Abstract
Background
Healthy lifestyle behaviours may improve outcomes for people with colorectal cancer (crc), but the intention to take action and to change those behaviours may vary with time and resource availability. We aimed to estimate the prevalence of current lifestyle behaviours in people with and without crc in our community, and to identify their desire to change and their resource preferences.
Methods
A mixed-methods survey was completed by people diagnosed with crc who were pre-treatment (n = 54), undergoing treatment (n = 62), or done with treatment for less than 6 months (n = 67) or for more than 6 months (n = 178), and by people without cancer (n = 83).
Results
Current lifestyle behaviours were similar in all groups, with the exception of vigorous physical activity levels, which were significantly lower in the pre-treatment and ongoing treatment respondents than in cancer-free respondents. Significantly more crc respondents than respondents without cancer had made lifestyle changes. Among the crc respondents, dietary change was the change most frequently made (39.3%), and increased physical activity was the change most frequently desired (39.1%). Respondents wanted to use complementary and alternative medicine (cam), reading materials, self-efficacy, and group activities to make future changes.
Conclusions
Resources for lifestyle change should be made available for people diagnosed with crc, and should be tailored to address physical activity, cam, and diet. Lifestyle programs offered throughout the cancer trajectory and beyond treatment completion might be well received by people with crc.
Keywords: Colorectal cancer, lifestyle support, lifestyle resources, health behaviours, mixed-methods
1. INTRODUCTION
Estimates suggest that 1 in 14 Canadians will be diagnosed with colorectal cancer (crc) in their lifetime; approximately 99,000 people have been diagnosed since the early 2000s in Canada alone1. People who have had cancer are at increased risk of developing secondary tumours and other chronic diseases, including osteoporosis, cardiovascular disease, and diabetes2–4. Although those outcomes may be a direct result of the disease and its treatment5–7, research suggests that lifestyle factors play an important role in maintaining quality of life for people who have completed cancer treatment5,6.
Lifestyle behaviours such as alcohol consumption, tobacco use, and poor diet have been linked to increased risk of developing new cancers and recurrences and to reduced survival8. The World Health Organization estimates that about 30% of cancer deaths are attributable to behavioural and dietary risks such as low intake of fruits and vegetables and lack of physical activity9. However, behavioural change often represents a significant obstacle for people3,10,11.
A cancer diagnosis may serve as a “teachable moment” for promoting lifestyle changes4,12. Satia et al. reported significant increases in physical activity, vegetable intake, and supplement use 2 years after colon cancer diagnosis13. Furthermore, a recent qualitative study of crc patients living in the United Kingdom suggested that successful lifestyle changes were often brought on by either the cancer diagnosis or other serious comorbidities11. It seems that there are opportunities for improvement in the lifestyles of people diagnosed with crc. A survey of crc survivors in Alberta revealed that Canadian dietary and physical activity guidelines were being met by only 58% and 26% of respondents respectively14.
Our study approach draws from the “stages of change” construct described by the trans-theoretical model (ttm) of change15–17. The ttm describes behavioural change as a process that, over time, progresses through six stages: pre-contemplation, contemplation, preparation, action, maintenance, and termination15–17. We focused our inquiry on the intention to take action—the “preparation stage” of the ttm. People at this stage have already progressed through the first two stages, pre-contemplation and contemplation, and typically have taken some action toward the behavioural change and are ready for action-oriented programs16. Developing interventions for people at the preparation stage might maximize the use of resources by obtaining results after a shorter period of time.
Interest in the development of lifestyle interventions for cancer survivors is growing. Randomized controlled trials suggest that diet and exercise interventions are safe and lead to improvements in diet, physical function, body weight, and biomarkers for positive disease outcomes18. Numerous health promotion interventions are also focusing on improving lifestyle in the general population19–21, and many of their recommendations are applicable to people with cancer, particularly after treatment completion. Indeed, the World Cancer Research Fund recommends that cancer survivors should use the same cancer-preventive lifestyle guidelines used by people in the general population22. We would argue that, if people with and without a crc diagnosis show no marked differences in lifestyle behaviours or preferred resources, referral to general community programs might be the best course of action. On the other hand, if certain lifestyle information needs are unique to people with a crc diagnosis, those needs might be best addressed by developing tailored information resources and programs.
Approximately 2700 people are diagnosed with crc in the province of British Columbia annually, and approximately 300 are treated at the Vancouver Island Cancer Centre, which is 1 of 6 regional centres in the province. The value of healthy lifestyle behaviours in people with cancer appears undeniable, but few studies have looked at the lifestyle behaviours of people diagnosed with crc13,14, and no information has been published about the intention to make changes. We surveyed people with and without crc to investigate their intention to take action and change lifestyle behaviours. We hypothesized that the relative proportion of people at the preparation stage described by the ttm would vary with their stage in the cancer care trajectory.
Our specific objectives were threefold:
Current lifestyle behaviours: To estimate the prevalence of current lifestyle behaviours in people with crc at multiple stages of care, and to identify any lifestyle behaviours whose prevalences differ from those in cancer-free controls
Lifestyle changes made and resources used: To identify lifestyle changes already made, and the resources participants used to make those changes.
Future lifestyle changes and resources desired: To identify lifestyle changes desired, and the resources participants wanted to use to make future changes.
2. METHODS
Our study used a cross-sectional survey design with purposive sampling. It included people with crc enrolled in the Personal Response Determinants in Cancer Therapy research database. Approximately half the crc patients treated annually at our clinic agree to be contacted for future research projects, and they enrol in the database at the time of their first clinic appointment. All living people who had a crc history and who were enrolled in the database before April 2011 (n = 734) were invited to participate. Specifically, our inclusion criteria were living English-speaking patients who had received a crc diagnosis and had come to the Vancouver Island Cancer Centre for at least 1 consultation. The survey was also mailed to people without a cancer diagnosis who belonged two local community groups (n = 105). The community groups were invited to participate in our study by a potential financial donor who expressed interest in research while visiting our centre. This potential donor was a member of the local community groups and asked other group members if they would be willing to participate. The group members lived in the same region, and their age and sex distributions were similar to those in the research database.
Surveys were mailed together with a cover letter introducing the study. Respondents were able to complete and return the survey anonymously. No interaction between respondents and the research team occurred, with the exception of four telephone calls made by participants to the research leader (two thanking the group for conducting the survey, and two requesting help to complete the survey). Mailing back the survey implied consent to participate. The study protocol was approved by the appropriate Research Ethics Committee.
2.1. Lifestyle Survey
Our mixed-methods survey assessed the lifestyle behaviours of participants (Appendix a). The first survey section contained a modified version of the fantastic Lifestyle Assessment tool23–28, which was adapted for our study with permission from its developers23, and has been adapted and validated for several populations25–30. The modified checklist used 6-point Likert scales to assess 22 current lifestyle behaviours.
The second section of the survey included questions about demographics, treatment history, lifestyle changes, and supportive resources. Checkbox questions were used to determine the prevalence of lifestyle changes made or desired in the future. Respondents with a cancer diagnosis recorded changes they had made since their diagnosis, and cancer-free control subjects recorded any lifestyle changes made in the preceding 3 years. Open-ended questions were used to gather information about support and resources that respondents used or wanted to use, plus any additional comments. Both sections of the modified survey were validated using a cognitive interviewing method with 10 people who had had crc31,32. The interviewer used a “thinking aloud technique”31 to assess the survey questions for comprehension, usefulness, and types of responses elicited. Changes were made to the survey based on the notes and feedback provided.
2.2. Data Analysis
2.2.1. Data Preparation
Survey respondents were separated into 5 groups:
Pre-treatment (P): The pre-treatment group included participants who had received no treatment, and those who had undergone surgery only and were waiting to start further treatment.
Ongoing (O): Participants undergoing one or more treatments at the time of response were assigned to the ongoing group.
Completed, 6 months or less (C≤6) and more than 6 months (C>6): Participants who had completed all scheduled treatments were assigned to one of the two completed groups, as appropriate.
Control (C): The control group included the participants without a cancer diagnosis.
Quantitative responses were entered into a database and uploaded to the Statistical Package for the Social Sciences (SPSS, version 14.0: SPSS, Chicago, IL, U.S.A.) and R Statistical Software (version 2.13: The R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was reported at p < 0.05 (two-tailed). Qualitative responses were transcribed verbatim, and the data were uploaded to the Coding Analysis Toolkit (Texifter, LLC, Amherst, MA, U.S.A.; http://cat.ucsur.pitt.edu/).
2.2.2. Quantitative Analysis
For the fantastic current lifestyle questions, multivariate imputations by chained equations33,34 were used to deal with missing or incomplete data. After a complete dataset was achieved, ordinal responses for each of the 22 fantastic questions were compared between the 5 groups using the Kruskal–Wallis rank sum test, which allows for pair-wise multiple comparisons when significant differences are found. Because the comparisons between groups found few significant differences, cancer-free control respondents were omitted, and the response frequencies of the respondents diagnosed with crc—that is, the remaining groups (P, O, C≤6, C>6)—were analyzed together. Response frequencies of the combined cancer respondents were calculated for each of the 22 behavioural questions, and chi-square goodness-of-fit tests were used to measure significant differences in responses.
For the checkbox questions, a Pearson chi-square test was used to compare differences in responses between all 5 groups. When significant associations were found, post-hoc pairwise chi-square analyses with Bonferroni correction were performed to determine pairwise differences between groups.
To prepare for the qualitative analysis of open-ended questions, Pearson chi-square tests were used to compare the demographic and treatment variables for respondents who provided written comments and those who did not.
2.2.3. Qualitative Analysis
The thematic analysis of the responses to the open-ended questions was conducted by the entire research team and used a combined inductive and deductive process as outlined by Fereday and Muir–Cochrane35. Qualitative responses were organized through coding. Code manuals for each question were developed based on the survey questions and a preliminary data review conducted by DLD and HMLD. The analysis was not limited to the original code frameworks, and new patterns and codes were allowed to emerge inductively during the process. As a result, the existing code manuals were modified as needed, and earlier coded data were revisited and re-coded for consistency. Two authors coded the responses to each question, compared findings, and resolved discrepancies through discussion.
After coding, text annotations from all sections were sorted by code. Existing codes were clustered, re-organized, and re-interpreted by each author separately. The authors compared interpretations and decided on important themes, which were cross-referenced back to the original text to ensure accurate reflection of the content.
3. RESULTS
3.1. Respondent Characteristics
Of 839 mailed surveys, 460 were returned (54.8%). Of the returned surveys, 10 were excluded because of incomplete demographic fields, and 6 were excluded because the respondents had experienced non-crc cancers, leaving 444 surveys for analysis.
Table i shows categorical demographic and treatment characteristics for the respondents. Mean age was 69 years (range: 36–91 years). Of the respondents with crc, 83% were 60 years of age or older, and 59% were men. Those data are comparable to the 2009 crc incidence data for B.C. residents, which indicate that 80% of people diagnosed with crc were 60 years of age or older, and 55% were men36. For respondents who had completed treatment (n = 245), the average elapsed time since treatment completion was 15 months (range: 0–46 months). Control respondents (n = 83) did not differ significantly from respondents diagnosed with crc (n = 361) in terms of sex [χ2(1, n = 444) = 0.589, p = 0.459) or age category [χ2(1, n = 444) = 3.01, p = 0.089].
Table I.
Demographics at the time of survey completion, by group
| Characteristic |
Patient group
|
||||
|---|---|---|---|---|---|
| Cancer-free control | Pre-treatment | Treatment ongoing |
Treatment completed
|
||
| ≤6 Months | >6 Months | ||||
| Patients (n) | 83 | 54 | 62 | 67 | 178 |
| Sex [n (%)] | |||||
| Men | 53 (64) | 37 (69) | 36 (58) | 41 (61) | 100 (56) |
| Women | 30 (36) | 17 (31) | 26 (42) | 26 (39) | 78 (44) |
| Age [n (%)] | |||||
| ≤70 Years | 37 (45) | 25 (46) | 47 (76) | 34 (51) | 93 (52) |
| >70 Years | 46 (55) | 29 (54) | 15 (24) | 33 (49) | 85 (48) |
| Ostomy status [n (%)] | |||||
| Yes | 0 (0) | 4 (7) | 8 (13) | 18 (27) | 25 (14) |
| No | 0 (0) | 48 (89) | 51 (82) | 43 (64) | 130 (73) |
| Formerly (reversed) | 0 (0) | 2 (4) | 3 (5) | 6 (9) | 22 (12) |
| Missing data | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Treatment type [n (%)] | |||||
| None | 83 (100) | 15 (28) | 0 (0) | 0 (0) | 0 (0) |
| Surgery | 0 (0) | 39 (72) | 1 (2) | 32 (48) | 62 (35) |
| Chemotherapy | 0 (0) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| Radiation therapy | 0 (0) | 0 (0) | 2 (3) | 1 (1) | 2 (1) |
| Combination | 0 (0) | 0 (0) | 54 (87) | 34 (51) | 114 (64.0) |
| Missing data | 0 (0) | 0 (0) | 4 (6) | 0 (0) | 0 (0) |
3.2. Current Lifestyle Behaviours
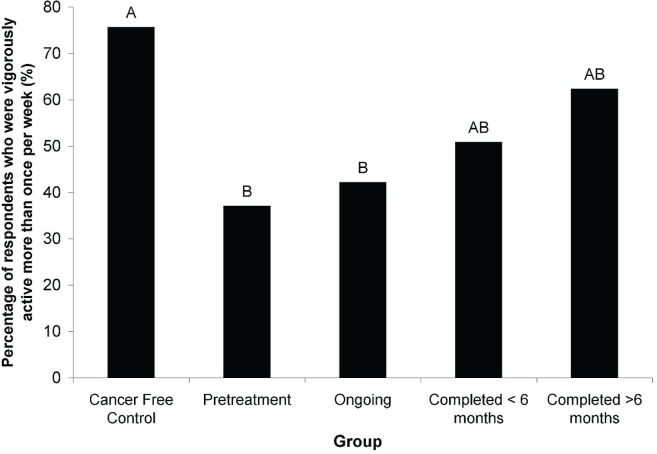
Of the 22 individual questions related to current lifestyle, only the vigorous physical activity question demonstrated significant differences between the 5 groups [Kruskal–Wallis t(4, n = 361) = 15.72, p = 0.0034; Figure 1]. All 5 groups, including cancer-free control respondents, reported similar behaviours in all other questions. As a result, we report only the results of the combined crc respondents (n = 361) for the remainder of the current lifestyle results. Differences in the frequency of selection of each response option by crc respondents were significant for all 22 questions (χ2 goodness-of-fit test: p < 0.001 for each question).
FIGURE 1.
Percentage of respondents who reported vigorously exercising for 30 minutes one or more times weekly, by group. Bars with different letters are significantly different (Kruskal–Wallis t, p < 0.05).
Most respondents regularly engaged in moderate physical activity, but very few engaged in vigorous physical activity. Of respondents with crc, 75% were moderately active (gardening, climbing stairs, walking, doing housework) 4 or more times per week, 17.5% were moderately active 1–3 times per week, and only 5.3% were moderately active less than 1 time per week. On the other hand, few crc respondents were engaging in 30 minutes of vigorous physical activity daily: 42.4% were vigorously active less than 1 time per week, 29.6% were vigorously active 1–3 times per week, and only 18.0% were vigorously active 4 or more times per week.
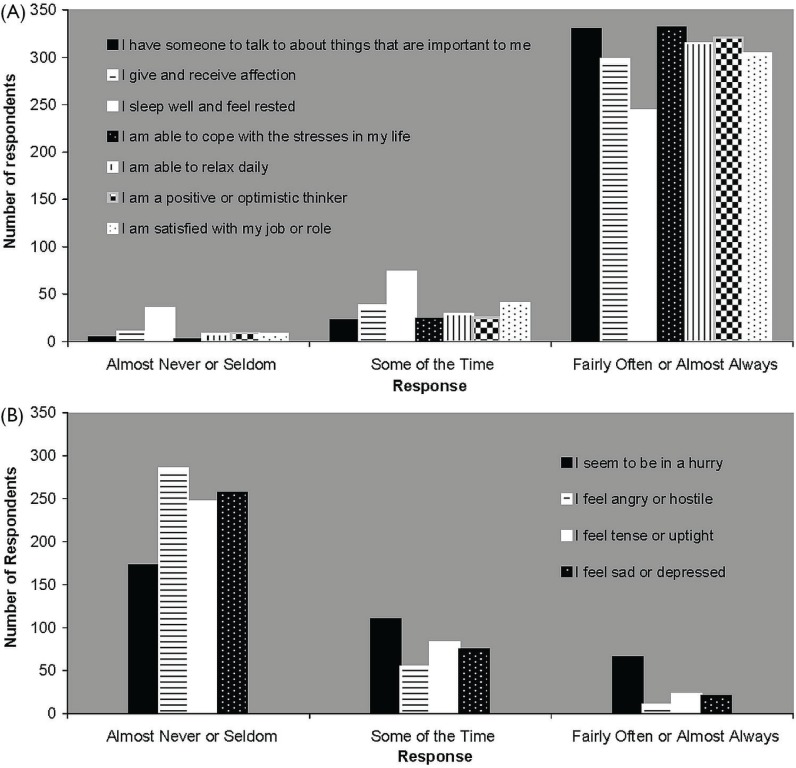
Figure 2 illustrates the psychosocial current lifestyle behaviours of crc respondents. Most respondents engaged in positive psychosocial behaviours fairly often or almost always [Figure 2(A)], and engaged in negative behaviours almost never or seldom [Figure 2(B)]. Table ii reports current lifestyle behaviours related to nutrition, alcohol intake, and tobacco use. The nutrition-related behaviours varied, but most crc respondents reported low levels of alcohol and tobacco use.
FIGURE 2.
Current psychosocial behaviours of people diagnosed with colorectal cancer (n = 361).
TABLE II.
Current nutrition, alcohol, and tobacco behaviours of people with colorectal cancer
| Question |
Response [n (%) of 361a]
|
|||
|---|---|---|---|---|
| Missing data | ||||
| I eat breakfast daily | Almost never or seldom | Some of the time | Fairly often or almost always | |
| 18 (5.0) | 14 (3.9) | 11 (3.0) | 318 (88.1) | |
| I eat a variety of fruits and vegetables daily | 0–2 Servings | 3–6 Servings | 7 or more servings | |
| 10 (2.8) | 50 (13.9) | 231 (64.0) | 70 (19.4) | |
| I eat processed meats | 2 Days per week or more | 1 Day per week | 2 Days per month or less | |
| 1 (0.3) | 91 (25.2) | 91 (25.2) | 178 (49.3) | |
| I drink water daily | 0–2 Cups | 3–5 Cups | >8 Cups | |
| 6 (1.7) | 90 (24.9) | 130 (36.0) | 135 (37.4) | |
| I take a vitamin D supplement daily | None | 100–400 IU | 1000 IU or more | |
| 15 (4.2) | 148 (41.0) | 71 (19.7) | 127 (35.2) | |
| My weight is | 15+ lb. over/under my ideal weight | 6–14 lb. over/under my ideal weight | At (or within 5 lb.) my ideal weight | |
| 6 (1.7) | 99 (27.4) | 119 (33.0) | 137 (38.0) | |
| I smoke tobacco | Once a week or more | None in the past 6 months or more | Never smoked | |
| 4 (1.1) | 29 (8.0) | 165 (45.7) | 163 (45.2) | |
| My average weekly alcohol intake is | >13 Servings | 8–12 Servings | 0–7 Servings | |
| 8 (2.2) | 30 (8.3) | 62 (17.2) | 261 (72.3) | |
| I drink more than four alcoholic beverages at one time | Almost daily or fairly often | Only on occasion | Never or almost never | |
| 5 (1.4) | 16 (4.4) | 39 (10.8) | 101 (28.0) | |
Cancer-free control respondents (n = 83) omitted.
3.3. Lifestyle Changes Made and Resources Used
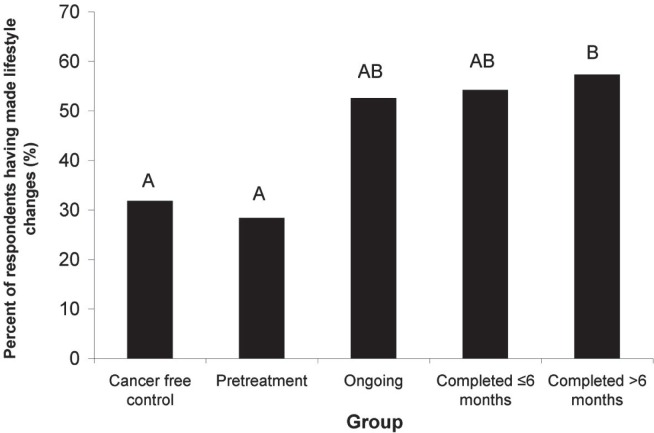
Lifestyle changes made and the resources used to make them were evaluated using a combination of checkbox and written responses (Appendix a). Almost all respondents (C: 82 of 83; P: 53 of 54; O: 61 of 62; C≤6: 61 of 67; C<6: 173 of 178) provided a response when asked “Since being diagnosed with cancer (or in the past 3 years for controls), have you made any lifestyle changes?” Many respondents reported having made changes, with marked differences being observed between the groups (Figure 3). We observed a significant relationship between the group (C, P, O, C≤6, or C<6) and the frequency of lifestyle changes made since diagnosis [χ2(4, n = 430) = 24.5, p = 0.000]. Pair-wise comparison revealed that significantly more C>6 respondents than P respondents [χ2(1, n = 226) = 13.6, p = 0.000] or C respondents [χ2(1, n = 255) = 14.5, p = 0.000] reported having made lifestyle changes.
FIGURE 3.
Percentage of respondents who reported having made lifestyle changes, by group. Bars with different letters are significantly different (pairwise χ2 with Bonferroni correction, p < 0.05)
The types of changes that respondents had made were evaluated using checkboxes (Appendix a). Healthier dietary choices were the most frequently reported change already made by people affected by crc (39.3%); the next most frequent changes were increased physical activity (21.1%), other changes (12.2%), stress management strategies (11.4%), and quitting smoking (4.2%). Cancer-free controls reported increased physical activity (18.1%), healthier dietary choices (16.9%), other changes (14.5%), and stress management strategies (3.6%). Written comments about “other changes” that had made were provided by 52 respondents (C: 11 of 83; P: 6 of 54; O: 6 of 62; C≤6: 5 of 67; C<6: 24 of 178). Common “other changes” were retirement, quitting or reducing alcohol intake, and participating in complementary and alternative medicine (cam) therapies including meditation, tai chi, and naturopathy.
Respondents who had made lifestyle changes were asked to indicate “What types of support and/or resources—if any—did you use to help you make these changes?” Table iii reports the response themes and sample quotes (response rates: C, 15 of 83; P, 14 of 54; O, 24 of 62; C≤6, 25 of 67; C<6, 80 of 178). The same themes (with the exception of cam) emerged from the written responses of all 5 groups; cam was a resource reported only by people affected by crc. Rather than mention external resources, many respondents referred to their internal ability to commit to lifestyle changes. We labelled that theme “self-efficacy” (the belief that one has mastery over events and the ability to meet challenges)37. Family and friends were also considered an important resource, particularly by respondents who were undergoing treatment or who had completed treatment. Health care providers were used by all groups, with the most commonly cited providers being physicians (general practitioners, oncologists, and specialists). Use of reading materials was also widely reported. Respondents from all groups mentioned books, articles, and pamphlets as resources used. Advice on healthy diet and nutrition was the most popular reading topic reported.
TABLE III.
Self-reported support and resources used to make lifestyle changes and desired by respondents for the future
|
Support and resources used
|
Support and resources desired
|
||
|---|---|---|---|
| Main theme | Sample quote (patient group) | Main theme | Sample quote (patient group) |
| Self-efficacy | I took charge of my own improvements (C>6 respondent) | Self-efficacy | I can do it without “outside” help—just need the determination (C respondent) |
| Complementary and alternative therapies | Am learning to meditate in order to stop feeling driven to rush around as I have too much to do (P respondent) | Complementary and alternative therapies | Add yoga and tai chi for stress management (C>6 respondent) |
| Reading materials | Books on healthy eating and health magazines from health stores (C>6 respondent) | Reading materials | I would like recommended reading materials on subjects I find embarrassing to discuss—sex (C≤6 respondent) |
| Family and friends | I had coaching by my family and friends. Without them I would have relied more on counsellors from the cancer agency (O respondent) | Programs, workshops, and classes | Community programs would be my preference as group programs seem to work best when dealing with stress management (C>6 respondent) |
| Health care providers | Physician at bcca suggested more fruit, veggie and less red meat; we are now eating fish and chicken more often (C≤6 respondent) | ||
C = treatment completed; >6 = more than 6 months; P = pre-treatment; ≤6 = 6 months or less; O = treatment ongoing.
3.4. Future Lifestyle Changes and Resources Desired
Lifestyle changes and resources desired for the future were evaluated using a combination of checkbox and written responses (Appendix a). Most respondents (C: 79 of 83; P: 51 of 54; O: 54 of 62; C≤6: 59 of 67; C<6: 151 of 178) provided a response when asked “Would you like to make any lifestyle changes in the future?” More than half the respondents in all groups reported a desire to make future lifestyle changes (C: 60.8%; P: 51.0%; O: 63.0%; C≤6: 64.4%; C<6: 63.6%). There were no significant differences between any of the groups [χ2(4, n = 394) = 2.93, p = 0.569].
The types of changes that respondents wanted to make were evaluated using checkboxes (Appendix a). Increasing physical activity was the most frequently desired change for people with a crc history (39.1%). Some crc respondents also wanted to make healthier dietary choices (21.6%), to apply stress management strategies (10%), to make other changes (7.8%), and to quit smoking (3.6%). Similarly, cancer-free controls reported that they wanted to increase physical activity (50.6%), to make healthier dietary choices (32.5%), to apply stress management strategies (7.2%), to make other changes (7.2%), and to quit smoking (1.2%). Written comments indicating “other changes” that respondents desired to make in the future were provided by 33 respondents (C: 8 of 83; P: 3 of 54; O: 5 of 62; C≤6: 4 of 67; C<6: 13 of 178); common themes were losing weight, being more social, and switching residence.
Respondents who wanted to make lifestyle changes were asked to indicate “What types of support and/or resources—if any—do you think would help you make these changes?” Table iii reports response themes and sample quotes (response rate: C, 34 of 83; P, 22 of 54; O, 24 of 62; C≤6, 27 of 67; C<6, 66 of 178). As with the results for resources used, the themes emerging from the written responses in all 5 groups were similar. The one exception was cam, which was mentioned only by people diagnosed with crc. Of the emerging themes for desired resources, 3 were the same as the themes that had emerged as resources used: reading materials, self-efficacy, and cam. The cam strategies most often cited as desired were yoga, meditation, and naturopathy.
Although respondents rarely mentioned group interventions such as “programs, workshops, and classes” as resources that they had already used to make changes, interventions of this kind were frequently mentioned by respondents from all groups as resources that they thought would help them to make future lifestyle changes. Many of the respondents indicated a need for “community programs” in general, with specific references to physical activity, relaxation, and stress management programs. Some respondents noted limitations to participation such as treatment-related fatigue, peripherally inserted central catheters, and ostomies.
3.5. Additional Themes and Comments
Additional comments were provided in a designated comment box by many respondents (response rate: C, 18 of 83; P, 11 of 54; O, 29 of 62; C≤6, 25 of 67; C<6, 91 of 178), and many respondents also added additional thoughts to other open-ended survey questions. Four recurrent themes emerged from the analysis of additional thoughts. People from all groups referred to their experiences with comorbidities such as diabetes, arthritis, heart conditions, knee and hip surgery, high blood pressure, and depression. Lifestyle changes and the ability to make them were often discussed within the limits of comorbidities:
Much less physical activity since I developed bursitis in my hip 4 years ago. There are still marked residual effects so that I am unable to walk or move as much as I used to.
— C respondent
From pre-treatment through post-treatment, respondents diagnosed with crc referred to continued bowel issues, including frequency, looseness, constipation, and poor reactions to certain foods. Those issues often limited their ability to make lifestyle changes:
Lack of exercise is due to the fact I am mainly housebound due to excessive and unexpected bowel movements due to ops and chemo. This is also the reason for not eating many fruits and vegetables.
— O respondent
Respondents affected by cancer often discussed their lifestyle before their diagnosis. Some wondered if they could have done anything else to prevent crc. Others speculated about the potential causes of their cancer, including genetics, stress, medications, and chemical exposure:
When I first was diagnosed with cancer I couldn’t believe it. After all I don’t drink alcohol, have never smoked and have grown all our own vegetables organically ... The only thing I could think of were the pcb’s I used to work with as an electrician years ago.
— C<6 respondent
A final recurrent theme was the use of integrative medicine by people affected by cancer. Respondents expressed frustration in their efforts to utilize cam therapies alongside conventional treatment:
I feel I’m covering all my “health care bases” if I use various professionals and thus healing modalities such as a naturopathic doctor who suggests herbal supplements and an acupuncturist who uses tcm [Traditional Chinese Medicine] methods. I get stressed at visits with pharmacists, oncologists or physicians when I need to defend my supplementary choices and these are not respected.
— C<6 respondent
4. DISCUSSION
Our study surveyed respondents at specified time points across the care continuum to gather information about current lifestyle, lifestyle changes made and resources already used, and lifestyle changes and resources desired for the future. More than half the respondents in all groups reported wanting to make future lifestyle changes, and many respondents indicated a desire for support and resources. Results from the survey might be useful in guiding the development of future lifestyle behavioural change programs for people with crc, including behaviours to be addressed, types of resources to be designed, and appropriate timing to implement the resources.
4.1. Lifestyle Behaviours to Be Addressed
Several lifestyle behaviours represent possible areas for future resource development. Of all the lifestyle behaviours assessed, the one with most opportunity for improvement was physical activity. Most crc respondents were not vigorously active on a regular basis, and fewer people with crc engaged in vigorous activity than did people without cancer. Furthermore, increase in physical activity was the lifestyle change most frequently desired by respondents (39% of respondents with cancer, 50.1% of respondents without cancer). Our results are consistent with those described by Grimmett et al.38, who reported that, of people diagnosed with crc and surveyed in London, United Kingdom, 82% were not physically active, a statistic that was associated with poor functional quality of life. Research consistently shows that regular physical activity protects against colon cancer22, and participation in physical activity programs reduces the risk of crc-specific mortality and death from other causes39. Taken together, those data strongly suggest the need to develop exercise programs for people with crc in our community. Such exercise programs must be sensitive to crc-specific issues such as ostomy and bowel symptoms and must teach people to navigate those challenges so that they can engage in safe physical activity after treatment.
The most frequently reported lifestyle change that had already been made by people with crc was “healthier dietary choices” (almost 40% of respondents). However, some of the current dietary behaviours reported by our respondents still failed to meet national guidelines. Health Canada recommends that people consume at least 7 servings of fruits and vegetable daily40, but only 20% of the crc respondents met that recommendation. People with crc commonly have problems eating and digesting food, and they experience symptoms such as diarrhea, stoma irritation, or gas after eating high-fibre foods and raw fruits and vegetables41. It is therefore likely that such symptoms restricted the consumption of the recommended servings of fruits and vegetable for that group. Health Canada recommendations for fruit and vegetable intake in the general population might not be appropriate for some individuals with crc. Development of evidence-based guidelines specifically for people with crc would be a valuable undertaking. In addition, the Canadian Cancer Society recommends consuming processed meats only on special occasions42, but more than half the respondents with crc reported eating processed meats at least once weekly. For the general population, Health Canada recommends a daily intake of 600–800 IU vitamin D in people more than 50 years of age43. In our study, only 35% of crc respondents (average age: 69 years) were meeting this requirement through supplement use, and approximately 41% were not taking any vitamin D supplements. Our research suggests that there is a need to provide education about healthy dietary behaviours for people with crc.
Our results also indicate the emerging importance of cam as a resource for people with cancer, but not for those without a cancer diagnosis. That finding is particularly interesting, because all groups reported experiencing comorbidities other than cancer, but only those with cancer appeared to have been triggered to use cam. Our results are corroborated by Mao et al.44, who found that U.S. cancer survivors are more likely than the general population to use cam, and by Molassiotis et al.45, who found that, in Europe, use of all types of cam increased dramatically after a crc diagnosis. Our survey responses also indicated that the perceived lack of physician acceptance was a common source of frustration for people considering cam options. Earlier studies have also reported that oncologists may have a negative perception of cam use46 and that their views may differ considerably from those of their patients47,48. It seems evident that people with crc are interested in cam, both to improve lifestyle behaviours and as adjunct to treatment. Future programs should offer guidance for making safe and informed cam choices, while respecting the opinions and autonomy of the patient.
4.2. Considerations for Designing Lifestyle Resources
In addition to lifestyle behaviours to be addressed, our qualitative survey results also revealed ideas about the structure and type of resources that might best lead to lifestyle improvements. Respondents from all stages of the cancer care continuum mentioned a desire for group activities such as programs, workshops, and classes. However, that desired resource was the only one that did not also emerge as a theme under resources already used. That finding may indicate either a lack of availability of, or a lack of awareness about, existing group programs. Given that many respondents requested such programs, we believe that promotion and development of group-oriented lifestyle interventions is an important way to encourage lifestyle change in our community. Group intervention formats allow people who are in similar situation to experience a sense of belonging49, and qualitative studies have suggested that group commitment49 or group cohesion50 can enhance the motivation of cancer patients to engage in healthy behaviours such as exercise.
Another frequently requested format for lifestyle support was print materials, which were reported both as a desired future resource and as a resource that participants were already using. Those findings may be surprising, given the rapid growth of Web-based health information and the emerging number of health care interventions being delivered over the Internet51. Nevertheless, our findings are consistent with those of Dowswell et al.11, who reported that 92% of their crc survey respondents would like booklets or leaflets to be included when planning diet and physical activity interventions. The production of reading materials as a resource for lifestyle support should be a priority.
A further consideration in the development of lifestyle resources is the involvement of a person’s existing social support network. In our data, crc and cancer-free respondents both emphasized the importance of family and friends in helping them with lifestyle changes. James et al.52 reported that perceived social support for a healthier diet was significantly higher for people with crc than for the general population, although the higher support was not associated with healthier behaviours. Another study by Bloom et al.53 reported that breast cancer patients with large social networks were more likely than those with small networks to report increased physical activity after a socio-educational intervention. Because family and friends were frequently cited as resources and might directly affect the lifestyle behaviours of people with cancer, we argue that lifestyle resources and programs should consider involving and providing information not only to people with cancer, but also to their friends and family. One potential medium for providing healthy lifestyle information to people with crc and their support networks is to harness the growing popularity of mobile technology and social networking Web sites. De la Torre–Diez et al.54 reported in July 2011 that there were already 171 crc groups on Facebook and Twitter, with 19% of them specifically aiming to support crc patients and their relatives.
An additional theme emerging from the qualitative data was the importance that many respondents placed on their own internal ability to commit to change and to control outcomes: that is, their perceived self-efficacy37. James et al.52 measured the extent to which self-efficacy influenced lifestyle behaviours and found that it predicted higher fruit and vegetable intake and increased physical activity in people with and without crc. Author Lev, alone and with colleagues37,55, noted that self-care and self-efficacy can be learned and that interventions including coping strategies and stress management have helped people with cancer. We did not directly measure self-efficacy, but respondents frequently used words such as “willpower,” “determination,” and “self-motivation” when asked about resources they used or wanted to use. Self-efficacy is considered one of the major ttm constructs; it reflects the degree of confidence a person has in maintaining desired behavioural change without relapsing16, and it tends to increase as people progress through the various stages of change56,57. Programs designed for people with cancer that teach such coping and self-management skills might increase the likelihood of successful change.
4.3. Timing of Lifestyle Resources
The individual lifestyle behaviours and types of lifestyle resources used and desired by respondents differed very little between the various crc groups. That finding was unexpected because previous studies had suggested that the timing of interventions and support might be important2,12,58. Furthermore, our results suggest that that the proportion of people at the preparation stage of the ttm may have been lower in the pre-treatment group than in the groups that had begun or completed treatment. Of pre-treatment respondents, 30% reported having already made lifestyle changes, but more than 50% of respondents whose treatment was ongoing and who had completed treatment had made changes (Figure 3). Furthermore, more than half the respondents in all crc groups (P, O, C≤6, C>6) reported wanting to make future lifestyle changes. Because people at the preparation stage have a desire to make changes and have typically taken some action toward behavioural change16, our results might suggest that a large proportion of people with crc are ready to participate in action-oriented programs once they have moved past the diagnosis and pre-treatment period.
Some important differences between the crc and cancer-free respondents have to be considered. Respondents from our population with a crc diagnosis reported lower levels of vigorous physical activity and a higher interest in cam than did cancer-free respondents. Furthermore, many crc respondents had ongoing disease-specific concerns such as bowel symptoms, and those symptoms often acted as barriers to lifestyle change. Finally, crc and control respondents differed in the number of lifestyle changes that they had made. With the exception of respondents in the pre-treatment phase, respondents with crc reported making more lifestyle changes than did cancer-free controls. It could be that a cancer diagnosis provides a “teachable moment” for lifestyle change, as has been suggested by other studies2,12,59. When considering the 6 stages of change described by the ttm17, a cancer diagnosis may work as a cue to move some people from the “contemplative stage” to the subsequent “preparation” stage.
Considering the data overall, we feel that the best approach is to make tailored resources available after diagnosis and to offer those resources throughout the entire crc trajectory (throughout treatment and after treatment completion). Action-oriented programs can address areas of particular need such as physical activity and cam, and can provide people with the confidence and specific information that they will need to participate in programs for a broader population after they complete treatment.
4.4. Limitations
When interpreting our results, it is important to keep in mind the context of the sampled population. Patients were recruited from an integrated cancer centre where they receive treatment and may participate in research. Survey recipients were of two types: people with cancer who had previously been approached and agreed to be contacted for research purposes, and people without a cancer diagnosis who were invited to participate by a potential financial donor who expressed interest in participating while visiting the cancer centre. As a result, the population contained many “self-selectors.” Furthermore, because the cancer-free respondents were all drawn from two community groups, they may represent a more select sample than a general cancer-free population. It should also be noted that respondents were limited to one geographic and regional location and one cancer care facility within the context of a publicly funded health care system.
It is possible that the retrospective design of our survey might have resulted in some recall bias when participants tried to remember their lifestyle behaviours. Also, not all respondents answered all survey questions, in particular the question regarding vigorous activity (10% blank responses). It is possible that respondents were less likely to answer questions if they felt embarrassed about a poor behaviour, which would suggest that the healthy behaviours assessed in questions with large amounts of missing data were actually less frequent than are reported in this article. However, we cannot accurately make that assumption.
Our use of open-ended questions had strengths and limitations. Written surveys provided time to contemplate a response and were not subject to interviewer influence60. However, we observed differences between those who provided written responses and those who did not. Respondents with cancer, women, respondents less than 70 years of age, respondents who had completed chemotherapy, and respondents who were more than 6 months out of treatment were more likely to provide written comments. The concerns of such individuals may therefore have been overrepresented in our qualitative data. Furthermore, the unequal sample sizes of the 5 groups limited our ability to compare themes between them.
One final consideration is that we focused our inquiry on healthy behavioural change; we did not directly identify whether respondents had made negative lifestyle changes. Our qualitative data did reveal that a few respondents had decreased their physical activity and fruit and vegetable intake as a result of symptoms from both crc and other comorbidities. Future research should consider both positive and negative lifestyle changes in this population.
5. CONCLUSIONS
People diagnosed with crc should be provided with information and opportunities to change behaviours through the introduction of action-oriented programs during the entire cancer trajectory, including during treatment and after treatment completion. Important lifestyle behaviours to be addressed include physical activity; dietary guidance related to the consumption of fruit, vegetables, and processed meats; vitamin D supplementation; and support for making informed cam decisions. Printed materials and programs such as workshops and classes appear to be a desirable delivery format, and resources should aim to increase self-efficacy and to engage each individual’s existing social support network. Finally, although referring people to programs for a broad audience may be feasible, we argue that people with crc should be directed to such programs after receiving adequate and tailored information during the treatment phase of their care continuum.
6. ACKNOWLEDGMENTS
We thank all respondents for volunteering their time and sharing their thoughts. We also thank Phil Brierley for his help with study and survey design, Eric Cormier for statistical guidance, and Heather Watson and Samantha Scott for their assistance with data extraction and review of earlier versions of the manuscript. Additional thanks to Douglas Wilson, developer of the original fantastic Checklist, for permission to use and modify the tool.
All patient participants were identified using the Personal Response Determinants in Cancer Therapy database. Our study was supported by Catalyst Grants awarded by the Vancouver Island Research Advisory and Development Committee (virad) and the BC Cancer Foundation as part of the virad Catalyst Program.
Preliminary survey data were presented as an abstract and poster at the bcca Annual Conference; Vancouver, British Columbia; November 25–27, 2010. Complete survey results have not previously been published as an abstract or full publication.
APPENDIX A: LIFESTYLE SURVEY FOR PEOPLE WITH COLORECTAL CANCER
FANTASTIC Lifestyle Checklista
Instructions: Unless otherwise specified, place an “X” beside the box which best describes your behavior or situation in the past month. Definitions and explanations on scoring are provided on the last page.

| FAMILY, FRIENDS | ||||
| I have someone to talk to about things that are important to me | ||||
| □ Almost never | □ Seldom | □ Some of the time | □ Fairly often | □ Almost always |
| I give and receive affection | ||||
| □ Almost never | □ Seldom | □ Some of the time | □ Fairly often | □ Almost always |
|
| ||||
| ACTIVITY | ||||
| I am vigorously active for at least 30 min. per day e.g., running, cycling, etc. | ||||
| □ Less than once per week | □ 1–2 Times weekly | □ 3 Times weekly | □ 4 Times weekly | □ 5 or more times weekly |
| I am moderately active (gardening, climbing stairs, walking, housework) | ||||
| □ Less than once per week | □ 1–2 Times weekly | □ 3 Times weekly | □ 4 Times weekly | □ 5 or more times weekly |
|
| ||||
| NUTRITION | ||||
| I eat breakfast daily | ||||
| □ Almost never | □ Seldom | □ Some of the time | □ Fairly often | □ Almost always |
| I eat a variety of fruits and vegetables daily (see explanation on last page) | ||||
| □ None | □ 1–2 Servings | □ 3–4 Servings | □ 5–6 Servings | □ 7 or more servings |
| I eat processed meats (see explanation) | ||||
| □ Daily | □ 2–3 Days per week | □ 1 Day per week | □ 1–2 Days per month | □ Never |
| My weight is (see weight chart) | ||||
| □ 20+ lb. Over or under my ideal weight | □ 15–19 lb. Over or under my ideal weight | □ 10–14 lb. Over or under my ideal weight | □ 6–9 lb. Over or under ideal weight | □ At (or within 5 lb. of) my ideal weight |
|
| ||||
| TOBACCO | ||||
| I smoke tobacco | ||||
| □ More than 10 times per week | □ 1–10 Times per week | □ None in the past 6–12 months | □ None in the past 1–5 years | □ Never smoked |
|
| ||||
| ALCOHOL AND WATER | ||||
| My average weekly alcohol intake is (see explanation on last page) | ||||
| □ More than 20 servings of alcohol | □ 13–20 Servings | □ 11–12 Servings | □ 8–10 Servings | □ 0–7 Servings |
| I drink more than four alcoholic drinks at one time | ||||
| □ Almost daily | □ Fairly often | □ Only on occasion | □ Almost never | □ Never |
| I drink water daily | ||||
| □ Almost never | □ 1–2 Cups | □ 3–5 Cups | □ 6–8 Cups | □ More than 8 cups |
|
| ||||
| SLEEP | ||||
| I sleep well and feel rested | ||||
| □ Almost never | □ Seldom | □ Some of the time | □ Fairly often | □ Almost always |
|
| ||||
| SUPPLEMENTS | ||||
| I take a vitamin D supplement daily | ||||
| □ None | □ 100IU | □ 200 IU | □ 400 IU | □ 1000 IU or more |
|
| ||||
| STRESS | ||||
| I am able to cope with the stresses in my life | ||||
| □ Almost never | □ Seldom | □ Some of the time | □ Fairly often | □ Almost always |
| I am able to relax daily | ||||
| □ Almost never | □ Seldom | □ Some of the time | □ Fairly often | □ Almost always |
|
| ||||
| TYPE OF BEHAVIOR | ||||
| I seem to be in a hurry | ||||
| □ Almost always | □ Fairly often | □ Some of the time | □ Seldom | □ Almost never |
| I feel angry or hostile | ||||
| □ Almost always | □ Fairly often | □ Some of the time | □ Seldom | □ Almost never |
|
| ||||
| INSIGHT | ||||
| I am a positive or optimistic thinker | ||||
| □ Almost never | □ Seldom | □ Some of the time | □ Fairly often | □ Almost always |
| I feel tense or uptight | ||||
| □ Almost always | □ Fairly often | □ Some of the time | □ Seldom | □ Almost never |
| I feel sad or depressed | ||||
| □ Almost always | □ Fairly often | □ Some of the time | □ Seldom | □ Almost never |
|
| ||||
| CAREER (or retirement) | ||||
| I am satisfied with my job or role | ||||
| □ Almost never | □ Seldom | □ Some of the time | □ Fairly often | □ Almost always |
|
| ||||
| STEP1 Total the Xs in each column | ||||
| → | ||||
| ______________ | ______________ | ______________ | ______________ | ______________ |
|
| ||||
| STEP2 Multiply the totals by the numbers indicated (write your answer in the box below) | ||||
| ×0 | ×1 | ×2 | ×3 | ×4 |
| ______________ | ______________ | ______________ | ______________ | ______________ |
|
| ||||
| STEP3 Add your scores across the bottom for your grand total | ||||
| 0 + | + | + | + | = |
| ______________ | ______________ | ______________ | ______________ | ______________ |
| GRAND TOTAL | ______________ | |||
Adapted with permission from the “fantastic Lifestyle Assessment” Copyright 1985, Dr. Douglas Wilson, Department of Family Medicine, McMaster University, Hamilton, Ontario L8N 3Z5.
Demographic and General Information
| 1. | Gender: | Male □ | Female □ |
| 2. | Year of birth: | ____________________________________________ | |
| 3. | I currently have an ostomy | Yes □ | No □ |
| Previously had an ostomy—now reversed | □ | ||
| Ostomy: An operation that makes it possible for stool to leave the body through an opening made in the abdomen. This surgery is sometimes medically required when a part of the intestines is removed. Colostomy and ileostomy are types of ostomies. Your Treatment History for Colorectal Cancer | |||
| 4. | Select one that applies to you: | Tickhere | |
| I have had surgery only | □ | ||
| I have had surgery and chemotherapy | □ | ||
| I have had surgery and radiation therapy | □ | ||
| I have had surgery with both chemotherapy and radiation therapy | □ | ||
| I have had chemotherapy and radiation therapy | □ | ||
| I have had chemotherapy only | □ | ||
| I have had radiation therapy only | □ | ||
| I have had no treatments | □ | ||
| I am currently having radiation | □ | ||
| I am currently having chemotherapy | □ | ||
| I am currently having both radiation therapy and chemotherapy | □ | ||
| 5. | I completed all of the above treatments OR made the decision not to have any treatments on this date: | ||
| Month: | Year: | ||
Lifestyle Changes
| 6. | Since being diagnosed with cancer, have you made any lifestyle changes (e.g. diet, smoking cessation, exercise, stress management)? | ||
| Yes □ | No □ | ||
| IF you answered YES to CHANGES—please answer Sections 7 and 9 below. | |||
| IF you answered NO to CHANGES—please skip to Sections 8 and 9 below. | |||
| 7. | a) Which type of lifestyle change(s) have you made? Check all that apply. | ||
| Tick here | |||
| Healthier dietary choices | □ | ||
| Increased exercise/physical activity | □ | ||
| Quit smoking | □ | ||
| Applied stress management strategies | □ | ||
| Other Changes – please describe below: | □ | ||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| b) What types of support and/or resources—if any—did you use to help you make these changes? | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| 8. | a) Would you like to make any lifestyle changes in future? | ||
| Yes □ | No □ | ||
| b) If YES, what type of lifestyle change(s) would you like to make? Check all that apply. | |||
| Tick here | |||
| Healthier dietary choices | □ | ||
| Increased exercise/physical activity | □ | ||
| Quit smoking | □ | ||
| Apply stress management strategies | □ | ||
| Other Changes—please describe below: | □ | ||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| c) What types of support and/or resources – if any - do you think would help you make these changes? (e.g. Community programs, recommended reading materials, internet/web-based resources, other) | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| 9. | Please add any additional comments you would like to share with our research team | ||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
| ____________________________________________________________________________________________ | |||
THANK YOU FOR COMPLETING OUR SURVEY!
By completing and returning this survey, you have provided your consent and agree that the BC Cancer Agency may use this information for research purposes. No identifying information will be attached to your responses. All information will be kept confidential.
BCCA Research Team
2008–2009 Catalyst Grant: Lifestyle Behaviours of People with Colorectal Cancer
Supported by the BC Cancer Foundation
Nutrition
| VEGETABLES AND FRUITS | ||
| According to Canada’s Food Guide to Healthy Eating, the amount of fruits and vegetables you need every day depends on your age, body size, activity level, and whether you are male or female. | ||
| Vegetables and fruits | Amounts | |
| Number of servings for adults 19–50 years: | ||
| Females | 7–8 servings daily | |
| Males | 8–10 servings daily | |
| Number of servings for adults 51+ years: | ||
| Females | 7 servings daily | |
| Males | 7 servings daily | |
| Examples of one serving: | ||
| 1 cup strawberries ; ½ cup 100% vegetable or fruit juice | ||
| ½ cup asparagus, beets, broccoli, yellow and green beans, carrots, corn, cucumber, lettuce, mushrooms, peas, peppers, radish, sprouts, squash, water chestnuts, zucchini, applesauce, diced pineapple, raspberries, blackberries, cherries, apricots, watermelon | ||
| 1 medium potato, yam, sweet potato, pear, apple, peach, orange, kiwi fruit; ½ medium tomato | ||
| 3 plums, ¼ cantaloupe or honeydew melon, ½ banana or papaya | ||
| PROCESSED MEATS | ||
| Examples of processed meats include ham, bologna, bacon, breakfast sausage, wieners or frankfurters, sandwich meats, salami, pepperoni, and beef jerky | ||
| ALCOHOL INTAKE | ||
| 1 drink equals: | Imperial/metric | |
| 1 bottle of beer | 5% alcohol | 12 oz./340.8 mL |
| 1 glass wine | 12% alcohol | 5 oz./142 mL |
| 1 shot spirits | 40% alcohol | 1.5 oz./42.6 mL |
Height and Weight Tables
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Height Feet Inches | Frame
|
Height Feet Inches | Frame
|
||||
| Small | Medium | Large | Small | Medium | Large | ||
| 4′ 11″ | 102–116 | 109–127 | 118–136 | 5′ 2″ | 128–139 | 131–146 | 138–155 |
| 5′ 0″ | 103–118 | 111–128 | 120–139 | 5′ 3″ | 130–141 | 133–148 | 140–158 |
| 5′ 1″ | 104–119 | 113–131 | 122–141 | 5′ 4″ | 132–143 | 135–150 | 142–161 |
| 5′ 2″ | 106–123 | 115–134 | 125–145 | 5′ 5″ | 134–145 | 137–153 | 144–165 |
| 5′ 3″ | 108–126 | 118–137 | 128–148 | 5′ 6″ | 136–147 | 139–156 | 146–169 |
| 5′ 4″ | 111–129 | 121–139 | 131–152 | 5′ 7″ | 138–150 | 142–159 | 149–173 |
| 5′ 5″ | 114–131 | 124–143 | 134–156 | 5′ 8″ | 140–153 | 145–162 | 152–177 |
| 5′ 6″ | 117–135 | 127–146 | 137–159 | 5′ 9″ | 142–156 | 148–165 | 155–181 |
| 5′ 7″ | 120–138 | 130–149 | 140–163 | 5′ 10″ | 144–159 | 151–168 | 158–185 |
| 5′ 8″ | 123–141 | 133–152 | 143–168 | 5′ 11″ | 146–162 | 154–171 | 161–185 |
| 5′ 9″ | 126–144 | 136–155 | 146–172 | 6′ 0″ | 149–165 | 157–175 | 164–193 |
| 5′ 10″ | 129–147 | 139–158 | 149–175 | 6′ 1″ | 152–169 | 160–179 | 168–197 |
| 5′ 11″ | 132–149 | 142–161 | 152–178 | 6′ 2″ | 155–173 | 164–182 | 172–202 |
| 6′ 0″ | 135–153 | 145–164 | 155–181 | 6′ 3″ | 158–177 | 167–187 | 176–207 |
| 6′ 1″ | 138–156 | 148–167 | 158–184 | 6′ 4″ | 162–181 | 171–192 | 181–212 |
Weights based on lowest mortality. Weight in pounds according to frame.
To calculate your frame type, place your thumb and index finger around your wrist. If your finger overlaps the thumb, your frame is a “small frame”. If they touch, your frame is a “medium frame”. If they do not touch, your frame is a “large frame.”
Chart adapted from Metropolitan Life Insurance Company: 1983 Metropolitan height and weight tables. Stat Bull 1983; 64:2–9.
What Does the Score Mean?
| 75–88 | 62–74 | 48–61 | 31–47 | 0–30 |
|
| ||||
| EXCELLENT | VERY GOOD | GOOD | FAIR | NEEDS IMPROVEMENT |
|
| ||||
| Note: A low total score does not mean that you have failed. There is always the chance to change your lifestyle – starting now. Look at the areas where you scored a 0 or 1 and decide which areas you want to work on first. | ||||
|
| ||||
TIPS for making lifestyle changes:
| ||||
7. CONFLICT OF INTEREST DISCLOSURES
The authors of this publication are not representing a product or company. The authors therefore have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2012. Toronto, ON: Canadian Cancer Society; 2012. [Google Scholar]
- 2.Demark–Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–84. doi: 10.1002/(SICI)1097-0142(20000201)88:3<674::AID-CNCR26>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 3.Arroyave WD, Clipp EC, Miller PE, et al. Childhood cancer survivors’ perceived barriers to improving exercise and dietary behaviors. Oncol Nurs Forum. 2008;35:121–30. doi: 10.1188/08.ONF.121-130. [DOI] [PubMed] [Google Scholar]
- 4.Demark–Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–30. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35:1846–52. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard CM, Courneya KS, Stein K, on behalf of the American Cancer Society’s scs-ii Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s scs-ii. J Clin Oncol. 2008;26:2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 7.Meyerhardt JA. Beyond standard adjuvant therapy for colon cancer: role of nonstandard interventions. Semin Oncol. 2011;38:533–41. doi: 10.1053/j.seminoncol.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rausch SM, Millay S, Scott C, et al. Health behaviors among cancer survivors receiving screening mammography. Am J Clin Oncol. 2012;35:22–31. doi: 10.1097/COC.0b013e318200598e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (who) Cancer Fact Sheet N297 [Web page] Geneva, Switzerland: WHO; 2012. [Available at: http://www.who.int/mediacentre/factsheets/fs297/en/index.html; cited October 30, 2012] [Google Scholar]
- 10.Lynch BM, Owen N, Hawkes AL, Aitken JF. Perceived barriers to physical activity for colorectal cancer survivors. Support Care Cancer. 2010;18:729–34. doi: 10.1007/s00520-009-0705-4. [DOI] [PubMed] [Google Scholar]
- 11.Dowswell G, Ryan A, Taylor A, et al. Designing an intervention to help people with colorectal adenomas reduce their intake of red and processed meat and increase their levels of physical activity: a qualitative study. BMC Cancer. 2012;12:255. doi: 10.1186/1471-2407-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humpel N, Magee C, Jones SC. The impact of a cancer diagnosis on the health behaviors of cancer survivors and their family and friends. Support Care Cancer. 2007;15:621–30. doi: 10.1007/s00520-006-0207-6. [DOI] [PubMed] [Google Scholar]
- 13.Satia JA, Campbell MK, Galanko JA, James A, Carr C, Sandler RS. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev. 2004;13:1022–31. [PubMed] [Google Scholar]
- 14.Stephenson LE, Bebb DG, Reimer RA, Culos–Reed SN. Physical activity and diet behaviour in colorectal cancer patients receiving chemotherapy: associations with quality of life. BMC Gastroenterol. 2009;9:60. doi: 10.1186/1471-230X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In: Glanz K, Rimer BK, Viswanath K, editors. Health Behavior and Health Education: Theory, Research and Practice. 4th ed. San Francisco, CA: Jossey–Bass; 2008. pp. 97–121. [Google Scholar]
- 17.Zimmerman GL, Olsen CG, Bosworth MF. A “stages of change” approach to helping patients change behavior. Am Fam Physician. 2000;61:1409–16. [PubMed] [Google Scholar]
- 18.Pekmezi DW, Demark–Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167–78. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SparkPeople . SparkTeams > Geographic Locations > Canada [Web page] Cincinnati, OH: SparkPeople; n.d. [Available at: http://www.sparkpeople.com/myspark/groups_subcategory.asp?CatId=560; cited October 25, 2012] [Google Scholar]
- 20.Weight Watchers Canada . WeightWatchers.ca home [Web page] Oakville, ON: Weight Watchers Canada; n.d. [Available at: http://www.weightwatchers.ca/index.aspx; cited October 25, 2012] [Google Scholar]
- 21.Parekh S, Vandelanotte C, King D, Boyle FM. Improving diet, physical activity and other lifestyle behaviours using computer-tailored advice in general practice: a randomised controlled trial. Int J Behav Nutr Phys Act. 2012;9:108. doi: 10.1186/1479-5868-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Cancer Research Fund, American Institute of Cancer Research . Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective [Web resource] Washington, DC: American Institute of Cancer Research; 2007. [Available at: http://www.dietandcancerreport.org/expert_report/index.php; cited October 1, 2012] [Google Scholar]
- 23.Wilson DMC, Ciliska D. Lifestyle assessment: development and use of the fantastic checklist. Can Fam Physician. 1984;30:1527–32. [Google Scholar]
- 24.Ciliska D, Wilson DMC. Lifestyle assessment: helping patients change health behaviors. Can Fam Physician. 1984;30:1665–70. [Google Scholar]
- 25.Wilson DMC, Ciliska D. Lifestyle assessment: testing the fantastic instrument. Can Fam Physician. 1984;30:1863–6. [Google Scholar]
- 26.Simpson R, Albert W, Wilson DMC, Ciliska D, Evans CE. Lifestyle assessment: part 4. The Halton Health Promotion Survey. Can Fam Physician. 1984;30:2147–55. [Google Scholar]
- 27.Sharratt JK, Sharratt MT, Smith DM, Howell MJ, Davenport L. fantastic lifestyle survey of University of Waterloo employees. Can Fam Physician. 1984;30:1869–72. [PMC free article] [PubMed] [Google Scholar]
- 28.Kason Y, Ylanko VJ. fantastic lifestyle assessment: part 5. Measuring lifestyle in family practice. Can Fam Physician. 1984;30:2379–83. [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez–Carmona JM, Rodriguez–Moctezuma R, Munguia–Miranda C, Hernandez–Santiago JL, Casas de la Torre E. Validity and reliability of fantastic an instrument for measuring the life style in Mexican patients with arterial hypertension [Spanish] Aten Primaria. 2000;26:542–9. doi: 10.1016/S0212-6567(00)78719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez Anez CR, Reis RS, Petroski EL. Brazilian version of a lifestyle questionnaire: translation and validation for young adults. Arq Bras Cardiol. 2008;91:92–8. doi: 10.1590/s0066-782x2008001400006. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed N, Bestall JC, Payne SA, Noble B, Ahmedzai SH. The use of cognitive interviewing methodology in the design and testing of a screening tool for supportive and palliative care needs. Support Care Cancer. 2009;17:665–73. doi: 10.1007/s00520-008-0521-2. [DOI] [PubMed] [Google Scholar]
- 32.Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12:229–38. doi: 10.1023/A:1023254226592. [DOI] [PubMed] [Google Scholar]
- 33.Van Buuren S, Brand JPL, Groothuis–Oudshoorn CGM, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76:1049–64. doi: 10.1080/10629360600810434. [DOI] [Google Scholar]
- 34.Graham JW, Hofer SM, Donaldson SI, MacKinnon DP, Schafer JL. Analysis with missing data in prevention research. In: Bryant K, Windle M, West S, editors. The Science of Prevention: Methodological Advances from Alcohol and Substance Abuse Research. Washington, DC: American Psychological Association; 1997. pp. 325–66. [Google Scholar]
- 35.Fereday J, Muir–Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Meth. 2006;5:1–11. [Google Scholar]
- 36.Cancer Surveillance and Outcomes, BC Cancer Agency (bcca) New Cancer Diagnoses for 2009 by Cancer Type, Age at Diagnosis and Gender [Web resource] Vancouver, BC: BCCA; 2011. [Available at: http://www.bccancer.bc.ca/HPI/CancerStatistics/FF/cancercases.htm; cited June 6, 2013] [Google Scholar]
- 37.Lev EL, Paul D, Owen SV. Age, self-efficacy, and change in patients’ adjustment to cancer. Cancer Pract. 1999;7:170–6. doi: 10.1046/j.1523-5394.1999.74004.x. [DOI] [PubMed] [Google Scholar]
- 38.Grimmett C, Bridgewater J, Steptoe A, Wardle J. Lifestyle and quality of life in colorectal cancer survivors. Qual Life Res. 2011;20:1237–45. doi: 10.1007/s11136-011-9855-1. [DOI] [PubMed] [Google Scholar]
- 39.Ballard–Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–40. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Health Canada . Eating Well with Canada’s Food Guide. Ottawa, ON: Health Canada; 2011. [Available online at: http://www.hcsc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/food-guide-aliment/print_eatwell_bienmang-eng.pdf; cited August 21, 2012] [Google Scholar]
- 41.Canadian Cancer Society . Nutrition and Colorectal Cancer [Web page] Toronto, ON: Canadian Cancer Society; 2013. [Available at: http://www.cancer.ca/en/cancer-information/cancer-type/colorectal/supportive-care/nutrition/?region=bc; cited June 7, 2013] [Google Scholar]
- 42.Canadian Cancer Society . Cancer information> Cancer 101 > What is a risk factor? > Diet > Cured, smoked, and saltpreserved foods [Web page] Toronto, ON: Canadian Cancer Society; n.d. [Available online at: http://www.cancer.ca/en/cancer-information/cancer-101/what-is-a-risk-factor/diet/cured-smoked-and-salt-preserved-foods/?region=ab; cited August 21, 2012] [Google Scholar]
- 43.Health Canada . Home > Food and Nutrition > Nutrition and Healthy Eating > Dietary Reference Intakes [Web resource] Ottawa, ON: Health Canada; 2011. [Current version available at: http://www.hc-sc.gc.ca/fn-an/nutrition/reference/index-eng.php; cited December 2, 2011] [Google Scholar]
- 44.Mao JJ, Palmer CS, Healy KE, Desai K, Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. J Cancer Surviv. 2011;5:8–17. doi: 10.1007/s11764-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molassiotis A, Fernandez–Ortega P, Pud D, et al. Complementary and alternative medicine use in colorectal cancer patients in seven European countries. Complement Ther Med. 2005;13:251–7. doi: 10.1016/j.ctim.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Hyodo I, Eguchi K, Nishina T, et al. Perceptions and attitudes of clinical oncologists on complementary and alternative medicine: a nationwide survey in Japan. Cancer. 2003;97:2861–8. doi: 10.1002/cncr.11402. [DOI] [PubMed] [Google Scholar]
- 47.Kim do Y, Kim BS, Lee KH, et al. Discrepant views of Korean medical oncologists and cancer patients on complementary and alternative medicine. Cancer Res Treat. 2008;40:87–92. doi: 10.4143/crt.2008.40.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson MA, Masse LC, Nanny K, Sanders C. Discrepant views of oncologists and cancer patients on complementary/alternative medicine. Support Care Cancer. 2004;12:797–804. doi: 10.1007/s00520-004-0677-3. [DOI] [PubMed] [Google Scholar]
- 49.Paltiel H, Solvoll E, Loge JH, Kaasa S, Oldervoll L. “The healthy me appears”: palliative cancer patients’ experiences of participation in a physical group exercise program. Palliat Support Care. 2009;7:459–67. doi: 10.1017/S1478951509990460. [DOI] [PubMed] [Google Scholar]
- 50.Midtgaard J, Rorth M, Stelter R, Adamsen L. The group matters: an explorative study of group cohesion and quality of life in cancer patients participating in physical exercise intervention during treatment. Eur J Cancer Care (Engl) 2006;15:25–33. doi: 10.1111/j.1365-2354.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 51.Griffiths F, Lindenmeyer A, Powell J, Lowe P, Thorogood M. Why are health care interventions delivered over the Internet? A systematic review of the published literature. J Med Internet Res. 2006;8:e10. doi: 10.2196/jmir.8.2.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.James AS, Campbell MK, DeVellis B, Reedy J, Carr C, Sandler RS. Health behavior correlates among colon cancer survivors: nc strides baseline results. Am J Health Behav. 2006;30:720–30. doi: 10.5993/AJHB.30.6.17. [DOI] [PubMed] [Google Scholar]
- 53.Bloom JR, Stewart SL, D’Onofrio CN, Luce J, Banks PJ. Addressing the needs of young breast cancer survivors at the 5 year milestone: can a short-term, low intensity intervention produce change? J Cancer Surviv. 2008;2:190–204. doi: 10.1007/s11764-008-0058-x. [DOI] [PubMed] [Google Scholar]
- 54.De la Torre–Diez I, Diaz–Pernas FJ, Anton–Rodriguez M. A content analysis of chronic diseases social groups on Facebook and Twitter. Telemed J E Health. 2012;18:404–8. doi: 10.1089/tmj.2011.0227. [DOI] [PubMed] [Google Scholar]
- 55.Lev EL. Bandura’s theory of self-efficacy: applications to oncology. Sch Inq Nurs Pract. 1997;11:21–37. [PubMed] [Google Scholar]
- 56.Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15:271–83. doi: 10.1016/0306-4603(90)90070-E. [DOI] [PubMed] [Google Scholar]
- 57.Kolundzija K, Gajic Z, Misic–Pavkov G, Maras JS. Core constructs of the transtheoretical model of behavior change. Curr Top Neurol Psychiatr Relat Discip. 2011;19:48–52. [Google Scholar]
- 58.Stull VB, Snyder DC, Demark–Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007;137(suppl):243S–8S. doi: 10.1093/jn/137.1.243S. [DOI] [PubMed] [Google Scholar]
- 59.Patterson RE, Neuhouser ML, Hedderson MM, Schwartz SM, Standish LJ, Bowen DJ. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J Am Diet Assoc. 2003;103:323–8. doi: 10.1053/jada.2003.50045. [DOI] [PubMed] [Google Scholar]
- 60.Morris BA, Shakespeare–Finch J, Scott JL. Posttraumatic growth after cancer: the importance of health-related benefits and newfound compassion for others. Support Care Cancer. 2011;20:749–56. doi: 10.1007/s00520-011-1143-7. [DOI] [PubMed] [Google Scholar]