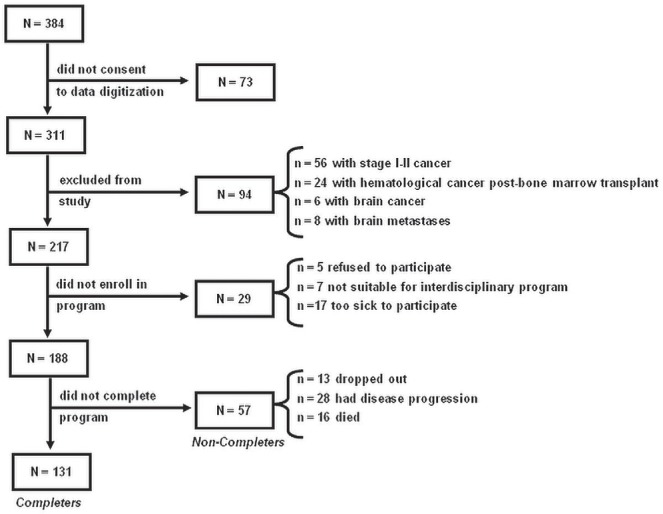
Figure 1 is the study inclusion diagram, which shows patients consecutively evaluated at the cnr clinic between January 10, 2007, and September 29, 2010. Of the 188 patients with advanced cancer who enrolled in the cnr program, 30% did not complete all sessions (“non-completers”: 13 dropped out; 28 experienced disease progression; 16 died). At program entry, non-completers were more likely than completers to have a poor Eastern Cooperative Oncology Group performance status (54.9% for non-completers vs. 34.8% for completers, p = 0.028), a crp concentration greater than 20 mg/L (41.5% in non-completers vs. 18.5% in completers, p = 0.003), poor nutrition status (39.3% in non-completers vs. 22.8% in completers, p = 0.022), and worse anorexia [mean: 5.3 ± 3.0 in non-completers vs. 4.1 ± 2.9 in completers, p = 0.012).
FIGURE 1.
Study inclusion diagram. Patients evaluated at the Cancer Nutrition–Rehabilitation clinic between January 10, 2007, and September 29, 2010.
Table i shows the baseline characteristics of patients who completed the program. Mean age was 63.4 ± 11.2 years, and approximately two thirds of the patients were men. Chemotherapy status was variable and most patients had an Eastern Cooperative Oncology Group performance status of 1 or 2. Most patients had serum crp concentrations of less than 10 mg/L and normal concentrations of lactate dehydrogenase; fewer than one quarter of the patients presented with poor nutrition status.
TABLE I.
Baseline characteristics of patients with advanced cancer who completed the Cancer Nutrition–Rehabilitation program
| Characteristic | Available data (n pts) | Value |
|---|---|---|
| Study cohort | 131 | |
| Mean age (years) | 131 | 59.9±13.0 |
| Sex [n (%)] | 131 | |
| Men | 66 (50.4) | |
| Women | 65 (49.6) | |
| Cancer site [n (%)] | 131 | |
| Head and neck | 20 (15.3) | |
| Breast | 13 (9.9) | |
| Lung | 8 (6.1) | |
| Upper gastrointestinal | 8 (6.1) | |
| Hepatobiliary | 10 (7.6) | |
| Pancreatic | 12 (9.2) | |
| Colorectal | 21 (16.0) | |
| Prostate | 5 (3.8) | |
| Gynecologic | 11(8.4) | |
| Hematologic | 13 (9.9) | |
| Other | 10 (7.6) | |
| Chemotherapy [n (%)] | 131 | |
| Current | ||
| No | 29 (22.1) | |
| Yes | 50 (38.2) | |
| Past | ||
| ≤4 Weeks | 5 (3.8) | |
| >4 Weeks | 47 (35.9) | |
| ecog performance status [n (%)] | 118 | |
| 0 | 10 (8.5) | |
| 1 | 67 (56.8) | |
| 2 | 33 (28.0) | |
| 3 | 8 (6.8) | |
| C-Reactive protein [n (%)] | 126 | |
| <10 mg/L | 88 (69.8) | |
| 10–20 mg/L | 15 (11.9) | |
| >20 mg/L | 23 (18.5) | |
| Lactate dehydrogenase [n (%)] | 128 | |
| <210 U/L | 111 (86.7) | |
| ≥210 U/L | 17 (13.3) | |
| Poor nutrition statusa | 127 | 29 (22.8) |
| BMI<20 kg/m2 | 127 | 25 (19.7) |
| Weight loss ≥10% in preceding 6 months | 109 | 35 (32.1) |
| Serum albumin < 38 g/L | 128 | 69 (53.9) |
Defined as two or more of weight loss of 10% or more in the preceding 6 months, body mass index less than 20 kg/m2, and serum albumin less than 38 g/L.
Pts = patients; ecog = Eastern Cooperative Oncology Group; bmi = body mass index.
Table ii outlines the interventions accepted by the patients who completed the program. Physicians most frequently prescribed pain medications, laxatives, dietary supplements, and psychotropic agents, and made referrals to psychosocial services. More than half the patients received teaching and follow-up from the nurse for pain and symptom management, medication management, sleep hygiene, and bowel care. Almost all patients received dietary counselling from the dietitian, most of whom also received supplement recommendations. The occupational therapist commonly provided instruction on energy conservation techniques and activity management, goal setting, and leisure or exercise, and also conducted training sessions on activities of daily living and work.
TABLE II.
Types of interventions provided by each Cancer Nutrition–Rehabilitation program health professional
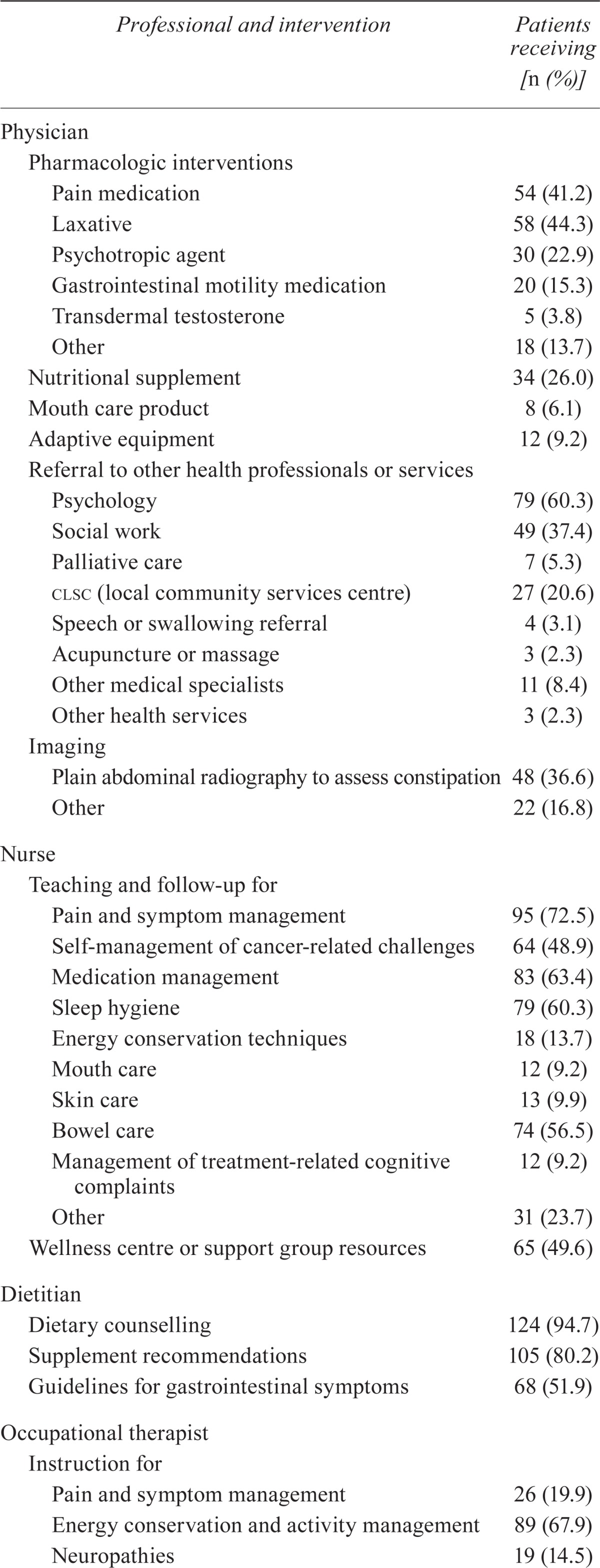
| Professional and intervention | Patients receiving [n (%)] |
|---|---|
| Physician | |
| Pharmacologic interventions | |
| Pain medication | 54 (41.2) |
| Laxative | 58 (44.3) |
| Psychotropic agent | 30 (22.9) |
| Gastrointestinal motility medication | 20 (15.3) |
| Transdermal testosterone | 5 (3.8) |
| Other | 18 (13.7) |
| Nutritional supplement | 34 (26.0) |
| Mouth care product | 8 (6.1) |
| Adaptive equipment | 12 (9.2) |
| Referral to other health professionals or services | |
| Psychology | 79 (60.3) |
| Social work | 49 (37.4) |
| Palliative care | 7 (5.3) |
| clsc (local community services centre) | 27 (20.6) |
| Speech or swallowing referral | 4 (3.1) |
| Acupuncture or massage | 3 (2.3) |
| Other medical specialists | 11 (8.4) |
| Other health services | 3 (2.3) |
| Imaging | |
| Plain abdominal radiography to assess constipation | 48 (36.6) |
| Other | 22 (16.8) |
| Nurse | |
| Teaching and follow-up for | |
| Pain and symptom management | 95 (72.5) |
| Self-management of cancer-related challenges | 64 (48.9) |
| Medication management | 83 (63.4) |
| Sleep hygiene | 79 (60.3) |
| Energy conservation techniques | 18 (13.7) |
| Mouth care | 12 (9.2) |
| Skin care | 13 (9.9) |
| Bowel care | 74 (56.5) |
| Management of treatment-related cognitive complaints | 12 (9.2) |
| Other | 31 (23.7) |
| Wellness centre or support group resources | 65 (49.6) |
| Dietitian | |
| Dietary counselling | 124 (94.7) |
| Supplement recommendations | 105 (80.2) |
| Guidelines for gastrointestinal symptoms | 68 (51.9) |
| Occupational therapist | |
| Instruction for | |
| Pain and symptom management | 26 (19.9) |
| Energy conservation and activity management | 89 (67.9) |
| Neuropathies | 19 (14.5) |
| Goal-setting | 52 (39.7) |
| Leisure or exercise | 40 (30.5) |
| Problem-solving | 14 (10.7) |
| Other | 9 (6.9) |
| Training sessions for | |
| Activities of daily living | 13 (9.9) |
| Instrumental activities of daily living and work | 43 (32.8) |
| Dysphagia management | 13 (9.9) |
| Cognition | 35 (26.7) |
| Positioning and equipment use | 18 (13.7) |
| Support or counselling | 33 (25.2) |
| Caregiver support | 13 (9.9) |
Patients who completed the program had a median of 5 in-person visits (interquartile range: 3–7 visits) and 3 telephone consultations with the nurse (interquartile range: 2–5 consultations). They also had a median of 2 visits with the physician (interquartile range: 1–4 visits), and 3 visits with both the dietitian and occupational therapist (interquartile range: 2–4 visits). The median number of exercise sessions with the physical therapist was 7 (interquartile range: 4–11 sessions). On average, patients attended 82% ± 15% (interquartile range: 75%–100%) of their scheduled exercise sessions with the physical therapist.
Table iii presents changes in symptom severity, fatigue, distress, coping and quality of life from baseline to the end of the program. The severity of all symptoms had significantly declined by the end of the program, with weakness, depression, nervousness, and shortness of breath showing moderate reductions (effect sizes: 0.5–0.7) and sleepiness, insomnia, pain, and anorexia showing small reductions (effect sizes: 0.4). Patients experienced strong improvement in activity and physical fatigue (effect sizes: 0.8–1.1) and a moderate reduction in general fatigue (effect size: 0.7), but only a small improvement in motivation and mental fatigue (effect size: 0.4). They also experienced a moderate reduction in distress and a moderate improvement in coping ability and overall quality of life (effect sizes: 0.5–0.7).
TABLE III.
Changes in symptom severity, fatigue, distress, coping, and quality of life
| Variable | Pts (n) | Baseline score (mean) |
Change in score
|
p Value | Effect sizea | |
|---|---|---|---|---|---|---|
| Mean | 95% ci | |||||
| Symptom severityb | ||||||
| Weakness | 129 | 5.5±2.0 | 1.5 | 1.1 to 1.8 | <0.0001 | 0.7 |
| Depression | 126 | 3.3±2.5 | 1.4 | 1.1 to 1.8 | <0.0001 | 0.7 |
| Nervousness | 124 | 3.7±2.6 | 1.3 | 0.9 to 1.7 | <0.0001 | 0.6 |
| Shortness of breath | 128 | 3.6±2.8 | 1.2 | 0.8 to 1.7 | <0.0001 | 0.5 |
| Sleepiness | 129 | 3.9±2.9 | 1.1 | 0.6 to 1.6 | <0.0001 | 0.4 |
| Insomnia | 129 | 4.1±2.7 | 1.0 | 0.5 to 1.4 | 0.0001 | 0.4 |
| Pain | 127 | 3.6±2.8 | 1.0 | 0.5 to 1.4 | <0.0001 | 0.4 |
| Anorexia | 127 | 4.0±2.9 | 1.0 | 0.5 to 1.4 | <0.0001 | 0.4 |
| Fatigue dimensionc | ||||||
| Reduced activity | 70 | 14.1±4.1 | 4.6 | 3.6 to 5.6 | <0.0001 | 1.1 |
| Physical fatigue | 72 | 14.5±4.3 | 3.7 | 2.6 to 4.7 | <0.001 | 0.8 |
| General fatigue | 71 | 13.8±3.8 | 2.8 | 1.8 to 3.8 | <0.0001 | 0.7 |
| Decreased motivation | 71 | 9.9±3.5 | 1.6 | 0.8 to 2.5 | 0.0004 | 0.4 |
| Mental fatigue | 71 | 10.0±4.1 | 1.7 | 0.8 to 2.6 | 0.0005 | 0.4 |
| Distressd | 126 | 4.2±2.6 | 1.4 | 0.9 to 1.9 | <0.0001 | 0.5 |
| Copinge | 77 | 4.1±2.7 | 1.8 | 1.2 to 2.4 | <0.0001 | 0.7 |
| Quality of lifef | 129 | 4.5±2.3 | 1.0 | 0.6 to 1.3 | <0.0001 | 0.5 |
Small: 0.2–0.5; moderate: 0.5–08; strong: >0.8.
Measured using the modified Edmonton Symptom Assessment System, which ranges from 0 (symptom is absent) to 10 (worst possible symptom severity).
Measured using the Multidimensional Fatigue Inventory, in which each fatigue dimension is scored out of 20, with higher scores indicating greater fatigue.
Measured using the Distress Thermometer, which ranges from 0 (no distress) to 10 (extreme distress).
Measured using the Coping Thermometer, which ranges from 0 (no difficulty coping) to 10 (great difficulty coping).
Measured using the modified Edmonton Symptom Assessment System, which ranges from 0 (excellent quality of life) to 10 (very bad quality of life).
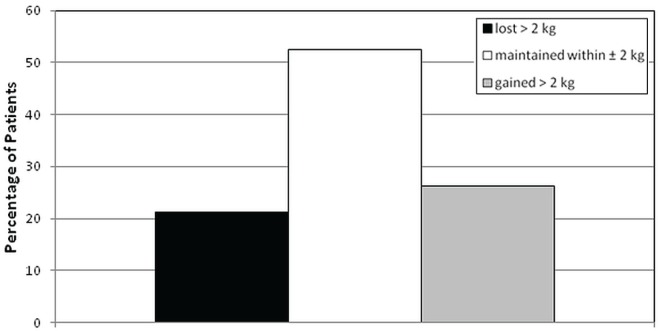
The average Six Minute Walk Test distance was 395 ± 111 m at baseline and improved by 41 m (95% confidence interval: 29 m to 52 m) by the end of the program (effect size: 0.7). Average maximal gait speed was 1.50 ± 0.44 m/s at baseline, which improved by 0.15 m/s (95% confidence interval: 0.09 m/s to 0.21 m/s) at the end of the program (effect size: 0.6). Furthermore, 77% of the patients either maintained their weight within 2 kg or gained more than 2 kg over the course of the program (Figure 2), and the percentage of patients with taste or smell alterations significantly declined to 28.8% from 56.1% (p < 0.0001).
FIGURE 2.
Percentage of patients who lost more than 2 kg, who maintained their weight within 2 kg, and who gained more than 2 kg during the Cancer Nutrition–Rehabilitation program.
4. DISCUSSION
We had hypothesized that an interdisciplinary approach, in step with enhanced symptom management, during or after therapies directly attacking the tumour represented a logical approach for the care of ambulatory patients with advanced cancer who are living at home. Our results suggest that our program can improve symptom control, physical function, psychological well-being, and quality of life.
Few studies to date have assessed the value of interdisciplinary cancer rehabilitation, especially in advanced cancer14,15. A recent randomized clinical trial in patients at the end of treatment for advanced recurrent hematologic or breast cancer showed that a rehabilitation intervention delivered by a multidisciplinary team in combination with usual care was superior to usual care alone in reducing psychological, physical, and patient care needs and in improving the patient’s self-reported health state16. The intervention was tailored to the patient and lasted approximately 3 months, but it was not clear which health professionals constituted the care team and which specific interventions they provided. Another study randomized advanced cancer patients undergoing radiation therapy to either 8 ninety-minute sessions of a structured, multidisciplinary intervention over 3 weeks or to standard care17,18. The intervention encompassed the cognitive, emotional, physical, social, and spiritual dimensions. Quality of life declined in the control group, but members of the intervention group were able to maintain quality of life during the study period. Furthermore, compared with control subjects, the intervention group experienced a greater improvement in subjective physical well-being. However, the difference did not persist at 8 and 27 weeks after the start of the intervention. Notably, the change in fatigue did not differ between the groups, and most patients reported an increase in fatigue. In contrast, although we had no control group for comparison, our study showed improvements in quality of life and in all dimensions of fatigue at the end of the program. This divergence in findings might be explained by differences in patient treatment status and intervention design; our patients were at various phases of the cancer trajectory, and our interventions were patient-tailored rather than structured.
Most multimodal intervention studies in cancer patients have assessed the effects of single elements of interdisciplinary care with an emphasis on nutrition or exercise. Expert panels commonly recommend nutrition guidance for advanced cancer patients19,20, but the research-based value of dietary counselling remains somewhat controversial today. A recent meta-analysis of 13 randomized controlled trials in cancer patients who were malnourished or deemed to be at risk of malnutrition concluded that dietary advice with or without dietary supplements improved some aspects of quality of life and nutritive intake. However, the interventions did not significantly affect weight or energy intake21. The reported studies were strictly dietary initiatives and not part of integrative exercise and palliative care programs, nor were they all specific to advanced-stage cancer.
The successful nutrition counselling trial of Ravasco et al.22 initially reported that nutrition counselling was superior to standard care or dietary supplements for colorectal patients undergoing neoadjuvant radiotherapy, surgery, and adjuvant chemotherapy. Improved outcomes included nutrition status, risk of radiotherapy toxicity, and quality of life. A follow-up study found that, after a median of 6.5 years, not only were the benefits of counselling long-lasting, but patient survival was also improved23. Nutrition counselling is operatordependent, relating to the bond between a counsellor and a patient. In articles on this topic, information is not provided on the relative skills of the dietitians, on their techniques, on the time they spend with patients, and on the intensity of follow-up. When done well, particularly as part of a comprehensive program, results similar to those in the Ravasco study might perhaps be achieved. In our study, most patients either maintained or increased their body weight, which might partly be explained by the integration of the nutrition interventions with exercise.
The benefits of exercise for patients with advanced cancer in a clinical setting remain uncertain. In 2009, Lowe and colleagues carried out a systematic review on exercise studies in the advanced cancer population24. They could find data for only 84 patients spread over six studies, three of which were case reports. All studies reported positive outcomes. A subsequent review described small studies that demonstrated improved functional status and, where measured, quality of life25. More recently, a randomized controlled trial showed that, compared with control subjects receiving usual care, patients who followed an 8-week exercise program showed improvement in physical performance and body weight, but not fatigue26. Another trial specific to stage iv lung and colorectal cancer patients showed that an 8-week home-based exercise program was superior to usual care in improving mobility, fatigue, and sleep quality27. However, that home-based program did not improve pain, quality of life, or ability to perform daily activities. The finding that patients improve in some, but not all, aspects of their well-being seems to suggest the need for more comprehensive care.
Although the ability to perform daily activities was not an outcome in our study, it was the target of some of the common occupational therapy interventions, including instruction in energy conservation techniques and activity management, and training sessions for instrumental activities of daily living and work. Hence, the exercise component of our program might be driving improvement in physical function, and the occupational therapist assists the patients in translating those benefits into everyday tasks and activities. The role of the occupational therapist in oncology has been outlined28,29, but intervention-based research in this area is lacking.
Nurses play a pivotal role in the care of advanced cancer patients30,31. Given the host of symptoms that affect patients and influence their energy intake, it is not surprising that most benefit from integrated nurse and physician instruction on pain and symptom management. All symptoms must be addressed by impeccable analysis and multidirectional therapies. If the full range of symptoms is not controlled, patients will not take in energy or be fit to exercise: someone in pain and fighting for every breath will not eat. It is important to factor the principles of palliative care into a nutrition–exercise program—including psychosocial interventions, which have consistently been shown to positively affect quality of life in patients with advanced cancer32. The symptom burden and psychological well-being of advanced cancer patients can be improved, thus increasing the opportunities of those patients to benefit from a comprehensive program.
Some aspects of a program of this type may be labour-intensive, but they do not depend on expensive technologic developments in modern medicine and could reduce overall health costs by maintaining function. The integrated approach provides an excellent background for carrying out interventional studies enlisting cancer patients early in the course of illness.
It is not surprising that patients with a poor performance status are less likely to complete a comprehensive interdisciplinary program with a nutrition–exercise component. The identification of high serum crp as a significant factor in determining program completion is novel. A raised crp concentration connotes the presence of a tumour promoting a chronic inflammatory state, which in advanced cancer patients is associated with a grim prognosis33,34. The causes of many symptoms—for example, pain, fatigue, insomnia, depression, and the anorexia–cachexia syndrome—are, in part, secondary to chronic inflammation and autonomic nervous system aberrations35–40. Our crp observation points to a need to reframe our program with a stronger emphasis on measures to control chronic inflammation.
Analyzing a pragmatic, nonrandomized, post-hoc study of prospectively collected data has its limitations. Chemotherapy could have contributed to the benefit seen in some patients, although the effect might not have been major, considering the risks and benefits of chemotherapy with respect to symptom control in a population receiving primarily second- to fourth-line regimens. Moreover, with the exception of weight stability or gain, the outcomes of patients receiving chemotherapy during or just before program enrolment were not significantly different from those for non-chemotherapy patients (data not shown).
We also do not have data on maintenance of effect after program completion. We do not know if patients will continue good dietary and exercise practices. Furthermore, because the program is viewed as a whole, from beginning to end, and because patient assessments and questionnaires are not completed at any other time point, we cannot report on any benefit that might have accrued to patients who did not complete the program.
5. CONCLUSIONS
We now accept that cancer control programs must integrate all phases of cancer prevention, including the fourth phase: prevention of suffering41. It is also clear that the treatment of cancer patients must no longer be episodic and sequential. Rather, multimodal therapy offered by interdisciplinary teams should be the norm for cancer care today42. Recently, a publication by Temel et al.43 concluded that early palliative care can improve quality of life in newly diagnosed stage iv lung cancer patients. They also found that survival was better in the randomized palliative care group than in the group simply receiving standard first-line chemotherapy.
Although our program was not initiated as a randomized study, our advanced cancer patients profited from a defined early palliative care program emphasizing nutrition and exercise. Our results suggest the need for well-designed clinical trials to confirm the benefits observed here for patients and their families.
We anticipate that cancer centres will introduce research programs incorporating the best principles of palliative care at the time of first diagnosis of a predictably fatal cancer. Palliative care is more than symptom control. Today, it should also include initiatives in nutrition and rehabilitation. Cancer care rightfully focuses on “personalized medicine,” which is more usually thought of as diagnosis and therapy related to an individual’s gene profile. We must couple those advances with programs incorporating early palliative care that is also linked to the patient’s unique needs. Then we will have a truly comprehensive program for cancer patients and their families.
6. ACKNOWLEDGMENTS
The McGill cnr program was started after receipt of a generous gift from the Riddell family, commemorating the life of Michael Riddell, and a grant from the Webster Foundation. The authors thank S. Schulman for her contribution to the success of the cnr program. The research presented here was funded partly by the Terry Fox Research Institute. BG is a recipient of a Chercheur–Boursier award from the Fonds de recherche du Québec–Santé.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Eades M, Murphy J, Carney S, et al. Effect of an interdisciplinary rehabilitation program on quality of life in patients with head and neck cancer: review of clinical experience. Head Neck. 2013;35:343–9. doi: 10.1002/hed.22972. [DOI] [PubMed] [Google Scholar]
- 2.Chasen MR, Bhargava R. A rehabilitation program for patients with gastroesophageal cancer—a pilot study. Support Care Cancer. 2010;18(suppl 2):S35–40. doi: 10.1007/s00520-010-0828-7. [DOI] [PubMed] [Google Scholar]
- 3.Chasen MR, Dippenaar AP. Cancer nutrition and rehabilitation—its time has come! Curr Oncol. 2008;15:117–22. doi: 10.3747/co.v15i3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemoignan J, Chasen M, Bhargava R. A retrospective study of the role of an occupational therapist in the cancer nutrition rehabilitation program. Support Care Cancer. 2010;18:1589–96. doi: 10.1007/s00520-009-0782-4. [DOI] [PubMed] [Google Scholar]
- 5.Townsend D, Accurso–Massana C, Lechman C, Duder S, Chasen M. Cancer nutrition rehabilitation program: the role of social work. Curr Oncol. 2010;17:12–17. doi: 10.3747/co.v17i6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (esas): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 7.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–71. doi: 10.1002/(SICI)1097-0142(20000501)88:9<2164::AID-CNCR24>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Smets EM, Garssen B, Bonke B, de Haes JC. The Multidimensional Fatigue Inventory (mfi) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- 9.Roth AJ, Kornblith AB, Batel–Copel L, Peabody E, Scher HI, Holland JC. Rapid screening for psychologic distress in men with prostate carcinoma. Cancer. 1998;82:1904–8. doi: 10.1002/(SICI)1097-0142(19980515)82:10<1904::AID-CNCR13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494–502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- 11.ats Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ats statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–17. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 12.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. New York, NY: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 13.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glare P, Jongs W, Zafiropoulos B. Establishing a cancer nutrition rehabilitation program (cnrp) for ambulatory patients attending an Australian cancer center. Support Care Cancer. 2011;19:445–54. doi: 10.1007/s00520-010-0834-9. [DOI] [PubMed] [Google Scholar]
- 15.Del Fabbro E, Hui D, Dalal S, Dev R, Nooruddin ZI, Bruera E. Clinical outcomes and contributors to weight loss in a cancer cachexia clinic. J Palliat Med. 2011;14:1004–8. doi: 10.1089/jpm.2011.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones L, Fitzgerald G, Leurent B, et al. Rehabilitation in advanced, progressive, recurrent cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;46:315–25.e3. doi: 10.1016/j.jpainsymman.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention: a randomized controlled trial. J Clin Oncol. 2006;24:635–42. doi: 10.1200/JCO.2006.06.209. [DOI] [PubMed] [Google Scholar]
- 18.Cheville AL, Girardi J, Clark MM, et al. Therapeutic exercise during outpatient radiation therapy for advanced cancer: feasibility and impact on physical well-being. Am J Phys Med Rehabil. 2010;89:611–19. doi: 10.1097/PHM.0b013e3181d3e782. [DOI] [PubMed] [Google Scholar]
- 19.Canadian Oncology Nutrition Clinical Practice Guideline (con-cpg) Initiative, Expert Panel on Nutrition Screening in Cancer . Systematic Review and Recommendations for Clinical Practice Screening for Malnutrition in Adult Cancer Patients Receiving Chemotherapy and/or Radiotherapy with Curative Intent. Hamilton, ON: CON-CPG Initiative; 2010. [Available online at: http://o.b5z.net/i/u/10020330/f/Screening_Guideline-11032010.pdf; cited January 14, 2013] [Google Scholar]
- 20.Radbruch L, Elsner F, Trottenberg P, Strasser F, Baracos V, Fearon K. Clinical Practice Guidelines on Cancer Cachexia in Advanced Cancer Patients. Aachen, Germany: Department of Palliative Medicine/European Palliative Care Research Collaborative; 2010. [Available online at http://www.epcrc.org/publication_listfiles.php?id=mWdBCMI5eXVlcNFk7Gnq; cited January 14, 2013] [Google Scholar]
- 21.Baldwin C, Spiro A, Ahern R, Emery PW. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:371–85. doi: 10.1093/jnci/djr556. [DOI] [PubMed] [Google Scholar]
- 22.Ravasco P, Monteiro–Grillo I, Vidal PM, Camilo ME. Dietary counseling improves patient outcomes: a prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J Clin Oncol. 2005;23:1431–8. doi: 10.1200/JCO.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 23.Ravasco P, Monteiro–Grillo I, Camilo M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. Am J Clin Nutr. 2012;96:1346–53. doi: 10.3945/ajcn.111.018838. [DOI] [PubMed] [Google Scholar]
- 24.Lowe SS, Watanabe SM, Courneya KS. Physical activity as a supportive care intervention in palliative cancer patients: a systematic review. J Support Oncol. 2009;7:27–34. [PubMed] [Google Scholar]
- 25.Albrecht TA, Taylor AG. Physical activity in patients with advanced-stage cancer: a systematic review of the literature. Clin J Oncol Nurs. 2012;16:293–300. doi: 10.1188/12.CJON.293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oldervoll LM, Loge JH, Lydersen S, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16:1649–57. doi: 10.1634/theoncologist.2011-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage iv lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45:811–21. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penfold SL. The role of the occupational therapist in oncology. Cancer Treat Rev. 1996;22:75–81. doi: 10.1016/S0305-7372(96)90016-X. [DOI] [PubMed] [Google Scholar]
- 29.Silver JK, Gilchrist LS. Cancer rehabilitation with a focus on evidence-based outpatient physical and occupational therapy interventions. Am J Phys Med Rehabil. 2011;90(suppl 1):S5–15. doi: 10.1097/PHM.0b013e31820be4ae. [DOI] [PubMed] [Google Scholar]
- 30.Corner J, Halliday D, Haviland J, et al. Exploring nursing outcomes for patients with advanced cancer following intervention by Macmillan specialist palliative care nurses. J Adv Nurs. 2003;41:561–74. doi: 10.1046/j.1365-2648.2003.02568.x. [DOI] [PubMed] [Google Scholar]
- 31.Sherwood P, Given BA, Given CW, et al. A cognitive behavioral intervention for symptom management in patients with advanced cancer. Oncol Nurs Forum. 2005;32:1190–8. doi: 10.1188/05.ONF.1190-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uitterhoeve RJ, Vernooy M, Litjens M, et al. Psychosocial interventions for patients with advanced cancer—a systematic review of the literature. Br J Cancer. 2004;91:1050–62. doi: 10.1038/sj.bjc.6602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–41. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mgps) predicts cancer survival independent of tumour site: a Glasgow Inflammation Outcome Study. Br J Cancer. 2011;104:726–34. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald N. Chronic inflammatory states: their relationship to cancer prognosis and symptoms. J R Coll Physicians Edinb. 2011;41:246–53. doi: 10.4997/JRCPE.2011.315. [DOI] [PubMed] [Google Scholar]
- 36.Laird BJ, Scott AC, Colvin LA, et al. Cancer pain and its relationship to systemic inflammation: an exploratory study. Pain. 2011;152:460–3. doi: 10.1016/j.pain.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 37.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65:S244–52. doi: 10.1301/nr.2007.dec.S244-S252. [DOI] [PubMed] [Google Scholar]
- 38.Giannousi Z, Gioulbasanis I, Pallis AG, et al. Nutritional status, acute phase response and depression in metastatic lung cancer patients: correlations and association prognosis. Support Care Cancer. 2012;20:1823–9. doi: 10.1007/s00520-011-1282-x. [DOI] [PubMed] [Google Scholar]
- 39.Fagundes CP, Murray DM, Hwang BS, et al. Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36:1137–47. doi: 10.1016/j.psyneuen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–99. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald N. Palliative care—the fourth phase of cancer prevention. Cancer Detect Prev. 1991;15:253–5. [PubMed] [Google Scholar]
- 42.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–7. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 43.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]