Abstract
Background
Prostate cancer (pca) is the most common non-skin cancer among men in Canada and other Western countries. Increased prevalence and higher cost of newer treatments have led to a significant rise in the economic burden of pca. The objectives of the present study were to systematically review the literature on direct costs for the initial management of pca, and to examine the methodologic considerations across studies.
Methods
Bibliographic databases were systematically searched for peer-reviewed articles in English. Studies were reviewed for methodologic considerations and mean direct cost of active surveillance or watchful waiting (as/ww) and initial treatments. Direct cost was standardized to 2011 Canadian dollars.
Results
After a review of abstracts and full-text papers, seventeen articles met the eligibility criteria and were included in the review. Studies were published during 1992–2010. The studies reported on health care systems in the United States, France, the United Kingdom, German, Italy, and Spain. Our review identified a lack of methodologic consensus, leading to variation in direct costs between studies. Nevertheless, results indicate a significant direct cost of pca treatments.
Conclusions
The existing literature lacks methodologically rigorous studies on the direct costs of pca treatments specific to publicly funded health care systems. Additional studies are required to appreciate the direct costs of newer treatments and the impact of their adoption on the growing economic burden of pca management.
Keywords: Prostate cancer, economic burden, direct costs, systematic review, health policy
1. INTRODUCTION
Prostate cancer (pca) is the cancer most commonly diagnosed in men in Canada, with an age-standardized incidence of 121 cases per 100,000 in 20111—about double the number of incident cases estimated for lung and colorectal cancer in 2011 (respectively the second and third leading cancers in men). The pca incidence rate has increased over the years, to 122.5 cases per 100,000 in 2011 from 77.9 per 100,000 in 19821. Introduction of prostate specific antigen–based early detection and more awareness in Canada and elsewhere explains the rise in pca incidence over the years. In contrast with the increasing incidence, the pca mortality rate has gradually declined over the years to 21 deaths per 100,000 in 2011 from 26 per 100,000 in 1982. Similar pca incidence and survival rates have been reported for other developed nations2. Early detection of pca and better treatments have led to those declines in the mortality rate. The increased detection of pca in men 60 years of age and older, combined with better survival, has led to an increase in the number of individuals living with pca. The aging population therefore magnifies the burden of pca on the health care system2–4.
For initial management of localized pca, patients might be monitored without radical treatment [using active surveillance (as) or watchful waiting (ww)], or they might undergo a radical treatment such as open radical prostatectomy (rp) or radiation therapy (rt), which might be administered together with androgen deprivation therapy (adt)5,6. Initial treatments for pca are resource-intensive, putting a significant economic burden on the health care system. Furthermore, management of complications associated with pca treatments increases the economic burden of the disease. The lifetime direct cost of pca management in a cohort of Canadian men 40–80 years of age was estimated at $9.76 billion in 19977. In 1998, hospitalization and drug costs associated with pca in Canada were $77.4 million and $25.7 million respectively8.
During the continuum of pca care, the initial treatment and terminal care periods accrue most of the direct costs7,9,10. The economic burden of pca management on the health care system continues to grow for various reasons, including rising incidence, early detection and treatment of low-risk cases, adoption of newer and more costly health technologies and pharmaceuticals, and an aging population. Adoption of newer health technologies such as minimally invasive radical prostatectomy (mirp) and advanced rt [that is, intensity-modulated rt (imrt)] increases the direct cost (that is, the health care expenditure) for pca management without clear evidence of a significant gain in health outcomes. It is imperative to assess and compare the costs of newer treatments with those of predecessors.
Studies on the costs of treatments quantify the absolute cost (that is, the economic burden) incurred by the health care system to provide a pca treatment without comparing it with other treatments. Such studies facilitate decision-making in health care planning and resource allocation by highlighting the economic impact on the health care system of adopting a treatment. In contrast, economic evaluation (that is, cost-effectiveness or cost–utility) informs choices by highlighting the marginal costs and health benefits associated with a treatment. Hence, cost-of-treatment studies and economic evaluations serve different purposes. The present review focuses on cost-of-treatment studies that reported a direct cost of pca treatments11–14. A systematic review of direct costs for the initial treatment of pca could assist decision-makers in appreciating the economic burden of as or ww compared with conventional and newer treatments, and in examining whether current evidence is enough to distinguish the direct costs of conventional and newer treatments.
The objectives of the present study were to systematically review the literature on direct costs for the initial management of pca and to examine the methodologic considerations of the studies.
2. METHODS
2.1. Literature Search and Article Selection
The bibliographic databases ovid (medline), embase, and the Web of Science were systematically searched for peer-reviewed articles reporting the direct costs of pca management. Studies published in 1992 through 2010 were reviewed. Additional articles were retrieved by reviewing the reference lists of peer-reviewed articles identified during the database search. The broad search strategy used the subject heading “prostate cancer” cross referenced with “cost,” “treatment cost,” “healthcare cost,” “direct cost,” “cost analysis,” “cost-of-illness,” “burden-of-illness,” and “economic burden”. Duplicate citations were identified and excluded using the EndNote bibliographic management software (Thomson Reuters, Carlsbad, CA, U.S.A.).
Two reviewers (CS, AD) independently searched the databases and screened the search results to identify potentially relevant studies. They reviewed the title, abstract, and full text of each article, reaching consensus during the screening process.
The articles were screened for relevance using these inclusion and exclusion criteria:
-
Inclusion criteria
Is a peer-reviewed article published in English
Addresses pca and as/ww or initial treatments such as rp, rt, and adt
Reports a monetary estimate of the direct costs of initial treatments for pca
-
Exclusion criteria
Is a conference abstract, comment, letter to the editor, review article without original data, or grey literature or report
Provides a cost-effectiveness or cost–utility analysis of pca management
Provides a cost estimate of pca screening
Cost-effectiveness and cost–utility studies were excluded because they compare health benefits and costs for treatment alternatives while reporting marginal cost, which is a focus different from that in cost-of-treatment studies reporting the absolute cost of treatments11–15.
For all potentially eligible articles identified during first-level screening, the full text was reviewed to ensure that the article met all eligibility criteria. The reference lists of eligible articles were reviewed for articles not identified by computerized searches.
2.2. Data Abstraction
The data abstracted independently by the two reviewers from each eligible article included author, country, year of study, population type, sample size, mean age in years, study design, data sources, year of costing, currency of valuation, source of unit cost, and mean direct cost by pca treatment. Given the methodologic heterogeneity across studies, results are summarized descriptively16.
2.3. Quality Assessment
The two reviewers appraised the quality of the studies included in the review by assessing methodologic considerations. Disagreements were discussed with co-authors and resolved by consensus. The methodologic considerations examined were definition of the study population, perspective of the direct cost analysis, health care resource utilization and related costs, valuation of as/ww and treatments, allocation of direct costs, contribution of cost components, and sensitivity analysis12–14.
2.4. Standardization of Direct Cost
Most of the studies used the first year of managing the disease as the time horizon for the analysis of mean direct cost. Wherever required to facilitate a comparison of estimates across studies, the direct costs reported by a study were adapted to reflect the first-year costs17. The direct costs of as/ww, rp, rt, and adt reported by the individual studies were standardized to account for differences in currency and to facilitate a comparison of cost estimates across studies16. A two-step procedure was adopted to standardize costs to 2011 Canadian dollars. First, the reported mean pca treatment costs were converted to Canadian dollars in the study period by using purchasing power parity for the year of cost valuation; the Canadian health care services price index was then used to convert the result into 2011 Canadian dollars18–20. An assumption for the year of costing was made for studies that did not state the year15. This standardization of direct costs is consistent with recommended guidelines16,21 and similar studies in the literature15,17,22,23.
3. RESULTS
The initial literature search identified 1495 articles as outlined in the prisma (Preferred Reporting Items for Systematic Reviews and Meta Analyses) diagram in Figure 1. Duplicate articles (n = 327) retrieved by the computerized search were excluded. On review of the article title and abstracts, 1168 articles were excluded because they were unrelated and did not meet the eligibility criteria. A full-text review of the remaining seventy-two articles identified fourteen studies for inclusion in the final review. A further three articles were identified by reviewing the reference lists of the eligible articles, yielding seventeen articles in total. Table i summarizes the study characteristics and the standardized mean direct costs for initial treatments and as/ww for pca.
FIGURE 1.
Study flow diagram.
TABLE I.
Study characteristics
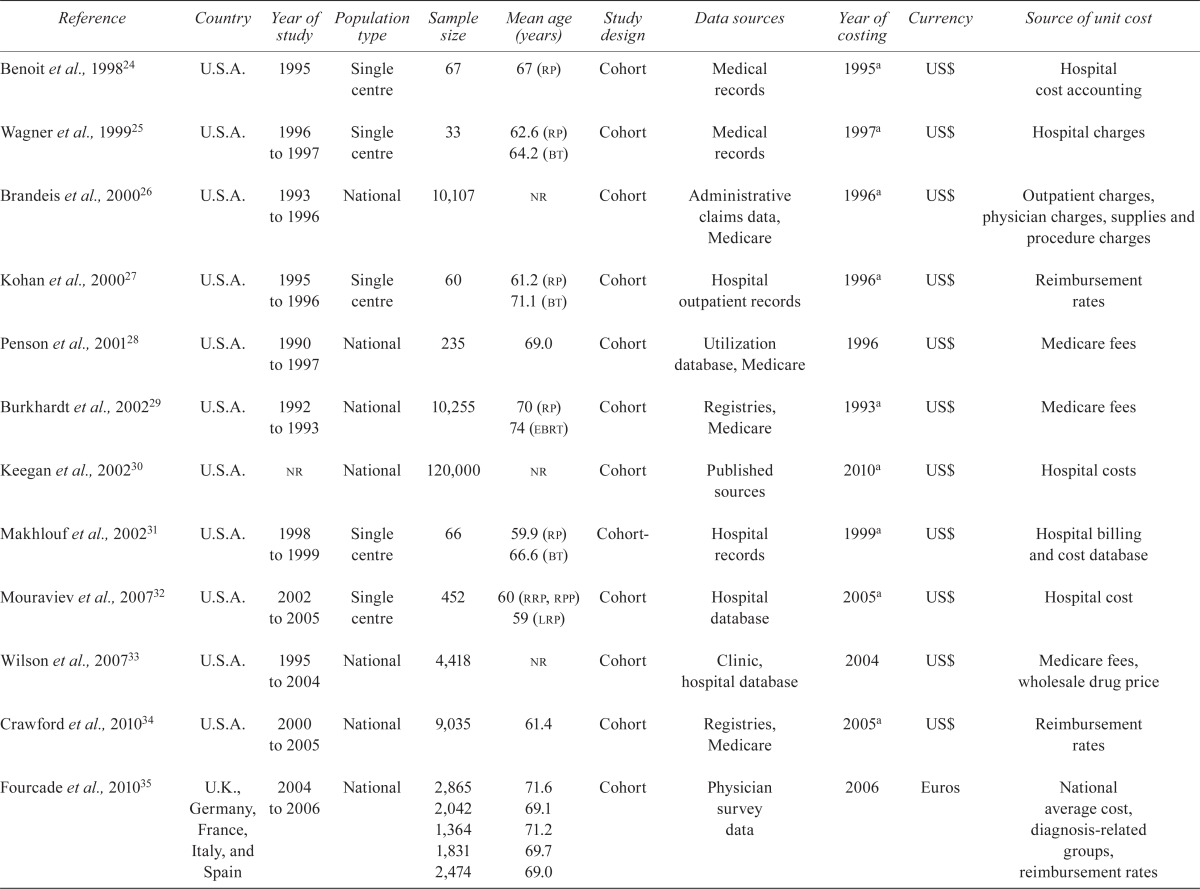
| Reference | Country | Year of study | Population type | Sample size | Mean age (years) | Study design | Data sources | Year of costing | Currency | Source of unit cost |
|---|---|---|---|---|---|---|---|---|---|---|
| Benoit et al., 199824 | U.S.A. | 1995 | Single centre | 67 | 67 (rp) | Cohort | Medical records | 1995a | US$ | Hospital cost accounting |
| Wagner et al., 199925 | U.S.A. | 1996 to 1997 | Single centre | 33 | 62.6 (rp) 64.2 (bt) |
Cohort | Medical records | 1997a | US$ | Hospital charges |
| Brandeis et al., 200026 | U.S.A. | 1993 to 1996 | National | 10,107 | nr | Cohort | Administrative claims data, Medicare | 1996a | US$ | Outpatient charges, physician charges, supplies and procedure charges |
| Kohan et al., 200027 | U.S.A. | 1995 to 1996 | Single centre | 60 | 61.2 (rp) 71.1 (bt) |
Cohort | Hospital outpatient records | 1996a | US$ | Reimbursement rates |
| Penson et al., 200128 | U.S.A. | 1990 to 1997 | National | 235 | 69.0 | Cohort | Utilization database, Medicare | 1996 | US$ | Medicare fees |
| Burkhardt et al., 200229 | U.S.A. | 1992 to 1993 | National | 10,255 | 70 (rp) 74 (ebrt) |
Cohort | Registries, Medicare | 1993a | US$ | Medicare fees |
| Keegan et al., 200230 | U.S.A. | nr | National | 120,000 | nr | Cohort | Published sources | 2010a | US$ | Hospital costs |
| Makhlouf et al., 200231 | U.S.A. | 1998 to 1999 | Single centre | 66 | 59.9 (rp) 66.6 (bt) |
Cohort- | Hospital records | 1999a | US$ | Hospital billing and cost database |
| Mouraviev et al., 200732 | U.S.A. | 2002 to 2005 | Single centre | 452 | 60 (rrp, rpp) 59 (lrp) |
Cohort | Hospital database | 2005a | US$ | Hospital cost |
| Wilson et al., 200733 | U.S.A. | 1995 to 2004 | National | 4,418 | nr | Cohort | Clinic, hospital database | 2004 | US$ | Medicare fees, wholesale drug price |
| Crawford et al., 201034 | U.S.A. | 2000 to 2005 | National | 9,035 | 61.4 | Cohort | Registries, Medicare | 2005a | US$ | Reimbursement rates |
| Fourcade et al., 201035 | U.K., Germany, France, Italy, and Spain | 2004 to 2006 | National | 2,865 2,042 1,364 1,831 2,474 |
71.6 69.1 71.2 69.7 69.0 |
Cohort | Physician survey data | 2006 | Euros | National average cost, diagnosis-related groups, reimbursement rates |
| Eldefrawy et al., 201136 | U.S.A. | nr | Single centre | Unknown | nr | Cohort | National, single-centre indicators | 2010 | US$ | Reimbursement rates, inpatient costs |
| Molinier et al., 201137 | France | 1995 | National | 879 | 71.2 | Cohort | Registries, medical records | 2008 | Euros | French diagnosis-related group, national unit cost, insurance cost |
| Nguyen et al., 201138 | U.S.A. | 2002 to 2005 | National | 45,636 | nr | Cohort | Registries, Medicare data | 2008 | US$ | Medicare fees |
| Lowrance et al., 201239 | U.S.A. | 2003 to 2006 | National | 5,445 | nr | Cohort | Registries, Medicare data | 2006 | US$ | Reimbursement rates |
| Tomaszewski et al., 201240 | U.S.A. | 2009 to 2010 | Single centre | 473 | nr | Cohort | Hospital records | 2010a | US$ | Hospital costs |
Year of costing assumed (not stated in article).
rp = radical prostatectomy; bt = brachytherapy; nr = not reported; ebrt = external-beam radiation therapy; rrp = radical retropubic prostatectomy; rpp = radical perineal prostatectomy; lrp = laparoscopic robotic prostatectomy.
3.1. Study Characteristics
The reviewed articles represent health care delivery systems in France35,37, Germany35, Italy35, Spain35, the United Kingdom35, and the United States24–34,36,38,39. The studies assessed health care resource use and associated costs for 1990–2010. Study subjects were drawn from national26,28–30,33–35,37–39 or single-centre24,25,27,31,32,36 populations, and sample sizes ranged between 33 and 120,000 patients25,30.
3.2. Methodologic Considerations
3.2.1. Definition of Study Population
Study subjects were classified into cohorts using International Classification of Diseases codes26,29,34,35,38,39 or grade and stage of disease24,25,27,28,30 –33,36,37,40. From a clinical perspective, precise grading and staging of pca or classification of the disease is critical in determining appropriate treatment. Furthermore, from a costestimation perspective, precise case definition aids in apportioning the direct costs associated with pca treatments (“sensitivity”) and non-pca treatments (“specificity”). In contrast, a generic definition would identify a cohort with varying degrees of sensitivity and specificity, leading to estimates that fail to isolate the direct costs solely associated with pca treatments41,42. For example, Cooper et al.43 reported 63.6% sensitivity of the Surveillance, Epidemiology and End Results database to detect pca. Similarly, cohorts constructed on grade and stage of disease will have varying degrees of sensitivity and specificity depending on the accuracy of the diagnostic tests available at the time44–47. Estimates of direct costs for pca treatments based on such definitions have varying degrees of accuracy.
3.2.2. Perspective of Direct Cost Analysis
The perspective taken for the cost estimation was clearly stated in eight studies24,26–28,31,35,37,39, with Medicare, private insurer, or health care payer perspectives being adopted for the United States24,26–28,34,39, and a public health care payer perspective being adopted for France, Germany, Italy, Spain, Sweden, and the United Kingdom35,37. Most of the studies failed to state the perspective of the analysis; however, the study designs suggested a health care system or institutional perspective.
3.2.3. Health Care Resource Utilization and Related Cost
Health care resource use was measured retrospectively in all the studies. Direct costs were quantified using either top-down24,28,30–33,37 or bottom-up25 –27,29,34,35,38,39 approaches. In the bottom-up approach, patient-specific health care resource utilization was multiplied by unit cost or charge25–27,29,34,35,38,39. In contrast, the top-down approach allocated portions of treatment costs (or charges) from diagnosis-related groups or national average costs24,28,30–33,37. In the absence of individual patient-level data to estimate cost, top-down is an alternative method used by researchers41,42.
Most studies reported estimates of direct costs in the national currency of the country whose system was being analyzed. In contrast, one study from Europe35 converted cost in U.K. pounds to euros. Seven studies28,33,35–39 reported the direct costs of pca treatment or as/ww adjusted to the price index of a specific year to account for increases in costs (that is, inflation) to the time of writing of the study.
3.2.4. Valuation of AS/WW and Initial Treatments
Either costs31,33 or charges24–30,32,34–39 were used in valuing health care resource use pertaining to as/ww and initial treatments. According to economic theory, cost reflects the opportunity cost of administering a treatment21,48. Valuation of health care resource use is better reflected by costs than by charges. Charges, a proxy for costs, include mark-ups and profit margins set by institutions for health care services provided33. One study distinguished valuation of direct costs by unit costs or unit charges. The direct cost of rp estimated by unit costs was less (CA$13,515) than that estimated by unit charges (CA$23,743). Similarly, the direct cost for brachytherapy (bt) estimated by unit costs was less (CA$22,072) than that estimated by unit charges (CA$33,890)31. The study that reported those differences indicated that direct cost of a treatment was greater when estimated by unit charges than by unit costs31. Hence, charges potentially overestimate the actual direct costs of health care resource use. In the absence of unit costs, the studies identified in our review generally considered unit charges for the valuation of treatments. From a public health care payer perspective, charges potentially represent the direct costs of providing pca treatments without profit margins.
3.2.5. Allocation of Direct Costs
Most of the studies in our review inadequately reported cost components considered in the valuation of pca treatment options. Further, studies lacked consensus on the cost components to be taken into account. The most commonly reported cost components were physician or specialist fees (n = 12), laboratory tests (n = 9), imaging (n = 8), hospitalization (n = 8), medications (n = 8), pharmacy (n = 7), anesthesia (n = 7), operating room (n = 6), supplies (n = 6), computed tomography imaging (n = 5), ultrasonography (n = 5), respiratory care (n = 5), electrocardiography (n = 4), and pathology (n = 4). Few studies reported the cost components specifically excluded from the cost estimation (such as hormonal therapy31, adjuvant hormonal therapy25, post-intervention complications35, and physician charges24). Notably, studies of mirp did not account for the acquisition and maintenance costs of robots in their estimations of direct costs32,36,38,39. Those studies therefore failed to highlight the actual increase in the direct cost associated with mirp compared with rp.
3.2.6. Contribution of Cost Components
In a cohort of pca patients, rp, bt, and external-beam rt respectively represented 78%, 19%, and 6% of inpatient costs26. For rp, more than 90% of the total direct cost was attributable to inpatient costs and about 5% to outpatient costs28. Inpatient costs of rp (such as the operating room) constituted 27% of the total direct cost, followed by ward care (27%), supplies (13%), anesthesia (9%), and pathology (8%). For bt, seeds (103Pd) contributed 50% of total direct cost, followed by radiology (17%), ultrasonography (16%), supplies (5%), and operating room (5%). The authors noted that replacing 103Pd with 125I seeds has the potential to reduce the total direct cost of bt by 5%31. Another study reported that 53% of the total direct cost was attributable to office visits, followed by medications (26%) and hospitalizations (21%), including emergency room visits33.
3.3. Sensitivity Analysis
Cost estimation involves a degree of uncertainty. Sensitivity analysis is therefore recommended to ensure the robustness of estimates. In sensitivity analysis, the original analysis is reworked with varying assumptions and estimates to examine the impact on the study findings16,21. Our review identified one study that performed a sensitivity analysis to assess the effect of disease stage on the estimates of direct cost35.
3.4. Direct Cost for Initial Management of PCa
Table ii reflects the variation in mean direct costs for initial pca treatments and as/ww, standardized to 2011 Canadian dollars. Despite considerable heterogeneity, the studies highlight consistent findings with respect to
choice of initial treatment (influenced by patient characteristics such as age, grade or stage of the disease, comorbidities, and region); and
health care expenditure to manage pca (influenced by choice of initial treatment).
TABLE II.
Standardized direct cost by active surveillance or watchful waiting (as/ww), or initial treatment for prostate cancer
| Reference | Country | as/ww |
Initial treatment (2011 Canadian dollars)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Androgen deprivation therapy | Radical prostatectomy (rp) | External-beam rt | Brachytherapy | Robot-assisted rp or minimally invasive prostatectomy | 3D conformal rt | Intensity-modulated rt | |||
| Benoit et al., 199824 | U.S.A. | — | — | $11,655 | — | — | — | — | — |
| Wagner et al., 199925 | U.S.A. | — | — | $29,398 | — | $40,941 | — | — | — |
| Brandeis et al., 200026 | U.S.A. | — | — | $38,189 | $32,001 | $30,724 | — | — | — |
| Kohan et al., 200027 | U.S.A. | — | — | $27,919 | — | $27,882 | — | — | — |
| Penson et al., 200128 | U.S.A. | $972 | $9,582 | $14,698 | $14,919 | — | — | — | — |
| Burkhardt et al., 200229 | U.S.A. | — | — | $37,146 | $30,293 | — | — | — | — |
| Keegan et al., 200230 | U.S.A. | $7,920 | $12,623 | $32,655 | — | $25,819 | — | — | $65,946 |
| Makhlouf et al., 200231 | U.S.A. | — | — | $40,966 | — | $47,583 | — | — | — |
| Mouraviev et al., 200732 | U.S.A. | — | — | $7,863 | — | — | $8,053 | — | — |
| Wilson et al., 200733 | U.S.A. | $8,794 | $23,491 | $23,209 | $43,593 | $16,249 | — | — | — |
| Crawford et al., 201034 | U.S.A. | $5,678 | $22,416 | $23,674 | $31,814 | — | — | — | — |
| Fourcade et al., 201035 | U.K. | — | $1,004 | $528 | $2,671 | — | — | — | — |
| Germany | — | $2,146 | $2,489 | $1,200 | — | — | — | — | |
| France | — | $2,340 | $3,355 | $2,923 | — | — | — | — | |
| Italy | — | $1,484 | $4,154 | $2,492 | — | — | — | — | |
| Spain | — | $1,760 | $1,924 | $1,031 | — | — | — | — | |
| Eldefrawy et al., 201136 | U.S.A. | $1,449 | — | $12,217 | $26,024 | $17,652 | $22,376 | — | — |
| Molinier et al., 201137 | France | $2,754 | $5,193 | $5,062 | $6,473 | — | — | — | — |
| Nguyen et al., 201138 | U.S.A. | — | — | $22,573 | — | $23,405 | $22,974 | $28,218 | $43,276 |
| Lowrance et al., 201239 | U.S.A. | — | — | $22,615 | — | — | $24,383 | — | — |
| Tomaszewski et al., 201240 | U.S.A. | — | — | $5,116 | — | — | $8,146 | — | — |
rt = radiation therapy; 3D = three-dimensional.
Very low– to low-risk patients and older patients with comorbidities and a short expected survival were primarily under observation (that is, as or ww)28,30,34,36,37. Patients who were relatively younger, with fewer comorbidities, and a low- to intermediate-risk profile, received rp (open or robot-assisted)24–29,31,33–39. Patients who were relatively older, with more comorbidities and an intermediate- to high-risk profile, received rt [that is, imrt or three-dimensional conformal rt (3D-crt)] 26,28,29,33–38. Brachytherapy alone was usually administered to low-risk patients25–27,31,33,36,38. Older patients and those with more comorbidities received adt alone28,33,35,37.
The studies indicate variation not only in patient characteristics, but also in clinical practice (that is, choice of initial treatment) by geographic region26,29,35,37–39. Studies consistently reported that choice of initial treatment influenced the total direct costs of initial treatments28,30,34,36–38, ranging between 49% and 82% depending on the treatment option37.
Studies elucidated a stage effect of pca on the direct cost of treatment. Costs were more for the high-risk group than for the intermediate- and low-risk groups28,33. In low-risk pca, as with delayed active treatment cost the least and at the same time favoured quality of life and minimized the risk of complications30,36. Results indicated that the direct cost of adt increased significantly during follow-up38. Further, multimodal treatments cost more than did treatments administered alone26,38. Use of newer health technologies [imrt, robotic-assisted rp (rarp)] in pca treatment has increased over the years, contributing to the rise in health care expenditure38,39. Variations in health care expenditure across the country to treat pca might arise from variation in clinical practice, case mix, and unit costs35.
4. DISCUSSION
Policymakers and health care payers require information about the direct costs (“absolute costs”) of pca treatments and as/ww so that they can quantify current health care expenditures and project future costs, assess the impact of health care policy, and realize the economic consequences of treatments. Depending on the policy context, studies of direct cost have the potential to facilitate decision-making about the efficient allocation of resources. Our study reviewed seventeen selected articles on initial treatment and as/ww in pca11–13. We focused on initial treatment because earlier studies noted that a substantial proportion of the direct cost is accrued during this treatment phase9,10.
Our review identified considerably methodologic heterogeneity between the studies. Most did not account for the costs of treating complications. Many lacked detail about the contributions of cost components to the total cost of a treatment and the direct cost by pca stage. Variations in methodologic considerations were likely to influence the precision of the estimates and hence the quality of the studies. Guidelines that standardize the methods for direct cost analysis would minimize heterogeneity across studies13. Caution should be exercised in comparing results across studies and generalizing them to other health care settings12–14.
Our results show variation in the direct costs reported by the analyzed studies within and between treatments (Table ii). The variations in direct cost between countries might be a result of differences in patient characteristics, health care delivery systems, equipment acquisition costs, year of cost valuation, clinical practice, cost components, and cost estimation methodologies16. Fewer studies have assessed the direct costs of newer health technologies, and thus further research on the direct costs of adopting newer health technologies is warranted.
Our study has several limitations. Articles published in languages other than English were not considered for the review, and it is possible that the broad search strategy failed to identify relevant studies. However, manual searches of the reference lists from the articles included in the study were conducted to identify potential candidate studies that were not retrieved by the database searches. Most of the studies that met the inclusion criteria did not report the direct costs of treatments by disease stage. Despite standardization of the direct costs (to 2011 Canadian dollars), estimates varied between the studies. Cost-effectiveness and cost–utility studies were not considered for our review, and so health benefits were not considered. Such limitations are akin to those in other reviews13,15.
5. CONCLUSIONS
The existing literature lacks studies specific to the Canadian health care system or other publicly funded health systems on the direct costs of initial treatments and of as/ww for pca. Additional studies are required to better appreciate the impact on the growing economic burden of pca management of adopting newer health technologies. Most of the studies reviewed here represent the U.S. health care system. Health care resource use and unit costs are sensitive to variations across health care systems and therefore limit the generalizability and transferability of cost estimates16. Hence, country-specific cost is essential so that decision-makers and health care planners can efficiently allocate competing health care resources. The aging population will substantially increase the clinical and economic burden of pca on the health care system. From a health care policy perspective, resources are limited, representing an opportunity cost21,48. The choice of initial treatment, which is related to the severity of pca at diagnosis, could potentially limit health care resource use and cost. Optimizing resource use might help to sustain the health care system.
6. ACKNOWLEDGMENTS
CS is a recipient of a Fonds de recherche du Québec–Santé doctoral research scholarship.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics. Canadian Cancer Statistics 2011. Toronto, ON: Canadian Cancer Society; 2011. [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [Erratum in: CA Cancer J Clin 2011;61:133–4] [DOI] [PubMed] [Google Scholar]
- 3.Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11:437–41. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Quon H, Loblaw A, Nam R. Dramatic increase in prostate cancer cases by 2021. BJU Int. 2011;108:1734–8. doi: 10.1111/j.1464-410X.2011.10197.x. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (nccn) NCCN Guidelines for Patients: Prostate Cancer. Fort Washington PA: NCCN; 2013. Ver 12013. [Available online at: http://www.nccn.org/patients/guidelines/prostate/index.html; cited May 19, 2013] [Google Scholar]
- 6.Heidenreich A, Bellmunt J, Bolla M, et al. on behalf of the European Association of Urology eau guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Grover SA, Coupal L, Zowall H, et al. The economic burden of prostate cancer in Canada: forecasts from the Montreal Prostate Cancer Model. CMAJ. 2000;162:987–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Health Canada, Population and Public Health Branch, Strategic Policy Directorate, Policy Research Division. Economic Burden of Illness in Canada, 1998. Ottawa, ON: Health Canada; 2002. [Available online at http://publications.gc.ca/collections/Collection/H21-136-1998E.pdf; cited October 18, 2012] [Google Scholar]
- 9.Krahn MD, Zagorski B, Laporte A, et al. Healthcare costs associated with prostate cancer: estimates from a population-based study. BJU Int. 2010;105:338–46. doi: 10.1111/j.1464-410X.2009.08758.x. [DOI] [PubMed] [Google Scholar]
- 10.Riley GF, Potosky AL, Lubitz JD, Kessler LG. Medicare payments from diagnosis to death for elderly cancer patients by stage at diagnosis. Med Care. 1995;33:828–41. doi: 10.1097/00005650-199508000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Druss BG, Marcus SC, Olfson M, Pincus HA. The most expensive medical conditions in America. Health Aff (Millwood) 2002;21:105–11. doi: 10.1377/hlthaff.21.4.105. [DOI] [PubMed] [Google Scholar]
- 12.Pagano E, Brunetti M, Tediosi F, Garattini L. Costs of diabetes. A methodological analysis of the literature. Pharmacoeconomics. 1999;15:583–95. doi: 10.2165/00019053-199915060-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bloom BS, Bruno DJ, Maman DY, Jayadevappa R. Usefulness of US cost-of-illness studies in healthcare decision making. Pharmacoeconomics. 2001;19:207–13. doi: 10.2165/00019053-200119020-00007. [DOI] [PubMed] [Google Scholar]
- 14.Cooper NJ. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 2000;39:28–33. doi: 10.1093/rheumatology/39.1.28. [DOI] [PubMed] [Google Scholar]
- 15.Tarride JE, Lim M, DesMeules M, et al. A review of the cost of cardiovascular disease. Can J Cardiol. 2009;25:195–202. doi: 10.1016/S0828-282X(09)70098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shemilt I, Mugford M, Byford S, et al. Chapter 15: incorporating economics evidence. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Oxford, UK: The Cochrane Collaboration; 2008. Ver. 5.0.1. [Current version available online at: http://www.cochrane-handbook.org; cited May 19, 2013] [Google Scholar]
- 17.Wodchis WP, Bhatia RS, Leblanc K, Meshkat N, Morra D. A review of the cost of atrial fibrillation. Value Health. 2012;15:240–8. doi: 10.1016/j.jval.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Organisation for Economic Co-operation and Development (oecd) StatExtracts. 4. PPPs and exchange rates [Web resource] Paris, France: OECD; n.d. [Available at: http://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4; cited May 19, 2013] [Google Scholar]
- 19.OANDA Corporation . Currency Converter [Web resource] New York, NY: OANDA Corporation; n.d. [Available at: http://www.oanda.com/ (click Currency Converter); cited May 19, 2013] [Google Scholar]
- 20.Statistics Canada. Home > CANSIM. Table 326-0021 Consumer Price Index (CPI), 2009 basket annual (2002=100) [Web resource] Ottawa, ON: Statistics Canada; 2013. [Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=3260021&paSer=&pattern=&stByVal=1&p1=1&p2=37&tabMode=dataTable&csid=; cited May 19, 2013] [Google Scholar]
- 21.Drummond MF, Sculpher MJ, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programs. 3rd ed. Oxford, UK: Oxford University Press; 1997. [Google Scholar]
- 22.Jonsson L, Wimo A. The cost of dementia in Europe: a review of the evidence, and methodological considerations. Pharmacoeconomics. 2009;27:391–403. doi: 10.2165/00019053-200927050-00004. [DOI] [PubMed] [Google Scholar]
- 23.Ray JA, Valentine WJ, Secnik K, et al. Review of the cost of diabetes complications in Australia, Canada, France. Curr Med Res Opin. 2005;21:1617–29. doi: 10.1185/030079905X65349. [DOI] [PubMed] [Google Scholar]
- 24.Benoit RM, Cohen JK, Miller RJ., Jr Comparison of the hospital costs for radical prostatectomy and cryosurgical ablation of the prostate. Urology. 1998;52:820–4. doi: 10.1016/S0090-4295(98)00300-8. [DOI] [PubMed] [Google Scholar]
- 25.Wagner TT, 3rd, Young D, Bahnson RR. Charge and length of hospital stay analysis of radical retropubic prostatectomy and transperineal prostate brachytherapy. J Urol. 1999;161:1216–18. doi: 10.1016/S0022-5347(01)61638-0. [DOI] [PubMed] [Google Scholar]
- 26.Brandeis J, Pashos CL, Henning JM, Litwin MS. A nationwide charge comparison of the principal treatments for early stage prostate carcinoma. Cancer. 2000;89:1792–9. doi: 10.1002/1097-0142(20001015)89:8<1792::AID-CNCR20>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Kohan AD, Armenakas NA, Fracchia JA. The perioperative charge equivalence of interstitial brachytherapy and radical prostatectomy with 1-year follow-up. J Urol. 2000;163:511–14. doi: 10.1016/S0022-5347(05)67913-X. [DOI] [PubMed] [Google Scholar]
- 28.Penson DF, Schonfeld WH, Flanders SC, et al. Relationship of first-year costs of treating localized prostate cancer to initial choice of therapy and stage at diagnosis: results from the capsure database. Urology. 2001;57:499–503. doi: 10.1016/S0090-4295(00)01033-5. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt JH, Litwin MS, Rose CM, et al. Comparing the costs of radiation therapy and radical prostatectomy for the initial treatment of early-stage prostate cancer. J Clin Oncol. 2002;20:2869–75. doi: 10.1200/JCO.2002.11.136. [DOI] [PubMed] [Google Scholar]
- 30.Keegan KA, Dall’Era MA, Durbin–Johnson B, Evans CP. Active surveillance for prostate cancer compared with immediate treatment. Cancer. 2012;118:3512–18. doi: 10.1002/cncr.26688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makhlouf AA, Boyd JC, Chapman TN, Theodorescu D. Perioperative costs and charges of prostate brachytherapy and prostatectomy. Urology. 2002;60:656–60. doi: 10.1016/S0090-4295(02)01859-9. [DOI] [PubMed] [Google Scholar]
- 32.Mouraviev V, Nosnik I, Sun L, et al. Financial comparative analysis of minimally invasive surgery to open surgery for localized prostate cancer: a single-institution experience. Urology. 2007;69:311–14. doi: 10.1016/j.urology.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer. 2007;109:518–27. doi: 10.1002/cncr.22433. [DOI] [PubMed] [Google Scholar]
- 34.Crawford ED, Black L, Eaddy M, Kruep EJ. A retrospective analysis illustrating the substantial clinical and economic burden of prostate cancer. Prostate Cancer Prostatic Dis. 2010;13:162–7. doi: 10.1038/pcan.2009.63. [DOI] [PubMed] [Google Scholar]
- 35.Fourcade RO, Benedict A, Black LK, Stokes ME, Alcaraz A, Castro R. Treatment costs of prostate cancer in the first year after diagnosis: a short-term cost of illness study for France, Germany, Italy, Spain and the UK. BJU Int. 2010;105:49–56. doi: 10.1111/j.1464-410X.2009.08716.x. [DOI] [PubMed] [Google Scholar]
- 36.Eldefrawy A, Katkoori D, Abramowitz M, Soloway MS, Manoharan M. Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol Oncol. 2013;31:576–80. doi: 10.1016/j.urolonc.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Molinier L, Castelli C, Bauvin E, et al. Cost study of the clinical management of prostate cancer in France: results on the basis of population-based data. Eur J Health Econ. 2011;12:363–71. doi: 10.1007/s10198-010-0250-6. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–24. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowrance WT, Eastham JA, Yee DS, et al. Costs of medical care after open or minimally invasive prostate cancer surgery: a population-based analysis. Cancer. 2012;118:3079–86. doi: 10.1002/cncr.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomaszewski JJ, Matchett JC, Davies BJ, Jackman SV, Hrebinko RL, Nelson JB. Comparative hospital cost-analysis of open and robotic-assisted radical prostatectomy. Urology. 2012;80:126–9. doi: 10.1016/j.urology.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Larg A, Moss JR. Cost-of-illness studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29:653–71. doi: 10.2165/11588380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Tarricone R. Cost-of-illness analysis. What room in health economics? Health Policy. 2006;77:51–63. doi: 10.1016/j.healthpol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Cooper GS, Yuan Z, Stange KC, Dennis LK, Amini SB, Rimm AA. The sensitivity of Medicare claims data for case ascertainment of six common cancers. Med Care. 1999;37:436–44. doi: 10.1097/00005650-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Bostwick DG. Gleason grading of prostatic needle biopsies. Correlation with grade in 316 matched prostatectomies. Am J Surg Pathol. 1994;18:796–803. doi: 10.1097/00000478-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Cookson MS, Fleshner NE, Soloway SM, Fair WR. Correlation between Gleason score of needle biopsy and radical prostatectomy specimen: accuracy and clinical implications. J Urol. 1997;157:559–62. doi: 10.1016/S0022-5347(01)65201-7. [DOI] [PubMed] [Google Scholar]
- 46.Spires SE, Cibull ML, Wood DP, Jr, Miller S, Spires SM, Banks ER. Gleason histologic grading in prostatic carcinoma. Correlation of 18-gauge core biopsy with prostatectomy. Arch Pathol Lab Med. 1994;118:705–8. [PubMed] [Google Scholar]
- 47.Steinberg DM, Sauvageot J, Piantadosi S, Epstein JI. Correlation of prostate needle biopsy and radical prostatectomy Gleason grade in academic and community settings. Am J Surg Pathol. 1997;21:566–76. doi: 10.1097/00000478-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Palmer S, Byford S, Raftery J. Economics notes: types of economic evaluation. BMJ. 1999;318:1349. doi: 10.1136/bmj.318.7194.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]



